Importance of Soil, Stand, and Mycorrhizal Fungi in Abies balsamea Establishment in the Boreal Forest
Abstract
1. Introduction
2. Materials and Methods
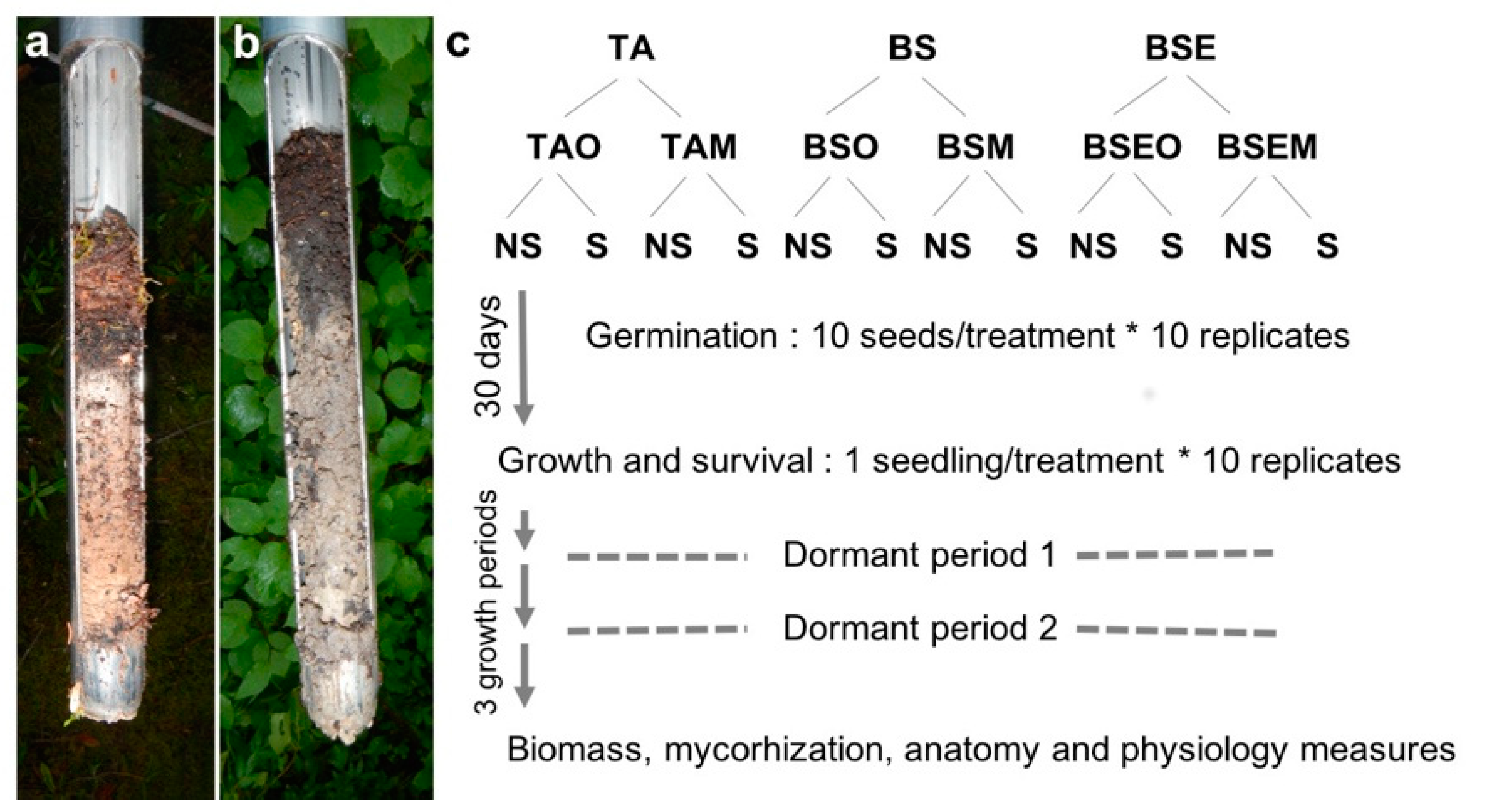
2.1. Soil Origin and Sterilization
2.2. Germination Process
2.3. Growing Period
2.4. Seedlings Mycorrhizal Status, Biomass, Anatomy, and Physiology
2.5. Statistical Analyses
3. Results
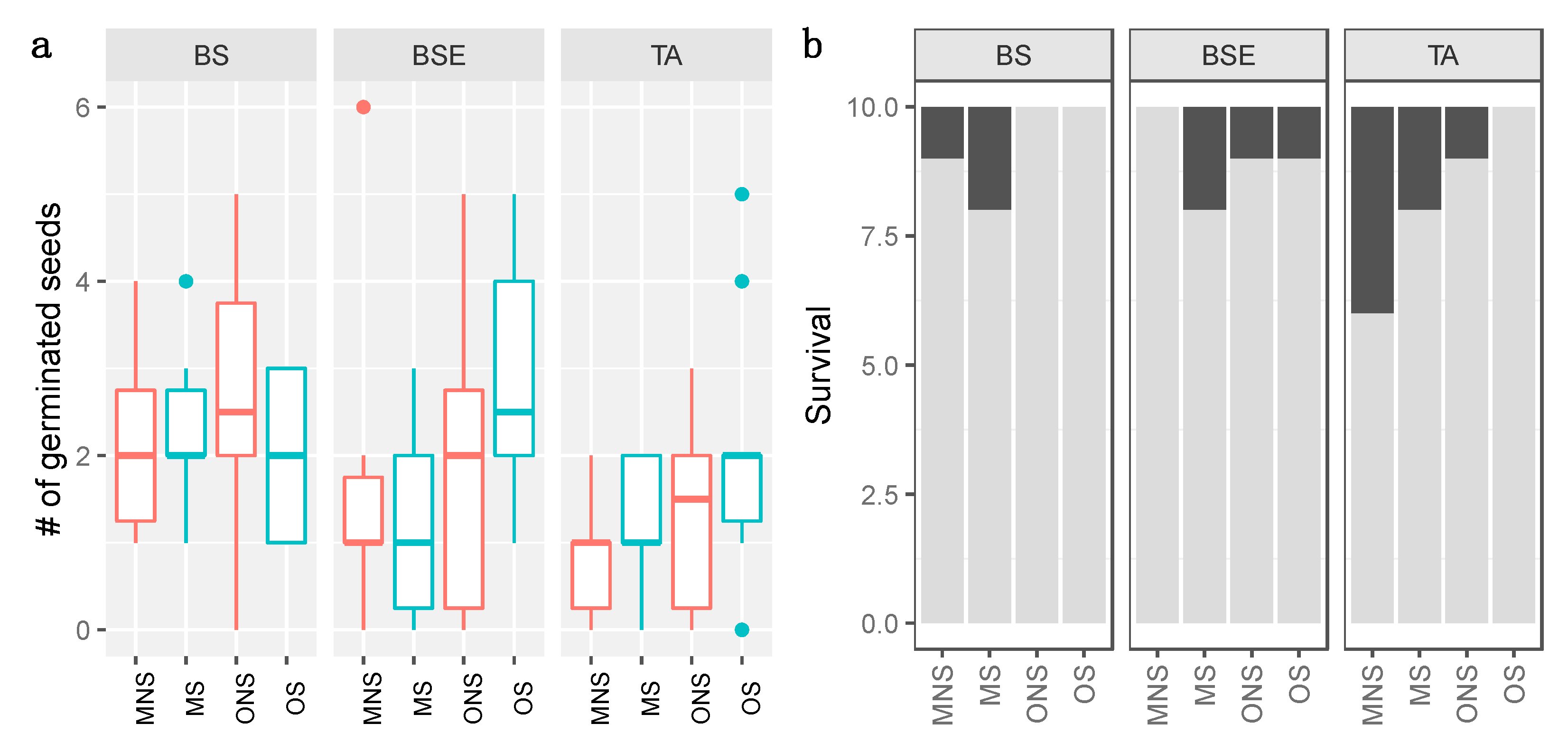
3.1. Germination
3.2. Survival
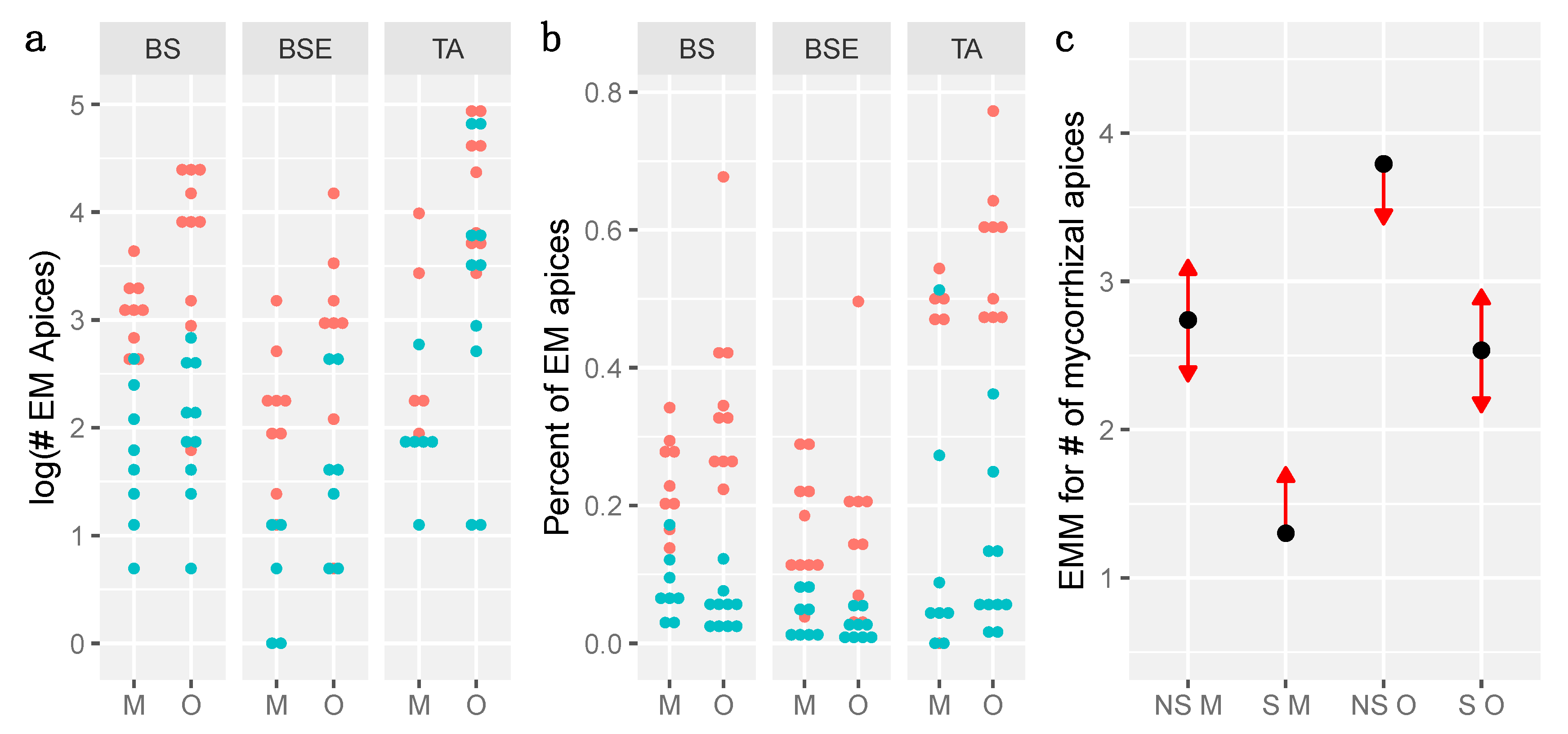
3.3. Mycorrhization
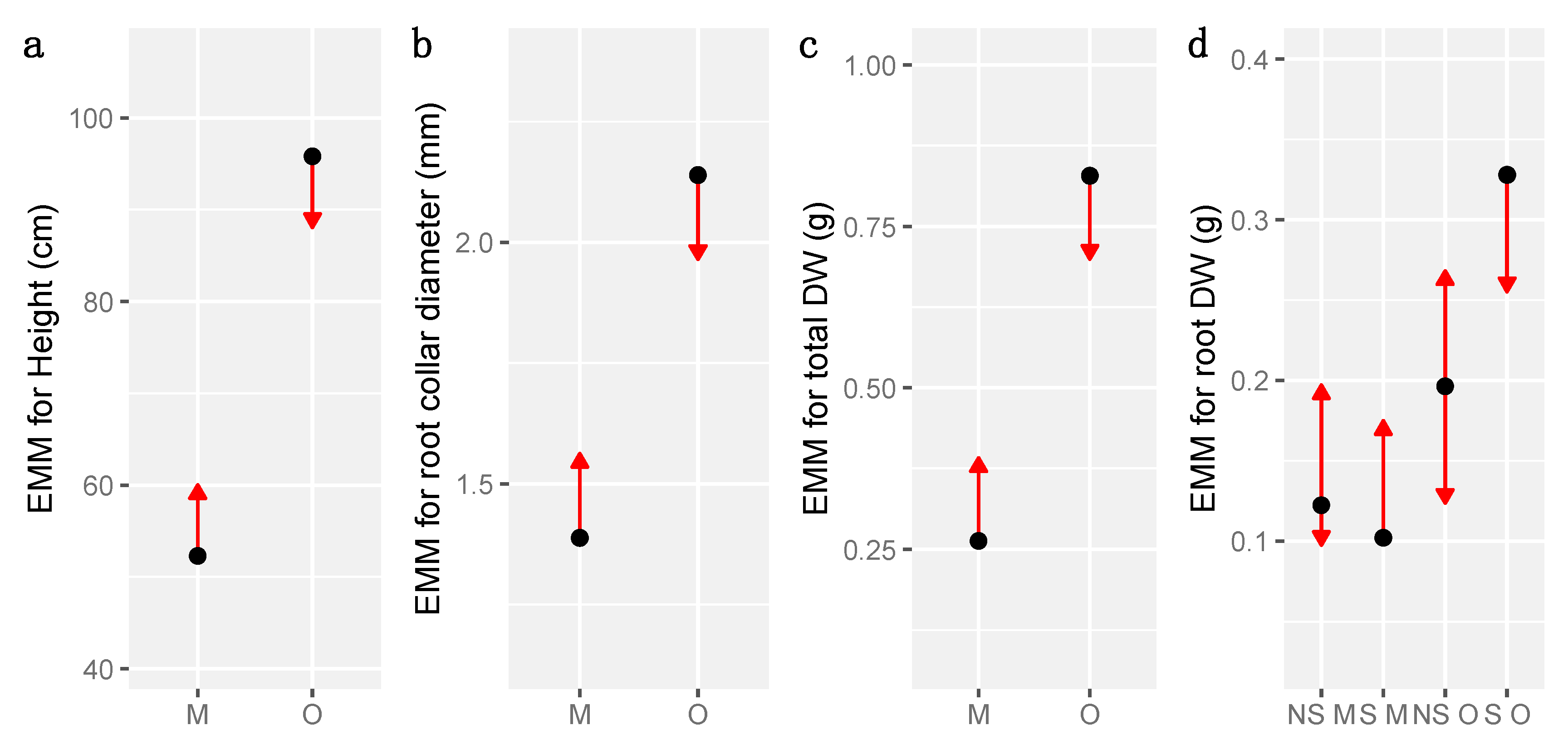
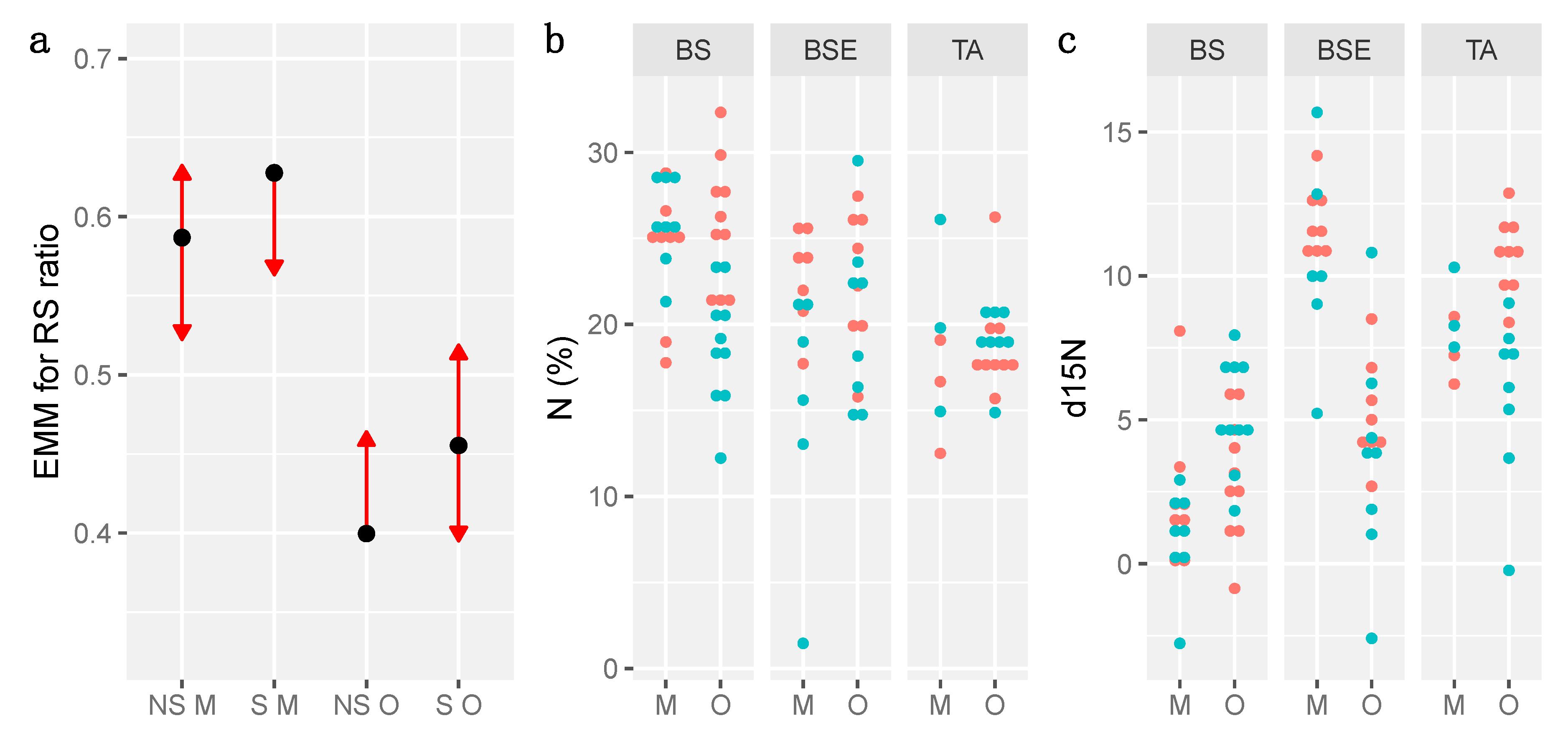
3.4. Growth and Biomass
3.5. Anatomy and Physiology
3.6. The Effects of Ericaceous Shrubs
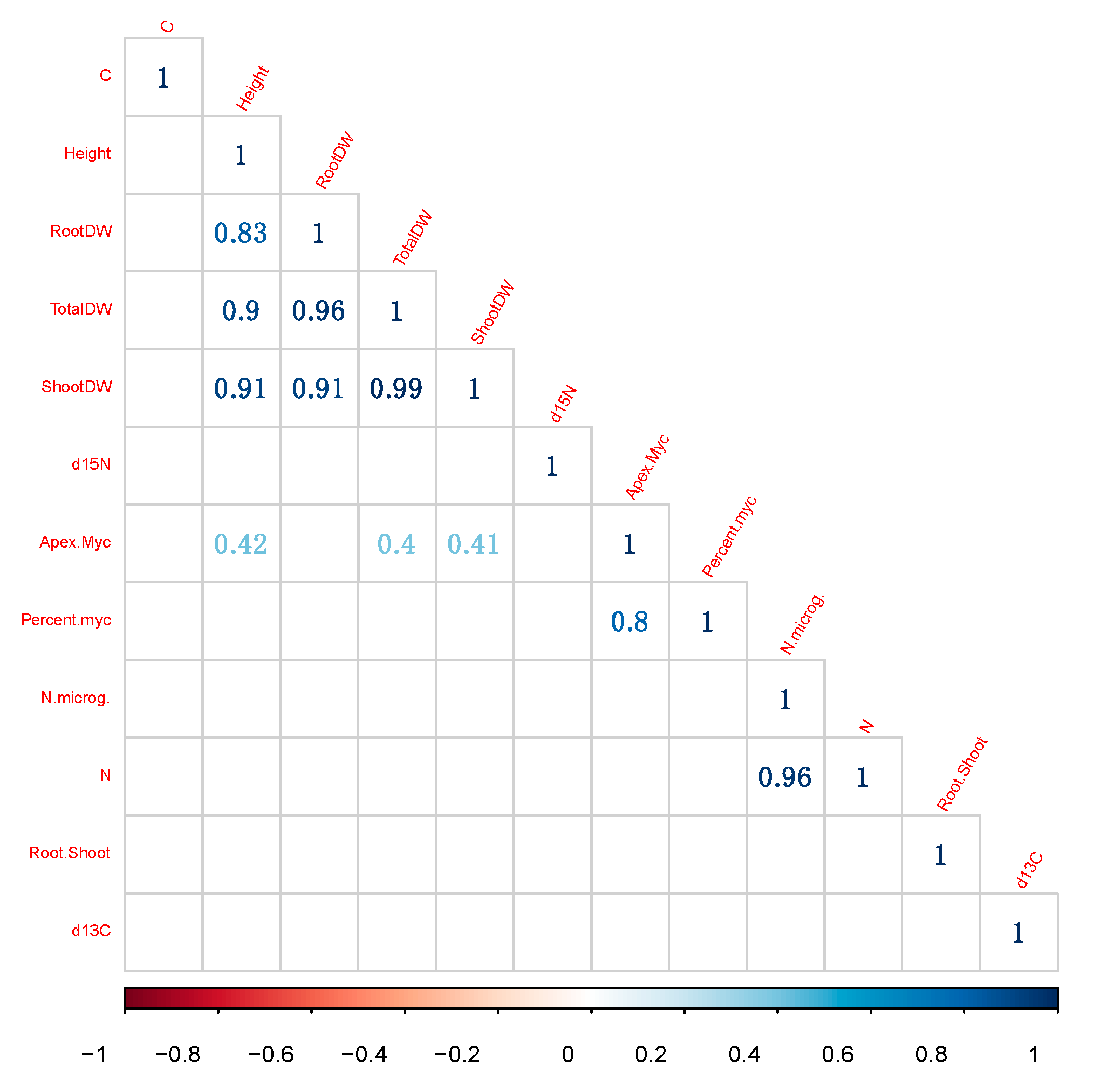
3.7. Correlations between Variables
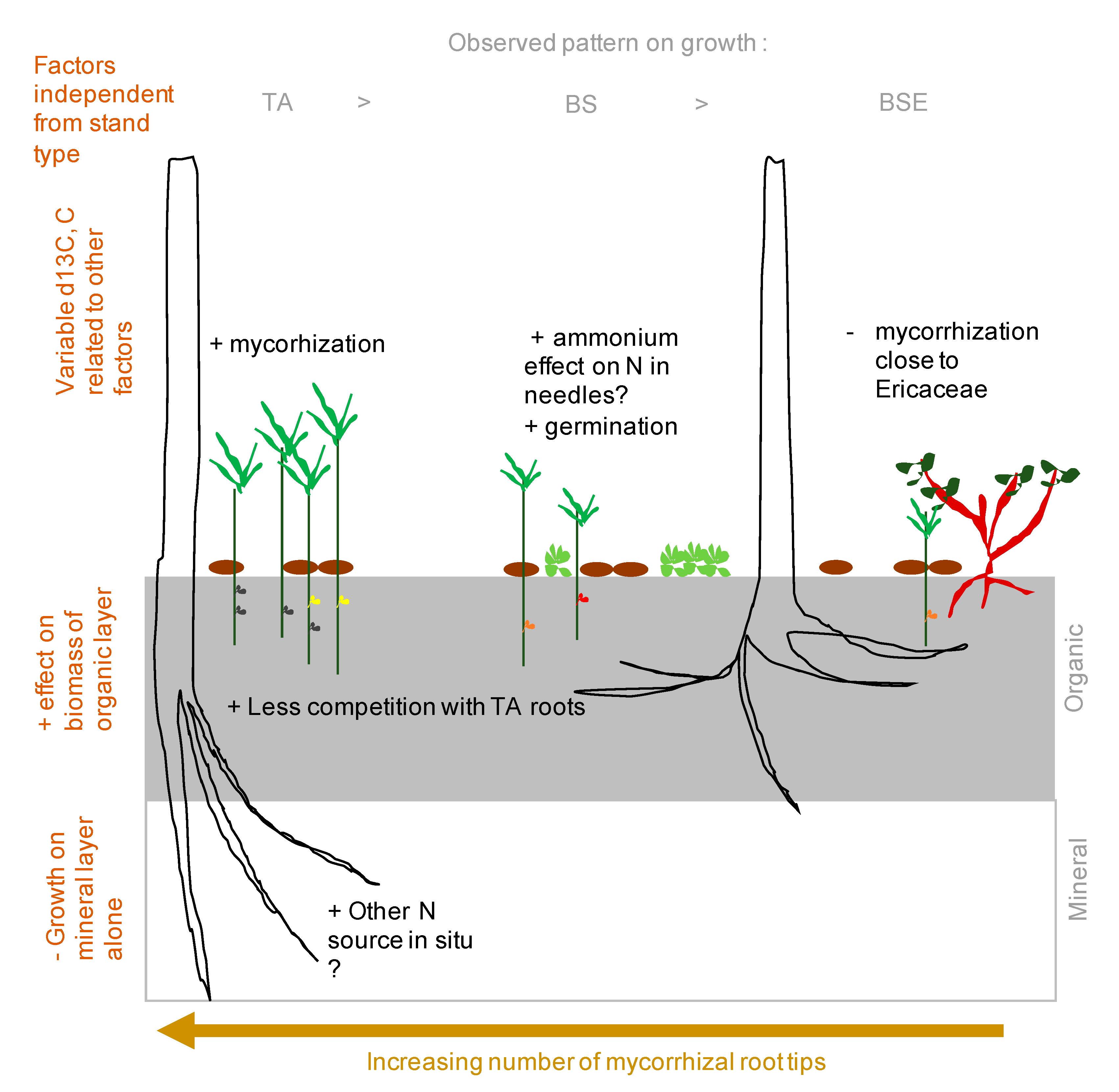
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Woodall, C.W.; Clark, J.S. Failure to migrate: Lack of tree range expansion in response to climate change. Glob. Chang. Biol. 2012, 18, 1042–1052. [Google Scholar] [CrossRef]
- Iverson, L.R.; McKenzie, D. Tree-species range shifts in a changing climate: Detecting, modeling, assisting. Landsc. Ecol. 2013, 28, 879–889. [Google Scholar] [CrossRef]
