Abstract
Extensive decline and mortality events of alder trees have recently been observed in several riparian ecosystems in Italy. Since there is little information about the aetiology of this disease and given the high ecological relevance of riparian ecosystems, an in-depth study was conducted in three sites spanning from the Mediterranean to Alpine regions. From spring 2019 to spring 2020, 261 samples of bleeding cankers, rhizosphere soil and leaves used as baits along waterways were collected and used for Phytophthora isolation. Based on morphology, colony appearance and DNA sequence data, 10 species belonging to 6 clades were identified. These included P. plurivora (84 isolates), P. pseudocryptogea (50), P. hydropathica (18), P. gonapodyides (14), P. bilorbang (13), P. pseudosyringae (12), P. lacustris (11), P. acerina (7), P. cactorum (1) and one isolate of the hybrid Phytophthora ×serendipita. In addition, two new Phytophthora species, one of which is described here as Phytophthora alpina sp. nov., were isolated. The pathogenicity of P. alpina and other species obtained from samples collected in the green alder stand was assessed on 3-year-old seedlings. All species proved to be pathogenic on green alder causing symptoms congruent with field observations. Results obtained have allowed us to expand knowledge about alder decline aetiology. The diversity of pathogenicity of Phytophthora species associated with symptomatic alder trees suggested that no single agent is responsible for the disease, but that it is the result of multiple infections of different Phytophthora species, variable in assemblages among sites.
1. Introduction
Alder is the common name for an anemophilous woody genus (Alnus Mill.) of forest trees and shrubs that encompasses about 30 species mainly distributed in the Mediterranean, temperate and boreal areas of the northern hemisphere [1,2]. Alders are actinorhizal plants that play an important ecological role in riparian ecosystems through atmospheric nitrogen fixation, filtration and purification of waterlogged soils as well as providing a refuge for terrestrial and aquatic organisms and helping to stabilize stream banks [3,4,5].
Over the past 20 years, different alder species in both Europe and North America have been severely impacted by decline and mortality events [6,7,8,9]. Declining alder trees are characterized by a complex symptomology including shoot blight, progressive canopy dieback, bleeding cankers, epicormic shoots on trunk, and root and collar rot [10].
In Europe initially, the main causal agent was identified as Phytophthora alni s. lat., a species complex the isolates of which have recently been renamed as Phytophthora uniformis, P. ×multiformis (resulted from the hybridization between two unknown Phytophthora species) and P. ×alni (from the hybridization between P. uniformis and P. ×multiformis) [11]. Phytophthora ×alni is considered more aggressive than P. ×multiformis and P. uniformis [12]. However, recent studies have demonstrated that other Phytophthora species are involved in the aetiology of alder decline. In particular, the plurivorous pathogen P. plurivora has recurrently been isolated from declining alders in different countries [13,14,15] and its pathogenicity towards alders has been demonstrated [15,16].
In North America Phytophthora chlamydospora, P. gonapodyides and P. siskiyouensis are considered the most important species involved in the aetiology of root and stem alder diseases [9] and the pathogenicity of these species was demonstrated on Italian, red and white alder [17,18]. At the same time, P. uniformis was reported in Alaskan soils around thin-leaf alder but not in association with declining trees [7].
In Italy four alder species occur naturally: black, green, grey and Italian alder. Black alder is widespread across the country and represents the key component of riparian vegetation along streams [19]. The other species have a more limited geographical distribution. In particular, Italian alder is naturally present in southern Italy whereas green and grey alder are common in the Alpine region [20,21,22].
Although symptoms of alder decline along riparian ecosystems have long been known in Italy, until now few studies have investigated the pathogens involved [23]. Recently, Phytophthora acerina, P. plurivora and P. pseudocryptogea have been reported as the main species associated with declining black alder trees along several torrential streams in Sardinia [15]. Further surveys have allowed the same symptoms to be discovered on green and grey alders in several natural ecosystems in the Alpine region along the border between Italy and Austria.
Therefore, given the growing expansion of alder decline events in riparian ecosystems in Italy and the limited information available about the pathogens involved, a study was conducted to isolate, identify and characterize the main pathogens associated.
2. Materials and Methods
2.1. Study Sites, Field Surveys and Sampling Procedure
From spring 2019 to spring 2020, the health status of black, green and grey alder trees was monitored in three natural ecosystems located in the Sardinia and Veneto regions (Table 1). The plants were checked for the presence of disease symptoms on branches and stem (dieback, exudates, bleeding cankers and epicormic shoots), and on the collar and root system (exudates and necrosis). In each site a 100 m long transect was established along the waterway and 9–18 symptomatic alder trees per transect were sampled for Phytophthora isolation (Table 1).

Table 1.
Study sites information and number of stem (S), rhizosphere (R) and leaf (L) samples used for Phytophthora isolation.
For each selected plant, a rhizosphere soil sample (about 300 g of soil and fine roots) was collected around the collar. In addition, on the same plants a bark sample was taken from the margin of an active bleeding canker on the stem. The water in the streams of site 1 and 2 was also monitored for Phytophthora following the method of Huberli et al. [24]. In particular, along the transect in each waterway from 5 to 13 nylon mesh bags containing 10 young elder (Sambucus nigra L.) leaves each to use as baits were positioned near the root systems of alder trees [15].
2.2. Phytophthora Isolation and Identification
In the laboratory rhizosphere soil samples were placed in plastic boxes and flooded with 2 L of distilled water. After 24 h, young elder leaves were placed on the water surface and used as baits [25]. Boxes were kept at 18–20 °C under natural daylight and after 3–5 days, leaves showing dark spots were cut in small pieces (5 mm2) and placed on Petri dishes containing potato dextrose agar (PDA 39 g/L, Oxoid Ltd., Basingstoke, UK) supplemented with 100 mL/L of carrot juice, 0.013 g/L of pimaricin and 0.05 g/L of hymexazol (PDA+) [25].
