Carbon Cycling in the World’s Mangrove Ecosystems Revisited: Significance of Non-Steady State Diagenesis and Subsurface Linkages between the Forest Floor and the Coastal Ocean
Abstract
1. Introduction
2. Rates of Surface and Subsurface Soil CORG Mineralization
2.1. Respiration at the Soil Surface
2.2. Rates of DIC Production within the Forest Floor
2.3. Comparing Surface and Subsurface CO2 Fluxes
3. Composition of Mangrove Tidal Waters
4. Estimates of Tidal Export of DIC, DOC, POC, CH4, and Alkalinity
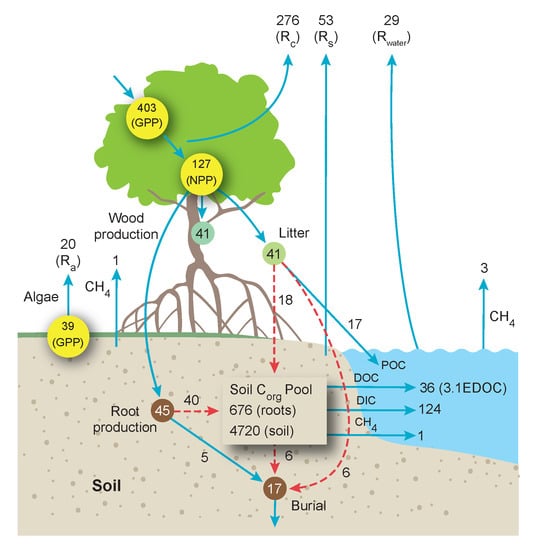
5. Carbon Flow through the World’s Mangrove Ecosystems and Contributions to the Coastal Ocean
6. Conclusions
Funding
Conflicts of Interest
References
- Donato, D.C.; Kauffman, J.B.; Murdiyarso, D.; Kurnianto, S.; Stidham, M.; Kanninen, M. Mangroves among the most carbon-rich forests in the tropics. Nat. Geosci. 2011, 4, 293–297. [Google Scholar] [CrossRef]
- Alongi, D.M. Carbon cycling and storage in mangrove forests. Annu. Rev. Mar. Sci. 2014, 6, 195–219. [Google Scholar] [CrossRef] [PubMed]
- Alongi, D.M. Global significance of mangrove blue carbon in climate change mitigation. Science 2020, 2, 57. [Google Scholar] [CrossRef]
- Lovelock, C.E.; Krauss, K.W.; Osland, M.J.; Reef, R.; Ball, M.C. The physiology of mangrove trees with changing climate. In Tropical Tree Physiology; Goldstein, G., Santiago, L.S., Eds.; Springer: Cham, Switzerland, 2016; pp. 149–179. [Google Scholar]
- Feller, I.C.; Lovelock, C.E.; Berger, U.; McKee, K.L.; Joye, S.B.; Ball, M.C. Biocomplexity in mangrove ecosystems. Annu. Rev. Mar. Sci. 2010, 2, 395–417. [Google Scholar] [CrossRef] [PubMed]
- Alongi, D.M. Mangroves. In Encyclopedia of Estuaries; Kennish, M., Ed.; Springer: Berlin, Germany, 2016; pp. 393–404. [Google Scholar]
- Adame, M.E.; Lovelock, C.E. Carbon and nutrient exchange of mangrove forests with the coastal ocean. Hydrobiologia 2011, 663, 23–50. [Google Scholar] [CrossRef]
- Mazda, Y.; Wolanski, E.; Ridd, P.V. The Role of Physical Processes in Mangrove Environments: Manual for the Preservation and Utilization of Mangrove Ecosystems; Terapub: Tokyo, Japan, 2007. [Google Scholar]
- Woodroffe, C.D.; Rogers, K.; McKee, K.L.; Lovelock, C.E.; Mendelssohn, I.A.; Saintilan, N. Mangrove sedimentation and response to relative sea-level rise. Annu. Rev. Mar. Sci. 2016, 8, 243–266. [Google Scholar] [CrossRef]
- Alongi, D.M. The Energetics of Mangrove Forests; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar]
- Alongi, D.M.; Mukhopadhyay, S.K. Contribution of mangroves to coastal carbon cycling in low latitude seas. Agric. For. Meteorol. 2015, 213, 266–272. [Google Scholar] [CrossRef]
- Jennerjahn, T.C.; Ittekot, V. Relevance of mangroves for the production and deposition of organic matter along tropical continental margins. Naturwissenschaften 2002, 89, 23–30. [Google Scholar] [CrossRef]
- Maher, D.T.; Call, M.; Santos, I.R.; Sanders, C.J. Beyond burial: Lateral exchange is a significant atmospheric carbon sink in mangrove forests. Biol. Lett. 2018, 14, 20180200. [Google Scholar] [CrossRef]
- Kristensen, E.; Connolly, R.M.; Otero, X.L.; Marchand, C.; Ferreira, T.O.; Rivera-Monroy, V.H. Biogeochemical cycles: Global approaches and perspectives. In Mangrove Ecosystems: A Global and Biogeographic Perspectives; Rivera-Monroy, V.H., Lee, S.Y., Kristensen, E., Twilley, R.R., Eds.; Springer: Cham, Switzerland, 2017; pp. 163–209. [Google Scholar]
- Alongi, D.M. Mangrove-microbe-soil relations. In Interactions between Macro- and Microorganisms in Marine Sediments; Kristensen, E., Haese, R.R., Kostka, J.K., Eds.; American Geophysical Union: Washington, DC, USA, 2005; pp. 85–103. [Google Scholar]
- Hopkinson, C.S.; Smith, E.M. Estuarine respiration: An overview of benthic, pelagic, and whole system respiration. In Respiration in Aquatic Ecosystems; del Georgio, P.A., Williams, P.J.l.B., Eds.; Oxford University Press: Oxford, UK, 2005; pp. 122–146. [Google Scholar]
- Castillo, J.A.A.; Apan, A.A.; Maraseni, T.N.; Salmo, S.G., III. Soil greenhouse gas fluxes in tropical mangrove forests and in land uses on deforested mangrove lands. Catena 2017, 159, 60–69. [Google Scholar] [CrossRef]
