Oak Taproot Growth Disruption Differentially Impacts Root Architecture during Nursery Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Taproot Pruning Study
2.2. Taproot Inhibition Study
2.3. Liner Production of Taproot-Disrupted and Inhibited Seedlings Study
3. Results and Discussion
3.1. Taproot Pruning
3.1.1. Taproot Regeneration
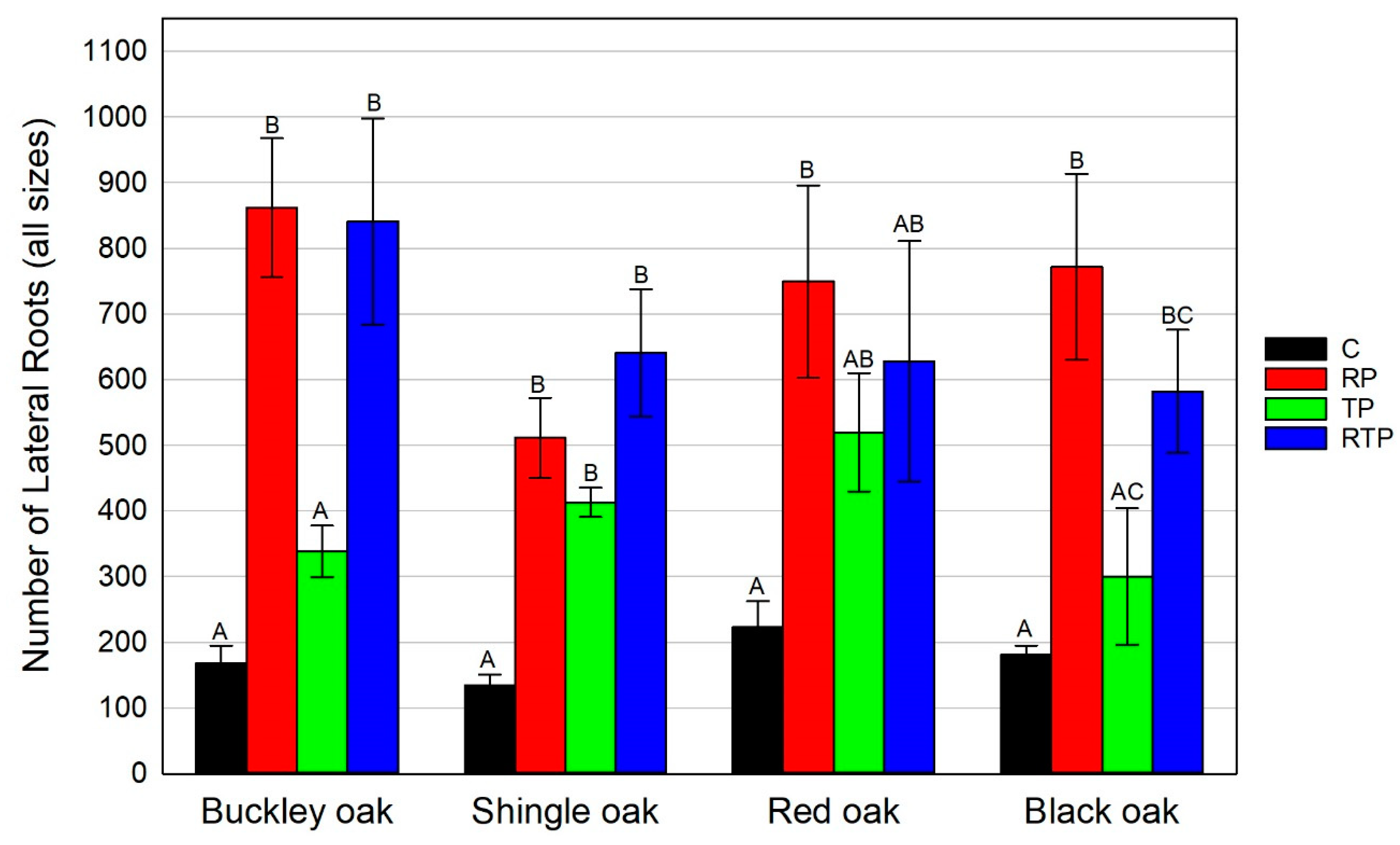
3.1.2. Lateral Root Development
- (1)
- Ultimate Number of Lateral Roots (All Sizes)
- (2)
- Vertical Distribution of Lateral Roots (All Sizes)
- (3)
- Total Number of Lateral Roots (Greater than 1 mm in Diameter)
3.2. Taproot Inhibition
3.3. Liner Production Using Taproot-Disrupted Seedlings
3.3.1. Root Collar Diameter
3.3.2. Root Regeneration Potential
- (1)
- Total Root Ball Surface
- (2)
- Lateral Root Ball Surface
- (3)
- Bottom Root Ball Surface
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pagès, L.; Serra, V. Growth and branching of the taproot of young oak trees—A dynamic study. J. Exp. Bot. 1994, 45, 1327–1334. [Google Scholar] [CrossRef]
- Bidel, L.P.R.; Pagès, L.; Rivière, L.M.; Pelloux, G.; Lorendeau, J.Y. MassFlowDyn I: A carbon transport and partitioning model for root system architecture. Ann. Bot. 2000, 85, 869–886. [Google Scholar] [CrossRef]
- Arnold, M.A.; Struve, D.K. Green ash establishment following transplant. J. Am. Soc. Hort. Sci. 1989, 114, 591–595. [Google Scholar]
- Bonner, F.T. The effect of damaged radicles of presprouted red oak acorns on seedling production. Tree Plant. Notes 1982, 33, 13–15. [Google Scholar]
- Caliskan, S. Germination and seedling growth of holm oak (Quercus ilex L.): Effects of provenance, temperature, and radicle pruning. iForest-Biogeosci. For. 2013, 7, 103–109. [Google Scholar] [CrossRef]
- Insley, H.; Buckley, G.P. The influence of desiccation and root pruning on the survival and growth of broadleaved seedlings. J. Hortic. Sci. 1985, 60, 377–387. [Google Scholar] [CrossRef]
- Lamond, M.; Takavol, R.; Riedacker, A. Influence d’un blocage de l’extremite du pivot d’un semis de chene, sur la morphogenese de son systeme racinaire. Ann. Des Sci. For. 1983, 40, 227–250. [Google Scholar] [CrossRef]
- McCreary, D.D. The effects of stock type and radicle pruning on blue oak morphology and field performance. Ann. Sci. For. 1996, 53, 641–648. [Google Scholar] [CrossRef][Green Version]
- Thaler, P.; Pagès, L. Competition within the root system of rubber seedlings (Hevea brasiliensis) studied by root pruning and blockage. J. Exp. Bot. 1997, 48, 1451–1459. [Google Scholar] [CrossRef]