Isolations of Phytophthora species were also directly attempted from inner bark samples taken from bleeding cankers. After removing the outer bark, small fragments were aseptically cut from the margin of each lesion with a sterile scalpel and placed onto 90 mm Petri dishes containing PDA+. The dishes were incubated in the dark at 20 °C and examined every 12 h. Hyphal tips from the emerging colonies were sub-cultured on carrot agar (CA) [26] and incubated at 20 °C in the dark.
After a week the mesh bags floating in the streams were collected and transferred to the laboratory to be processed. Leaves with necrotic spots were cleaned with distilled water, placed to dry on sterile papers, cut in small fragments (5 mm2) and used for Phytophthora isolations as described above.
All Phytophthora isolates were initially grouped in morphotypes on the basis of colony growth characteristics including surface and reverse colony appearance observed after 7 days of incubation on PDA and CA at 20 °C in the dark and morpho-biometric data of oogonia and sporangia. To enhance sporangia production, CA plugs (5 mm diameter) of each isolate were placed in Petri dishes containing unsterile pond water and three fine root alder samples (1 cm long). Petri dishes were kept at 20 °C in the dark and sporangia production was assessed every 12 h for 4 days.
For the putative new species colony morphology was determined on 7-day-old cultures incubated at 20 °C in the dark on CA, PDA and malt extract agar (MEA, 20 g/L, Oxoid Ltd.). Cardinal temperatures for growth were evaluated on CA plates incubated at 2, 5, 10, 15, 20, 25, 30 and 35 °C (± 0.5 °C) in the dark. Five replicate plates of each isolate were made and colony diameter was measured after 7 days. Size and shape of fifty chlamydospores, hyphal swellings, sporangia and pedicels were recorded for each isolate. Fifty gametangia were examined after 10–20 days on CA at 20 °C. Measurements and photos of the main morphological structures were taken at 400× and 600× magnification and recorded using the software Motic Images Plus 3.0 paired with a Moticam 10+ camera connected to a Motic BA410E microscope. Sporangia dimensions are presented as mean values ± standard deviation.
Representative isolates of each species were stored on PDA and CA slants under oil in the culture collection of the Dipartimento Territorio e Sistemi Agro-Forestali, Università degli Studi di Padova.
Two representative cultures of the new species including the ex-type culture were deposited at the Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands, and nomenclatural data in MycoBank (www.MycoBank.org) [27]. The holotype was lodged with the herbarium of Westerdijk Fungal Biodiversity Institute as a dried culture on CA.
2.3. DNA Extraction, Polymerase Chain Reaction (PCR) Amplification, and Sequencing
Genomic DNA was extracted from mycelium of 5-day-old cultures grown on PDA at 20 °C using Instagene Matrix (BioRad Laboratories, Hercules, CA, USA). For all isolates the entire internal transcribed spacer (ITS) region of the ribosomal DNA, including the 5.8S rRNA gene, was amplified and sequenced using primers ITS1 and ITS4 [28]. ITS sequences were used to confirm the identification at the species level. For six isolates belonging to the ITS clade 1 [29] including those of the new species, another two DNA regions namely ß-tubulin (Btub) and cytochrome c oxidase subunit I (cox1), were amplified and sequenced using the primer-pairs TUBUF2/TUBUR1 and FM84/FM83 [30,31], respectively. Polymerase chain reactions (PCR) were performed in 25 mL reaction mixtures using the GoTaq® Hot Start Green Master Mix (Promega, Milano, Italy) and a SimpliAmp Thermal Cycler (Thermo Fisher Scientific Inc.). For ITS and Btub regions, amplification conditions were as described in Linaldeddu et al. [32], whereas for the cox1 were used the following thermal conditions: initial denaturation at 95 °C for 2 min followed by 35 cycles at 94 °C for 40 s, 54 °C for 50 s, 72 °C for 65 s and a final extension step of 8 min at 72 °C.
PCR products were purified using a EUROGOLD gel extraction kit according to the manufacturer’s instructions (EuroClone S.p.A., Pero, Italy). Both strands were sequenced by BMR Genomics DNA sequencing service (www.bmr-genomics.it). Sequences were edited with FinchTV v1.4.0 (Geospiza, Inc., http://www.geospiza.com/finchtv) and compared with sequences of ex-type culture deposited in GenBank (http://blast.ncbi.nlm.nih.gov). New sequences were deposited in GenBank (Table 2 and Table 3). Alignments and trees are available in TreeBase (study ID S26587).

Table 2.
Details of Phytophthora isolates belonging to the clade 1 included in the phylogenetic analyses. Ex-type cultures are given in bold typeface and newly generated sequences are indicated in italics.

Table 3.
Number of isolates of Phytophthora species obtained from stem (S), rhizosphere (R) and water (W) samples in the investigated sites.
2.4. Phylogenetic Analysis
ITS, Btub and cox1 sequences of six isolates obtained from green alders were compiled in a dataset together with 60 sequences of other 20 Phytophthora species representative of the formally described species in the ITS clade 1 (including ex-type culture) and for which DNA sequences are available in GenBank (Table 2).
Sequence alignments were performed with ClustalX v. 1.83 [33], using the following parameters: pairwise alignment parameters (gap opening = 10, gap extension = 0.1) and multiple alignment parameters (gap opening = 10, gap extension = 0.2, transition weight = 0.5, delay divergent sequences = 25%). Alignments were checked and edited with BioEdit Alignment Editor v. 7.2.5 [34]. Phylogenetic analyses were done with MEGA-X 10.1.8 [35]. All gaps were included in the analyses. The best model of DNA sequence evolution was determined automatically by the software. Maximum likelihood (ML) analyses were performed on a neighbor-joining (NJ) starting tree generated by the software. A bootstrap analysis (1000 replicates) was used to estimate the robustness of nodes.