- Sea, M.A.; Garcias-Bonet, N.; Saderne, V.; Duarte, C.M. Carbon dioxide and methane emissions from Red Sea mangrove sediments. Biogeosci. Discuss. 2018. [CrossRef]
- Rodda, S.R.; Thumaty, K.C.; Jha, C.S.; Dadhwal, V.K. Seasonal variations of carbon dioxide, water vapor and energy fluxes in tropical Indian mangroves. Forests 2016, 7, 35. [Google Scholar] [CrossRef]
- Cameron, C.; Hutley, L.B.; Friess, D.A.; Brown, B. High greenhouse gas emissions mitigation benefits from mangrove rehabilitation in Sulawesi, Indonesia. Ecosyst. Serv. 2019, 40, 101035. [Google Scholar] [CrossRef]
- Lovelock, C.E.; Feller, I.C.; Reef, R.; Ruess, R.W. Variable effects of nutrient enrichment on soil respiration in mangrove forests. Plant Soil 2014, 379, 135–148. [Google Scholar] [CrossRef]
- Middelburg, J.J.; Nieuwenhuize, J.; Slim, F.J.; Ohowa, B. Sediment biogeochemistry in an East African mangrove forest (Gazi Bay, Kenya). Biogeochemistry 1996, 34, 133–155. [Google Scholar] [CrossRef]
- Alongi, D.M. The role of soft-bottom benthic communities in tropical mangrove and coral reef ecosystems. Rev. Aquat. Sci. 1989, 1, 243–280. [Google Scholar]
- Alongi, D.M. Zonation and seasonality of benthic primary production and community respiration in tropical mangrove forests. Oecologia 1994, 98, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Alongi, D.M.; Christoffersen, P.; Tirendi, F. The influence of forest type on microbial-nutrient relationships in tropical mangrove sediments. J. Exp. Mar. Biol. Ecol. 1993, 171, 201–223. [Google Scholar] [CrossRef]
- Blackburn, T.H.; Christensen, D.; Fanger, A.M.; Henriksen, K.; Iizumi, H.; Ivensen, N.; Limpsaichol, P. Mineralization processes in mangrove and seagrass sediments. In Ao Yon—A Mangrove in the Andaman Sea; Hylleberg, J., Ed.; Institute of Ecology and Genetics, University of Aarhus: Aarhus, Denmark, 1987; pp. 22–32. [Google Scholar]
- Dye, A.H. Oxygen consumption by sediments in a Southern African mangrove swamp. Estuar. Coast. Shelf Sci. 1983, 17, 473–478. [Google Scholar] [CrossRef]
- Kristensen, E.; Andersen, F.Ø.; Kofoed, L.H. Preliminary assessment of benthic metabolism in a south-east Asian mangrove swamp. Mar. Ecol. Prog. Ser. 1988, 48, 137–145. [Google Scholar] [CrossRef]
- Kristensen, E.; Holmer, M.; Bussarawit, N. Benthic metabolism and sulfate reduction in a south-east Asian mangrove swamp. Mar. Ecol. Prog. Ser. 1991, 73, 93–103. [Google Scholar] [CrossRef]
- Kristensen, E.; Devol, A.H.; Ahmed, S.I.; Saleem, M. Preliminary study of benthic metabolism and sulfate reduction in a mangrove swamp in the Indus delta, Pakistan. Mar. Ecol. Prog. Ser. 1992, 90, 287–297. [Google Scholar] [CrossRef]
- Kristensen, E.; King, G.M.; Holmer, M.; Banta, G.T.; Jensen, M.H.; Hansen, K.; Bussarawit, N. Sulfate reduction, acetate turnover and carbon metabolism in sediments of Ao Nam Bor mangrove, Phuket, Thailand. Mar. Ecol. Prog. Ser. 1994, 109, 245–255. [Google Scholar] [CrossRef]
- Nedwell, D.B.; Blackburn, T.H.; Wiebe, W.J. Dynamic nature of the turnover of organic carbon, nitrogen and sulphur in the sediments of a Jamaican mangrove forest. Mar. Ecol. Prog. Ser. 1994, 110, 223–231. [Google Scholar] [CrossRef]
- Padhy, S.R.; Bhattacharyya, P.; Dash, P.K.; Reddy, C.S.; Chakraborty, A.; Pathak, H. Seasonal fluctuation in the three mode of greenhouse gases emission in relation to soil labile carbon pools in degraded mangrove, Sundarbans, India. Sci. Total Environ. 2020, 705, 135909. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, E.; Flindt, M.R.; Ulomi, S.; Borges, A.V.; Abril, G.; Bouillon, S. Emission of CO2 and CH4 to the atmosphere by sediments and open waters in two Tanzanian mangrove forests. Mar. Ecol. Prog. Ser. 2008, 370, 53–67. [Google Scholar] [CrossRef]
- Leopold, A.; Marchand, C.; Deborde, J.; Chaduteau, C.; Allenbach, M. Influence of mangrove zonation on CO2 fluxes at the sediment-air interface (New Caledonia). Geoderma 2013, 202, 62–70. [Google Scholar] [CrossRef]