- Tilki, F.; Unal Alptekin, C. Germination and seedling growth of Quercus vulcanica effects of stratification, desiccation, radicle pruning, and season of sowing. New For. 2006, 32, 243–251. [Google Scholar] [CrossRef]
- Wilson, E.R.; Vitols, K.C.; Park, A. Root characteristics and growth potential of container and bare-root seedlings of red oak (Quercus rubra L.) in Ontario, Canada. New For. 2007, 34, 163–176. [Google Scholar] [CrossRef]
- Esau, K. Anatomy of Seed Plants, 2nd ed.; Wiley: New York, NY, USA, 1977; p. 176. [Google Scholar]
- McCraw, B.; Smith, M. Root pruning and soil type affect pecan root regeneration. HortTechnology 1998, 8, 573–575. [Google Scholar] [CrossRef]
- Harmer, R.; Walder, K.E. The growth of shoots and lateral roots of Quercus robur L. seedlings following simulated undercutting. New For. 1994, 8, 351–362. [Google Scholar] [CrossRef]
- Coutts, M.P. Developmental processes in tree root systems. Can. J. For. Res. 1987, 17, 761–767. [Google Scholar] [CrossRef]
- Thaler, P.; Pagès, L. Root apical diameter and root elongation rate of rubber seedlings (Hevea brasiliensis) show parallel responses to photoassimilate availability. Physiol. Plant. 1996, 97, 365–371. [Google Scholar] [CrossRef]
- Lecompte, F.; Ozier-Lafontaine, H.; Pagès, L. The relationship between static and dynamic variables in the description of root growth. Consequences for field interpretation of rooting variability. Plant Soil 2001, 236, 19–31. [Google Scholar] [CrossRef]
- May, L.H.; Randdes, F.H.; Aspinall, D.; Paleg, L.G. Quantitative studies of root development II. Growth in the early stages of root development. Aust. J. Biol. Sci. 1967, 20, 273–283. [Google Scholar] [CrossRef]
- Pagès, L.; Kervella, J.; Chadoeuf, J. Development of the root system of young peach trees (Prunus persica Batsch): A morphometrical analysis. Ann. Bot. 1993, 71, 369–375. [Google Scholar] [CrossRef]
- Wightman, F.; Thimann, K.V. Hormonal factors controlling the initiation and development of lateral roots. I. Sources of primordia-inducing substances in the primary root of pea seedlings. Physiol. Plant. 1980, 49, 13–20. [Google Scholar] [CrossRef]
- Johnson, P.S. Growth Potential and Field Performance of Planted oaks. In Proceedings: Regenerating Oaks in Upland Hardwood Forests, the 1979 John S Wright Forestry Conference; Holt, H.A., Fisher, B.C., Eds.; Purdue Research Foundation: West Lafayette, IN, USA, 1979; pp. 113–119. [Google Scholar]
- Pagès, L.; Pierre, N.; Petit, P. Growth correlations within the root system of young oak trees. In Root Ecology and its Practical Applications; Kutschera, L., Hobl, E., Lichtenegger, E., Persson, H., Sobotik, M., Eds.; Verein fur Wurzelvorschung: Klagenfurt, Austria, 1992; pp. 505–508. [Google Scholar]