- Neilson, R.P.; Pitelka, L.F.; Solomon, A.M.; Nathan, R.A.N.; Midgley, G.F.; Fragoso, J.M.; Lischke, H.; Thompson, K.E.N. Forecasting regional to global plant migration in response to climate change. Bioscience 2005, 55, 749–759. [Google Scholar] [CrossRef]
- Peay, K.G. The mutualistic niche: Mycorrhizal symbiosis and community dynamics. Annu. Rev. Ecol. Evol. Syst. 2016, 47, 143–164. [Google Scholar] [CrossRef]
- Karst, J.; Burns, C.; Cale, J.A.; Antunes, P.M.; Woods, M.; Lamit, L.J.; Hoeksema, J.D.; Zabinski, C.; Gehring, C.A.; La Flèche, M. Tree species with limited geographical ranges show extreme responses to ectomycorrhizas. Glob. Ecol. Biogeogr. 2018, 27, 839–848. [Google Scholar] [CrossRef]
- Meier, E.S.; Lischke, H.; Schmatz, D.R.; Zimmermann, N.E. Climate, competition and connectivity affect future migration and ranges of European trees. Glob. Ecol. Biogeogr. 2012, 21, 164–178. [Google Scholar] [CrossRef]
- Goldblum, D.; Rigg, L.S. The deciduous forest–boreal forest ecotone. Geogr. Compass 2010, 4, 701–717. [Google Scholar] [CrossRef]
- Brandt, J.P.; Flannigan, M.D.; Maynard, D.G.; Thompson, I.D.; Volney, W.J.A. An introduction to Canada’s boreal zone: Ecosystem processes, health, sustainability, and environmental issues. Environ. Rev. 2013, 21, 207–226. [Google Scholar] [CrossRef]
