The Nutrient Status of Plant Roots Reveals Competition Intensities in Rubber Agroforestry Systems
Abstract
1. Introduction
2. Materials and Methods
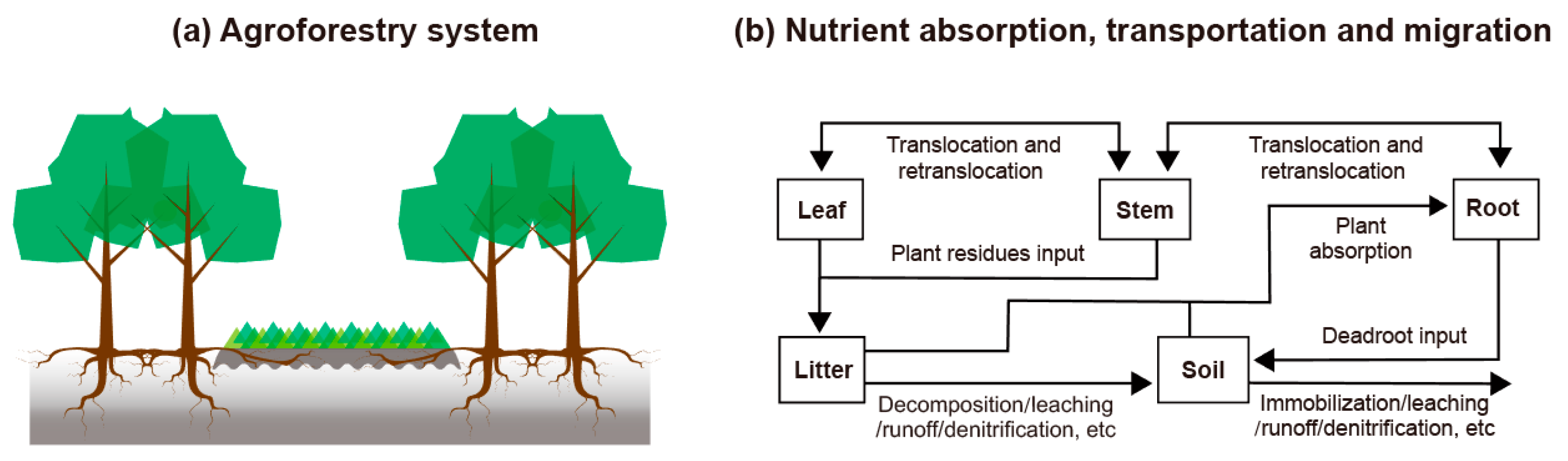
2.1. Description of Study Areas
2.2. Sampling and Measurement Methods
2.3. Calculations and Statistical Analysis
3. Results
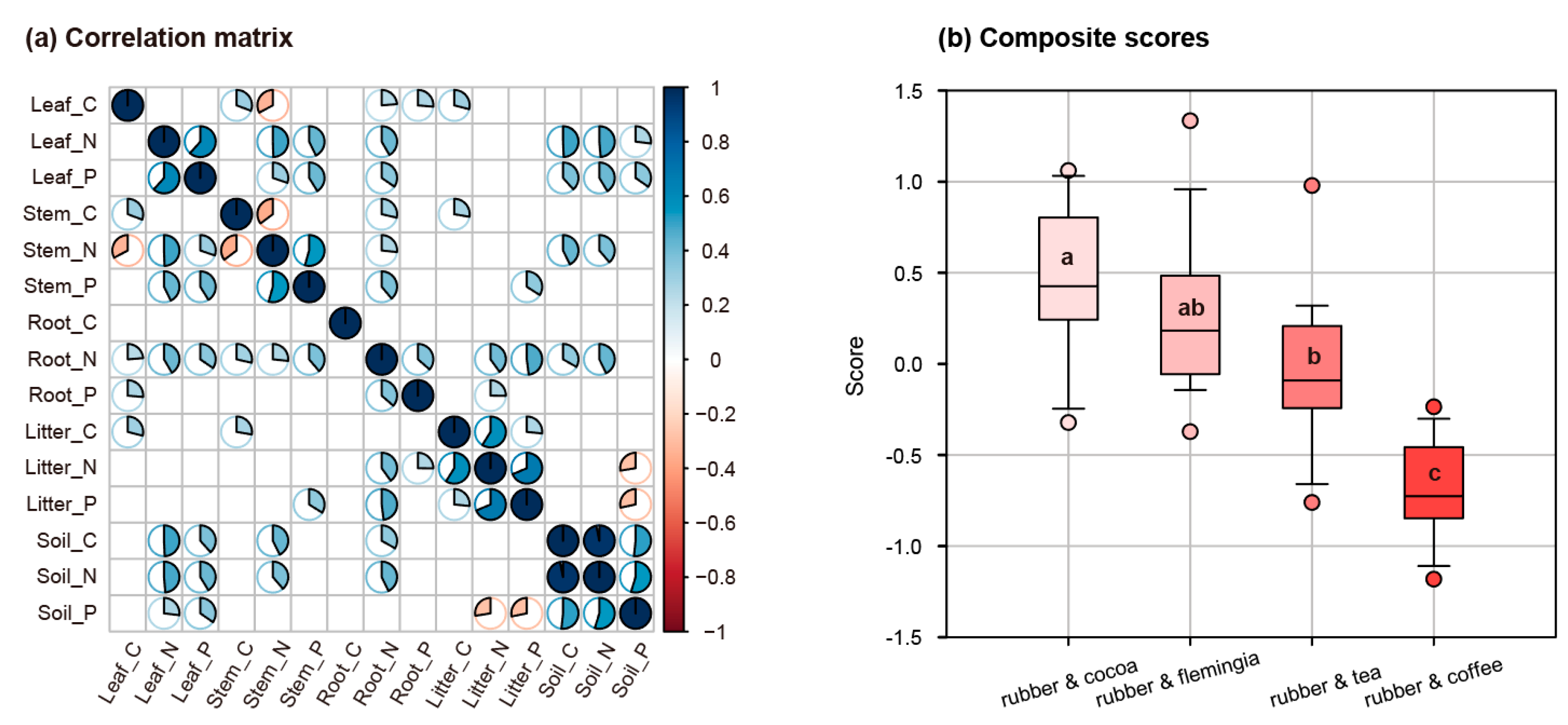
3.1. Competition Intensity of Each AFS
3.2. C, N and P Concentrations of Plant Organs, Litter and Soil