- Röhrig, E. Wurzelschnitt an Eichensamlingen. Forstarchiv 1977, 48, 25–28. [Google Scholar]
- Wheeler, B. lmPerm: Permutation Tests for Linear Models. R Package Version 2.1.0. 2016. Available online: https://CRAN.R-project.org/package=lmPerm (accessed on 6 May 2020).
- RStudio Team. RStudio: Integrated Development for R. RStudio; RStudio Team: Boston, MA, USA, 2015; Available online: http://www.rstudio.com/ (accessed on 6 May 2020).
- Cahn, M.D.; Zobel, R.W.; Bouldin, D.R. Relationship between root elongation rate and diameter and duration of growth of lateral roots of maize. Plant Soil 1989, 119, 271–279. [Google Scholar] [CrossRef]
- Pagès, L. Growth patterns of the lateral roots of young oak (Quercus robur) tree seedlings. Relationship with apical diameter. New Phytol. 1995, 130, 503–509. [Google Scholar] [CrossRef]
- Lecompte, F.; Pagès, L. Apical diameter and branching density affect lateral root elongation rates in banana. Environ. Exp. Bot. 2007, 59, 243–251. [Google Scholar] [CrossRef]
- McCormack, M.L.; Adams, T.S.; Smithwick, E.A.H.; Eissenstat, D.M. Predicting fine root lifespan from plant functional traits in temperate trees. New Phytol. 2012, 195, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Watson, G.; Hewitt, A. Changes in tree root architecture resulting from field nursery production practices. J. Environ. Hortic. 2020, 38, 22–28. [Google Scholar]
- Hankin, S.; Lo, M.; Balestri, F.; Watson, G. Tree seedling root architecture alteration by tap root pruning. J. Environ. Hortic. 2019, 2, 37–50. [Google Scholar] [CrossRef]
- Zhang, R.; Peng, F.-R.; Yan, P.; Cao, F.; Liu, Z.-Z. Effects of root pruning on germinated pecan seedlings. Hortscience 2015, 50, 1549–1552. [Google Scholar] [CrossRef]
- Harris, J.R.; Niemiera, A.; Fanelli, J.; Wright, R. Root pruning pin oak liners affects growth and root morphology. HortTechnology 2001, 11, 49–52. [Google Scholar] [CrossRef]
- Coutts, M.P. Development of the structural root system of sitka spruce. Forestry 1983, 56, 1–16. [Google Scholar] [CrossRef]
- Day, S.D.; Wiseman, P.E.; Dickinson, S.B.; Harris, J.R. Contemporary concepts of root system architecture of urban trees. Arboric. Urban For. 2010, 36, 149–159. [Google Scholar]
- Kormanik, P.P.; Ruehle, J.L.; Muse, H.D. Frequency Distribution of Lateral Roots of 1−0 Bare-Root White Oak Seedlings; USDA Forest Service Southeastern Forest Experiment Station Research Note SE-353; USDA Forest Service Southeastern Forest Experiment Station: Asheville, NC, USA, 1989; p. 5.
