Morphology and Material Composition of the Mouthparts of Stromatium unicolor Olivier 1795 (Coleoptera: Cerambycidae) for Bionic Application
Abstract
1. Introduction
2. Materials and Methods
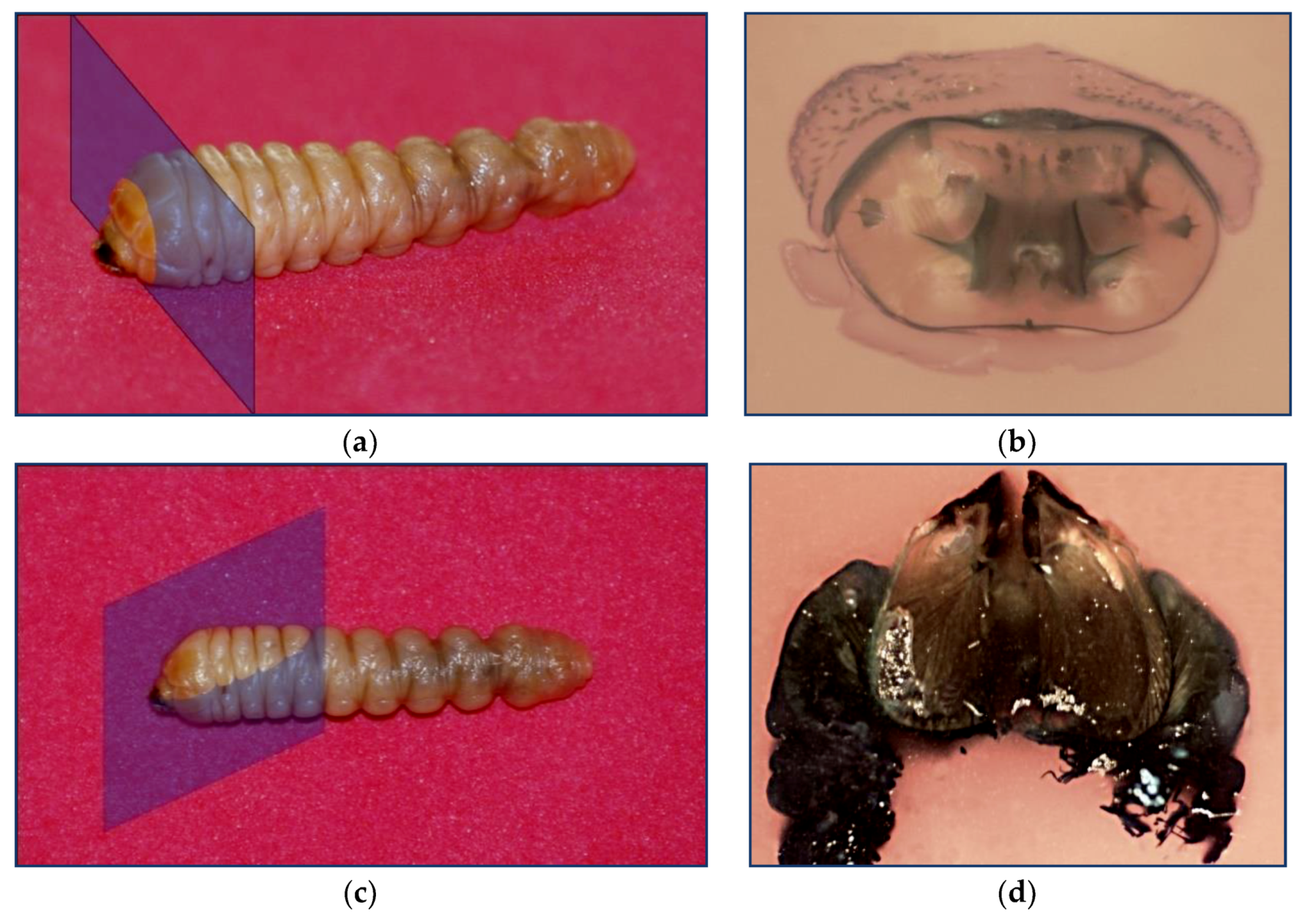
2.1. Obtaining The Profile of The Jaw and Perpendicular Cut of The Musculature. Paraffin Inclusion
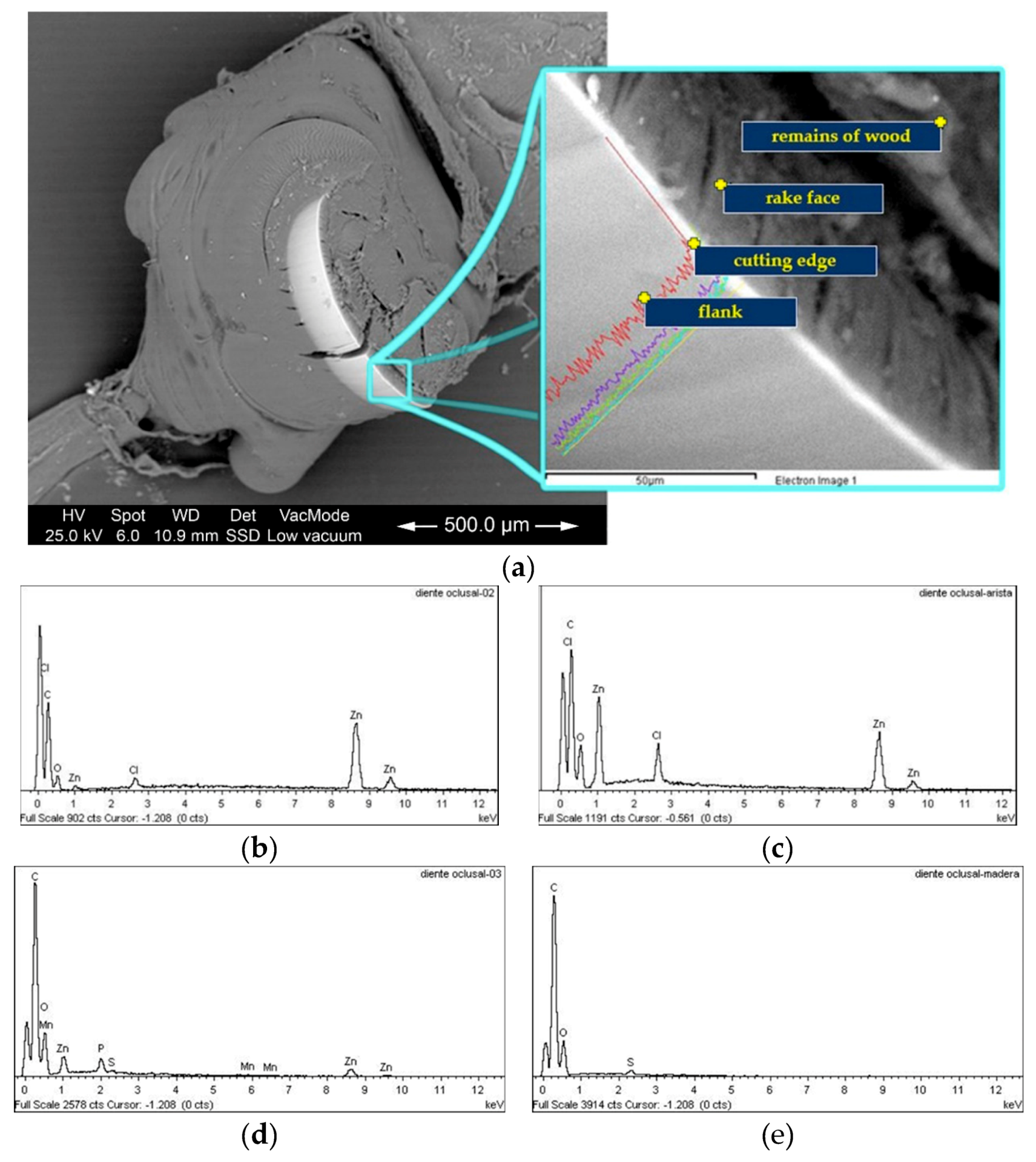
2.2. Surface and Elemental Composition Analysis
2.3. Measurement Methodology
3. Results and Discussion
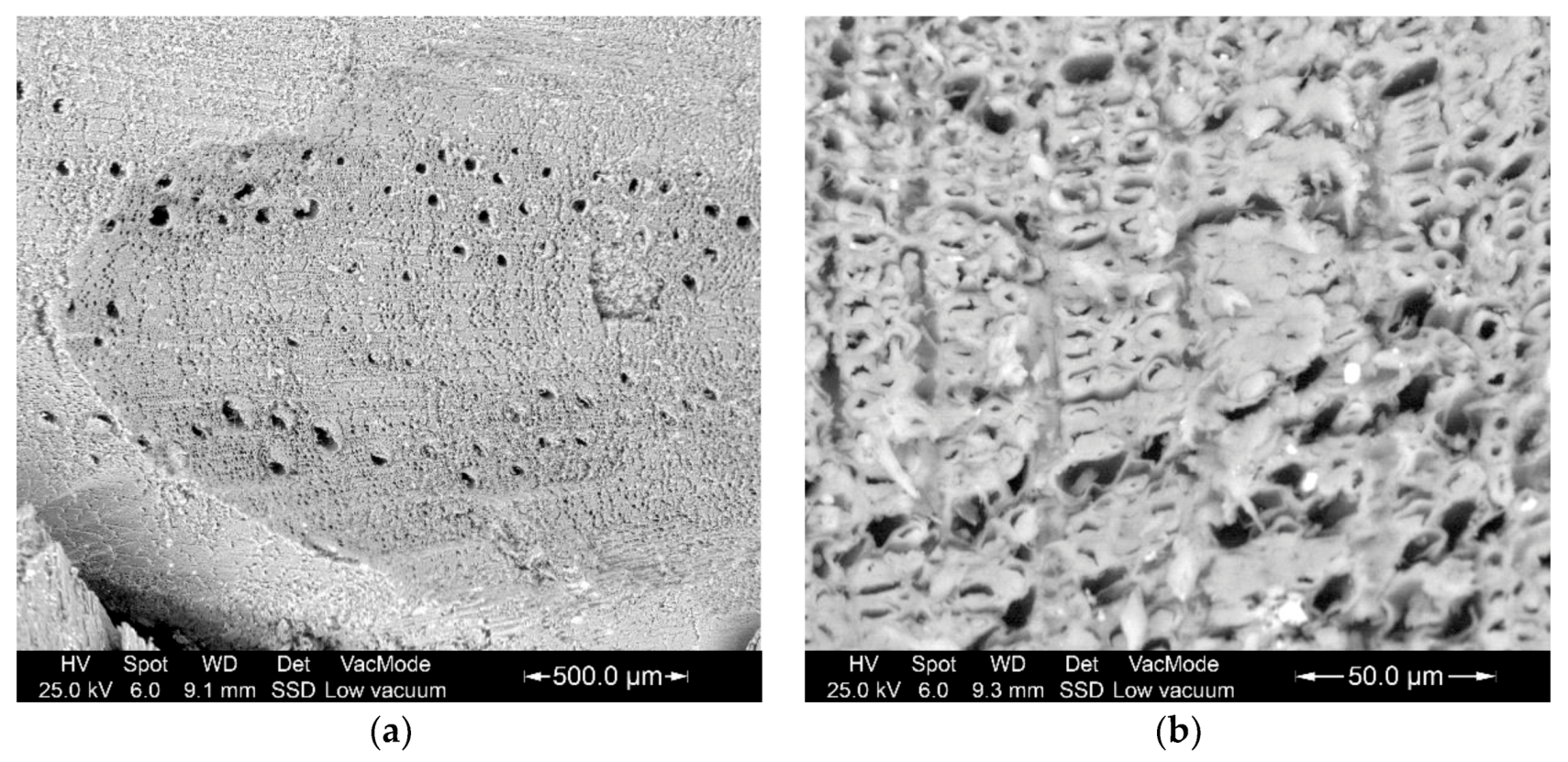
3.1. Elemental Composition of The Mandibles
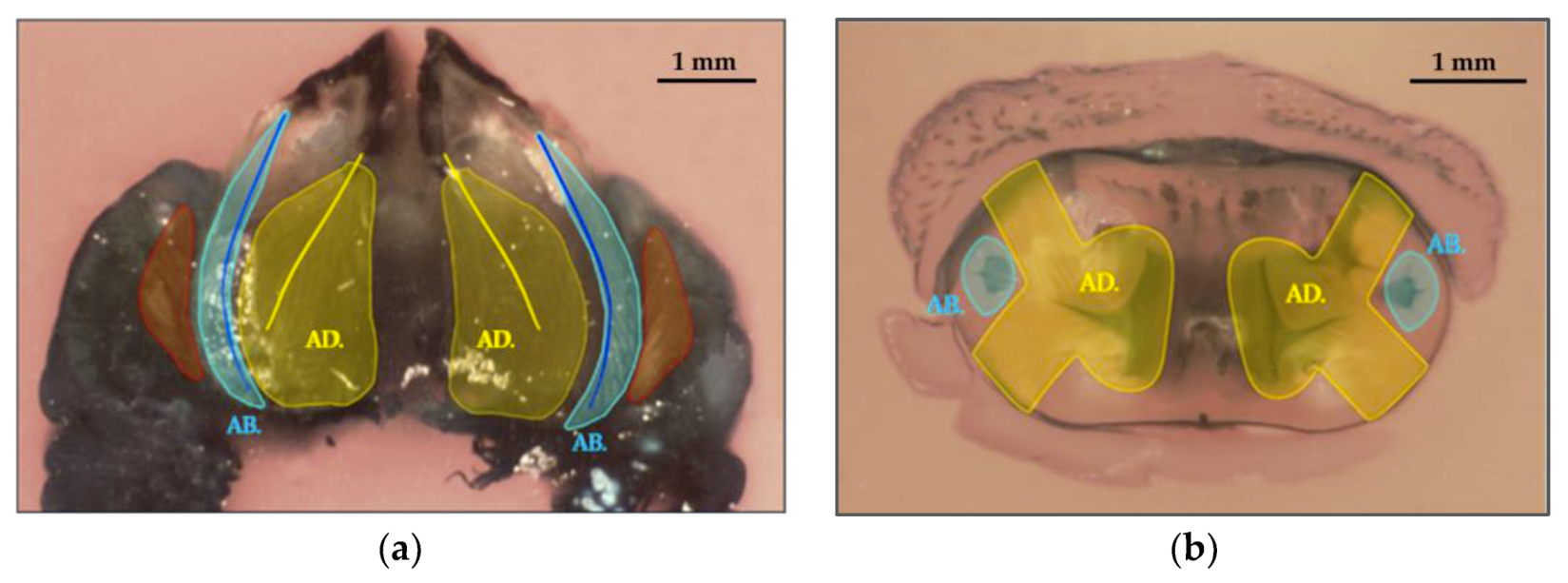
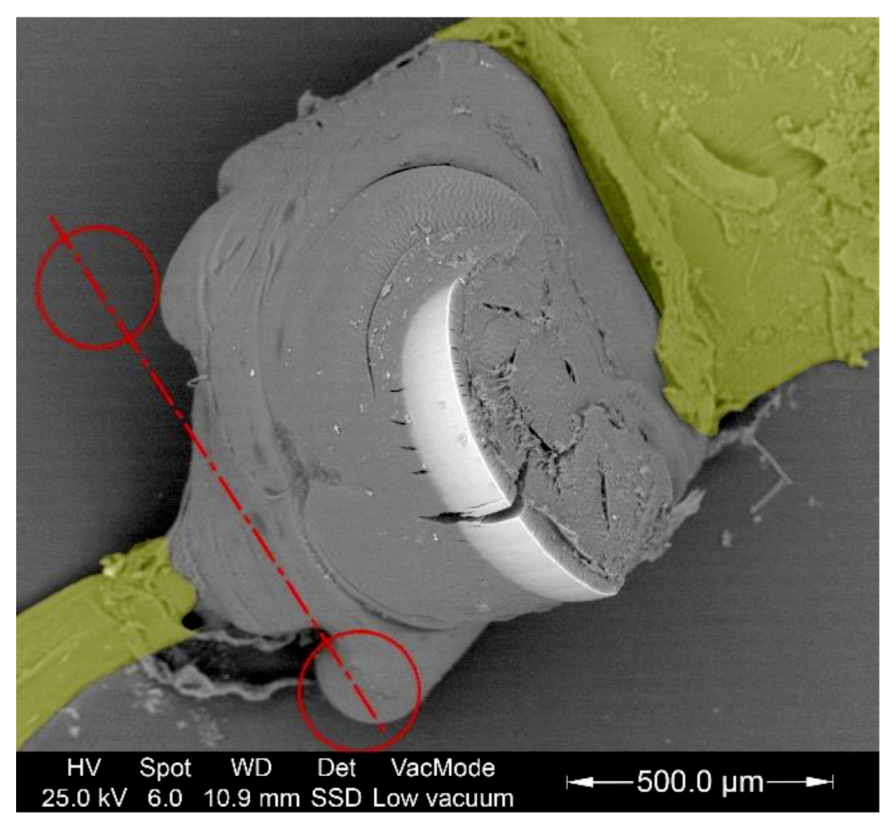