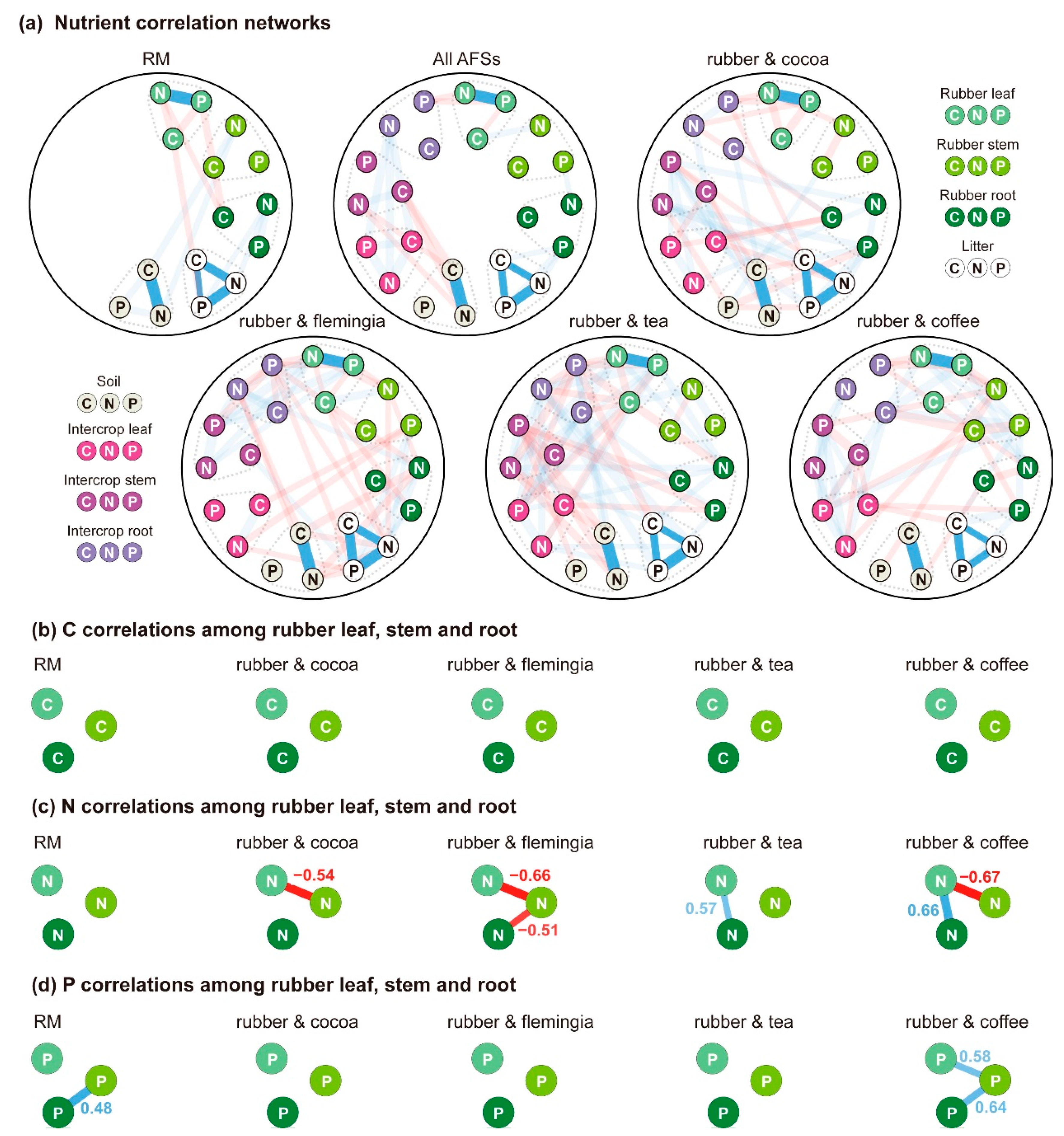
3.3. Correlations among Plant Organs, Litter and Soil
3.4. The Bayesian Network of the Competition Effects
4. Discussion
4.1. Competition Reduced the Nutrient Concentrations in the Soil and Litter
4.2. Competition Changed the Nutrient Allocations of Rubber Trees
4.3. The Linkage Effects of Nutrient Competition
4.4. The Mechanism of Nutrient Competition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Craine, J.M.; Dybzinski, R. Mechanisms of plant competition for nutrients, water and light. Funct. Ecol. 2013, 27, 833–840. [Google Scholar] [CrossRef]
- FAO; IAEA. Management of agroforestry systems for enhancing resource use efficiency and crop productivity. Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture, Vienna. 2008. Available online: https://www-pub.iaea.org/MTCD/Publications/PDF/te_1606_web.pdf (accessed on 7 October 2020).