2.5. Pathogenicity Test
The pathogenicity of the new Phytophthora species and of the others obtained from the green alder stand was tested on 3-year-old green alder seedlings grown in plastic pots (10 cm diameter, 1 L volume). Six seedlings were inoculated with each isolate, and four seedlings were used as control. The inoculated region of the collar was surface-disinfected with 70% ethanol and a small piece of outer and inner bark (5 mm diameter) was removed with a flamed cork borer and replaced with an agar-mycelium plug, of the same size, taken from the margin of an actively growing colony on CA. The inoculation site was covered with cotton wool soaked in sterile water and wrapped in a piece of aluminium foil. Controls were inoculated with a sterile CA plug applied as described above.
All inoculated seedlings were kept in field conditions at 10 to 33 °C and watered regularly for 30 days. At the end of the experimental period, seedlings were checked for the presence of disease symptoms, the outer bark was carefully removed with a scalpel and the length of necrotic lesion surrounding each inoculation point was measured.
Re-isolation of isolates was attempted by transferring 10 pieces of inner bark taken around the margin of the necrotic lesions onto PDA+. Growing colonies were sub-cultured onto CA and PDA, incubated in the dark at 20 °C and identified by morphological and molecular analysis (ITS region).
2.6. Data Analysis
Pathogenicity assay data were checked for normality, then subjected to analysis of variance (ANOVA). Significant differences among mean values were determined using Fisher’s least significant differences multiple range test (p = 0.05) after one-way ANOVA using XLSTAT 2008 software (Addinsoft).
Similarities in fungal taxonomic assemblage among host species and substrates used for isolation were summarized in Venn diagrams using GeneVenn software (http://genevenn.sourceforge.net/).
3. Results
3.1. Field Survey
Field surveys conducted in three riparian ecosystems spanning from the Mediterranean to the Alpine climate region showed the widespread presence of typical Phytophthora disease symptoms on three of the most important alder species in Italy: black, green and grey alder. Disease incidence (estimated as the number of symptomatic plants out of the total number of plants in each transect) ranged from 62.7 (site 1) to 100% (sites 2 and 3) and the rate of mortality from 13.7 (site 1) to 38.9% (site 3). Declining alder showed the same symptomology regardless of species, including wilting foliage, shoot blight, dieback, bleeding cankers and epicormic shoots on stem and branches (Figure 1). Interestingly, on green alder extensive aerial bleeding cankers were observed on the whole canopy including small branches and twigs whereas on black alder exudations were more evident at the collar. Bleeding lesions often girdled the entirely circumference of twigs and branches, causing crown dieback and dying foliage. Epicormic shoots near bleeding cankers on the stem progressively died during the growing season. At the same time, leaves of epicormic shoots on the lower stem often displayed necrotic spots.

Figure 1.
Branch dieback and bleeding cankers on collar and branches of black alder (a–c), grey alder (d–f) and green alder (g–i) trees in the investigated sites. Colony morphology of Phytophthora acerina (j), Phytophthora alpina (k), Phytophthora bilorbang (l), Phytophthora cactorum (m), Phytophthora gonapodyides (n), Phytophthora hydropathica (o), Phytophthora lacustris (p), Phytophthora plurivora (q), Phytophthora pseudocryptogea (r), Phytophthora pseudosyringae (s), Phytophthora ×serendipita (t) and Phytophthora sp. (u) after 7 days growth at 20 °C on CA in the dark.
3.2. Phytophthora Diversity in Declining Alder Stands
From the 261 samples collected in three declining alder stands, 232 Phytophthora isolates belonging to 6 different ITS clades were obtained (Table 3). Of these, 43 isolates were obtained from bleeding cankers, 82 from rhizosphere soil samples and 107 from necrotic spots detected on leaves used as baits along the waterways. Based on morphology, colony appearance and ITS sequence data, Phytophthora isolates were identified as P. plurivora (84 isolates), P. pseudocryptogea (50), P. hydropathica (18), P. gonapodyides (14), P. bilorbang (13), P. pseudosyringae (12), P. lacustris (11), P. acerina (7), P. cactorum (1) and Phytophthora ×serendipita (1) (Table 3).
In addition, 21 isolates on the basis of morphological features and DNA sequence data could not be assigned to any known species of Phytophthora. Five isolates obtained from bleeding cankers of grey alder belong to a new species closely related to P. megasperma and P. crassamura (further morphological and phylogenetic analysis are currently in progress to clarify their taxonomic status), whereas 16 isolates obtained from bleeding cankers and rhizosphere soil samples of green alder were here described as Phytophthora alpina sp. nov.
The most common Phytophthora species detected in this study was P. plurivora, and isolates were obtained from cankers, rhizosphere and waterways of both black and grey alder stands. Phytophthora pseudocryptogea was the second most frequent species and the only one to be isolated from bleeding cankers on all three alder species (Figure 2a). On four black alder trees P. plurivora and P. pseudocryptogea were isolated from the same bark lesions whereas in the rhizosphere the two Phytophthora species were found to cohabit on all 13 trees studied. The other species were isolated less frequently and often preferentially from a single substrate (bark, rhizosphere or water) (Figure 2b). In particular, P. bilorbang, P. gonapodyides, P. hydropathica and P. lacustris were isolated more frequently from waterways, whereas P. cactorum and Phytophthora sp. only from bleeding cankers.
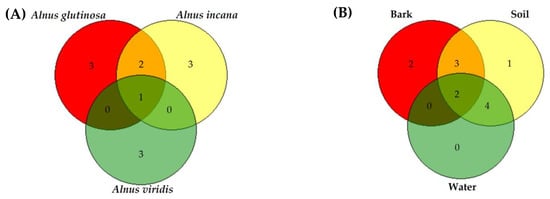
Figure 2.
Venn diagrams illustrating the number of unique and shared Phytophthora species among alder species (A) and substrates used for isolations (B).
The distribution of Phytophthora spp. was variable among the three riparian ecosystems suggesting that site specific factors could influence pathogen assemblages.