- Grondin, P.; Ansseau, C.; Bélanger, L.; Bergeron, Y.; De Grandpré, L.; Gagnon, G.; Lavoie, C. Cadre Bioclimatique de Référence des Régions Ecologiques du Québec; Manuel de Foresterie; Les Presses de l’Université Laval: Québec City, QC, Canada, 1996; pp. 134–279. [Google Scholar]
- Laquerre, S.; Leduc, A.; Harvey, B.D. Augmentation du couvert en peuplier faux-tremble dans les pessières noires du nord-ouest du Québec après coupe totale. Ecoscience 2009, 16, 483–491. [Google Scholar] [CrossRef]
- Messaoud, Y.; Bergeron, Y.; Asselin, H. Reproductive potential of balsam fir (Abies balsamea), white spruce (Picea glauca), and black spruce (P. mariana) at the ecotone between mixedwood and coniferous forests in the boreal zone of western Quebec. Am. J. Bot. 2007, 94, 746–754. [Google Scholar] [CrossRef]
- Arbour, M.-L.; Bergeron, Y. Effect of increased Populus cover on Abies regeneration in the Picea–feathermoss boreal forest. J. Veg. Sci. 2011, 22, 1132–1142. [Google Scholar] [CrossRef]
- Cavard, X.; Bergeron, Y.; Chen, H.Y.; Paré, D. Effect of forest canopy composition on soil nutrients and dynamics of the understorey: Mixed canopies serve neither vascular nor bryophyte strata. J. Veg. Sci. 2011, 22, 1105–1119. [Google Scholar] [CrossRef]
