Effects of Temperature Factors on Resistance against Pine Wood Nematodes in Pinus thunbergii, Based on Multiple Location Sites Nematode Inoculation Tests
Abstract
1. Introduction
2. Materials and Methods
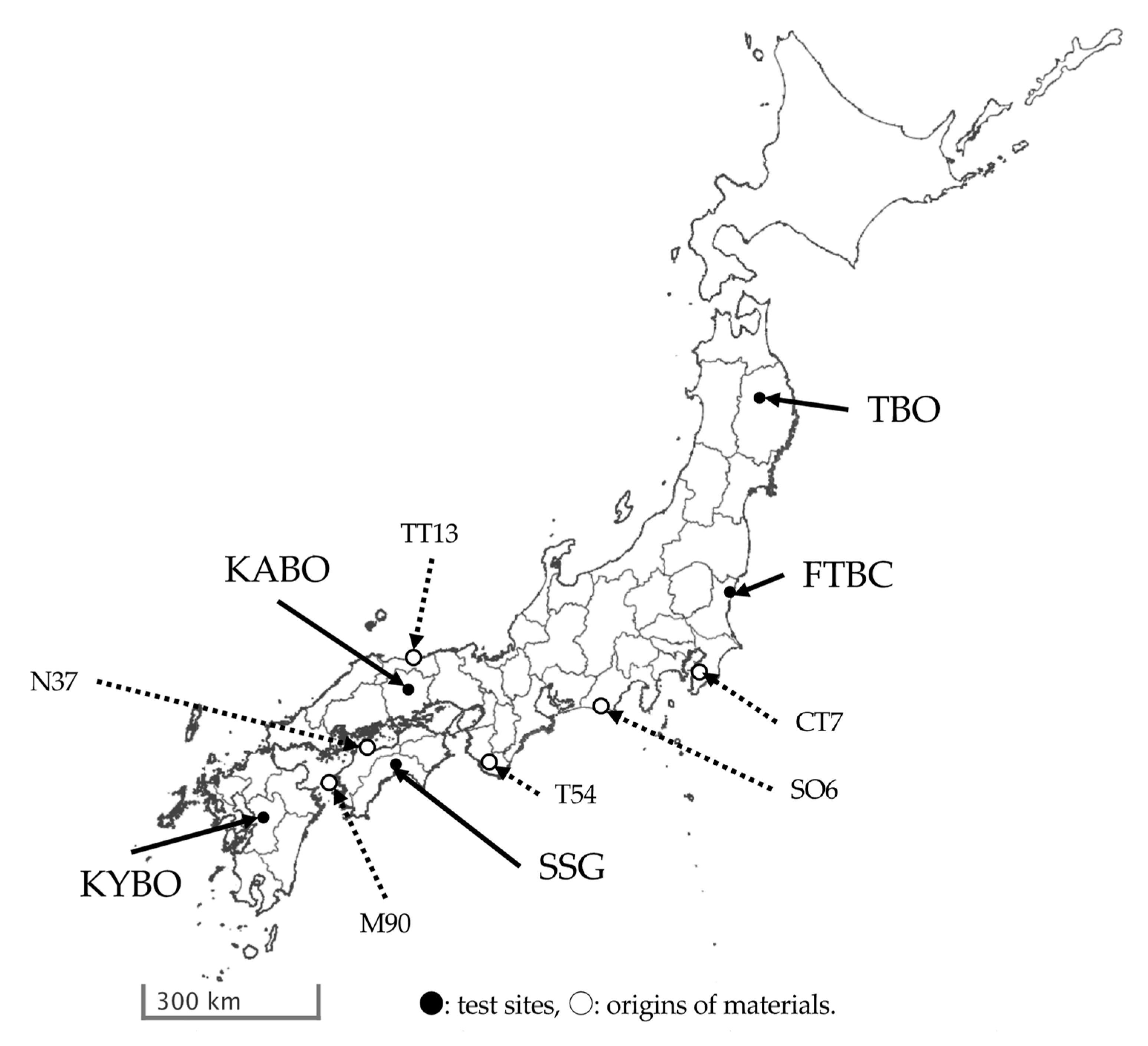
2.1. Test Sites
2.2. Plant Material and PWN Inoculation
2.3. Symptom Observation
2.4. Temperature Data
2.5. Statistical Analysis
3. Results
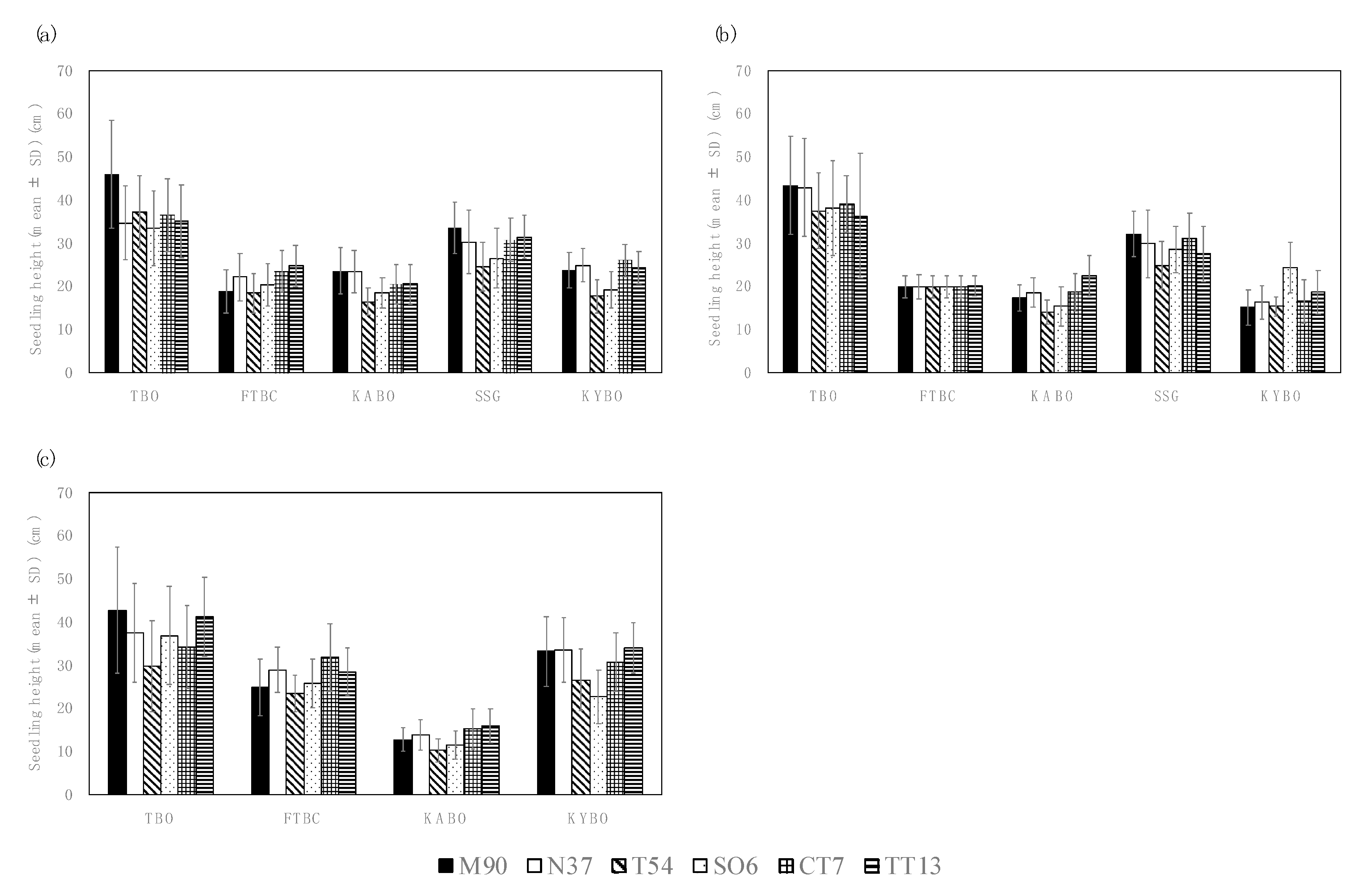
3.1. Seedling Heights
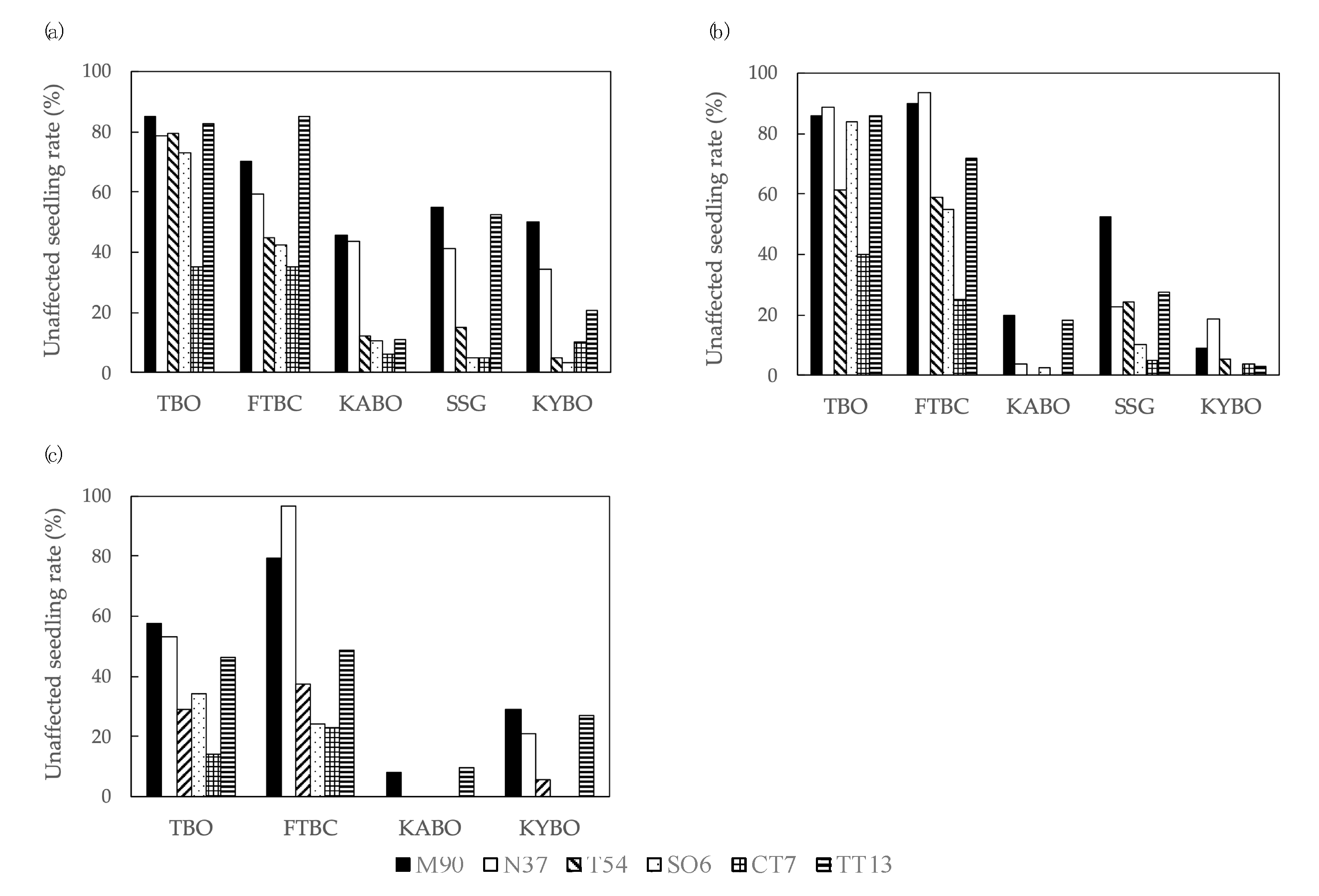
3.2. Unaffected Seedling Rates
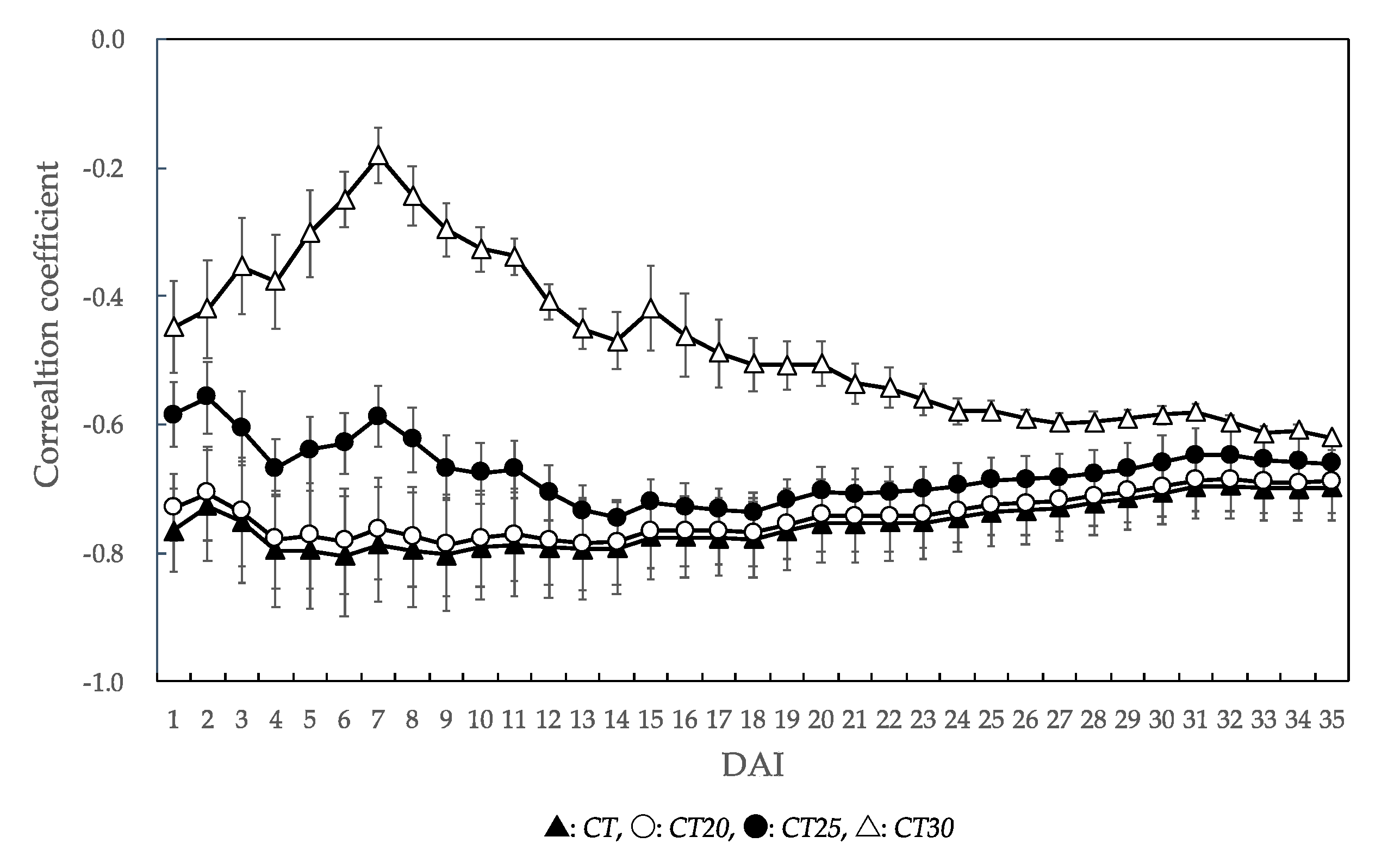
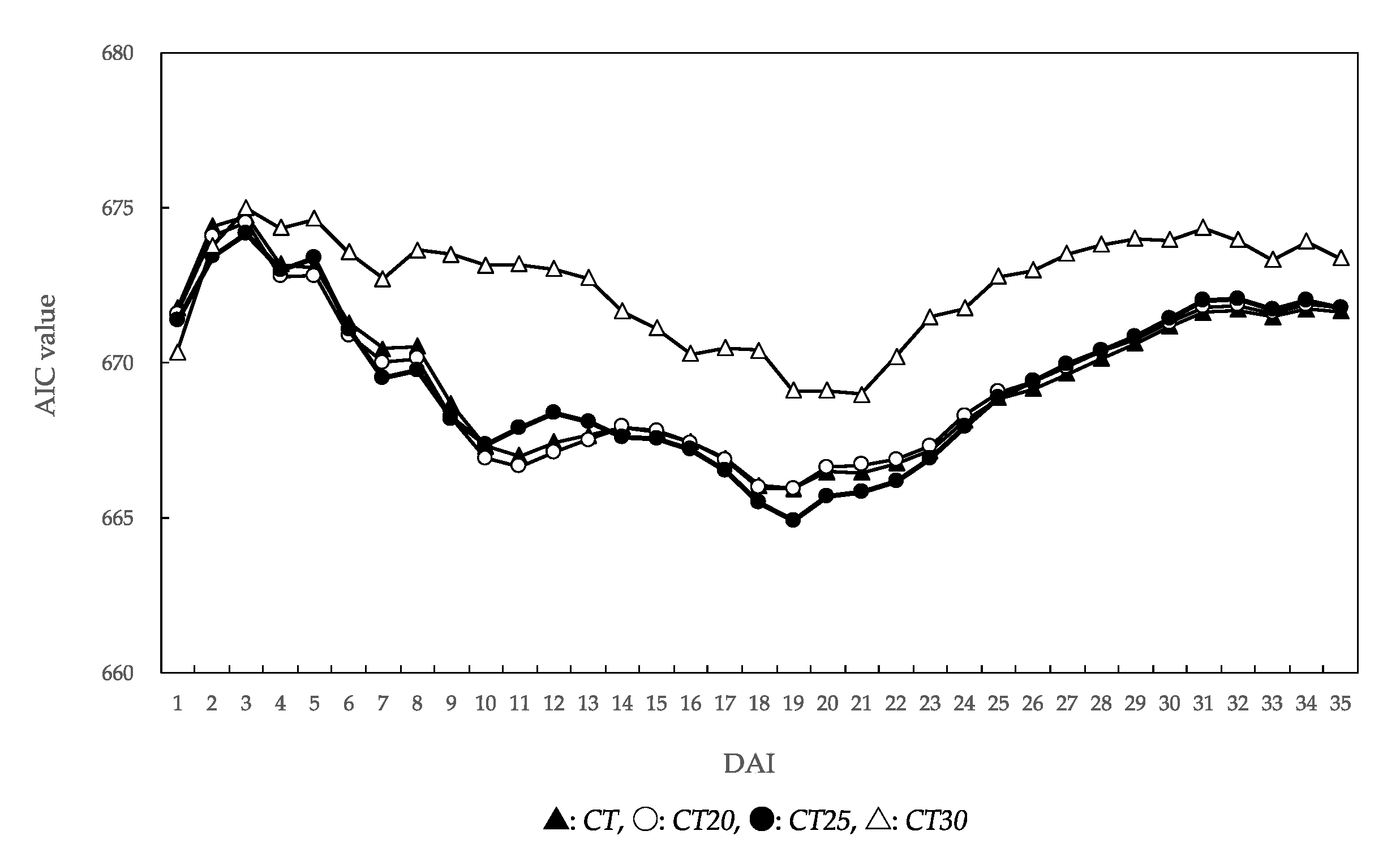
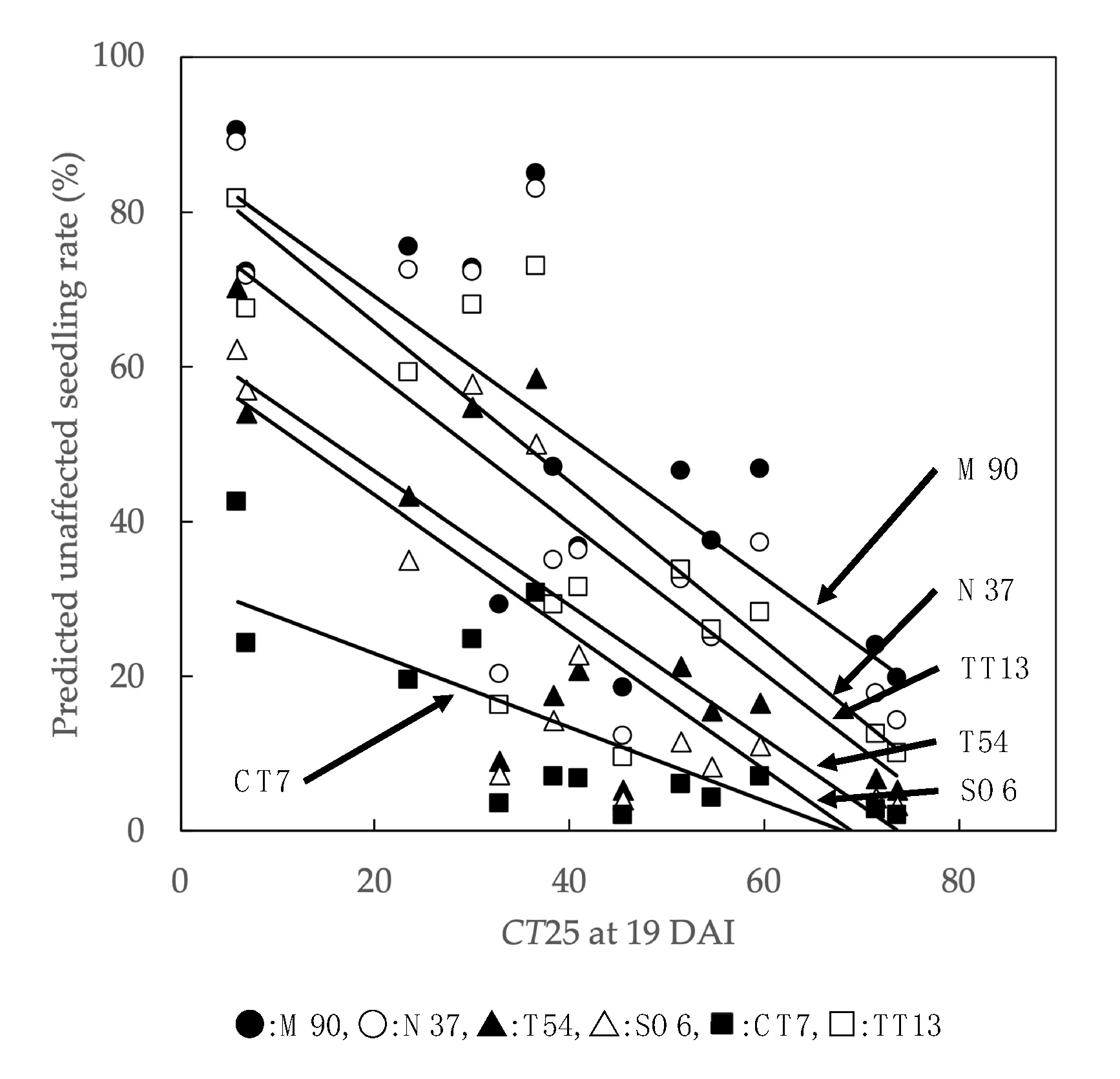
3.3. Analysis of Temperature Factors Affecting Unaffected Seedling Rate
4. Discussion
4.1. Differences in PWN-Resistance among Test Sites and Inoculation Years
4.2. Temperature Factors Affecting PWN-Resistance