3.3. DNA Phylogeny of Phytophthora Clade 1
The phylogenetic relationships among six representative isolates obtained in this study from green alder and the known Phytophthora species belonging to ITS clade 1 were clarified using nuclear (ITS and Btub) and mitochondrial (cox1) gene regions.
Fragments of approximately 800, 920 and 1070 bp were obtained for ITS, Btub and cox1 regions, respectively. Individual gene phylogenies revealed no major conflicts, indicating that the three loci could be combined (data not shown). In particular, the six isolates studied were distributed into two sub-clades placed in clade 1a (Figure 3). The isolate (OV4) clustered with P. ×serendipita, whereas the remaining five isolates clustered together in a well-supported clade (ML bootstrap = 99%) and were considered to represent a new Phytophthora species.
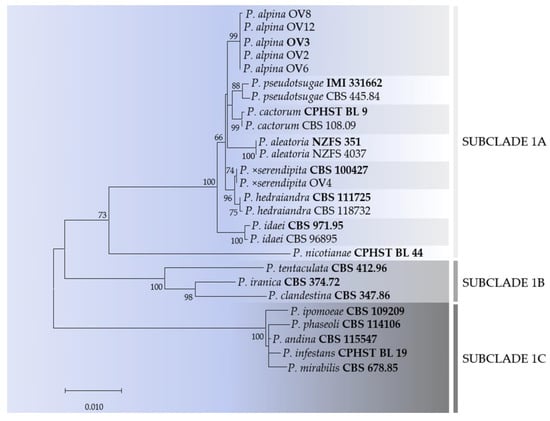
Figure 3.
Maximum likelihood tree obtained from concatenated internal transcribed spacer (ITS), ß-tubulin (Btub) and cytochrome c oxidase subunit I (cox1) sequences of Phytophthora species belonging to ITS clade 1. Data are based on the General Time Reversible model. A discrete Gamma distribution was used to model evolutionary rate differences among sites. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. Bootstrap support values in percentage (1000 replicates) are given at the nodes. Ex-type cultures are in bold.
Phylogenetically, the new Phytophthora species is closely related to P. cactorum, from which it can be distinguished on the basis of 3, 3 and 5 bp in ITS, Btub and cox1 loci, respectively. The other close species is P. hedraiandra from which it differs by a total of 12 nucleotides in the DNA regions studied.
3.4. Taxonomy
Phytophthora alpina Bregant, Montecchio and Linaldeddu sp. nov. (Figure 4).
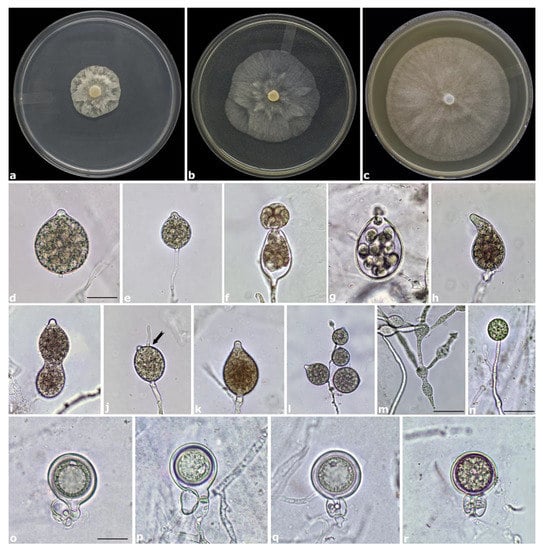
Figure 4.
Colony morphology of Phytophthora alpina (OV3) after 7 days growth at 20 °C on potato dextrose agar (PDA) (a), malt extract agar (MEA) (b) and carrot agar (CA) (c). Papillate sporangia on CA after 24–48 h flooding in nonsterile pond water: caducous with a short pedicel, globose to ovoid (d), persistent (e), releasing zoospores (f,g), distorted (h,i), with a hyphal extension (arrow) (j). Sporangia on simple (k) and sympodial sporangiophores (l). Intercalary hyphal swellings (m). A terminal chlamydospore (n). Mature oogonia with paragynous (o,p) and amphigynous antheridia (q,r) and slight aplerotic oospores. Scale bars = 20 μm. Photo (l) at 400× magnification.
MycoBank: MB836109.
Etymology: the epithet refers to the Alpine environment, where the species was originally found.
Holotype: CBS H-24392
Host/distribution: On bleeding cankers and rhizosphere of declining green alder trees in Italy.
Description: papillate and mainly caducous sporangia were produced rarely on solid agar whereas abundantly in unsterile pond water after 24–36 h. Sporangia were mostly globose to ovoid and only occasionally pyriform, distorted or with a curved apex (Figure 4d–l). Sporangia were terminal or more often formed on sympodial sporangiophores (Figure 4l). Caducous sporangia with a short pedicel, length < 5 µm (av. 3.5 ± 0.8 µm) were observed in all isolates. Sporangia size ranged from 18.3 to 54.3 µm in length (av. 32.7 ± 8.1 µm) and from 13.7 to 38.7 µm in breadth (av. 26.6 ± 6.5 µm) with a length/breadth ratio of 1.2 ± 0.1 (n = 50). Irregular hyphal swellings sometimes with radiating hyphae were abundantly produced in CA and water (Figure 4m). Spherical chlamydospores were mostly terminal and only occasionally intercalary (Figure 4n). Chlamydospores ranged from 14.6 to 33.4 µm in diameter (av. 23.3 ± 3.5 µm). Phytophthora alpina is homothallic. Smooth wall oogonia (av. 30.9 ± 2.4 µm) borne mainly terminally, often on short side hyphae, were abundantly produced by all isolates on CA after 7–10 days. Oospores were spherical and usually aplerotic 25.8 ± 2.1 µm in diameter. Both paragynous and amphigynous antheridia were regularly observed in all isolates (Figure 4o–r). They were hyaline, rounded, club-shaped or irregular, attached near the oogonial stalk, one per oogonium (av. 12.6 ± 1.8 × 10.4 ± 1.6 µm).