3.2. Study of The Mandible Movement: Joints, Musculature and Footprint of The Bite
3.3. Tool Footprint (Mandible)
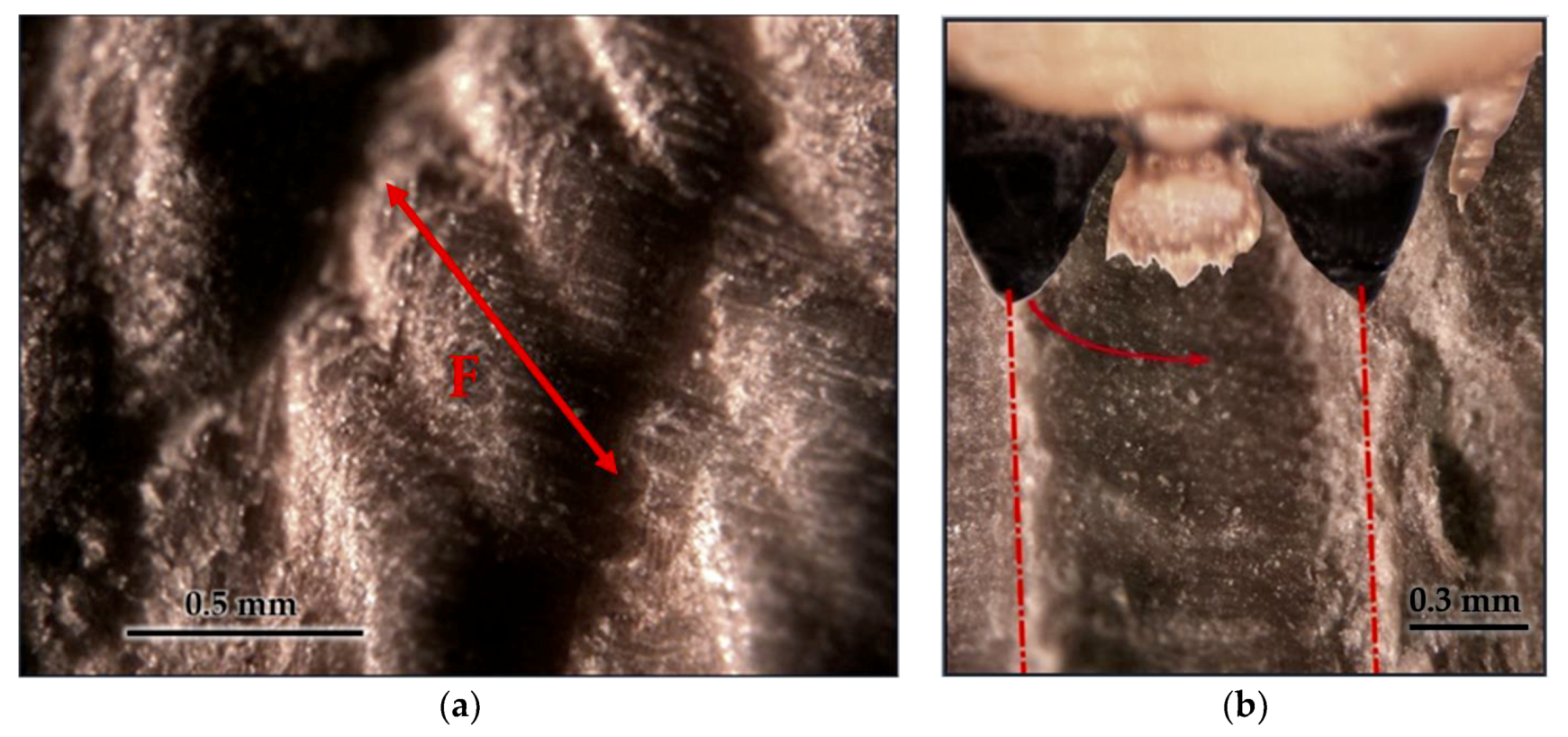
3.4. Cut Type
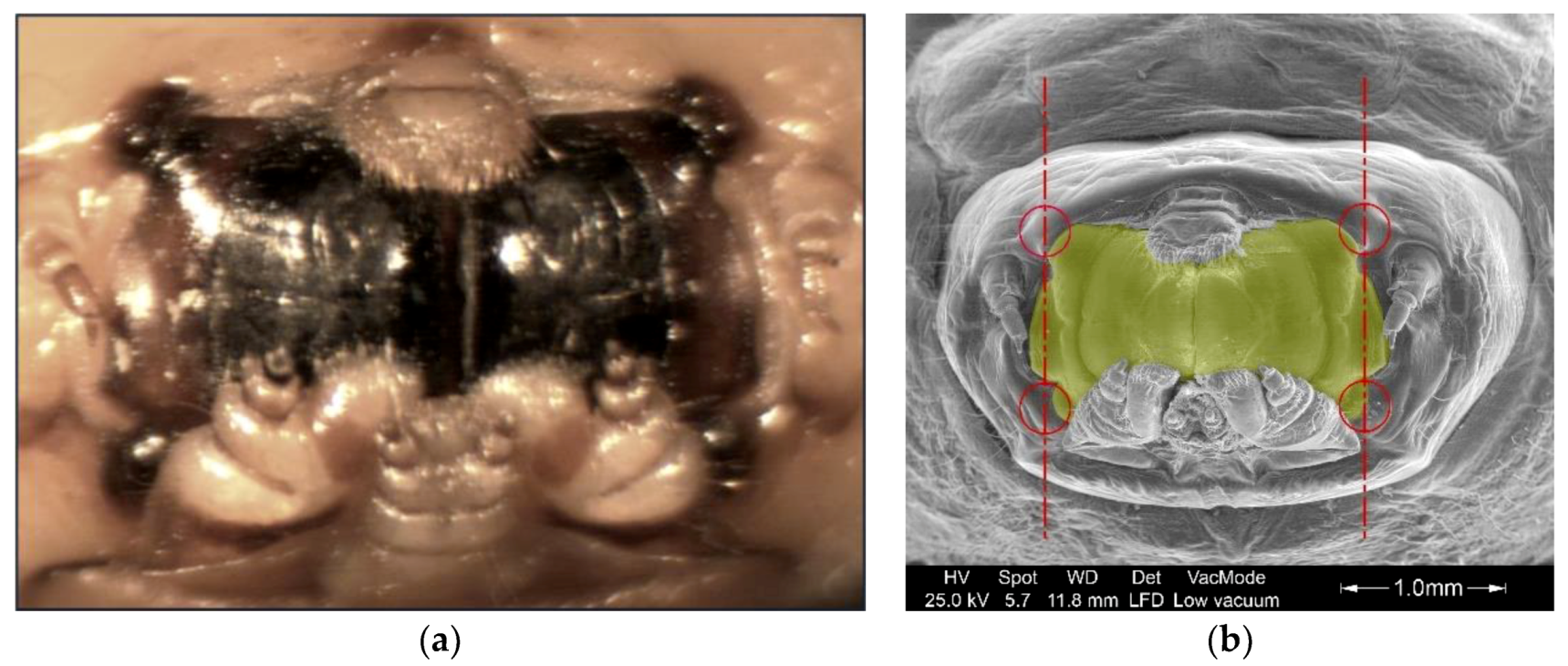
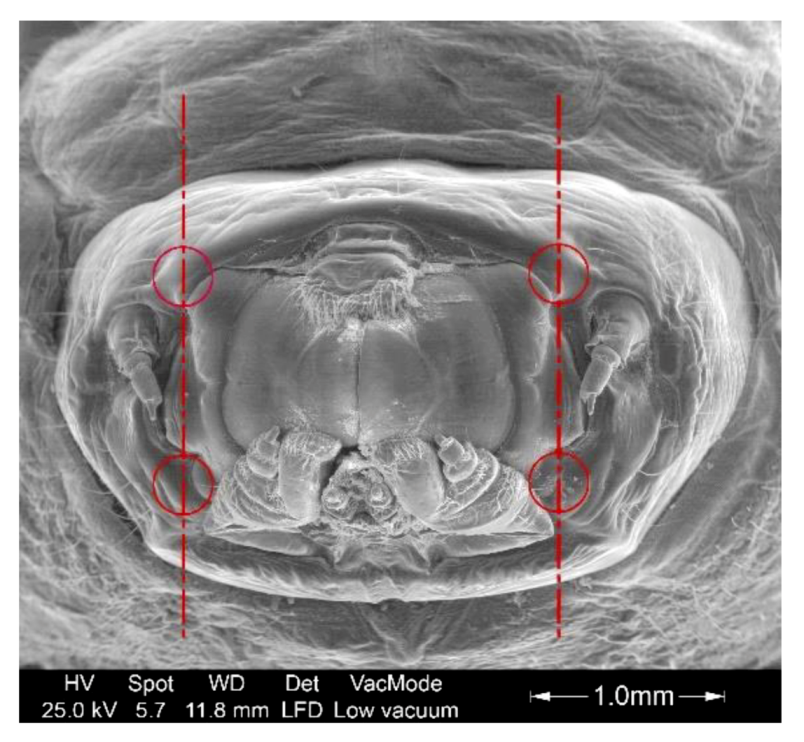
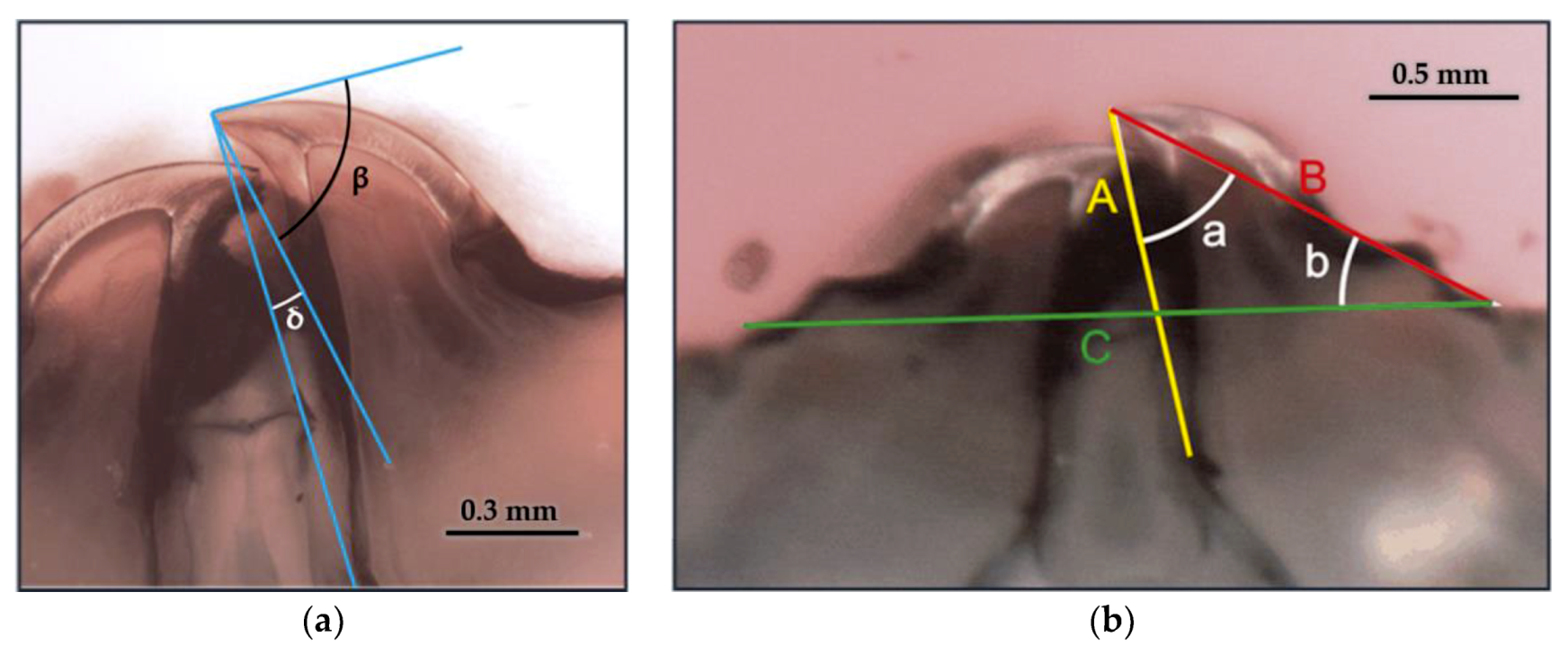
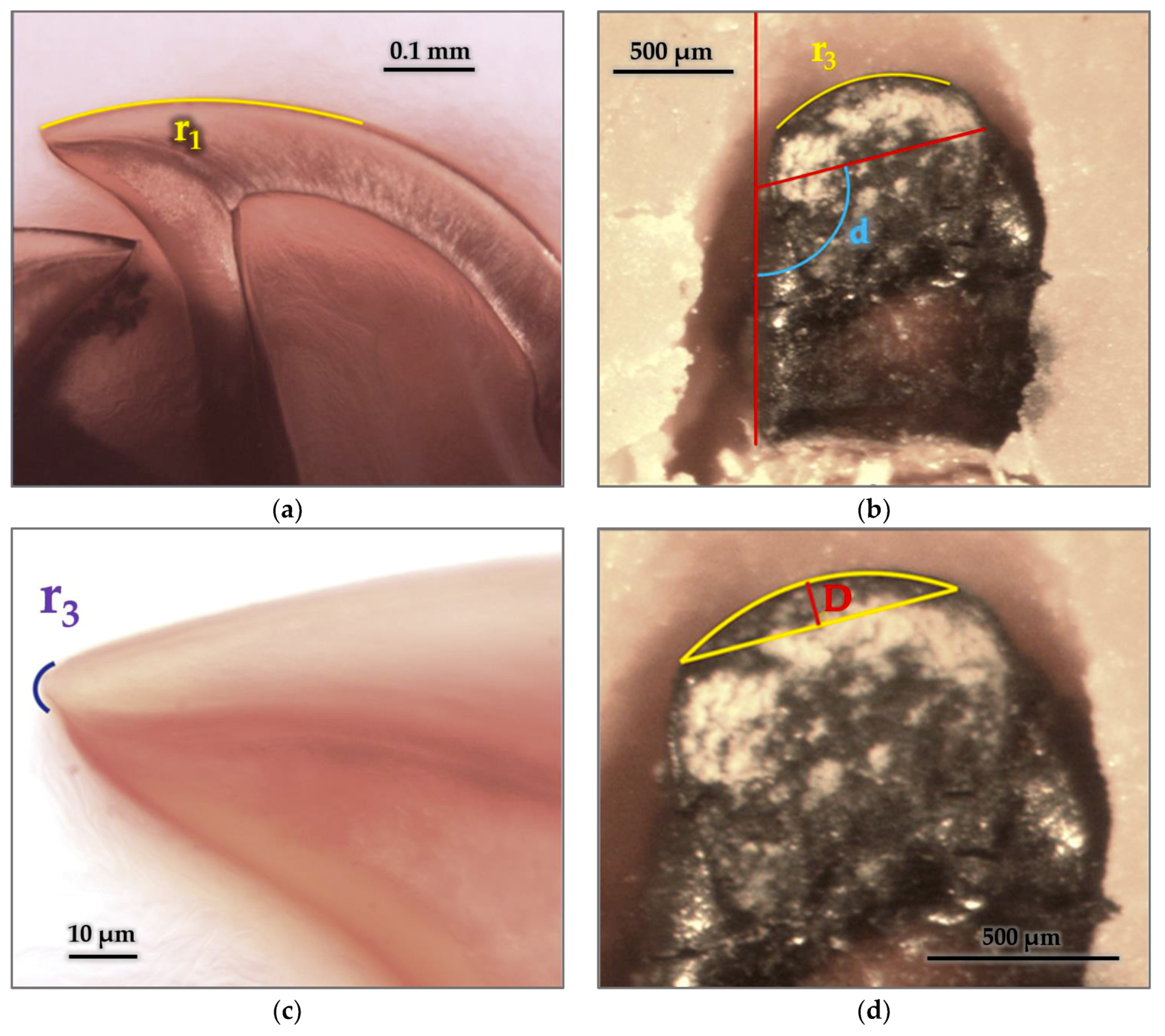
3.5. Geometric Characterization of The Mandibles
3.5.1. Geometric Parameters
3.5.2. Radius and Arches
3.5.3. Cutting Edge
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thompson, D.W. On Growth and Form; Cambridge University Press: Cambridge, UK, 1942. [Google Scholar]
