Organic Carbon Stabilization Mechanisms in Mangrove Soils: A Review
Abstract
1. Introduction
2. Organic Matter Stabilization Mechanisms in Terrestrial Soils and Marine Sediments
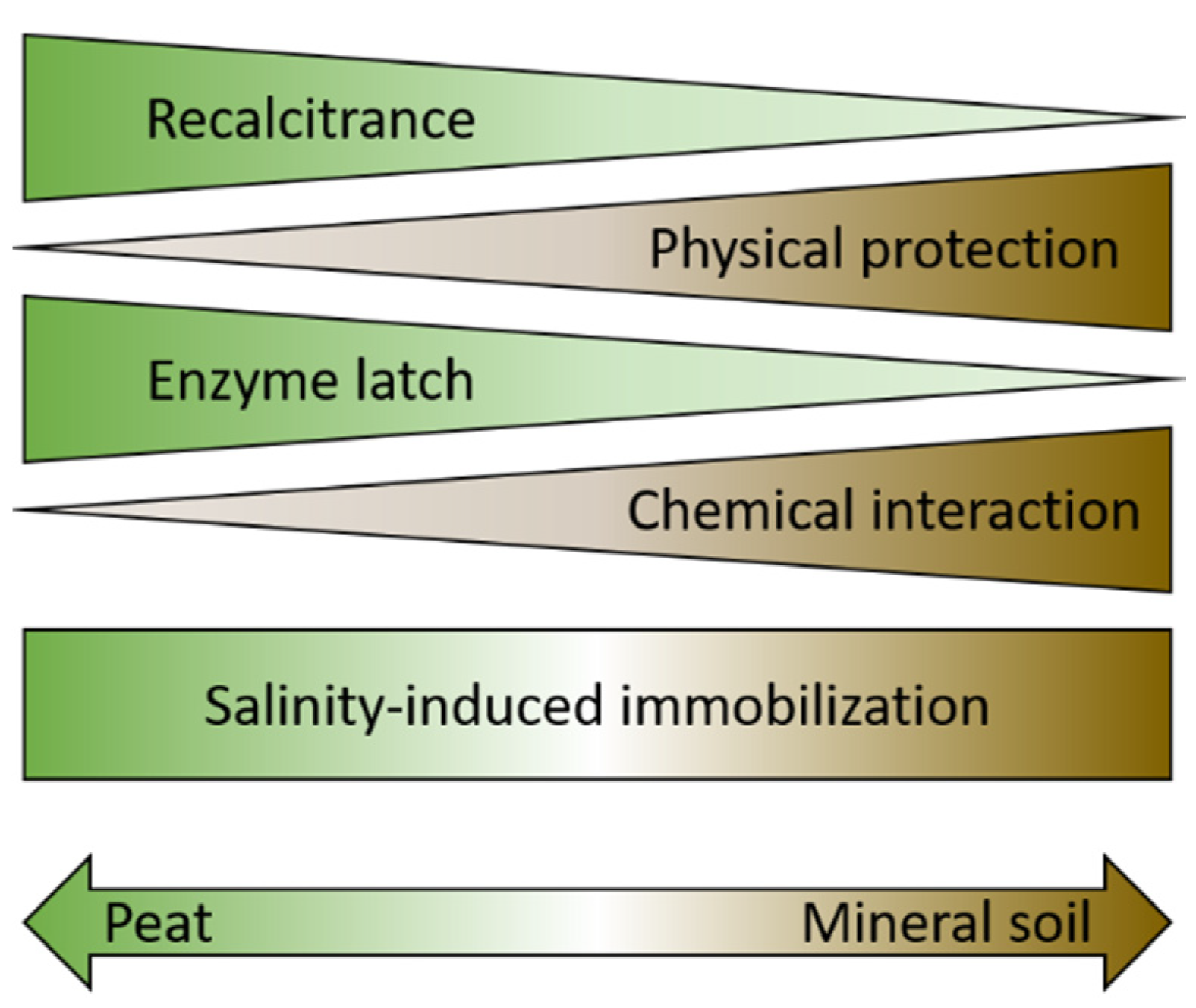
3. Mangrove Soils: Mineral or Peaty
3.1. Mineral Soils
3.1.1. Mineral Soils: Interactions with Soil Minerals or Metals
3.1.2. Mineral Soils: Physical Protection
3.2. Peaty Soils: Inherent Recalcitrance of Roots and “Enzyme Latch” Hypothesis
3.3. Both Type of Soils: Salinity-Induced Immobilization
4. Slow Decomposition or Higher Inputs?
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McLeod, E.; Chmura, G.L.; Bouillon, S.; Salm, R.; Björk, M.; Duarte, C.M.; Lovelock, C.E.; Schlesinger, W.H.; Silliman, B.R. A blueprint for blue carbon: Toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 2011, 9, 552–560. [Google Scholar] [CrossRef]
- Giri, C.; Ochieng, E.; Tieszen, L.L.; Zhu, Z.; Singh, A.; Loveland, T.; Masek, J.; Duke, N. Status and distribution of mangrove forests of the world using earth observation satellite data. Glob. Ecol. Biogeogr. 2011, 20, 154–159. [Google Scholar] [CrossRef]
- Alongi, D.M. Carbon Cycling and Storage in Mangrove Forests. Ann. Rev. Mar. Sci. 2014, 6, 195–219. [Google Scholar] [CrossRef]
- Granek, E.; Ruttenberg, B.I. Changes in biotic and abiotic processes following mangrove clearing. Estuar. Coast. Shelf Sci. 2008, 80, 555–562. [Google Scholar] [CrossRef]
- Grellier, S.; Janeau, J.-L.; Dang Hoai, N.; Nguyen Thi Kim, C.; Le Thi Phuong, Q.; Pham Thi Thu, T.; Tran-Thi, N.-T.; Marchand, C. Changes in soil characteristics and C dynamics after mangrove clearing (Vietnam). Sci. Total Environ. 2017, 593–594, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Richards, D.R.; Friess, D.A. Rates and drivers of mangrove deforestation in Southeast Asia, 2000–2012. Proc. Natl. Acad. Sci. USA 2016, 113, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Pérez, A.; Libardoni, B.G.; Sanders, C.J. Factors influencing organic carbon accumulation in mangrove ecosystems. Biol. Lett. 2018, 14, 20180237. [Google Scholar] [CrossRef]
- Sasmito, S.D.; Taillardat, P.; Clendenning, J.N.; Cameron, C.; Friess, D.A.; Murdiyarso, D.; Hutley, L.B. Effect of land-use and land-cover change on mangrove blue carbon: A systematic review. Glob. Chang. Biol. 2019, 25, 4291–4302. [Google Scholar] [CrossRef]
- Kida, M.; Tomotsune, M.; Iimura, Y.; Kinjo, K.; Ohtsuka, T.; Fujitake, N. High salinity leads to accumulation of soil organic carbon in mangrove soil. Chemosphere 2017, 177, 51–55. [Google Scholar] [CrossRef]
- Li, S.-B.; Chen, P.-H.; Huang, J.-S.; Hsueh, M.-L.; Hsieh, L.-Y.; Lee, C.-L.; Lin, H.-J. Factors regulating carbon sinks in mangrove ecosystems. Glob. Chang. Biol. 2018, 24, 4195–4210. [Google Scholar] [CrossRef]
- Friesen, S.D.; Dunn, C.; Freeman, C. Decomposition as a regulator of carbon accretion in mangroves: A review. Ecol. Eng. 2018, 114, 173–178. [Google Scholar] [CrossRef]