- Nagati, M.; Roy, M.; Manzi, S.; Richard, F.; Desrochers, A.; Gardes, M.; Bergeron, Y. Impact of local forest composition on soil fungal communities in a mixed boreal forest. Plant Soil 2018, 432, 1–13. [Google Scholar] [CrossRef]
- Taylor, D.L.; Hollingsworth, T.N.; McFarland, J.W.; Lennon, N.J.; Nusbaum, C.; Ruess, R.W. A first comprehensive census of fungi in soil reveals both hyperdiversity and fine-scale niche partitioning. Ecol. Monogr. 2014, 84, 3–20. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: New York, NY, USA, 2008; p. 605. ISBN 440026354. [Google Scholar]
- Wang, B.; Qiu, Y.-L. Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 2006, 16, 299–363. [Google Scholar] [CrossRef]
- Nagati, M.; Roy, M.; Desrochers, A.; Manzi, S.; Bergeron, Y.; Gardes, M. Facilitation of balsam fir by trembling aspen in the boreal forest: Do ectomycorrhizal communities matter? Front. Plant Sci. 2019, 10, 932. [Google Scholar] [CrossRef]
- Mallik, A.U. Conifer Regeneration Problems in Boreal and Temperate Forests with Ericaceous Understory: Role of Disturbance, Seedbed Limitation, and Keytsone Species Change. Crit. Rev. Plant Sci. 2003, 22, 341–366. [Google Scholar] [CrossRef]