- Ouyang, X.; Lee, S.Y.; Connolly, R.M. Structural equation modelling reveals factors regulating surface sediment organic carbon content and CO2 efflux in a subtropical mangrove. Sci. Total Environ. 2017, 578, 513–522. [Google Scholar] [CrossRef]
- Lovelock, C.E. Soil respiration and belowground carbon allocation in mangrove forests. Ecosystems 2008, 11, 342–354. [Google Scholar] [CrossRef]
- Gillis, L.G.; Belshe, E.F.; Narayan, G.R. Deforested mangroves affect the potential for carbon linkages between connected ecosystems. Estuar. Coast. 2017, 40, 1207–1213. [Google Scholar] [CrossRef]
- Bulmer, R.H.; Schwendenmann, L.; Lohrer, A.M.; Lundquist, C.J. Sediment carbon and nutrient fluxes from cleared and intact temperate mangrove ecosystems and adjacent sandflats. Sci. Total Environ. 2017, 599, 1874–1884. [Google Scholar] [CrossRef] [PubMed]
- Grellier, S.; Janeau, J.-L.; Hoai, N.D.; Kim, C.N.T.; Phuong, Q.L.T.; Thu, T.P.T.; Tran-Thi, N.-T.; Marchand, C. Changes in soil characteristics and C dynamics after mangrove clearing (Vietnam). Sci. Total Environ. 2017, 593–594, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.-F.; Zhang, X.-M.; Li, P.-F.; Simon, M.; Shen, Z.-J.; Chen, J.; Gao, C.-H.; Zheng, H.-L. Examining soil carbon gas (CO2, CH4) emissions and the effect on functional microbial abundances in the Zhangjiang Estuary Mangrove Reserve. J. Coast. Res. 2020, 36, 54–62. [Google Scholar] [CrossRef]
- Alongi, D.M.; Ramanathan, A.L.; Kannan, L.; Tirendi, F.; Trott., L.A.; Bala Krishna Prasad, M. Influence of human-induced disturbance on benthic microbial metabolism in the Pichavaram mangroves, Vellar-Coleroon complex, India. Mar. Biol 2005, 147, 1033–1044. [Google Scholar] [CrossRef]
- Poungpam, S.; Komiyama, A.; Tanaka, A.; Sangtiean, T.; Maknual, C.; Kato, S.; Tanapermpool, P.; Patanaponpaiboon, P. Carbon dioxide emission through soil respiration in a secondary mangrove forest of eastern Thailand. J. Trop. Ecol. 2009, 25, 393–400. [Google Scholar] [CrossRef]
- Gocke, K.; Vitola, M.; Rojas, G. Oxygen consumption patterns in a mangrove swamp on the Pacific coast of Costa Rica. Rev. Biol. Trop. 1981, 29, 143–154. [Google Scholar]
- Golley, F.B.; Odum, H.T.; Wilson, R.F. The structure and metabolism of a Puerto Rican red mangrove forest in May. Ecology 1962, 43, 9–19. [Google Scholar] [CrossRef]
- Chen, G.; Chen, B.; Yu, D.; Tam, N.F.Y.; Ye, Y.; Chen, S. Soil greenhouse gas emissions reduce the contribution of mangrove plants to the atmospheric cooling effect. Environ. Res. Lett 2016, 11, 124019. [Google Scholar] [CrossRef]
- Chen, S.; Chmura, G.L.; Wang, Y.; Yu, D.; Ou, D.; Chen, B.; Ye, Y.; Chen, G. Benthic microalgae offset the sediment carbon dioxide emission in subtropical mangrove in cold seasons. Limnol. Oceanogr. 2019, 64, 11116. [Google Scholar] [CrossRef]
- Chen, G.C.; Ulumuddin, Y.I.; Pramudji, S.; Chen, S.Y.; Chen, B.; Ye, Y.; Ou, D.Y.; Ma, Z.Y.; Huang, H.; Wang, J.K. Rich soil carbon and nitrogen but low atmospheric greenhouse gas fluxes from North Sulawesi mangrove swamps in Indonesia. Sci. Total Environ. 2014, 487, 91–96. [Google Scholar] [CrossRef]
- Holmer, M.; Andersen, F.Ø.; Kristensen, E.; Thongham, N. Transformation in the Bangrong mangrove forest-seagrass bed system, Thailand: Seasonal and spatial variations in benthic metabolism and sulfur biogeochemistry. Aquat. Microb. Ecol. 1999, 20, 203–212. [Google Scholar] [CrossRef]
- Alongi, D.M.; Sasekumar, A.; Tirendi, F.; Dixon, P. The influence of stand age on benthic decomposition and recycling of organic matter in managed mangrove forests in Malaysia. J. Exp. Mar. Biol. Ecol. 1998, 225, 197–218. [Google Scholar] [CrossRef]
- Lovelock, C.E.; Ruess, R.W.; Feller, I.C. CO2 efflux from cleared mangrove peat. PLoS ONE 2011, 6, e21279. [Google Scholar] [CrossRef] [PubMed]
- Ray, R.; Chowdhury, C.; Majumder, N.; Dutta, M.K.; Mukhopadhyay, S.K.; Jana, T.K. Improved model calculation of atmospheric CO2 increment in affecting carbon stock of tropical mangrove forest. Tellus B Chem. Phys. Meteorol. 2013, 65, 18981. [Google Scholar] [CrossRef]
- Kristensen, E.; Mangion, P.; Tang, M.; Flindt, M.R.; Holmer, M.; Ulomi, S. Microbial carbon oxidation and pathways in sediments in two Tanzanian mangrove forests. Biogeochemistry 2011, 103, 143–158. [Google Scholar] [CrossRef]