- Yarwood, S.A. The role of wetland microorganisms in plant-litter decomposition and soil organic matter formation: A critical review. FEMS Microbiol. Ecol. 2018, 94, fiy175. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, S.; Borges, A.V.; Castañeda-Moya, E.; Diele, K.; Dittmar, T.; Duke, N.C.; Kristensen, E.; Lee, S.Y.; Marchand, C.; Middelburg, J.J.; et al. Mangrove production and carbon sinks: A revision of global budget estimates. Glob. Biogeochem. Cycles 2008, 22, 12. [Google Scholar] [CrossRef]
- Murdiyarso, D.; Purbopuspito, J.; Kauffman, J.B.; Warren, M.W.; Sasmito, S.D.; Donato, D.C.; Manuri, S.; Krisnawati, H.; Taberima, S.; Kurnianto, S. The potential of Indonesian mangrove forests for global climate change mitigation. Nat. Clim. Chang. 2015, 5, 1089–1092. [Google Scholar] [CrossRef]
- Atwood, T.B.; Connolly, R.M.; Almahasheer, H.; Carnell, P.E.; Duarte, C.M.; Lewis, C.J.E.; Irigoien, X.; Kelleway, J.J.; Lavery, P.S.; Macreadie, P.I.; et al. Global patterns in mangrove soil carbon stocks and losses. Nat. Clim. Chang. 2017, 7, 523–528. [Google Scholar] [CrossRef]
- Ouyang, X.; Lee, S.Y. Improved estimates on global carbon stock and carbon pools in tidal wetlands. Nat. Commun. 2020, 11, 1–7. [Google Scholar] [CrossRef]
- Rovai, A.S.; Twilley, R.R.; Castañeda-Moya, E.; Riul, P.; Cifuentes-Jara, M.; Manrow-Villalobos, M.; Horta, P.A.; Simonassi, J.C.; Fonseca, A.L.; Pagliosa, P.R. Global controls on carbon storage in mangrove soils. Nat. Clim. Chang. 2018, 8, 534–538. [Google Scholar] [CrossRef]
- Maher, D.T.; Santos, I.R.; Golsby-Smith, L.; Gleeson, J.; Eyre, B.D. Groundwater-derived dissolved inorganic and organic carbon exports from a mangrove tidal creek: The missing mangrove carbon sink? Limnol. Oceanogr. 2013, 58, 475–488. [Google Scholar] [CrossRef]
- Alongi, D.M.; de Carvalho, N.A.; Amaral, A.L.; da Costa, A.; Trott, L.; Tirendi, F. Uncoupled surface and below-ground soil respiration in mangroves: Implications for estimates of dissolved inorganic carbon export. Biogeochemistry 2012, 109, 151–162. [Google Scholar] [CrossRef]
- Robertson, A.I.; Alongi, D.M. Massive turnover rates of fine root detrital carbon in tropical Australian mangroves. Oecologia 2016, 180, 841–851. [Google Scholar] [CrossRef]
- Ouyang, X.; Lee, S.Y.; Connolly, R.M. The role of root decomposition in global mangrove and saltmarsh carbon budgets. Earth-Sci. Rev. 2017, 166, 53–63. [Google Scholar] [CrossRef]
- Pisani, O.; Gao, M.; Maie, N.; Miyoshi, T.; Childers, D.L.; Jaffé, R. Compositional aspects of herbaceous litter decomposition in the freshwater marshes of the Florida Everglades. Plant Soil 2018, 423, 87–98. [Google Scholar] [CrossRef]
- Huxham, M.; Langat, J.; Tamooh, F.; Kennedy, H.; Mencuccini, M.; Skov, M.W.; Kairo, J. Decomposition of mangrove roots: Effects of location, nutrients, species identity and mix in a Kenyan forest. Estuar. Coast. Shelf Sci. 2010, 88, 135–142. [Google Scholar] [CrossRef]