- Hwang, J.; Jeong, Y.; Park, J.M.; Lee, K.H.; Hong, J.W.; Choi, J. Biomimetics: Forecasting the future of science, engineering, and medicine. Int. J. Nanomed. 2015, 10, 5701. [Google Scholar] [CrossRef]
- Nachtigall, W.; Wisser, A. Bionics by Examples. 250 Scenarios from Classical to Modern Times; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Tong, J.; Moayad, B.Z.; Ma, Y.H.; Sun, J.Y.; Chen, D.H.; Jia, H.L.; Ren, L.Q. Effects of biomimetic surface designs on furrow opener performance. J. Bionic Eng. 2009, 6, 280–289. [Google Scholar] [CrossRef]
- Betz, O.; Wegst, U.; Weide, D.; Heethoff, M.; Helfen, L.; LEE, W.K.; Cloetens, P. Imaging applications of synchrotron X-ray phase-contrast microtomography in biological morphology and biomaterials science. I. General aspects of the technique and its advantages in the analysis of millimetre-sized arthropod structure. J. Microsc. 2007, 227, 51–71. [Google Scholar] [CrossRef]
- Michels, J.; Gorb, S.N. Detailed three-dimensional visualisation of resilin in the exoskeleton of arthropods using confocal laser scanning microscopy. J. Microsc. 2012, 245, 1–16. [Google Scholar] [CrossRef]
- Liu, C.; Liu, J.; Xu, L.; Xiang, W. Recent achievements in bionic implementations of insect structure and functions. Kybernetes 2014, 43, 307–324. [Google Scholar] [CrossRef]