- Mallik, A.; Kravchenko, D. Recruitment and ontogenic patterns of stunting and growth release of black spruce (Picea mariana) in post-fire Kalmia heaths. For. Ecol. Manag. 2018, 407, 135–144. [Google Scholar] [CrossRef]
- Kennedy, P.G.; Smith, D.P.; Horton, T.R.; Molina, R.J. Arbutus menziesii (Ericaceae) facilitates regeneration dynamics in mixed evergreen forests by promoting mycorrhizal fungal diversity and host connectivity. Am. J. Bot. 2012, 99, 1691–1701. [Google Scholar] [CrossRef]
- Sietiö, O.-M.; Tuomivirta, T.; Santalahti, M.; Kiheri, H.; Timonen, S.; Sun, H.; Fritze, H.; Heinonsalo, J. Ericoid plant species and Pinus sylvestris shape fungal communities in their roots and surrounding soil. New Phytol. 2018, 218, 738–751. [Google Scholar] [CrossRef] [PubMed]
- Richard, F.; Selosse, M.-A.; Gardes, M. Facilitated establishment of Quercus ilex in shrub-dominated communities within a Mediterranean ecosystem: Do mycorrhizal partners matter? FEMS Microbiol. Ecol. 2009, 68, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Booth, M.G.; Hoeksema, J.D. Mycorrhizal networks counteract competitive effects of canopy trees on seedling survival. Ecology 2010, 91, 2294–2302. [Google Scholar] [CrossRef] [PubMed]
- McLaren, B.E.; Janke, R.A. Seedbed and canopy cover effects on balsam fir seedling establishment in Isle Royale National Park. Can. J. For. Res. 1996, 26, 782–793. [Google Scholar] [CrossRef]
- Trevors, J.T. Sterilization and inhibition of microbial activity in soil. J. Microbiol. Methods 1996, 26, 53–59. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Schielzeth, H.; Dingemanse, N.J.; Nakagawa, S.; Westneat, D.F.; Allegue, H.; Teplitsky, C.; Réale, D.; Dochtermann, N.A.; Garamszegi, L.Z.; Araya-Ajoy, Y.G. Robustness of linear mixed-effects models to violations of distributional assumptions. Methods Ecol. Evol. 2020. [Google Scholar] [CrossRef]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. R Package Version 0.1. 2017, Volume 5. Available online: https://cran.r-project.org/web/packages/DHARMa/vignettes/DHARMa.html (accessed on 30 June 2020).
- Lenth, R.; Singmann, H.; Love, J.; Buerkner, P.; Herve, M. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Package Version. 2018, Volume 1, p. 3. Available online: https://rdrr.io/cran/emmeans/ (accessed on 30 June 2020).
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; Team, R.C. nlme: Linear and Nonlinear Mixed Effects Models. R Package Version. 2013, Volume 3, p. 111. Available online: https://cran.r-project.org/web/packages/nlme/index.html (accessed on 30 June 2020).