- Jia, B.; Niu, Z.; Wu, Y.; Kuzyakov, Y.; Li, X.G. Waterlogging increases organic carbon decomposition in grassland soils. Soil Biol. Biochem. 2020, 148, 107927. [Google Scholar] [CrossRef]
- Huang, W.; Ye, C.; Hockaday, W.C.; Hall, S.J. Trade-offs in soil carbon protection mechanisms under aerobic and anaerobic conditions. Glob. Chang. Biol. 2020, 26, 3726–3737. [Google Scholar] [CrossRef]
- Chen, C.; Hall, S.J.; Coward, E.; Thompson, A. Iron-mediated organic matter decomposition in humid soils can counteract protection. Nat. Commun. 2020, 11, 2255. [Google Scholar] [CrossRef]
- Sollins, P.; Homann, P.; Caldwell, B.A. Stabilization and destabilization of soil organic matter: Mechanisms and controls. Geoderma 1996, 74, 65–105. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kögel-Knabner, I.; Lehmann, J.; Manning, D.A.C.; et al. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef]
- Keil, R.G.; Mayer, L.M. Mineral matrices and organic matter. In Treatise on Geochemistry; Elsevier: Amsterdam, Netherlands, 2014; Volume 12, pp. 337–359. ISBN 9780080983004. [Google Scholar]
- Hedges, J.I.; Oades, J.M. Comparative organic geochemistries of soils and marine sediments. Org. Geochem. 1997, 27, 319–361. [Google Scholar] [CrossRef]
- Marschner, B.; Brodowski, S.; Dreves, A.; Gleixner, G.; Gude, A.; Grootes, P.M.; Hamer, U.; Heim, A.; Jandl, G.; Ji, R.; et al. How relevant is recalcitrance for the stabilization of organic matter in soils? J. Plant Nutr. Soil Sci. 2008, 171, 91–110. [Google Scholar] [CrossRef]
- Lutzow, M.V.; Kogel-Knabner, I.; Ekschmitt, K.; Matzner, E.; Guggenberger, G.; Marschner, B.; Flessa, H. Stabilization of organic matter in temperate soils: Mechanisms and their relevance under different soil conditions—A review. Eur. J. Soil Sci. 2006, 57, 426–445. [Google Scholar] [CrossRef]
- Rothman, D.H.; Forney, D.C. Physical model for the decay and preservation of marine organic carbon. Science 2007, 316, 1325–1328. [Google Scholar] [CrossRef] [PubMed]
- Rasse, D.P.; Rumpel, C.; Dignac, M.-F. Is soil carbon mostly root carbon? Mechanisms for a specific stabilisation. Plant Soil 2005, 269, 341–356. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef]
- Kida, M.; Kondo, M.; Tomotsune, M.; Kinjo, K.; Ohtsuka, T.; Fujitake, N. Molecular composition and decomposition stages of organic matter in a mangrove mineral soil with time. Estuar. Coast. Shelf Sci. 2019, 231, 106478. [Google Scholar] [CrossRef]
- Schwertmann, U.; Murad, E. The nature of an iron oxide—Organic iron association in a peaty environment. Clay Miner. 1988, 23, 291–299. [Google Scholar] [CrossRef]
- Keil, R.G.; Montluçon, D.B.; Prahl, F.G.; Hedges, J.I. Sorptive preservation of labile organic matter in marine sediments. Nature 1994, 370, 549–552. [Google Scholar] [CrossRef]
- Dicen, G.P.; Navarrete, I.A.; Rallos, R.V.; Salmo, S.G.; Garcia, M.C.A. The role of reactive iron in long-term carbon sequestration in mangrove sediments. J. Soils Sediments 2019, 19, 501–510. [Google Scholar] [CrossRef]