- Alongi, D.M.; de Carvalho, N.A.; Amaral, A.L.; da Costa, A.; Trott, L.A.; Tirendi, F. Uncoupled surface and below-ground soil respiration in mangroves: Implications for estimates of dissolved inorganic carbon export. Biogeochemistry 2012, 109, 151–162. [Google Scholar] [CrossRef]
- Kristensen, E.; Andersen, F.Ø.; Holmboe, N.; Holmer, M.; Thongtham, N. Carbon and nitrogen mineralization in sediments of the Bangrong mangrove area, Phuket, Thailand. Aquat. Microb. Ecol. 2000, 22, 199–213. [Google Scholar] [CrossRef]
- Alongi, D.M.; Sasekumar, A.; Chong, V.C.; Pfitzner, J.; Trott., L.A.; Dixon, P.; Brunskill, G.J. Sediment accumulation and organic material flux in a managed mangrove ecosystem: Estimates of land-ocean-atmosphere exchange in peninsular Malaysia. Mar. Geol. 2004, 208, 383–402. [Google Scholar] [CrossRef]
- Alongi, D.M.; Pfitzner, J.; Trott, L.A.; Tirendi, F.; Dixon, P.; Klumpp, D.W. Rapid sediment accumulation and microbial mineralization in forests of the mangrove Kandelia candel in the Jiulongjiang estuary, China. Estuar. Coast. Shelf Sci. 2005, 63, 605–618. [Google Scholar] [CrossRef]
- Alongi, D.M. Carbon dynamics in Southeast Asian mangroves. (Unpublished work).
- Alongi, D.M.; Trott, L.A.; Rachmansyah, R.; Tirendi, F.; McKinnon, A.D.; Undu, M.C. Growth and development of mangrove forests overlying smothered coral reefs, Sulawesi and Sumatra, Indonesia. Mar. Ecol. Prog. Ser. 2008, 370, 97–109. [Google Scholar] [CrossRef][Green Version]
- Alongi, D.M.; Tirendi, F.; Dixon, P.; Trott, L.A.; Brunskill, G.J. Mineralization of organic matter in intertidal sediments of a tropical semi-enclosed delta. Estuar. Coast. Shelf Sci. 1999, 48, 451–467. [Google Scholar] [CrossRef]
- Alongi, D.M.; Tirendi, F.; Clough, B.F. Below-ground decomposition of organic matter in forests of the mangroves Rhizophora stylosa and Avicennia marina along the arid coast of Western Australia. Aquat. Bot. 2000, 68, 97–122. [Google Scholar] [CrossRef]
- Alongi, D.M.; Tirendi, F.; Trott, L.A.; Xuan, T.T. Benthic decomposition rates and pathways in plantations of the mangrove Rhizophora apiculata in the Mekong delta, Vietnam. Mar. Ecol. Prog. Ser. 2000, 194, 87–101. [Google Scholar] [CrossRef]
- Alongi, D.M.; Wattayakorn, G.; Pfitzner, J.; Tirendi, F.; Zagorskis, I.; Brunskill, G.J.; Davidson, A.; Clough, B.F. Organic carbon accumulation and metabolic pathways in sediments of mangrove forests in southern Thailand. Mar. Geol. 2001, 179, 85–103. [Google Scholar] [CrossRef]
- Chu, S.N.; Wang, Z.A.; Gonneea, M.E.; Kroeger, K.D.; Ganju, N.K. Deciphering the dynamics of inorganic carbon export from intertidal salt marshes using high-frequency measurements. Mar. Chem. 2018, 206, 7–18. [Google Scholar] [CrossRef]
- Tobias, C.; Neubauer, S.C. Salt marsh biogeochemistry—An overview. In Coastal Wetlands; Perillo, G.M.E., Wolanski, E., Cahoon, D.R., Hopkinson, C.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 539–596. [Google Scholar]
- Troxler, T.G.; Barr, J.G.; Fuentes, J.D.; Engel, V.; Anderson, G.; Sanchez, C.; Lagomasino, D.; Price, R.; Davis, S.E. Component-specific dynamics of riverine mangrove CO2 efflux in the Florida Everglades. Agric. For. Meteorol. 2015, 213, 273–282. [Google Scholar] [CrossRef]
- Ralison, O.H.; Borges, A.V.; Dehairs, F.; Middelburg, J.J.; Bouillon, S. Carbon biogeochemistry of the Betsiboka estuary (north-western Madagascar). Org. Geochem. 2008, 39, 1649–1658. [Google Scholar] [CrossRef]
- Miyajima, T.; Tsuboi, Y.; Tanaka, Y.; Koike, I. Export of inorganic carbon from two Southeast Asian mangrove forests to adjacent estuaries as estimated by the stable isotope composition of dissolved inorganic carbon. J. Geophys. Res. 2009, 114, G01024. [Google Scholar] [CrossRef]
- Rosentreter, J.A.; Maher, D.T.; Erler, D.V.; Murray, R.; Eyre, B.D. Seasonal and temporal CO2 dynamics in three tropical mangrove creeks—A revision of global CO2 emissions. Geochim. Cosmochim. Acta 2018, 222, 729–745. [Google Scholar] [CrossRef]