- Parent, S.; Simard, M.-J.; Morin, H.; Messier, C. Establishment and dynamics of the balsam fir seedling bank in old forests of northeastern Quebec. Can. J. For. Res. 2003, 33, 597–603. [Google Scholar] [CrossRef]
- Laganière, J.; Angers, D.A.; Paré, D.; Bergeron, Y.; Chen, H.Y. Black spruce soils accumulate more uncomplexed organic matter than aspen soils. Soil Sci. Soc. Am. J. 2011, 75, 1125–1132. [Google Scholar] [CrossRef]
- Greene, D.F.; Noël, J.; Bergeron, Y.; Rousseau, M.; Gauthier, S. Recruitment of Picea mariana, Pinus banksiana, and Populus tremuloides across a burn severity gradient following wildfire in the southern boreal forest of Quebec. Can. J. For. Res. 2004, 34, 1845–1857. [Google Scholar] [CrossRef]
- Johnstone, J.F.; Chapin, F.S. Effects of soil burn severity on post-fire tree recruitment in boreal forest. Ecosystems 2006, 9, 14–31. [Google Scholar] [CrossRef]
- Johnstone, J.F.; Hollingsworth, T.N.; Chapin, F.S., III; Mack, M.C. Changes in fire regime break the legacy lock on successional trajectories in Alaskan boreal forest. Glob. Chang. Biol. 2010, 16, 1281–1295. [Google Scholar] [CrossRef]
- Lafleur, B.; Fenton, N.; Paré, D.; Simard, M.; Bergeron, Y. Contrasting effects of season and method of harvest on soil properties and the growth of black spruce regeneration in the boreal forested peatlands of eastern Canada. Silva Fenn. 2010, 44, 799–813. [Google Scholar] [CrossRef]
- Lafleur, B.; Cazal, A.; Leduc, A.; Bergeron, Y. Soil organic layer thickness influences the establishment and growth of trembling aspen (Populus tremuloides) in boreal forests. For. Ecol. Manag. 2015, 347, 209–216. [Google Scholar] [CrossRef]
- Trugman, A.T.; Fenton, N.J.; Bergeron, Y.; Xu, X.; Welp, L.R.; Medvigy, D. Climate, soil organic layer, and nitrogen jointly drive forest development after fire in the North American boreal zone. J. Adv. Model. Earth Syst. 2016, 8, 1180–1209. [Google Scholar] [CrossRef]
- De Grandpré, L.; Morissette, J.; Gauthier, S. Long-term post-fire changes in the northeastern boreal forest of Quebec. J. Veg. Sci. 2000, 11, 791–800. [Google Scholar] [CrossRef]
- Légaré, S.; Paré, D.; Bergeron, Y. Influence of aspen on forest floor properties in black spruce-dominated stands. Plant Soil 2005, 275, 207–220. [Google Scholar] [CrossRef]
- Stottlemyer, A.D.; Wang, G.G.; Wells, C.E.; Stottlemyer, D.W.; Waldrop, T.A. Reducing airborne ectomycorrhizal fungi and growing non-mycorrhizal loblolly pine (Pinus taeda L.) seedlings in a greenhouse. Mycorrhiza 2008, 18, 269–275. [Google Scholar] [CrossRef]
- Hoeksema, J.D.; Chaudhary, V.B.; Gehring, C.A.; Johnson, N.C.; Karst, J.; Koide, R.T.; Pringle, A.; Zabinski, C.; Bever, J.D.; Moore, J.C. A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecol. Lett. 2010, 13, 394–407. [Google Scholar] [CrossRef] [PubMed]
- Nara, K. Ectomycorrhizal networks and seedling establishment during early primary succession. New Phytol. 2006, 169, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Torres Aquino, M.; Plassard, C. Dynamics of ectomycorrhizal mycelial growth and P transfer to the host plant in response to low and high soil P availability. FEMS Microbiol. Ecol. 2004, 48, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Vallano, D.M.; Sparks, J.P. Foliar δ15N is affected by foliar nitrogen uptake, soil nitrogen, and mycorrhizae along a nitrogen deposition gradient. Oecologia 2013, 172, 47–58. [Google Scholar] [CrossRef]
- Mayor, J.R.; Schuur, E.A.G.; Mack, M.C.; Hollingsworth, T.N.; Bååth, E. Nitrogen Isotope Patterns in Alaskan Black Spruce Reflect Organic Nitrogen Sources and the Activity of Ectomycorrhizal Fungi. Ecosystems 2012, 15, 819–831. [Google Scholar] [CrossRef]
- DeLuca, T.H.; Zackrisson, O.; Gentili, F.; Sellstedt, A.; Nilsson, M.-C. Ecosystem controls on nitrogen fixation in boreal feather moss communities. Oecologia 2007, 152, 121–130. [Google Scholar] [CrossRef]
- Houle, D.; Moore, J.-D.; Ouimet, R.; Marty, C. Tree species partition N uptake by soil depth in boreal forests. Ecology 2014, 95, 1127–1133. [Google Scholar] [CrossRef]
- Kronzucker, H.J.; Siddiqi, M.Y.; Glass, A.D.M. Conifer root discrimination against soil nitrate and the ecology of forest succession. Nature 1997, 385, 59–61. [Google Scholar] [CrossRef]
- Kronzucker, H.J.; Siddiqi, M.Y.; Glass, A.D.M.; Britto, D.T. Root ammonium transport efficiency as a determinant in forest colonization patterns: An hypothesis. Physiol. Plant. 2003, 117, 164–170. [Google Scholar] [CrossRef]
- Ghotsa Mekontchou, C.; Houle, D.; Bergeron, Y.; Drobyshev, I. Contrasting Root System Structure and Belowground Interactions between Black Spruce (Picea mariana (Mill.) B.S.P) and Trembling Aspen (Populus tremuloides Michx) in Boreal Mixedwoods of Eastern Canada. Forests 2020, 11, 127. [Google Scholar] [CrossRef]
- Lavoie, M.; Paré, D.; Bergeron, Y. Quality of growth substrates of post-disturbed lowland black spruce sites for black spruce (Picea mariana) seedling growth. New For. 2007, 33, 207–216. [Google Scholar] [CrossRef]
- Simard, S.W.; Perry, D.A.; Jones, M.D.; Myrold, D.D.; Durall, D.M.; Molina, R. Net transfer of carbon between ectomycorrhizal tree species in the field. Nature 1997, 388, 579–582. [Google Scholar] [CrossRef]
- Simard, S.W.; Jones, M.D.; Durall, D.M. Carbon and nutrient fluxes within and between mycorrhizal plants. In Mycorrhizal Ecology; Springer: Berlin, Germany, 2003; pp. 33–74. [Google Scholar]
- Simard, S.W.; Beiler, K.J.; Bingham, M.A.; Deslippe, J.R.; Philip, L.J.; Teste, F.P. Mycorrhizal networks: Mechanisms, ecology and modelling. Fungal Biol. Rev. 2012, 26, 39–60. [Google Scholar] [CrossRef]