- Kormanik, P.P.; Sung, S.S.; Kass, D.J.; Schlarbaum, S. Effect of Seedling Size and First-Order-Lateral Roots on Early Development of Northern Red Oak on Mesic Sites. In Proceedings, 9th Biennial Southern Silvicultural Research Conference; Waldrop, T.A., Ed.; USDA Forest Service: Southern Research Station General Technical Report SRS-20; USDA Forest Service Southeastern Forest Experiment Station: Asheville, NC, USA, 1998; pp. 247–252. [Google Scholar] [CrossRef]
- Ruehle, J.L.; Kormanik, P.P. Lateral Root Morphology: A Potential Indicator of Seedling Quality in Northern Red Oak; USDA Forest Service Southeastern Forest Experiment Station Research Note SE-344; USDA Forest Service Southeastern Forest Experiment Station: Asheville, NC, USA, 1986; p. 6.
- Schultz, R.C.; Thompson, J.R. Effect of density control and undercutting on root morphology of 1+0 bare root hardwood seedlings: Five-year field performance of rootgraded stock in the central USA. New For. 1997, 13, 301–314. [Google Scholar] [CrossRef]
- Reiger, R.; Whitcomb, C.E. A root control system for growing and transplanting trees. Arboric. J. 1985, 9, 33–38. [Google Scholar] [CrossRef]
- Gilman, E.; Beeson, R.C.; Black, R. Comparing root balls on laurel oak transplanted from the wild with those of nursery and container grown trees. J. Arboric. 1992, 18, 124–129. [Google Scholar]
- Whitcomb, C.E. Establishment and Maintenance of Landscape Plants; Lacebark Publications: Stillwater, OK, USA, 1987; p. 47. [Google Scholar]
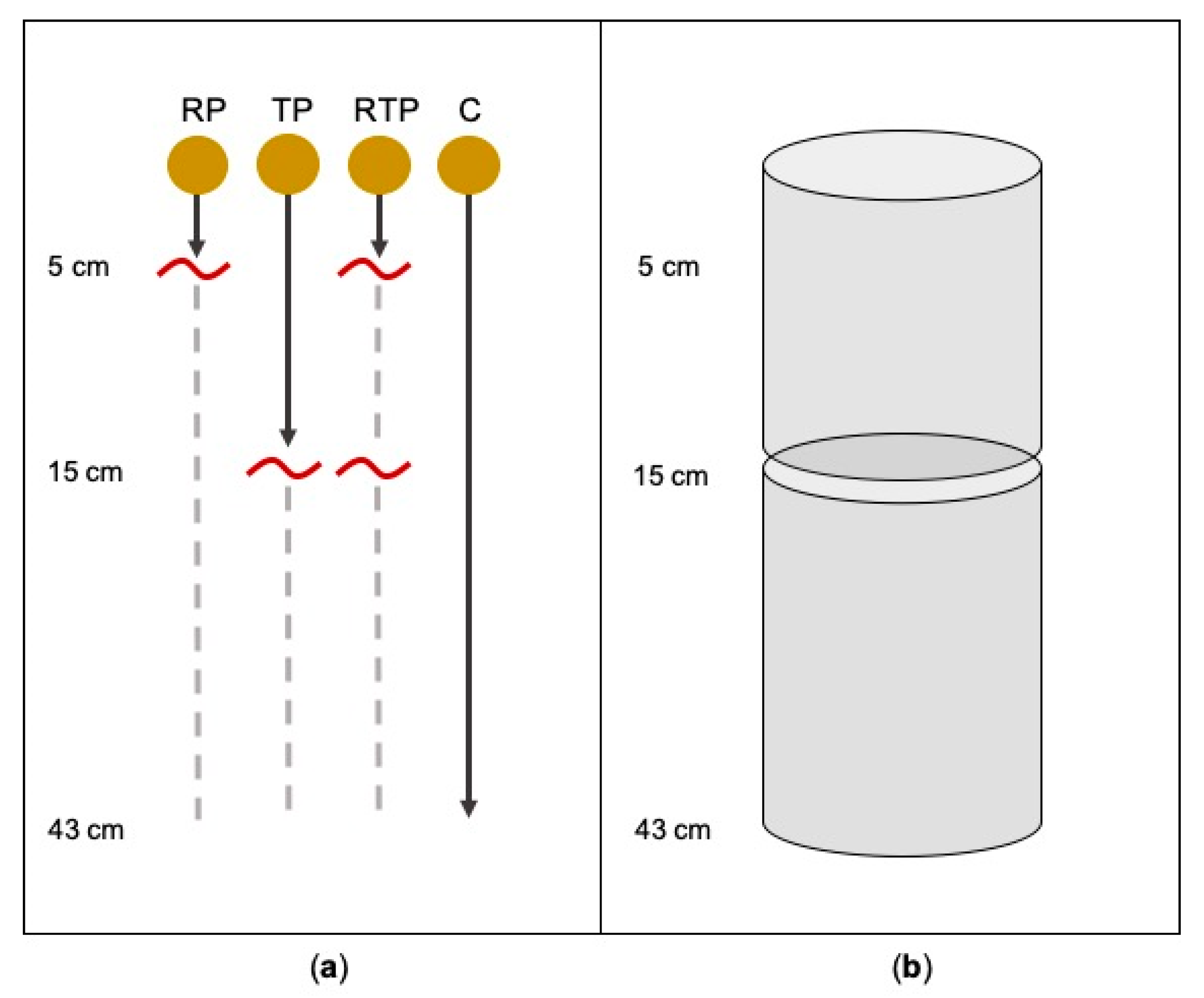




| Species | Treatment | Number of Lateral Roots | Lateral Root Diameter (mm) | ||
|---|---|---|---|---|---|
| Full Length | 15 cm Length | Full Length | 15 cm Length | ||
| White oak | AP | 14.8 a | 14.8 a | 2.4 a | 2.4 a |
| C | 31.2 a | 10.8 a | 2.2 a | 2.3 a | |