- Arnarson, T.S.; Keil, R.G. Mechanisms of pore water organic matter adsorption to montmorillonite. Mar. Chem. 2000, 71, 309–320. [Google Scholar] [CrossRef]
- Kooner, Z.S.; Jardine, P.M.; Feldman, S. Competitive surface complexation reactions of sulfate and natural organic carbon on soil. J. Environ. Qual. 1995, 24, 656–662. [Google Scholar] [CrossRef]
- Sayles, F.L.; Mangelsdorf, P.C. Cation-exchange characteristics of Amazon River suspended sediment and its reaction with seawater. Geochim. Cosmochim. Acta 1979, 43, 767–779. [Google Scholar] [CrossRef]
- Kalinichev, A.G.; Kirkpatrick, R.J. Molecular dynamics simulation of cationic complexation with natural organic matter. Eur. J. Soil Sci. 2007, 58, 909–917. [Google Scholar] [CrossRef]
- Rashid, M.; Buckley, D.; Robertson, K. Interactions of a marine humic acid with clay minerals and a natural sediment. Geoderma 1972, 8, 11–27. [Google Scholar] [CrossRef]
- Preston, M.R.; Riley, J.P. The interactions of humic compounds with electrolytes and three clay minerals under simulated estuarine conditions. Estuar. Coast. Shelf Sci. 1982, 14, 567–576. [Google Scholar] [CrossRef]
- Zhou, J.L.; Rowland, S.; Fauzi, R.; Mantoura, C.; Braven, J. The formation of humic coatings on mineral particles under simulated estuarine conditions—A mechanistic study. Water Res. 1994, 28, 571–579. [Google Scholar] [CrossRef]
- Mayer, L.M. Aggregation of colloidal iron during estuarine mixing: Kinetics, mechanism, and seasonality. Geochim. Cosmochim. Acta 1982, 46, 2527–2535. [Google Scholar] [CrossRef]
- Sutton, R.; Sposito, G. Molecular simulation of humic substance–Ca-montmorillonite complexes. Geochim. Cosmochim. Acta 2006, 70, 3566–3581. [Google Scholar] [CrossRef]
- Rowley, M.C.; Grand, S.; Verrecchia, É.P. Calcium-mediated stabilisation of soil organic carbon. Biogeochemistry 2018, 137, 27–49. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Peak, D.; Tang, Y.; Feng, X.; Zhu, M. Quantification of coexisting inner- and outer-sphere complexation of sulfate on hematite surfaces. ACS Earth Space Chem. 2018, 2, 387–398. [Google Scholar] [CrossRef]
- Mikutta, R.; Mikutta, C.; Kalbitz, K.; Scheel, T.; Kaiser, K.; Jahn, R. Biodegradation of forest floor organic matter bound to minerals via different binding mechanisms. Geochim. Cosmochim. Acta 2007, 71, 2569–2590. [Google Scholar] [CrossRef]
- Komiyama, A.; Poungparn, S.; Umnouysin, S.; Rodtassana, C.; Pravinvongvuthi, T.; Noda, T.; Kato, S. Occurrence of seasonal water replacement in mangrove soil and the trunk growth response of Avicennia alba related to salinity changes in a tropical monsoon climate. Ecol. Res. 2019, 34, 428–439. [Google Scholar] [CrossRef]
- Wagai, R.; Mayer, L.M. Sorptive stabilization of organic matter in soils by hydrous iron oxides. Geochim. Cosmochim. Acta 2007, 71, 25–35. [Google Scholar] [CrossRef]