- Hoeksema, J.D. Experimentally Testing Effects of Mycorrhizal Networks on Plant-Plant Interactions and Distinguishing Among Mechanisms. In Mycorrhizal Networks; Springer: Berlin, Germany, 2015; pp. 255–277. [Google Scholar]
- Gélinas-Pouliot, M. The Fate of 15N-Labeled Ammonium and Nitrate Applied on Trees Canopy in a Mature Balsam-Fir Stand. Ph.D. Thesis, Université du Québec ą Chicoutimi, Saguenay, QC, Canada, 2013. [Google Scholar]
- Bent, E.; Kiekel, P.; Brenton, R.; Taylor, D.L. Root-Associated Ectomycorrhizal Fungi Shared by Various Boreal Forest Seedlings Naturally Regenerating after a Fire in Interior Alaska and Correlation of Different Fungi with Host Growth Responses. Appl. Environ. Microbiol. 2011, 77, 3351–3359. [Google Scholar] [CrossRef]
- Marchais, M.; Arseneault, D.; Bergeron, Y. Composition changes in the boreal mixedwood forest of western Quebec since Euro-Canadian settlement. Front. Plant Sci. 2020. In Press. [Google Scholar] [CrossRef]
- Danneyrolles, V.; Dupuis, S.; Fortin, G.; Leroyer, M.; de Römer, A.; Terrail, R.; Vellend, M.; Boucher, Y.; Laflamme, J.; Bergeron, Y. Stronger influence of anthropogenic disturbance than climate change on century-scale compositional changes in northern forests. Nat. Commun. 2019, 10, 1–7. [Google Scholar] [CrossRef]
- Van der Putten, W.H.; Macel, M.; Visser, M.E. Predicting species distribution and abundance responses to climate change: Why it is essential to include biotic interactions across trophic levels. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2025–2034. [Google Scholar] [CrossRef]
- Urban, M.C.; Zarnetske, P.L.; Skelly, D.K. Moving forward: Dispersal and species interactions determine biotic responses to climate change: Dispersal and species interactions. Ann. N. Y. Acad. Sci. 2013, 1297, 44–60. [Google Scholar] [CrossRef]
- Pither, J.; Pickles, B.J.; Simard, S.W.; Ordonez, A.; Williams, J.W. Below-ground biotic interactions moderated the postglacial range dynamics of trees. New Phytol. 2018, 220, 1148–1160. [Google Scholar] [CrossRef]
- Hewitt, R.E.; Bennett, A.P.; Breen, A.L.; Hollingsworth, T.N.; Taylor, D.L.; Chapin, F.S.; Rupp, T.S. Getting to the root of the matter: Landscape implications of plant-fungal interactions for tree migration in Alaska. Landsc. Ecol. 2016, 31, 895–911. [Google Scholar] [CrossRef]
- Carteron, A.; Parasquive, V.; Blanchard, F.; Guilbeault-Mayers, X.; Turner, B.L.; Vellend, M.; Laliberté, E. Soil abiotic and biotic properties constrain the establishment of a dominant temperate tree into boreal forests. J. Ecol. 2020, 108, 931–944. [Google Scholar] [CrossRef]
| #EM Apices (GLMM) | % of EM Apices (LMM) | ||||||
|---|---|---|---|---|---|---|---|
| Factor | Estimate | Std. Error | Significance | Estimate | Std. Error | Significance | |
| Fixed effects | Intercept (Mineral) | 2.7393 | 0.3879 | *** | 0.27064 | 0.05809 | ** |
| Organic Layer | 1.0532 | 0.3122 | *** | 0.09448 | 0.05049 | ||
| Layer M: Sterile | −1.4386 | 0.3310 | *** | −0.18923 | 0.05116 | ** | |
| Layer O: Sterile | −1.2573 | 0.3138 | *** | −0.30239 | 0.04971 | *** | |
| Variance | Std. Error | Variance | Std. Error | ||||
| Random effects | Treatment:Stand | 0.1406 | 0.375 | 0.002488 | 0.04988 | ||
| Stand | 0.3025 | 0.550 | 0.006203 | 0.07876 | |||
| Residuals | 0.011555 | 0.10750 | |||||
| Height | RC diameter | Total DW | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Factor | Estimate | Std. Error | Sig. | Estimate | Std. Error | Sig. | Estimate | Std. Error | Sig. | |
| Fixed effects | Intercept (Mineral) | 56.374 | 6.089 | *** | 1.4646 | 0.1350 | *** | 0.29900 | 0.09784 | ** |
| Organic Layer | 31.051 | 8.378 | *** | 0.5410 | 0.1869 | * | 0.35967 | 0.13836 | * | |
| Layer M: Sterile | −8.146 | 8.701 | −0.1534 | 0.1918 | −0.07233 | 0.13836 | ||||
| Layer O: Sterile | 16.795 | 8.067 | * | 0.2673 | 0.1818 | 0.33967 | 0.13836 | * | ||
| Variance | Std. Error | Variance | Std. Error | Variance | Std. Error | |||||
| Random effects | Treatment: Stand | 0 | 0.00 | 0.01639 | 0.128 | 0.01401 | 0.1183 | |||
| Stand | 0 | 0.00 | 0.00000 | 0.000 | 0.00000 | 0.0000 | ||||
| Residuals | 927 | 30.45 | 0.31469 | 0.561 | 0.14709 | 0.3835 | ||||
| Stand | Germination | # EM Apex | %EM Apex | d15N | N (µg) | %N | R/S Ratio |
|---|---|---|---|---|---|---|---|
| BS | 0.1314 | 0.1526 | −0.0124 | −2.3987 | 5.1111 | 1.7067 | −0.0178 |
| BSE | 0.0142 | −0.6867 | −0.0829 | 1.0662 | −0.5412 | −0.4002 | −0.0081 |
| TA | −0.1382 | 0.5500 | 0.0953 | 1.3319 | −4.5698 | −1.3064 | 0.02600 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagati, M.; Roy, M.; DesRochers, A.; Bergeron, Y.; Gardes, M. Importance of Soil, Stand, and Mycorrhizal Fungi in Abies balsamea Establishment in the Boreal Forest. Forests 2020, 11, 815. https://doi.org/10.3390/f11080815
Nagati M, Roy M, DesRochers A, Bergeron Y, Gardes M. Importance of Soil, Stand, and Mycorrhizal Fungi in Abies balsamea Establishment in the Boreal Forest. Forests. 2020; 11(8):815. https://doi.org/10.3390/f11080815
Chicago/Turabian StyleNagati, Mélissande, Mélanie Roy, Annie DesRochers, Yves Bergeron, and Monique Gardes. 2020. "Importance of Soil, Stand, and Mycorrhizal Fungi in Abies balsamea Establishment in the Boreal Forest" Forests 11, no. 8: 815. https://doi.org/10.3390/f11080815
APA StyleNagati, M., Roy, M., DesRochers, A., Bergeron, Y., & Gardes, M. (2020). Importance of Soil, Stand, and Mycorrhizal Fungi in Abies balsamea Establishment in the Boreal Forest. Forests, 11(8), 815. https://doi.org/10.3390/f11080815