Cultural characteristics: All three-culture media supported growth of the species. Colony growth pattern was slight stellate on PDA and MEA whereas without a distinct pattern and with a regular margin on CA. On PDA and MEA colony growth was slow, whereas on CA the colony reached 53 mm diameter in 7 d at 20 °C.
Cardinal temperatures for growth: minimum <2 °C, maximum 30–35 °C and optimum 20 °C. All isolates failed to grow at 35 °C and mycelium did not resume growth when plates were moved to 20 °C.
Material examined: ITALY: Vigo di Cadore, isolated from rhizosphere of a declining green alder tree, 14 October 2019, collected by Carlo Bregant, HOLOTYPE CBS H-24392, a dried culture on CA, culture ex-holotype OV3 = CBS 146801. ITALY: Vigo di Cadore, isolated from a bleeding canker on green alder, 17 December 2019, collected by Carlo Bregant (culture OV2 = CBS 146802). ITALY: Vigo di Cadore, isolated from a bleeding canker on green alder, 26 May 2020, collected by Carlo Bregant (culture OV23).
Notes: Phytophthora alpina is morphologically similar to P. cactorum with which it shares some of the typical features of species belonging to sub-clade 1a, but it differs from P. cactorum through a combination of unique morphological and molecular features such as the presence of hyphal swellings and amphigynous antheridia, a lower minimum temperature for growth, smaller oospores and a total of 11 fixed differences in the ITS, Btub and cox1 sequences.
3.5. Pathogenicity
All four Phytophthora species proved to be pathogenic on green alder. At the end of the experimental period, all seedlings inoculated with P. alpina, P. pseudocryptogea, P. pseudosyringae and P. ×serendipita displayed dark brown inner bark lesions that spread up and down from the inoculation site (Figure 5).

Figure 5.
Symptoms observed on green alder seedlings 30 days after inoculation with Phytophthora alpina (a,b), Phytophthora pseudocryptogea (c,d), Phytophthora pseudosyringae (e,f) and Phytophthora ×serendipita (g,h). Control seedlings (i,j).
The average lesion length differed significantly among species (Table 4). The lesions caused by P. pseudosyringae were significantly larger than those caused by other species. Lesions caused by P. pseudosyringae progressively girdled the stem causing wilting symptoms. In addition, seedlings inoculated with P. pseudosyringae displayed stem exudates, congruent with field observations. The two isolates of P. alpina caused only small necrotic lesions confined to the inoculation site which did not differ statistically from the control.

Table 4.
Mean lesion length ± standard deviation caused by each Phytophthora species on green alder seedlings.
Control seedlings inoculated with sterile CA plugs remained symptomless, only a small light brown discoloration was observed restricted to the inoculation point. All four species were successfully re-isolated from the margin on necrotic inner bark lesions of all seedlings, thus fulfilling Koch’s postulates.
4. Discussion
Results obtained in this study have allowed us to clarify both symptomatology and aetiology related to severe decline affecting alder species in three natural ecosystems in Italy. The three investigated sites are characterized by different environmental conditions ranging from the Mediterranean climate of site 1 located in Sardinia to the extremely cold Alpine habitat of site 3, at 1803 m a. s. l. in the centre of the Dolomite mountains.
On all alder species, the most frequent disease symptoms consisted of canopy dieback associated with bleeding cankers on stem and branches. In particular, green alder was the species more severely impacted by the disease. The symptoms observed in this study were similar to those previously reported by various authors on different alder species in Europe and North America [8,10,15,17].
Twelve Phytophthora species belonging to 6 different phylogenetic clades were isolated and identified by means of morphological characters and DNA sequence data. These included P. acerina, P. alpina, P. bilorbang, P. cactorum, P. gonapodyides, P. hydropathica, P. lacustris, P. plurivora, P. pseudocryptogea, P. pseudosyringae, P. ×serendipita and unidentified Phytophthora species belonging to ITS clade 6. In particular, six different species were isolated in both sites 1 and 2 whereas four in site 3. An interesting issue in this study is related to the absence of Phytophthora uniformis, P. ×alni and P. ×multiformis. This result is in accordance with Phytophthora assemblages detected in recent studies conducted in various alder ecosystems in North America and Europe [9,14,15], in which none of these species was associated with declining alder trees.
Phytophthora plurivora was the dominant species detected in this study, followed by P. pseudocryptogea. The high isolation frequency of P. plurivora especially in site 1, is in accordance with results of previous studies conducted on declining black alder trees in Italy and Turkey [14,15]. Phytophthora plurivora is a widespread pathogen in natural environments and agriculture ecosystems in Europe [36,37]. It has also been reported from natural environments (streams and forest soil) in the US [38]. Molecular analyses based on microsatellite data seem to support the possible introduction of P. plurivora into the US from Europe [36]. They also emphasize that Germany could represent the centre of origin of P. plurivora populations in the Alps, Balkans and Eastern Europe [36].
Phytophthora pseudocryptogea was the second most common species and the only one detected in all three sites from bleeding cankers of all three alder species. Phytophthora pseudocryptogea was originally described from roots of dying Isopogon buxifolius, a small shrub endemic to the south coast of Western Australia [39], and subsequently from various natural niches [40,41] and diseased crops [42] in different countries. On black alder trees, P. plurivora and P. pseudocryptogea were often isolated from the same samples suggesting a potential synergistic interaction in the pathogenesis process.
Apart from P. plurivora and P. pseudocryptogea the other 10 Phytophthora species obtained in this study were, in most cases, isolated from a single site, suggesting that various site-specific factors such as microclimate conditions, human disturbance and alternative hosts surrounding riparian ecosystems may influence the presence/absence of these species. In particular, the Alpine green alder ecosystem (site 3) with average minimum, mean and maximum annual temperatures of −8 °C, 4 °C and 18 °C, respectively (extreme low temperature −21 °C), is a site probably inhospitable for several Phytophthora species. The low cardinal temperature for growth <2 °C of P. alpina suggest that it is well adapted to survive in this cold environment. The minimum temperature for growth of P. alpina is lower than other species in clade 1a. Phytophthora alpina is characterized by having principally caducous sporangia, a feature that could explain the multiple aerial bark lesions observed on green alder trees. Except for the isolate OV8, all isolates of P. alpina were genetically uniform sharing identical ITS, Btub and cox1 sequences. The isolate OV8 presented a single polymorphism in the Btub sequence.