- Mehra, O.P.; Jackson, M.L. Iron oxide removal from soils and clays by a dithionite-citrate system buffered with sodium bicarbonate. Clays Clay Miner. 1958, 7, 317–327. [Google Scholar] [CrossRef]
- Lalonde, K.; Mucci, A.; Ouellet, A.; Ge, Y. Preservation of organic matter in sediments promoted by iron. Nature 2012, 483, 8–10. [Google Scholar] [CrossRef]
- Barber, A.; Brandes, J.; Leri, A.; Lalonde, K.; Balind, K.; Wirick, S.; Wang, J.; Gélinas, Y. Preservation of organic matter in marine sediments by inner-sphere interactions with reactive iron. Sci. Rep. 2017, 7, 366. [Google Scholar] [CrossRef]
- Shields, M.R.; Bianchi, T.S.; Gélinas, Y.; Allison, M.A.; Twilley, R.R. Enhanced terrestrial carbon preservation promoted by reactive iron in deltaic sediments. Geophys. Res. Lett. 2016, 43, 1149–1157. [Google Scholar] [CrossRef]
- Zhao, B.; Yao, P.; Bianchi, T.S.; Shields, M.R.; Cui, X.Q.; Zhang, X.W.; Huang, X.Y.; Schröeder, C.; Zhao, J.; Yu, Z.G. The role of reactive iron in the preservation of terrestrial organic carbon in estuarine sediments. J. Geophys. Res. Biogeosci. 2018, 123, 3556–3569. [Google Scholar] [CrossRef]
- Cheng, H.; Chen, D.-T.; Tam, N.F.-Y.; Chen, G.-Z.; Li, S.-Y.; Ye, Z.-H. Interactions among Fe2+, S2−, and Zn2+ tolerance, root anatomy, and radial oxygen loss in mangrove plants. J. Exp. Bot. 2012, 63, 2619–2630. [Google Scholar] [CrossRef]
- Kristensen, E.; Alongi, D.M. Control by fiddler crabs (Uca vocans) and plant roots (Avicennia marina) on carbon, iron, and sulfur biogeochemistry in mangrove sediment. Limnol. Oceanogr. 2006, 51, 1557–1571. [Google Scholar] [CrossRef]
- Boudot, J.P.; Bel Hadj Brahim, A.; Steiman, R.; Seigle-Murandi, F. Biodegradation of synthetic organo-metallic complexes of iron and aluminium with selected metal to carbon ratios. Soil Biol. Biochem. 1989, 21, 961–966. [Google Scholar] [CrossRef]
- Riedel, T.; Zak, D.; Biester, H.; Dittmar, T. Iron traps terrestrially derived dissolved organic matter at redox interfaces. Proc. Natl. Acad. Sci. USA 2013, 110, 10101–10105. [Google Scholar] [CrossRef] [PubMed]
- Nierop, K.G.J.; Jansen, B.; Verstraten, J.M. Dissolved organic matter, aluminium and iron interactions: Precipitation induced by metal/carbon ratio, pH and competition. Sci. Total Environ. 2002, 300, 201–211. [Google Scholar] [CrossRef]
- Chen, C.; Dynes, J.J.; Wang, J.; Sparks, D.L. Properties of Fe-organic matter associations via coprecipitation versus adsorption. Environ. Sci. Technol. 2014, 48, 13751–13759. [Google Scholar] [CrossRef] [PubMed]
- McKee, K.L.; Cahoon, D.R.; Feller, I.C. Caribbean mangroves adjust to rising sea level through biotic controls on change in soil elevation. Glob. Ecol. Biogeogr. 2007, 16, 545–556. [Google Scholar] [CrossRef]
- Saraswati, S.; Dunn, C.; Mitsch, W.J.; Freeman, C. Is peat accumulation in mangrove swamps influenced by the “enzymic latch” mechanism? Wetl. Ecol. Manag. 2016, 24, 641–650. [Google Scholar] [CrossRef]