4.3. Application to the Resistance Breeding Program
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kiyohara, T.; Tokushige, Y. Inoculation experiments of a nematode, Bursaphelenchus sp., onto pine trees. J. Jpn. For. Soc. 1971, 5, 210–218, (In Japanese with English summary). [Google Scholar]
- Mamiya, Y.; Endo, N. Transmission of Bursaphelenchus lignicolus (Nematoda: Aphelenchoididae) by Monochamus alternatus (Coleoptera: Cerambycidae). Nematologica 1972, 18, 159–162. [Google Scholar] [CrossRef]
- Zhao, B.G.; Futai, K.; Sutherland, J.R.; Takeuchi, Y. Pine Wilt Disease; Springer: New York, NY, USA, 2008. [Google Scholar]
- Hirata, A.; Nakamura, K.; Nakao, K.; Kominami, Y.; Tanaka, N.; Ohashi, H.; Takano, K.T.; Takeuchi, W.; Matsui, T. Potential distribution of pine wilt disease under future climate change scenarios. PLoS ONE 2017, 12, e0182837. [Google Scholar] [CrossRef] [PubMed]
- Futai, K.; Furuno, T. The variety of resistance among pine-species to pine wood nematode, Bursaphelenchus lignicolus. Bull. Kyoto Univ. For. 1979, 51, 23–36, (In Japanese with English summary). [Google Scholar]
- Yano, M. Investigation on the causes of pine mortality in Nagasaki Prefecture. Sanrinkoho 1913, 4, 1–14. (In Japanese) [Google Scholar]
- Nakamura, K.; Otsuka, I. Forest Pest Management Leading to Establishment of Forestry Business: Pine Wilt Disease Unites the Locality; Japan Forest Investigation Company: Tokyo, Japan, 2019. (In Japanese) [Google Scholar]
- Fujimoto, Y.; Toda, T.; Nishimura, K.; Yamate, H.; Fuyuno, S. Breeding project on resistance to the pine Wood nematode—An outline of the research and the achievement of the project for ten years. Bull. For. Tree. Breed. Center. 1989, 7, 1–84, (In Japanese with English summary). [Google Scholar]