| Buckley oak | AP | 13.9 a | 13.9 a | 2.3 a | 2.3 a |
| C | 23.9 a | 9.9 a | 2.3 a | 2.4 a | |
| Shingle oak | AP | 7.7 a | 7.7 a | 3.0 a | 3.0 a |
| C | 5.9 a | 2.2 b | 2.4 b | 2.2 b | |
| Red oak | AP | 13.3 a | 13.3 a | 2.9 a | 2.9 a |
| C | 10.2 a | 4.8 b | 2.5 a | 2.5 a | |
| Black oak | AP | 13.7 a | 13.7 a | 3.3 a | 3.3 a |
| C | 7.1 a | 4.8 a | 2.4 a | 2.6 a | |
| Species | Seedling Production Treatment | Root Regeneration Potential (mm) | Root Collar Diameter (mm) | ||
| Total | Lateral | Bottom | |||
| Red Oak | BR | 528 b | 429 a | 99 b | 24.7 b |
| CG | 729 a | 460 a | 268 a | 30.2 a | |
| Bur Oak | BR | 438 a | 372 a | 66 b | 21.1 a |
| CG | 452 a | 153 b | 298 a | 19.3 a | |
| Species | Liner Production Treatment | Root Regeneration Potential (mm) | Root Collar Diameter (mm) | ||
| Total | Lateral | Bottom | |||
| Red Oak | Bag | 700 a | 469 a | 231 a | 28.6 a |
| Field | 565 a | 422 a | 143 b | 26.5 a | |
| Bur Oak | Bag | 538 a | 283 a | 256 a | 20.3 a |
| Field | 353 b | 231 a | 122 b | 20.0 a | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hankin, S.; Watson, G. Oak Taproot Growth Disruption Differentially Impacts Root Architecture during Nursery Production. Forests 2020, 11, 798. https://doi.org/10.3390/f11080798
Hankin S, Watson G. Oak Taproot Growth Disruption Differentially Impacts Root Architecture during Nursery Production. Forests. 2020; 11(8):798. https://doi.org/10.3390/f11080798
Chicago/Turabian StyleHankin, Shanon, and Gary Watson. 2020. "Oak Taproot Growth Disruption Differentially Impacts Root Architecture during Nursery Production" Forests 11, no. 8: 798. https://doi.org/10.3390/f11080798
APA StyleHankin, S., & Watson, G. (2020). Oak Taproot Growth Disruption Differentially Impacts Root Architecture during Nursery Production. Forests, 11(8), 798. https://doi.org/10.3390/f11080798