- van Noordwijk, M.V.; Cadisch, G.; Ong, C.K. Below-Ground Interactions in Tropical Agroecosystems: Concepts and Models with Multiple Plant Components; Centre for Agriculture and Biosciences International: Wallingford, UK, 2004. [Google Scholar]
- Langenberger, G.; Cadisch, G.; Martin, K.; Min, S.; Waibel, H. Rubber intercropping: A viable concept for the 21st century? Agrofor. Syst. 2017, 91, 577–596. [Google Scholar] [CrossRef]
- Grace, J.B. A Clarification of the Debate Between Grime and Tilman. Funct. Ecol. 1991, 5, 583. [Google Scholar] [CrossRef]
- Craine, J.M. Reconciling plant strategy theories of Grime and Tilman. J. Ecol. 2005, 93, 1041–1052. [Google Scholar] [CrossRef]
- Grime, J.P. Plant strategy theories: A comment on Craine (2005). J. Ecol. 2007, 95, 227–230. [Google Scholar] [CrossRef]
- Tilman, D. Resource competition and plant traits: A response to Craine et al. 2005. J. Ecol. 2007, 95, 231–234. [Google Scholar] [CrossRef]
- Craine, J.M. Plant strategy theories: Replies to Grime and Tilman. J. Ecol. 2007, 95, 235–240. [Google Scholar] [CrossRef]
- Trinder, C.J.; Brooker, R.W.; Robinson, D. Plant ecology’s guilty little secret: Understanding the dynamics of plant competition. Funct. Ecol. 2013, 27, 918–929. [Google Scholar] [CrossRef]
- Zechmeister-Boltenstern, S.; Keiblinger, K.M.; Mooshammer, M.; Peñuelas, J.; Richter, A.; Sardans, J.; Wanek, W. The application of ecological stoichiometry to plant–microbial–soil organic matter transformations. Ecol. Monogr. 2015, 85, 133–155. [Google Scholar] [CrossRef]
- Feng, Y.Z. Man-Made Community; Yunnan Science and Technology Press: Kunming, China, 2007. [Google Scholar]
- DiTommaso, A.; Aarssen, L.W. Effect of nutrient level on competition intensity in the field for three coexisting grass species. J. Veg. Sci. 1991, 2, 513–522. [Google Scholar] [CrossRef]
- Mamolos, A.P.; Veresoglou, D.S. Patterns of root activity and responses of species to nutrients in vegetation of fertile alluvial soil. Plant Ecol. 2000, 148, 245–253. [Google Scholar] [CrossRef]
- Güsewell, S.; Koerselman, W.; Verhoeven, J.T.A. Biomass N:P ratios as indicators of nutrient limitation for plant populations in wetlands. Ecol. Appl. 2003, 13, 372–384. [Google Scholar] [CrossRef]
- Vrignon-Brenas, S.; Gay, F.; Ricard, S.; Snoeck, D.; Perron, T.; Mareschal, L.; Malagoli, P. Nutrient management of immature rubber plantations. A review. Agron. Sustain. Dev. 2019, 39, 11. [Google Scholar] [CrossRef]
- Hobbie, S.E. Effects of plant species on nutrient cycling. Trends Ecol. Evol. 1992, 7, 336–339. [Google Scholar] [CrossRef]
- Chapin, F.S., III; Matson, P.A.; Mooney, H.A. Principles of Terrestrial Ecosystem Ecology; Springer: New York, NY, USA, 2011. [Google Scholar]
- Berhongaray, G.; King, J.S.; Janssens, I.A.; Ceulemans, R. An optimized fine root sampling methodology balancing accuracy and time investment. Plant Soil 2013, 366, 351–361. [Google Scholar] [CrossRef]
- Lukac, M. Fine Root Turnover. In Measuring Roots; Mancuso, S., Ed.; Springer: New York, NY, USA, 2012; pp. 363–373. [Google Scholar]
- Rewald, B.; Meinen, C.; Trockenbrodt, M.; Ephrath, J.E.; Rachmilevitch, S. Root taxa identification in plant mixtures-current techniques and future challenges. Plant Soil 2012, 359, 165–182. [Google Scholar] [CrossRef]
- McCormack, M.L.; Dickie, I.A.; Eissenstat, D.M.; Fahey, T.J.; Fernandez, C.W.; Guo, D.; Helmisaari, H.; Hobbie, E.A.; Jackson, R.B.; Leppälammi-Kujansuu, J.; et al. Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytol. 2015, 207, 505–518. [Google Scholar] [CrossRef]
- Gordon, W.S.; Jackson, R.B. Nutrient Concentrations in Fine Roots. Ecology 2000, 81, 275–280. [Google Scholar] [CrossRef]
- Pregitzer, K.S.; DeForest, J.L.; Burton, A.J.; Allen, M.F.; Ruess, R.W.; Hendrick, R.L. Fine Root Architecture of Nine North American Trees. Ecol. Monogr. 2002, 72, 293–309. [Google Scholar] [CrossRef]
- Van Noordwijk, M.; Lawson, G.; Hairiah, K.; Wilson, J. Root distribution of trees and crops: Competition and/or complementarity. In Tree-Crop Interactions: Agroforestry in a Changing Climate; Ong, C.K., Black, C., Wilson, J., Eds.; Centre for Agriculture and Biosciences International: Wallingford, UK, 2015; pp. 221–257. [Google Scholar]