- Toda, T. Studies on the breeding for resistance to the pine wilt disease in Pinus densiflora and P. thunbergii. Bull. For. Tree. Breed. Inst. 2004, 20, 83–217, (In Japanese with English summary). [Google Scholar]
- Kurinobu, S. Current status of resistance breeding of Japanese pine species to pine wilt disease. For. Sci. Tec. 2008, 4, 51–57. [Google Scholar] [CrossRef]
- Forest Tree Breeding Center. Annual Report; Forest Tree Breeding Center: Hitachi, Japan, 2020. (In Japanese) [Google Scholar]
- Kiyohara, T. Effect of temperature on the disease incidence of pine seedlings inoculated with Bursaphelenchus lignicolus. Trans. Ann. Meet. Jpn. For. Soc. 1973, 84, 334–335, (In Japanese with English summary). [Google Scholar]
- Ichihara, Y.; Fukuda, K.; Suzuki, K. Early symptom development and histological changes associated with migration of Bursaphelenchus xylophilus in seedling tissues of Pinus thunbergii. Plant Dis. 2000, 84, 675–680. [Google Scholar] [CrossRef]
- Yamaguchi, R.; Matsunaga, K.; Watanabe, A. Influence of temperature on pine wilt disease progression in Pinus thunbergii seedlings. Eur. J. Plant. Pathol. 2020, 156, 581–590. [Google Scholar] [CrossRef]
- Suzuki, K.; Kiyohara, T. Influence of water stress on development of pine wilting disease caused by Bursaphelenchus lignicolus. Eur. J. For. Pathol. 1978, 8, 97–107. [Google Scholar] [CrossRef]
- Kaneko, S. Effect of light intensity on the development of pine wilt disease. Can. J. Bot. 1989, 67, 1861–1864. [Google Scholar] [CrossRef]
- Rutherford, T.; Webster, J. Distribution of pine wilt disease with respect to temperature in North America, Japan, and Europe. Can. J. For. Res. 1987, 17, 1050–1059. [Google Scholar] [CrossRef]