The other species consistently detected from aerial bark lesions in site 3 was P. pseudosyringae, a plurivorous pathogen involved in the aetiology of several diseases in low-temperature habitats [43,44]. Phylogenetically P. pseudosyringae falls within phylogenetic clade 3, together with other species such as P. nemorosa and P. ilicis typically associated with aerial infections on stems, leaves and shoots [45,46]. It is also widespread along forest streams in North America, including Alaska [47]. In this study, P. pseudosyringae proved to be a very aggressive pathogen of green alder. Also P. ×serendipita caused extensive necrosis on inoculated green alder seedlings. Recently, two other hybrids closely related to P. ×serendipita were isolated in site 3 (Linaldeddu, unpublished). The low cardinal temperature for growth, the aggressiveness and polyphagous nature of the Phytophthora species detected in site 3 could represent a serious risk for the health of different ecosystems in the Alpine region. The diversity and ecological impact of Phytophthora species associated with ecological niches of Alpine environments and low temperature habitats is still poorly understood. In a recent study, two new species, namely Phytophthora cacuminis and P. oreophila, were discovered in alpine formations in Australia and Tasmania [40].
In this study a cryptic Phytophthora species was isolated from bleeding cankers of grey alder. Phylogenetically, it is closely related to P. megasperma but the two species differed by 1 fixed polymorph in the ITS region and 4 in the Btub as well as several morphological features (data not shown). Pathogenicity trials on grey alder seedlings are currently in progress to evaluate the aggressiveness of this undescribed Phytophthora species.
The other most frequent species isolated in this study were P. bilorbang, P. gonapodyides and P. lacustris, all belonging to ITS clade 6. All three species have been isolated mainly from leaves used as baits along waterways. This agrees with results of previous studies, confirming the wide spread of these species in aquatic environments where they can act as opportunistic pathogens [48,49,50]. The other species frequently isolated along the black alder waterway was P. hydropathica, a pathogenic species known to occur principally in the irrigation water of forest nurseries [51,52].
Finally, P. acerina was isolated from both black and grey alder. The ITS sequences of the isolates from grey alder were identical to those of the ex-type culture (CBS 133931) but differ by 1 nucleotide in the ITS2 region from isolates obtained from black alder. This difference among P. acerina isolates was also reported in the original description of Italian and Japanese isolates obtained from prairie gentian [53]. The isolates from black alder conformed to Japanese isolates, whereas all isolates were identical morphologically.
In recent years, several extensive studies have been conducted throughout North America and Europe on alder decline caused by Phytophthora species [8,9,10]. However, relatively few of these research studies have focused on riparian ecosystems in Italy [15]. This is the most comprehensive investigation on Phytophthora species involved in the aetiology of alder decline to date in this country.
An extensive survey is currently in progress to map the sites affected by this disease along the Alps and in the mountainous areas of central Italy including Sardinia, where black alder characterizes the landscape of several mountain streams.
5. Conclusions
The diversity and pathogenicity of Phytophthora species associated with declining alder trees detected in this study and in other similar research [9,15] suggest that no single agent is responsible for the disease, but that it is the result of multiple infections of different Phytophthora species, variable in assemblages among sites.
Author Contributions
B.T.L. conceptualization; B.T.L., C.B., G.P.S. and A.B. field survey, sample collection and assay; B.T.L., C.B., L.M. (Lucio Montecchio) and L.M. (Lucia Maddau) data analysis; B.T.L. and L.M. (Lucio Montecchio) funding acquisition; B.T.L. draft writing; B.T.L., L.M. (Lucio Montecchio) and L.M. (Lucia Maddau) review and manuscript editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, Z.D.; Li, J.H. Phylogenetics and biogeography of Alnus (Betulaceae) inferred from sequences of nuclear ribosomal DNA its region. Int. J. Plant Sci. 2004, 165, 325–335. [Google Scholar] [CrossRef]
- Ren, B.Q.; Xiang, X.G.; Chen, Z.D. Species identification of Alnus (Betulaceae) using nrDNA and cpDNA genetic markers. Mol. Ecol. Resour. 2010, 10, 594–605. [Google Scholar] [CrossRef]
- Wipfli, M.; Musslewhite, J. Density of red alder (Alnus rubra) in headwaters influences invertebrate and detritus subsidies to downstream fish habitats in Alaska. Hydrobiologia 2004, 520, 153–163. [Google Scholar] [CrossRef]
- Claessens, H.; Oosterbaan, A.; Savill, P.; Rondeux, J. A review of the characteristics of black alder (Alnus glutinosa (L.) Gaertn.) and their implications for silvicultural practices. Forestry 2010, 83, 163–175. [Google Scholar] [CrossRef]