- Wu, J.; Liu, W.; Chen, C. Below-ground interspecific competition for water in a rubber agroforestry system may enhance water utilization in plants. Sci. Rep. 2016, 6, 19502. [Google Scholar] [CrossRef]
- Wu, J.; Liu, W.; Chen, C. Can intercropping with the world’s three major beverage plants help improve the water use of rubber trees? J. Appl. Ecol. 2016, 53, 1787–1799. [Google Scholar] [CrossRef]
- Grace, J.B. On the measurement of plant competition intensity. Ecology 1995, 76, 305–308. [Google Scholar] [CrossRef]
- Weigelt, A.; Jolliffe, P. Indices of plant competition. J. Ecol. 2003, 91, 707–720. [Google Scholar] [CrossRef]
- R Core Team. R: A language and environment for statistical computing. 2019. Available online: https://www.r-project.org/ (accessed on 7 November 2019).
- Revelle, W. Psych: Procedures for Personality and Psychological Research. 2020. Available online: https://cran.r-project.org/web/packages/psych/ (accessed on 8 January 2020).
- Wei, T.; Simko, V. R package "corrplot": Visualization of a Correlation Matrix (Version 0.84). 2017. Available online: https://cran.r-project.org/web/packages/corrplot/index.html (accessed on 16 October 2017).
- Hair, J.F.; Black, W.C.; Babin, B.J.; Anderson, R.E. Multivariate Data Analysis, 7th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2010. [Google Scholar]
- Epskamp, S.; Cramer, A.O.; Waldorp, L.J.; Schmittmann, V.D.; Borsboom, D. Qgraph: Network visualizations of relationships in psychometric data. J. Stat. Softw. 2012, 48, 1–18. [Google Scholar] [CrossRef]
- Druzdzel, M.J. SMILE: Structural Modeling, Inference, and Learning Engine and GeNIe: A development environment for graphical decision-theoretic models. In Proceedings of the 16th National Conference on Artificial Intelligence, Orlando, FL, USA, 18–22 July 1999; pp. 902–903. [Google Scholar]
- Chen, C.; Liu, W.; Jiang, X.; Wu, J. Effects of rubber-based agroforestry systems on soil aggregation and associated soil organic carbon: Implications for land use. Geoderma 2017, 299, 13–24. [Google Scholar] [CrossRef]
- Schulten, H.R.; Schnitzer, M. The chemistry of soil organic nitrogen: A review. Biol. Fertil. Soils 1997, 26, 1–15. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, W.; Wu, J.; Wang, P.; Liu, C.; Yuan, Z. Land degradation controlled and mitigated by rubber-based agroforestry systems through optimizing soil physical conditions and water supply mechanisms: A case study in Xishuangbanna, China. Land Degrad. Dev. 2017, 28, 2277–2289. [Google Scholar] [CrossRef]
- Huston, M.A.; Deangelis, D.L. Competition and coexistence: The effects of resource transport and supply rates. Am. Nat. 1994, 144, 954–977. [Google Scholar] [CrossRef]
- Hodge, A.; Robinson, D.; Griffiths, B.S.; Fitter, A.H. Why plants bother: Root proliferation results in increased nitrogen capture from an organic patch when two grasses compete. Plant Cell Environ. 2010, 22, 811–820. [Google Scholar] [CrossRef]
- Mengel, K.; Kirkby, E.A.; Kosegarten, H.; Appel, T. Principles of Plant Nutrition; Springer: New York, NY, USA, 2001. [Google Scholar]
- Tilman, D.; Wedin, D. Plant traits and resource reduction for five grasses growing on a nitrogen gradient. Ecology 1991, 72, 685–700. [Google Scholar] [CrossRef]
- Killingbeck, K.T. Nutrients in Senesced Leaves: Keys to the Search for Potential Resorption and Resorption Proficiency. Ecology 1996, 77, 1716–1727. [Google Scholar] [CrossRef]
- Bennett, E.; Roberts, J.A.; Wagstaff, C. Manipulating resource allocation in plants. J. Exp. Bot. 2012, 63, 3391–3400. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshan, P.M. Biology of Hevea Rubber; Centre for Agriculture and Biosciences International: Wallingford, UK, 2011. [Google Scholar]
- Gal, Y.; Ghahramani, Z. Dropout as a bayesian approximation: Representing model uncertainty in deep learning. Available online: http://proceedings.mlr.press/v48/gal16.pdf (accessed on 14 August 2020).