- Kögel-Knabner, I. The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter. Soil Biol. Biochem. 2002, 34, 139–162. [Google Scholar] [CrossRef]
- Middleton, B.A.; McKee, K.L. Degradation of mangrove tissues and implications for peat formation in Belizean island forests. J. Ecol. 2001, 89, 818–828. [Google Scholar] [CrossRef]
- Liu, X.; Xiong, Y.; Liao, B. Relative contributions of leaf litter and fine roots to soil organic matter accumulation in mangrove forests. Plant Soil 2017, 421, 493–503. [Google Scholar] [CrossRef]
- Keuskamp, J.A.; Schmitt, H.; Laanbroek, H.J.; Verhoeven, J.T.A.; Hefting, M.M. Nutrient amendment does not increase mineralisation of sequestered carbon during incubation of a nitrogen limited mangrove soil. Soil Biol. Biochem. 2013, 57, 822–829. [Google Scholar] [CrossRef]
- Lovelock, C.E.; Feller, I.C.; Reef, R.; Ruess, R.W. Variable effects of nutrient enrichment on soil respiration in mangrove forests. Plant Soil 2014, 379, 135–148. [Google Scholar] [CrossRef]
- Freeman, C.; Ostle, N.J.; Fenner, N.; Kang, H. A regulatory role for phenol oxidase during decomposition in peatlands. Soil Biol. Biochem. 2004, 36, 1663–1667. [Google Scholar] [CrossRef]
- Bianchi, T.S.; Schreiner, K.M.; Smith, R.W.; Burdige, D.J.; Woodard, S.; Conley, D.J. Redox effects on organic matter storage in coastal sediments during the Holocene: A biomarker/proxy perspective. Annu. Rev. Earth Planet. Sci. 2016, 44, 295–319. [Google Scholar] [CrossRef]
- Dittmar, T.; Hertkorn, N.; Kattner, G.; Lara, R.J. Mangroves, a major source of dissolved organic carbon to the oceans. Glob. Biogeochem. Cycles 2006, 20, 7. [Google Scholar] [CrossRef]
- Sholkovitz, E.R. Flocculation of dissolved organic and inorganic matter during the mixing of river water and seawater. Geochim. Cosmochim. Acta 1976, 40, 831–845. [Google Scholar] [CrossRef]
- Ardón, M.; Helton, A.M.; Bernhardt, E.S. Drought and saltwater incursion synergistically reduce dissolved organic carbon export from coastal freshwater wetlands. Biogeochemistry 2016, 127, 411–426. [Google Scholar] [CrossRef]
- Hapsari, K.A.; Jennerjahn, T.C.; Lukas, M.C.; Karius, V.; Behling, H. Intertwined effects of climate and land use change on environmental dynamics and carbon accumulation in a mangrove-fringed coastal lagoon in Java, Indonesia. Glob. Chang. Biol. 2020, 26, 1414–1431. [Google Scholar] [CrossRef] [PubMed]
- Kellerman, A.M.; Arellano, A.; Podgorski, D.C.; Martin, E.E.; Martin, J.B.; Deuerling, K.M.; Bianchi, T.S.; Spencer, R.G.M. Fundamental drivers of dissolved organic matter composition across an Arctic effective precipitation gradient. Limnol. Oceanogr. 2020, 65, 1217–1234. [Google Scholar] [CrossRef]
- Kida, M.; Tanabe, M.; Tomotsune, M.; Yoshitake, S.; Kinjo, K.; Ohtsuka, T.; Fujitake, N. Changes in dissolved organic matter composition and dynamics in a subtropical mangrove river driven by rainfall. Estuar. Coast. Shelf Sci. 2019, 223, 6–17. [Google Scholar] [CrossRef]