- Toda, T.; Kurinobu, S. Genetic improvement in pine wilt disease resistance in Pinus thunbergii: The effectiveness of pre-screening with an artificial inoculation at the nursery. J. For. Res. 2001, 6, 197–201. [Google Scholar] [CrossRef]
- Toda, T.; Kurinobu, S. Realized genetic gains observed in progeny tolerance of selected red pine (Pinus densiflora) and black pine (P. thunbergii) to pine wilt disease. Silvae. Gent. 2002, 51, 42–44. [Google Scholar]
- Hakamata, T. Studies on efficient production technique of Pinus thunbergii seedlings to Bursaphelenchus xylophilus. Tech. Bull. Shizuoka. Res. Ins. Agric. For. 2013, 10, 1–46, (In Japanese with English summary). [Google Scholar]
- Kuroda, K.; Ohira, M.; Okamura, M.; Fujisawa, Y. Migration and population growth of the pine wood nematode (Bursaphelenchus xylophilus) related to the symptom development in the seedlings of Japanese black pine (Pinus thunbergii) families selected as resistant to pine wilt. J. Jpn. For. Soc. 2007, 89, 241–248, (In Japanese with English summary). [Google Scholar] [CrossRef][Green Version]
- Aikawa, T.; Kikuchi, T.; Kosaka, H. Demonstration of interbreeding between virulent and avirulent populations of Bursaphelenchus xylophilus (Nematoda: Aphelenchoididae) by PCR-RFLP method. Appl. Entomol. Zool. 2003, 38, 565–569. [Google Scholar] [CrossRef]
- Japan Meteorological Agency. 2020 Climate Statistics. Available online: http://www.data.jma.go.jp/obd/stats/etrn/index.php (accessed on 10 December 2017).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using LME4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2018, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Bartoń, K. MuMIn: Multi-Model Inference; R Package Version 1.43.17; 2020. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 1 June 2020).