- Callaway, R.M. The detection of neighbors by plants. Trends Ecol. Evol. 2002, 17, 104–105. [Google Scholar] [CrossRef]
- Kong, C.H.; Zhang, S.Z.; Li, Y.H.; Xia, Z.C.; Yang, X.F.; Meiners, S.J.; Wang, P. Plant neighbor detection and allelochemical response are driven by root-secreted signaling chemicals. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cahill, J.F. Fertilization effects on interactions between above-and belowground competition in an old field. Ecology 1999, 80, 466–480. [Google Scholar] [CrossRef]
- Aschehoug, E.T.; Brooker, R.; Atwater, D.Z.; Maron, J.L.; Callaway, R.M. The mechanisms and consequences of interspecific competition among plants. Annu. Rev. Ecol. Evol. Syst. 2016, 47, 263–281. [Google Scholar] [CrossRef]
- Craine, J.M.; Fargione, J.; Sugita, S. Supply pre-emption, not concentration reduction, is the mechanism of competition for nutrients. New Phytol. 2005, 166, 933–940. [Google Scholar] [CrossRef]
- Tinker, P.B.; Nye, P.H. Solute Movement in the Rhizosphere; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Schwinning, S.; Kelly, C.K. Plant competition, temporal niches and implications for productivity and adaptability to climate change in water-limited environments. Funct. Ecol. 2013, 27, 886–897. [Google Scholar] [CrossRef]
- Hautier, Y.; Niklaus, P.A.; Hector, A. Competition for light causes plant biodiversity loss after eutrophication. Science 2009, 324, 636–638. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Zeng, H.; Zhao, F.; Chen, C.; Jiang, X.; Zhu, X.; Wang, P.; Wu, Z.; Liu, W. The Nutrient Status of Plant Roots Reveals Competition Intensities in Rubber Agroforestry Systems. Forests 2020, 11, 1163. https://doi.org/10.3390/f11111163
Wu J, Zeng H, Zhao F, Chen C, Jiang X, Zhu X, Wang P, Wu Z, Liu W. The Nutrient Status of Plant Roots Reveals Competition Intensities in Rubber Agroforestry Systems. Forests. 2020; 11(11):1163. https://doi.org/10.3390/f11111163
Chicago/Turabian StyleWu, Junen, Huanhuan Zeng, Fan Zhao, Chunfeng Chen, Xiaojin Jiang, Xiai Zhu, Pingyuan Wang, Zhixiang Wu, and Wenjie Liu. 2020. "The Nutrient Status of Plant Roots Reveals Competition Intensities in Rubber Agroforestry Systems" Forests 11, no. 11: 1163. https://doi.org/10.3390/f11111163
APA StyleWu, J., Zeng, H., Zhao, F., Chen, C., Jiang, X., Zhu, X., Wang, P., Wu, Z., & Liu, W. (2020). The Nutrient Status of Plant Roots Reveals Competition Intensities in Rubber Agroforestry Systems. Forests, 11(11), 1163. https://doi.org/10.3390/f11111163