- Call, M.; Santos, I.R.; Dittmar, T.; de Rezende, C.E.; Asp, N.E.; Maher, D.T. High pore-water derived CO2 and CH4 emissions from a macro-tidal mangrove creek in the Amazon region. Geochim. Cosmochim. Acta 2019, 247, 106–120. [Google Scholar] [CrossRef]
- Macklin, P.A.; Suryaputra, I.G.N.A.; Maher, D.T.; Murdiyarso, D.; Santos, I.R. Drivers of CO2 along a mangrove-seagrass transect in a tropical bay: Delayed groundwater seepage and seagrass uptake. Cont. Shelf Res. 2019, 172, 57–67. [Google Scholar] [CrossRef]
- Ho, D.T.; Ferrόn, S.; Engel, V.C.; Larsen, L.G.; Barr, J.G. Air-water gas exchange and CO2 flux in a mangrove-dominated estuary. Geophys. Res. Lett. 2014, 41, 108–113. [Google Scholar] [CrossRef]
- Koné, Y.J.-M.; Borges, A.V. Dissolved inorganic carbon dynamics in the waters surrounding forested mangroves of the Ca Mau Province (Vietnam). Estuar. Coast. Shelf Sci. 2008, 77, 409–421. [Google Scholar] [CrossRef]
- Maher, D.T.; Cowley, K.; Santos, I.R.; Macklin, P.; Eyre, B.D. Methane and carbon dioxide dynamics in a subtropical estuary over a diel cycle: Insights from automated in situ radioactive and stable isotope measurements. Mar. Chem. 2015, 168, 69–79. [Google Scholar] [CrossRef]
- Taillardat, P.; Ziegler, A.D.; Friess, D.A.; Widory, D.R.; Van Vinh, T.; David, F.; Nguyên, T.-N.; Marchand, C. Carbon dynamics and inconstant porewater input in a mangrove tidal creek over contrasting seasons and tidal amplitudes. Geochim. Cosmochim. Acta 2018, 237, 32–48. [Google Scholar] [CrossRef]
- Atkins, M.L.; Santos, I.R.; Ruiz-Halpern, S.; Maher, D.T. Carbon dioxide dynamics driven by groundwater discharge in a coastal floodplain creek. J. Hydrol. 2013, 493, 30–42. [Google Scholar] [CrossRef]
- David, F.; Meziane, T.; Tran-Thi, N.-T.; Van, V.T.; Thanh-Nho, N.; Taillardat, P.; Marchand, C. Carbon biogeochemistry and CO2 emissions in a human impacted and mangrove dominated tropical estuary (Can Gio, Vietnam). Biogeochemistry 2018, 138, 261–275. [Google Scholar] [CrossRef]
- Bartlett, D.S.; Bartlett, K.B.; Hartman, J.M.; Harriss, R.C.; Sebacher, D.I.; Pelletier-Travis, R.; Dow, D.D.; Brannon, D.P. Methane emissions from the Florida Everglades: Patterns of variability in a regional wetland ecosystem. Glob. Biogeochem. Cycles 1989, 3, 363–374. [Google Scholar] [CrossRef]
- Dutta, M.K.; Kumar, S.; Mukherjee, R.; Sharma, N.; Acharya, A.; Sanyal, P.; Bhusan, R.; Mukhopadhyay, S.K. Diurnal carbon dynamics in a mangrove-dominated tropical estuary (Sundarbans, India). Estuar. Coast. Shelf Sci. 2019, 229, 106426. [Google Scholar] [CrossRef]
- Borges, A.V.; Djenidi, S.; Lacroix, G.; Théate, J.; Delille, B.; Frankignoulle, M. Atmospheric CO2 flux from mangrove surrounding waters. Geophys. Res. Lett. 2003, 30, 1558. [Google Scholar] [CrossRef]
- Ghosh, S.; Jana, T.K.; Singh, B.N.; Choudhury, A. Comparative study of carbon dioxide system in virgin and reclaimed mangrove waters of Sundarbans during freshnet. Mahasagar. Bull. Nat. Instit. Oceanogr. 1987, 20, 155–161. [Google Scholar]
- Ovalle, A.R.C.; Rezende, C.E.; Lacerda, L.D.; Silva, C.A.R. Factors affecting the hydrochemistry of a mangrove creek, Sepetiba Bay, Brazil. Estuar. Coast. Shelf Sci. 1990, 31, 639–650. [Google Scholar] [CrossRef]
- Bouillon, S.; Frankignoulle, M.; Dehairs, F.; Velimirov, B.; Eiler, A.; Abril, G.; Etcheber, H.; Borges, A.V. Inorganic and organic carbon biogeochemistry in the Gautami Godavari estuary (Andhra Pradesh, India) during pre-monsoon: The local impact of extensive mangrove forests. Glob. Biogeochem. Cycles 2003, 17, 1114. [Google Scholar] [CrossRef]
- Call, M.; Maher, D.T.; Santos, I.R.; Ruiz-Halpern, S.; Mangion, P.; Sanders, C.J.; Erler, D.V.; Oakes, J.M.; Rosentreter, R.; Eyre, B.D. Spatial and temporal variability of carbon dioxide and methane fluxes over semi-diurnal and spring-neap-spring timescales in a mangrove creek. Geochim. Cosmochim. Acta 2015, 150, 211–225. [Google Scholar] [CrossRef]
- Biswas, H.; Mukhopadhyay, S.K.; De, T.K. Biogenic controls in the air-water carbon dioxide exchange in the Sundarbans mangrove environment, northeast coast of Bay of Bengal, India. Limnol. Oceanogr. 2004, 49, 95–101. [Google Scholar] [CrossRef]