- Hirao, T.; Fukatsu, E.; Watanabe, A. Characterization of resistance to pine wood nematode infection in Pinus thunbergii using suppression subtractive hybridization. BMC Plant. Biol. 2012, 12, 13. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, D.; Yonemichi, T.; Inoue, H.; Hirao, T.; Watanabe, A.; Yamada, T. Comparison of histological responses and tissue damage expansion between resistant and susceptible Pine thunbergii infected with pine wood nematode Bursaphelenchus xylophilus. J. For. Res. 2014, 19, 285–294. [Google Scholar] [CrossRef]
- Kuroda, K. Inhibiting factors of symptom development in several Japanese red pine (Pinus densiflora) families selected as resistant to pine wilt. J. For. Res. 2004, 9, 217–224. [Google Scholar] [CrossRef]
- Nakajima, G.; Iki, T.; Yamanobe, T.; Nakamura, K.; Aikawa, T. Spatial and temporal distribution of Bursaphelenchus xylophilus inoculated in grafts of a resistant clone of Pinus thunbergii. J. For. Res. 2019, 24, 93–99. [Google Scholar] [CrossRef]
- Matsunaga, K.; Watanabe, A. Outline of the results of the “Project to advance the development of technology for varieties of Japanese black pine and red pine resistant to the pine wood nematode” commissioned by the Forestry Agency, Ministry of Agriculture, Forestry and Fisheries of Japan—Characterization of pine wood nematode and development of 2nd generation resistant varieties. For. Genet. Breed. 2018, 7, 115–119. (In Japanese) [Google Scholar]
- Iwaizumi, M.G. The development of 2nd generation resistant Japanese red pine varieties in collaboration with Prefecture. For. Genet. Breed. 2018, 7, 159–161. (In Japanese) [Google Scholar]
| Family Name | Resistance Ranking * | Number of Seedlings Used in the Inoculation Tests at Each Study Site in Each Year | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2015 | 2016 | 2017 | |||||||||||||
| TBO | FTBC | KABO | SSG | KYBO | TBO | FTBC | KABO | SSG | KYBO | TBO | FTBC | KABO | KYBO | ||
| M90 | 4 | 27 | 37 | 22 | 31 | 32 | 35 | 40 | 20 | 40 | 22 | 33 | 34 | 25 | 24 |
| N37 | 4 | 28 | 32 | 23 | 29 | 32 | 27 | 32 | 28 | 35 | 16 | 30 | 29 | 23 | 19 |
| T54 | 2 | 44 | 40 | 33 | 40 | 39 | 57 | 39 | 41 | 37 | 37 | 55 | 40 | 21 | 36 |
| SO6 | - | 37 | 40 | 28 | 40 | 32 | 50 | 40 | 37 | 40 | 34 | 44 | 37 | 28 | 17 |
| CT7 | - | 37 | 37 | 32 | 40 | 40 | 35 | 40 | 7 | 40 | 27 | 35 | 39 | 32 | 29 |
| TT13 | - | 40 | 40 | 27 | 19 | 39 | 50 | 39 | 11 | 40 | 32 | 28 | 39 | 31 | 26 |
| Deviance | Degree of Freedom | Significance Pr (>χ2) | ||
|---|---|---|---|---|
| Seedling height | 10.369 | 1 | 0.0013 | p < 0.01 |
| Test site | 242.816 | 4 | <2.2 × 10−16 | p < 0.01 |
| Year | 101.763 | 2 | <2.2 × 10−16 | p < 0.01 |
| Test site × Year | 29.450 | 7 | 0.0001 | p < 0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iki, T.; Matsunaga, K.; Hirao, T.; Ohira, M.; Yamanobe, T.; Iwaizumi, M.G.; Miura, M.; Isoda, K.; Kurita, M.; Takahashi, M.; et al. Effects of Temperature Factors on Resistance against Pine Wood Nematodes in Pinus thunbergii, Based on Multiple Location Sites Nematode Inoculation Tests. Forests 2020, 11, 922. https://doi.org/10.3390/f11090922
Iki T, Matsunaga K, Hirao T, Ohira M, Yamanobe T, Iwaizumi MG, Miura M, Isoda K, Kurita M, Takahashi M, et al. Effects of Temperature Factors on Resistance against Pine Wood Nematodes in Pinus thunbergii, Based on Multiple Location Sites Nematode Inoculation Tests. Forests. 2020; 11(9):922. https://doi.org/10.3390/f11090922
Chicago/Turabian StyleIki, Taiichi, Koji Matsunaga, Tomonori Hirao, Mineko Ohira, Taro Yamanobe, Masakazu G Iwaizumi, Masahiro Miura, Keiya Isoda, Manabu Kurita, Makoto Takahashi, and et al. 2020. "Effects of Temperature Factors on Resistance against Pine Wood Nematodes in Pinus thunbergii, Based on Multiple Location Sites Nematode Inoculation Tests" Forests 11, no. 9: 922. https://doi.org/10.3390/f11090922
APA StyleIki, T., Matsunaga, K., Hirao, T., Ohira, M., Yamanobe, T., Iwaizumi, M. G., Miura, M., Isoda, K., Kurita, M., Takahashi, M., & Watanabe, A. (2020). Effects of Temperature Factors on Resistance against Pine Wood Nematodes in Pinus thunbergii, Based on Multiple Location Sites Nematode Inoculation Tests. Forests, 11(9), 922. https://doi.org/10.3390/f11090922