- Handa, I.T.; Aerts, R.; Berendse, F.; Berg, M.P.; Bruder, A.; Butenschoen, O.; Chauvet, E.; Gessner, M.O.; Jabiol, J.; Makkonen, M.; et al. Consequences of biodiversity loss for litter decomposition across biomes. Nature 2014, 509, 218–221. [Google Scholar] [CrossRef]
- Brasier, C.; Rose, J.; Gibbs, J. An unusual Phytophthora associated with widespread alder mortality in Britain. Plant Pathol. 1995, 44, 999–1007. [Google Scholar] [CrossRef]
- Adams, G.C.; Catal, M.; Trummer, L.; Hansen, E.M.; Reeser, P.; Worrall, J.J. Phytophthora alni subsp. uniformis found in Alaska beneath thinleaf alders. Plant Health Prog. 2008, 9, 38. [Google Scholar] [CrossRef]
- Cerny, K.; Strnadova, V. Phytophthora alni decline: Disease symptoms, causal agent and its distribution in the Czech Republic. Plant Prot. Sci. 2010, 46, 12–18. [Google Scholar] [CrossRef]
- Sims, L.L.; Sutton, W.; Reeser, P.; Hansen, E.M. The Phytophthora species assemblage and diversity in riparian alder ecosystems of western Oregon, USA. Mycologia 2015, 107, 889–902. [Google Scholar] [CrossRef]
- Bjelke, U.; Boberg, J.; Oliva, J.; Tattersdill, K.; McKie, B.G. Dieback of riparian alder caused by the Phytophthora alni complex: Projected consequences for stream ecosystems. Freshw. Biol. 2016, 61, 565–579. [Google Scholar] [CrossRef]
- Husson, C.; Aguayo, J.; Revellin, C.; Frey, P.; Ioos, R.; Marcais, B. Evidence for homoploid speciation in Phytophthora alni supports taxonomic reclassification in this species complex. Fungal Genet. Biol. 2015, 77, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Brasier, C.M.; Kirk, S.A. Comparative aggressiveness of standard and variant hybrid alder phytophthoras, Phytophthora cambivora and other Phytophthora species on bark of Alnus, Quercus and other woody hosts. Plant Pathol. 2001, 50, 218–229. [Google Scholar] [CrossRef]
- Trzewik, A.; Orlikowski, L.B.; Oszako, T.; Nowakowska, J.A.; Orlikowska, T. The characterization of Phytophthora isolates obtained from diseased Alnus glutinosa in Poland. Balt. For. 2015, 21, 44–50. [Google Scholar]
- Aday Kaya, A.G.; Lehtijärvi, A.; Şaşmaz, Y.; Nowakowska, J.A.; Oszako, T.; Doğmuş Lehtijärvi, H.T.; Woodward, S. Phytophthora species detected in the rhizosphere of Alnus glutinosa stands in the floodplain forests of Western Turkey. For. Pathol. 2018, 48, 11–14. [Google Scholar] [CrossRef]
- Seddaiu, S.; Linaldeddu, B.T. First Report of Phytophthora acerina, P. plurivora, and P. pseudocryptogea associated with declining common alder trees in Italy. Plant Dis. 2020, 104, 1874. [Google Scholar] [CrossRef]
- Zamora-Ballesteros, C.; Haque, M.M.U.; Diez, J.J.; Martín-García, J. Pathogenicity of Phytophthora alni complex and P. plurivora in Alnus glutinosa seedlings. For. Pathol. 2017, 47, e12299. [Google Scholar] [CrossRef]
- Rooney-Latham, S.; Blomquist, C.L.; Pastalka, T.; Costello, L. Collar rot on Italian alder trees in California caused by Phytophthora siskiyouensis. Plant Health Prog. 2009, 10, 20. [Google Scholar] [CrossRef]
- Navarro, S.; Sims, L.; Hansen, E. Pathogenicity to alder of Phytophthora species from riparian ecosystems in western Oregon. For. Pathol. 2015, 45, 358–366. [Google Scholar] [CrossRef]
- Kajba, D.; Gračan, J. EUFORGEN Technical Guidelines for Genetic Conservation and Use for Black Alder (Alnus glutinosa); International Plant Genetic Resources Institute: Rome, Italy, 2003; p. 4. [Google Scholar]
- Ducci, F.; Tani, A. EUFORGEN Technical Guidelines for Genetic Conservation and Use of Italian Alder (Alnus cordata); Bioversity International: Rome, Italy, 2009; p. 6. [Google Scholar]
- Mauri, A.; Caudullo, G. Alnus viridis in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publications Office of the European Union: Luxembourg, 2016; p. e01f0e4+. [Google Scholar]
- Houston Durrant, T.; de Rigo, D.; Caudullo, G. Alnus incana in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publications Office of the European Union: Luxembourg, 2016; p. e01ff87+. [Google Scholar]
- Pisetta, M.; Montecchio, L.; Longa, C.M.O.; Salvadori, C.; Zottele, F.; Maresi, G. Green alder decline in the Italian Alps. For. Ecol. Manag. 2012, 281, 75–83. [Google Scholar] [CrossRef]
- Huberli, D.; Hardy, G.E.S.J.; White, D.; Williams, N.; Burgess, T.I. Fishing for Phytophthora from Western Australia’s waterways: A distribution and diversity survey. Australas. Plant Pathol. 2013, 42, 251–260. [Google Scholar] [CrossRef]
- Linaldeddu, B.T.; Mulas, A.A.; Bregant, C.; Piras, G.; Montecchio, L. First Report of Phytophthora pistaciae causing root and collar rot on nursery plants of Pistacia lentiscus in Italy. Plant Dis. 2020, 104, 1564. [Google Scholar] [CrossRef]
- Erwin, C.D.; Ribeiro, O.K. Phytophthora Diseases Worldwide; APS Press: Saint Paul, MN, USA, 1996. [Google Scholar]
- Crous, P.W.; Gams, W.; Stalpers, J.A.; Robert, V.; Stegehuis, G. MycoBank: An online initiative to launch mycology into the 21st century. Stud. Mycol. 2004, 50, 19–22. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols, a Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Cooke, D.E.L.; Drenth, A.; Duncan, J.M.; Wagels, G.; Brasier, C.M. A molecular phylogeny of Phytophthora and related oomycetes. Fungal Genet. Biol. 2000, 30, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.N.; Tooley, P.W. Phylogenetic relationships among Phytophthora species inferred from sequence analysis of mitochondrially encoded cytochrome oxidase I and II genes. Mycologia 2003, 95, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Kroon, L.P.N.M.; Bakker, F.T.; Van Den Bosch, G.B.M.; Bonants, P.J.M.; Flier, W.G. Phylogenetic analysis of Phytophthora species based on mitochondrial and nuclear DNA sequences. Fungal Genet. Biol. 2004, 41, 766–782. [Google Scholar] [CrossRef] [PubMed]