- Sun, J.; Ling, M.; Wu, W.; Bhushan, B.; Tong, J. The hydraulic mechanism of the unfolding of hind wings in Dorcus titanus platymelus (order: Coleoptera). Int. J. Mol. Sci. 2014, 15, 6009. [Google Scholar] [CrossRef]
- William, E.G.; Paul, Y.O.; Geoffrey, B. Flying insect inspired vision for autonomous aerial robot manoeuvres in near-earth environments. In Proceedings of the IEEE International Conference on Robotics & Automation, New Orleans, LA, USA, 26 April–1 May 2004. [Google Scholar]
- Du, J.; Hao, P. Investigation on microstructure of beetle elytra and energy absorption properties of bio-inspired honeycomb thin-walled structure under axial dynamic crushing. Nanomaterials 2018, 8, 667. [Google Scholar] [CrossRef]
- Chen, B.; Peng, X.H.; Fan, J.H. Microstructure of natural biocomposites and research of biomimetic composites. Acta Mater. Comp. Sin. 2000, 17, 59–62. [Google Scholar]
- Schleicher, S.; Kontominas, G.; Makker, T.; Tatli, I.; Yavaribajestani, Y. Studio One: A New Teaching Model for Exploring Bio-Inspired Design and Fabrication. Biomimetics 2019, 4, 34. [Google Scholar] [CrossRef]
- Hörnschemeyer, T.; Bond, J.; Young, P.G. Analysis of the functional morphology of mouthparts of the