- Biswas, H.; Mukopadhyay, S.K.; Jana, T.K. Spatial and temporal patterns of methane dynamics in the tropical mangrove dominated estuary, NE coast of Bay of Bengal, India. J. Mar. Syst. 2007, 68, 55–64. [Google Scholar] [CrossRef]
- Bouillon, S.; Middelburg, J.J.; Dehairs, F.; Borges, A.V.; Abril, G.; Flindt, M.R.; Ulomi, S.; Kristensen, E. Importance of intertidal sediment processes and porewater exchange on the water column biogeochemistry in a pristine mangrove creek (Ras Dege, Tanzania). Biogeosciences 2007, 4, 311–322. [Google Scholar] [CrossRef]
- Bouillon, S.; Dehairs, F.; Velimirov, B.; Abril, G.; Borges, A.V. Dynamics of organic and inorganic carbon across contiguous mangrove and seagrass systems (Gazi Bay, Kenya). J. Geophys. Res. Biogeosci. 2007, 112, G02018. [Google Scholar] [CrossRef]
- Linto, N.; Barnes, J.; Ramachandran, R.; Divia, J.; Ramachandran, P.; Upstill-Goddard, R.C. Carbon dioxide and methane emissions from mangrove-associated waters of the Andaman Islands, Bay of Bengal. Estuar. Coast. 2014, 37, 381–398. [Google Scholar] [CrossRef]
- Millero, F.J.; Hiscock, W.T.; Huang, F.; Roche, M.; Zhang, J.Z. Seasonal variation of the carbonate system in Florida Bay. Bull. Mar. Sci. 2001, 68, 101–123. [Google Scholar]
- Ramesh, R.; Purvaja, R.; Neetha, V.; Divia, J.; Barnes, J.; Upstill-Goddard, R.C. CO2 and CH4 emissions from Indian mangroves and its surrounding waters. In Greenhouse Gas and Carbon Balances in Mangrove Coastal Ecosystems; Tateda, Y., Upstill-Goddard, R.C., Goreau, T., Alongi, D.M., Kristensen, E., Wattayakorn, G., Eds.; Gendai Tosho: Kanagawa, Japan, 2007; pp. 139–151. [Google Scholar]
- Borges, A.V.; Abril, G.; Bouillon, S. Carbon dynamics and CO2 and CH4 outgassing in the Mekong delta. Biogeosciences 2018, 15, 1093–1114. [Google Scholar] [CrossRef]
- Zablocki, J.A.; Andersson, A.J.; Bates, N.R. Diel aquatic CO2 system dynamics of a Bermudian mangrove environment. Aquat. Geochem. 2011, 11, 841. [Google Scholar] [CrossRef]
- Maher, D.T.; Santos, I.R.; Golsby-Smith, L.; Gleeson, J.; Eyre, B.D. Groundwater-derived dissolved inorganic and organic carbon exports from a mangrove tidal creek: The missing carbon sink? Limnol. Oceanogr. 2013, 58, 475–488. [Google Scholar] [CrossRef]
- Santos, I.R.; Maher, D.T.; Larkin, R.; Webb, J.R.; Sanders, C.J. Carbon outwelling and outgassing vs. burial in an estuarine tidal creek surrounded by mangrove and saltmarsh wetlands. Limnol. Oceanogr. 2019, 64, 996–1013. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, F.; lao, Y.; Wang, X.; Du, J.; Santos, I.R. Submarine groundwater discharge-derived carbon fluxes in mangroves: An important component of blue carbon budgets? J. Geophys. Res. Ocean. 2018, 123, 6962–6979. [Google Scholar] [CrossRef]
- Sippo, J.Z.; Maher, D.T.; Schult, K.G.; Sanders, C.J.; McMahon, A.; Tucker, J.; Santos, I.R. Carbon outwelling across the shelf following a massive mangrove dieback in Australia: Insights from radium isotopes. Geochim. Cosmochim. Acta 2019, 253, 142–158. [Google Scholar] [CrossRef]
- Taillardat, P.; Willemsen, P.; Marchand, C.; Friess, D.A.; Widory, D.; Baudron, P.; Truong, V.V.; Nguyên, T.-N.; Ziegler, A.D. Assessing the contribution of porewater discharge in carbon export and CO2 evasion in a mangrove tidal creek (Can Gio, Vietnam). J. Hydrol. 2018, 563, 303–318. [Google Scholar] [CrossRef]
- Sippo, J.Z.; Maher, D.T.; Tait, D.R.; Holloway, C.; Santos, I.R. Are mangroves drivers or buffers of coastal acidification? Insights from alkalinity and dissolved inorganic carbon export estimates across a latitudinal gradient. Glob. Biogeochem. Cyles 2016, 30, 753–766. [Google Scholar] [CrossRef]
- Call, M.; Sanders, C.J.; Macklin, P.A.; Santos, I.R.; Maher, D.T. Carbon outwelling and emissions from two contrasting mangrove creeks during the monsoon storm season in Palau, Micronesia. Estuar. Coast. Shelf Sci. 2019, 218, 340–348. [Google Scholar] [CrossRef]
- Ray, R.; Bsum, A.; Rixen, T.; Gleixner, G.; Jana, T.K. Exportation of dissolved (inorganic and organic) and particulate carbon from mangroves and its implication to the carbon budget in the Indian Sundarbans. Sci. Total Environ. 2018, 621, 535–547. [Google Scholar] [CrossRef]