- Linaldeddu, B.T.; Franceschini, A.; Alves, A.; Phillips, A.J.L. Diplodia quercivora sp. nov.: A new species of Diplodia found on declining Quercus canariensis trees in Tunisia. Mycologia 2013, 105, 1266–1274. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. In Nucleic Acids Symposium Series; Oxfrod University Press: Oxford, UK, 1999; Volume 41, pp. 95–98. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Schoebel, C.N.; Stewart, J.; Gruenwald, N.J.; Rigling, D.; Prospero, S. Population history and pathways of spread of the plant pathogen Phytophthora plurivora. PLoS ONE 2014, 9, e85368. [Google Scholar] [CrossRef]
- Linaldeddu, B.T.; Bregant, C.; Montecchio, L.; Favaron, F.; Sella, L. First report of Phytophthora acerina, P. pini and P. plurivora causing root rot and sudden death on olive trees in Italy. Plant Dis. 2020, 104, 996. [Google Scholar] [CrossRef]
- Balci, Y.; Balci, S.; Eggers, J.; MacDonald, W.L.; Juzwik, J.; Long, R.P.; Gottschalk, K.W. Phytophthora spp. associated with forest soils in eastern and north-central US oak ecosystems. Plant Dis. 2007, 91, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Safaiefarahani, B.; Mostowfizadeh-Ghalamfarsa, R.; Hardy, G.E.S.J.; Burgess, T.I. Re-evaluation of the Phytophthora cryptogea species complex and the description of a new species, Phytophthora pseudocryptogea sp. nov. Mycol. Prog. 2015, 14, 108. [Google Scholar] [CrossRef]
- Khaliq, I.; Hardy, G.E.S.J.; Mc Dougall, K.L.; Burgess, T.I. Phytophthora species isolated from alpine and sub-alpine regions of Australia, including the description of two new species; Phytophthora cacuminis sp. nov. and Phytophthora oreophila sp. nov. Fungal Biol. 2018, 123, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Bose, T.; Wingfield, M.J.; Roux, J.; Vivas, M.; Burgess, T.I. Community composition and distribution of Phytophthora species across adjacent native and non-native forests of South Africa. Fungal Ecol. 2018, 36, 17–25. [Google Scholar] [CrossRef]
- Linaldeddu, B.T.; Bregant, C.; Ruzzon, B.; Montecchio, L. Coniella granati and Phytophthora palmivora the main pathogens involved in pomegranate dieback and mortality in north-eastern Italy. Ital. J. Mycol. 2020, 49, 92–100. [Google Scholar]
- Scanu, B.; Linaldeddu, B.T.; Franceschini, A. First report of Phytophthora pseudosyringae associated with ink disease of Castanea sativa in Italy. Plant Dis. 2010, 94, 1068. [Google Scholar] [CrossRef]
- Fajardo, S.N.A.; Valenzuela, S.B.; Dos Santos, A.F.C.; González, M.P.A.; Sanfuentes, E.A. Phytophthora pseudosyringae associated with the mortality of Nothofagus obliqua in a pure stand in central-southern Chile. For. Pathol. 2017, 47, e12361. [Google Scholar] [CrossRef]
- Wickland, A.C.; Jensen, C.E.; Rizzo, D.M. Geographic distribution, disease symptoms and pathogenicity of Phytophthora nemorosa and Phytophthora pseudosyringae in California, USA. For. Pathol. 2008, 38, 288–298. [Google Scholar] [CrossRef]
- Hansen, E.M.; Reeser, P.W.; Sutton, W. Ecology and pathology of Phytophthora ITS clade 3 species in forests in western Oregon, USA. Mycologia 2017, 109, 100–114. [Google Scholar] [CrossRef]
- Reeser, P.W.; Hansen, E.M.; Sutton, W.; Remigi, P.; Adams, G.C. Phytophthora species in forest streams in Oregon and Alaska. Mycologia 2011, 103, 22–35. [Google Scholar] [CrossRef]
- Aghighi, S.; Hardy, G.E.S.J.; Scott, J.K.; Burgess, T.I. Phytophthora bilorbang sp. nov., a new species associated with the decline of Rubus anglocandicans (European blackberry) in Western Australia. Eur. J. Plant Pathol. 2012, 133, 841–855. [Google Scholar] [CrossRef]
- Nagel, J.H.; Slippers, B.; Wingfield, M.J.; Gryzenhout, M. Multiple Phytophthora species associated with a single riparian ecosystem in South Africa. Mycologia 2015, 107, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Matsiakh, I.; Oszako, T.; Kramarets, V.; Nowakowska, J.A. Phytophthora and Pythium species detected in rivers of the Polish-Ukrainian border areas. Balt. For. 2016, 22, 230–238. [Google Scholar]
- Hong, C.X.; Gallegly, M.E.; Richardson, P.A.; Kong, P.; Moorman, J.D.; Lea-Cox, J.D.; Ross, D.S. Phytophthora hydropathica, a new pathogen identified from irrigation water, Rhododendron catawbiense and Kalmia latifolia. Plant Pathol. 2010, 59, 913–921. [Google Scholar] [CrossRef]
- Hulvey, J.; Gobena, D.; Finley, L.; Lamour, K. Co-occurrence and genotypic distribution of Phytophthora species recovered from watersheds and plant nurseries of eastern Tennessee. Mycologia 2010, 102, 1127–1133. [Google Scholar] [CrossRef]
- Ginetti, B.; Moricca, S.; Squires, J.N.; Cooke, D.E.L.; Ragazzi, A.; Jung, T. Phytophthora acerina sp. nov., a new species causing bleeding cankers and dieback of Acer pseudoplatanus trees in planted forests in northern Italy. Plant Pathol. 2014, 63, 858–876. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).