- Cawley, K.M.; Yamashita, Y.; Maie, N.; Jaffé, R. Using optical properties to quantify fringe mangrove inputs to the dissolved organic matter (DOM) pool in a subtropical estuary. Estuaries Coasts 2014, 37, 399–410. [Google Scholar] [CrossRef]
- Wagai, R.; Mayer, L.M.; Kitayama, K.; Knicker, H. Climate and parent material controls on organic matter storage in surface soils: A three-pool, density-separation approach. Geoderma 2008, 147, 23–33. [Google Scholar] [CrossRef]
- Alongi, D.M.; Mukhopadhyay, S.K. Contribution of mangroves to coastal carbon cycling in low latitude seas. Agric. For. Meteorol. 2015, 213, 266–272. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Tomotsune, M.; Suchewaboripont, V.; Iimura, Y.; Kida, M.; Yoshitake, S.; Kondo, M.; Kinjo, K. Stand dynamics and aboveground net primary productivity of a mature subtropical mangrove forest on Ishigaki Island, south-western Japan. Reg. Stud. Mar. Sci. 2019, 27, 100516. [Google Scholar] [CrossRef]
- Muhammad-Nor, S.M.; Huxham, M.; Salmon, Y.; Duddy, S.J.; Mazars-Simon, A.; Mencuccini, M.; Meir, P.; Jackson, G. Exceptionally high mangrove root production rates in the Kelantan Delta, Malaysia; An experimental and comparative study. For. Ecol. Manag. 2019, 444, 214–224. [Google Scholar] [CrossRef]
- Xiong, Y.; Liu, X.; Guan, W.; Liao, B.; Chen, Y.; Li, M.; Zhong, C. Fine root functional group based estimates of fine root production and turnover rate in natural mangrove forests. Plant Soil 2017, 413, 83–95. [Google Scholar] [CrossRef]
- Poungparn, S.; Charoenphonphakdi, T.; Sangtiean, T.; Patanaponpaiboon, P. Fine root production in three zones of secondary mangrove forest in eastern Thailand. Trees 2016, 30, 467–474. [Google Scholar] [CrossRef]
- Alongi, D.M. Carbon sequestration in mangrove forests. Carbon Manag. 2016, 3, 313–322. [Google Scholar] [CrossRef]
- Torres, J.R.; Barba, E.; Choix, F.J. Production and biomass of mangrove roots in relation to hydroperiod and physico-chemical properties of sediment and water in the Mecoacan Lagoon, Gulf of Mexico. Wetl. Ecol. Manag. 2019, 27, 427–442. [Google Scholar] [CrossRef]
- Wilkens, J.F.; Huth, F.; Berger, U.; Devlin, D.; Popow, F.; Wagner, S. Spatial explicit distribution of individual fine root biomass of Rhizophora mangle L. (Red Mangrove) in South Florida. Wetl. Ecol. Manag. 2018, 26, 775–788. [Google Scholar] [CrossRef]
- Furukawa, K.; Wolanski, E.; Mueller, H. Currents and sediment transport in mangrove forests. Estuar. Coast. Shelf Sci. 1997, 44, 301–310. [Google Scholar] [CrossRef]
- Wolanski, E. Transport of sediment in mangrove swamps. Hydrobiologia 1995, 295, 31–42. [Google Scholar] [CrossRef]
- Tue, N.T.; Hamaoka, H.; Sogabe, A.; Quy, T.D.; Nhuan, M.T.; Omori, K. The application of δ13C and C/N ratios as indicators of organic carbon sources and paleoenvironmental change of the mangrove ecosystem from Ba Lat Estuary, Red River, Vietnam. Environ. Earth Sci. 2011, 64, 1475–1486. [Google Scholar] [CrossRef]