- Sadat-Noori, M.; Maher, D.T.; Santos, I.R. Groundwater discharge as a source of dissolved carbon and greenhouse gases in a subtropical estuary. Estuar. Coast. 2016, 39, 639–656. [Google Scholar] [CrossRef]
- Ho, D.T.; Ferrόn, S.; Engel, V.C.; Anderson, W.T.; Swart, P.K.; Price, R.M.; Barbero, L. Dissolved carbon biogeochemistry and export in mangrove-dominated rivers of the Florida Everglades. Biogeosciences 2017, 14, 2543–2559. [Google Scholar] [CrossRef]
- Bouillon, S.; Borges, A.V.; Castañeda-Moya, E.; Diele, K.; Dittmat, T.; Duke, N.C.; Kristensen, E.; Lee, S.Y.; Marchand, C.; Middelburg, J.J.; et al. Mangrove production and carbon sinks: A revision of global budget estimates. Glob. Biogeochem. Cycles 2008, 22, GB2013. [Google Scholar] [CrossRef]
- Beithaupt, J.L.; Smoak, J.M.; Smith, T.J., III; Sanders, C.J.; Hoare, A. Organic carbon burial rates in mangrove sediments: Strengthening the global budget. Glob. Biogeochem. Cycles 2012, 26, GB3011. [Google Scholar] [CrossRef]
- Ouyang, X.; Lee, S.Y.; Connolly, R.M. The role of root decomposition in global mangrove and saltmarsh carbon budgets. Earth-Sci. Rev. 2017, 166, 53–63. [Google Scholar] [CrossRef]
- Twilley, R.R.; Castañeda-Moya, E.; Rivera-Monroy, V.H.; Rovai, A. Productivity and carbon dynamics in mangrove wetlands. In Mangrove Ecosystems: A Global and Biogeographic Perspectives; Rivera-Monroy, V.H., Lee, S.Y., Kristensen, E., Twilley, R.R., Eds.; Springer: Cham, Switzerland, 2017; pp. 113–162. [Google Scholar]
- Barr, J.G.; Engel, V.; Fuentes, J.D.; Fuller, D.O.; Kwon, H. Modeling light-use efficiency in a subtropical mangrove forest equipped with CO2 eddy covariance. Biogeosciences 2013, 10, 2145–2158. [Google Scholar] [CrossRef]
- Chen, H.; Lu, W.; Yan, G.; Yang, S.; Lin, G. Typhoons exert significant but differential impacts on net ecosystem carbon exchange of subtropical mangrove forests in China. Biogeosciences 2014, 11, 5323–5333. [Google Scholar] [CrossRef]
- Lovelock, C.E.; Simpson, L.T.; Duckett, L.J.; Feller, I.C. Carbon budgets for Caribbean mangrove forests of varying structure and with phosphorus enrichment. Forests 2015, 6, 3528–3546. [Google Scholar] [CrossRef]
- Umayangani, M.A.D.; Perera, K.A.R.S. Contribution of vegetation structure on carbon assimilation capacity of mangrove ecosystem: A case study from Negombo estuary, Sri Lanka. Int. J. Mar. Sci. 2017, 7, 439–446. [Google Scholar] [CrossRef]
- Cui, X.; Liang, J.; Lu, W.; Chen, H.; Liu, F.; Lin, G.; Xu, F.; Luo, Y.; Lin, G. Stronger ecosystem carbon sequestration potential of mangrove wetlands with respect to terrestrial forests in subtropical China. Agric. For. Meteorol. 2018, 249, 71–80. [Google Scholar] [CrossRef]
- Zhu, X.; Song, L.; Weng, Q.; Huang, G. Linking in situ photochemical reflectance index measurements with mangrove carbon dynamics in a subtropical coastal wetland. J. Geophys. Res. Biogeosci. 2019, 124. [Google Scholar] [CrossRef]
- Gnanamoorthy, P.; Selvam, V.; Burman, P.K.D.; Chakraborty, S.; Karipot, A.; Nagarajan, R.; Ramasubramanian, R.; Song, Q.; Zhang, Y.; Grace, J. Seasonal variations of net ecosystem (CO2) exchange in the Indian tropical mangrove forest of Pichavaram. Estuar. Coast. Shelf Sci. 2020, 243, 106828. [Google Scholar] [CrossRef]
- Hamilton, S.E.; Casey, D. Creation of a high spatio-temporal resolution global database of continuous mangrove forest cover for the 21st century. Glob. Ecol. Biogeogr. 2016, 25, 729–738. [Google Scholar] [CrossRef]
- Marchand, C.; Lallier-Vergès, E.; Disnar, J.R.; Kéravis, D. Organic carbon sources and transformations in mangrove sediments: A Rock-Eval pyrolysis approach. Org. Geochem. 2008, 39, 408–421. [Google Scholar] [CrossRef]
- Marchand, C.; Marchand, J.; Disnar, J.R.; Lallier-Vergès, E.; Lottier, N. Early diagenesis of carbohydrates and lignin in mangrove sediments subject to variable redox conditions (French Guiana). Geochim. Cosmochim. Acta 2005, 69, 131–142. [Google Scholar] [CrossRef]
- Kristensen, E.; Bouillon, S.; Dittmar, T.; Marchand, C. Organic carbon dynamics in mangrove ecosystems: A review. Aquat. Bot. 2008, 89, 201–219. [Google Scholar] [CrossRef]