- Gonneea, M.E.; Paytan, A.; Herrera-Silveira, J.a. Tracing organic matter sources and carbon burial in mangrove sediments over the past 160 years. Estuar. Coast. Shelf Sci. 2004, 61, 211–227. [Google Scholar] [CrossRef]
- Bouillon, S.; Dahdouh-Guebas, F.; Rao, A.; Koedam, N.; Dehairs, F. Sources of organic carbon in mangrove sediments: Variability and possible ecological implications. Hydrobiologia 2003, 495, 33–39. [Google Scholar] [CrossRef]
- Ray, R.; Baum, A.; Rixen, T.; Gleixner, G.; Jana, T.K. Exportation of dissolved (inorganic and organic) and particulate carbon from mangroves and its implication to the carbon budget in the Indian Sundarbans. Sci. Total Environ. 2018, 621, 535–547. [Google Scholar] [CrossRef]
- Hamilton, S.E.; Casey, D. Creation of a high spatio-temporal resolution global database of continuous mangrove forest cover for the 21st century (CGMFC-21). Glob. Ecol. Biogeogr. 2016, 25, 729–738. [Google Scholar] [CrossRef]
| Mechanism | Type of Interaction | Nature | Influence of Inorganic Ions | Reference | |
|---|---|---|---|---|---|
| Ligand exchange a | Inner-sphere | Covalent to ionic bond | Negative b | Competitive effect by SO42− | [40,41] |
| Ion exchange | Outer-sphere | Ionic bond | Negative | Competitive effect by inorganic ions | [40] |
| Cation bridging | Inner-sphere | Ionic bond (Direct cation bridging) c | Negative | Partial replacement of Ca2+ for Na+, K+, Mg2+; Monovalent cations are not good at bridging, and Mg2+ does not form inner-sphere bridge | [42,43] |
| Outer-sphere | Hydrogen bond (Exchangeable/water bridging) c | Negative | Partial replacement of Ca2+ for Na+ and K+; Monovalent cations are not good at bridging | [42] | |
| Van der Waals forces | Outer-sphere | Dipole–dipole force | Positive | The compression of the double-layers of both clays and organic matter at high ionic strength allows closer approach | [40,44,45,46] |
| Hydrogen bonding | Outer-sphere | Hydrogen bond | Negative d | Replacement of H with cations | [47] |
| Hydrophobic interactions | Outer-sphere | Entropy-driven | Negative d | The presence of hydrated Ca2+ between OM and mineral surfaces impedes the formation of direct hydrophobic interactions, as opposed to the H+-saturated system | [48] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kida, M.; Fujitake, N. Organic Carbon Stabilization Mechanisms in Mangrove Soils: A Review. Forests 2020, 11, 981. https://doi.org/10.3390/f11090981
Kida M, Fujitake N. Organic Carbon Stabilization Mechanisms in Mangrove Soils: A Review. Forests. 2020; 11(9):981. https://doi.org/10.3390/f11090981
Chicago/Turabian StyleKida, Morimaru, and Nobuhide Fujitake. 2020. "Organic Carbon Stabilization Mechanisms in Mangrove Soils: A Review" Forests 11, no. 9: 981. https://doi.org/10.3390/f11090981
APA StyleKida, M., & Fujitake, N. (2020). Organic Carbon Stabilization Mechanisms in Mangrove Soils: A Review. Forests, 11(9), 981. https://doi.org/10.3390/f11090981