- Robertson, A.I.; Daniel, P.A. Decomposition and the annual flux of detritus from fallen timber in tropical mangrove forests. Limnol. Oceanogr. 1989, 34, 640–646. [Google Scholar] [CrossRef]
- Maher, D.T.; Santos, I.R.; Schulz, K.G.; Call, M.; Jacobsen, G.E.; Sanders, C.J. Blue carbon oxidation revealed by radiogenic and stable isotopes in a mangrove system. Geophys. Res. Lett. 2017, 44, 4889–4896. [Google Scholar] [CrossRef]
- Huang, T.-H.; Fu, Y.-H.; Pan, P.Y.; Chen, C.-T.A. Fluvial carbon fluxes in tropical rivers. Curr. Opin. Environ. Sustain. 2012, 4, 162–169. [Google Scholar] [CrossRef]
- Chen, C.-T.A.; Huang, T.-H.; Chen, Y.-C.; Bai, Y.; He, X.; Kang, Y. Air-sea exchanges of CO2 in the world’s coastal seas. Biogeosciences 2013, 10, 6509–6544. [Google Scholar] [CrossRef]
- Bauer, J.E.; Cai, W.-J.; Raymond, P.A.; Bianchi, T.S.; Hopkinson, C.S.; Regnier, P.A.G. The changing carbon cycle of the coastal ocean. Nature 2013, 504, 61–70. [Google Scholar] [CrossRef] [PubMed]





| Parameters | Mean | Median | Number of Measurements |
|---|---|---|---|
| Total alkalinity | 1317.6 ± 153.3 | 1211.0 | 41 |
| pCO2 | 3202.8 ± 306.6 | 1233.0 | 219 |
| CO2 | 1446.3 ± 162.3 | 1270.5 | 44 |
| DIC | 1838.0 ± 71.4 | 2024.0 | 83 |
| CH4 | 244.7 ± 49.1 | 135.5 | 60 |
| CO2 fluxes | 78.9 ± 8.0 | 50.0 | 143 |
| CH4 fluxes | 530.4 ± 78.5 | 260.1 | 134 |
| Location | DIC Export | Alkalinity Export | DOC Export | CH4 Export | Reference |
|---|---|---|---|---|---|
| Southern Moreton Bay, Queensland, Australia | 186 ± 37 205 ± 41 247 ± 49 | 69 ± 14 88 ±18 132 ± 26 | 72 ±15 65 ±13 40 ± 8 | 0.014 ± 0.0023 0.0056 ± 0.0011 0.017 ± 0.0034 | [94] |
| Evans River estuary, New South Wales, Australia | 125 ± 82 559 ± 292 165 ± 102 177 ± 100 | 51 ± 34 321 ± 172 59 ± 37 67 ± 39 | 136 ± 89 562 ± 293 202 ± 125 233 ± 132 | 0.6 ± 0.4 2.3 ± 1.4 0.8 ± 0.6 0.9 ± 0.6 | [95] |
| Maowei Sea, Guangxi Province, south China | 700 ± 820 250 ± 240 | ND | 310 ± 300 250 ± 230 | ND | [96] |
| Eastern Gulf of Carpentaria, Australia | 440.4 ± 232.6 146.2 ± 125.1 610.9 ± 342.0 774.3 ± 223.5 730.0 ± 416.5 | 365.5 ± 177.2 896.4 ± 459.3 564.0 ± 383.5 726.9 ± 261.0 679.2 ± 367.3 | 846.1 ± 936.5 176.2 ± 529.9 2110.5 ± 1762.8 294.2 ± 1276.2 153.4 ± 626.7 | ND | [97] |
| Can Gio, Saigon River, southern Vietnam | 351.6 ± 33.9 480.0 ± 35.6 677.7 ± 79.0 612.2 ± 71.4 338.9 ± 39.5 | ND | 20.6 ± 1.9 31.0 ± 2.3 67.7 ± 7.9 60.0 ± 3.7 33.3 ± 2.0 | ND | [98] |
| Six mangrove estuaries, north, northeast, and southeast Australia | 85 22 −97 83 77 −3 | 116 21 81 12 116 −1 | 7.5 ± 0.2 3.3 ± 0.4 5.1 ± 0.5 4.2 ± 0.2 | ND | [99] |
| Badeldaob Island, Palau | 79 ± 28 10 ± 4 | 48 ± 17 2 ± 1 | 35 ± 12 8 ± 3 | ND | [100] |
| Indian Sundarbans | 202.3 | ND | 162.1 | ND | [101] |
| Korogoro Creek, New South Wales, Australia | 687 | 23 | 294.8 | ND | [102] |
| Harney and Shark Rivers, Florida Everglades | 574.5 1031.5 ± 291.3 | ND | ND | ND | [103] |
| Mean ± 1SE | 339.6 ± 51.5 | 211.5 ± 58.2 | 229.1 ± 78 | 0.66 ± 0.29 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alongi, D.M. Carbon Cycling in the World’s Mangrove Ecosystems Revisited: Significance of Non-Steady State Diagenesis and Subsurface Linkages between the Forest Floor and the Coastal Ocean. Forests 2020, 11, 977. https://doi.org/10.3390/f11090977
Alongi DM. Carbon Cycling in the World’s Mangrove Ecosystems Revisited: Significance of Non-Steady State Diagenesis and Subsurface Linkages between the Forest Floor and the Coastal Ocean. Forests. 2020; 11(9):977. https://doi.org/10.3390/f11090977
Chicago/Turabian StyleAlongi, Daniel M. 2020. "Carbon Cycling in the World’s Mangrove Ecosystems Revisited: Significance of Non-Steady State Diagenesis and Subsurface Linkages between the Forest Floor and the Coastal Ocean" Forests 11, no. 9: 977. https://doi.org/10.3390/f11090977
APA StyleAlongi, D. M. (2020). Carbon Cycling in the World’s Mangrove Ecosystems Revisited: Significance of Non-Steady State Diagenesis and Subsurface Linkages between the Forest Floor and the Coastal Ocean. Forests, 11(9), 977. https://doi.org/10.3390/f11090977