beetle Priacma serrata, and a discussion of possible food sources. J. Insect Sci. 2013, 13, 126. [Google Scholar] [CrossRef]
- Ball, G.E.; Acorn, J.H.; Shpeley, D. Mandibles and labrum-epipharynx of tiger beetles: Basic structure and evolution (Coleoptera, Carabidae, Cicindelitae). ZooKeys 2011, 147, 39–83. [Google Scholar] [CrossRef]
- Angelini, D.R.; Smith, F.W.; Aspiras, A.C.; Kikuchi, M.; Jockusch, E.L. Patterning of the adult mandibulate mouthparts in the red flour beetle, Tribolium castaneum. Genetics 2012, 190, 639–654. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Ji, B.Z.; Liu, S.W.; Qing, Z.H. Primary Research of Bionic Design on Tools with Mouthpart of Larvae Long Horned Beetles. Adv. Mater. Res. 2011, 142, 139–142. [Google Scholar] [CrossRef]
- Meyers, M.A.; Lin, A.Y.M.; Lin, Y.S.; Olevsky, E.A.; Georgalis, S. The cutting edge: Sharp biological materials. JOM 2008, 60, 19–24. [Google Scholar] [CrossRef]
- Zhang, K.; Zhong, B.; Lui, S.W.; Hua, Z. Research of Bionic Design on Tools with Chewing Mouthparts of Insects. Adv. Mater. Res. 2012, 426, 270–274. [Google Scholar] [CrossRef]
- Zhao, J.; Guo, M.; Lu, Y.; Huang, D.; Zhuang, J. Design of bionic locust mouthparts stubble cutting device. Int. J. Agric. Biol. Eng. 2020, 13, 20–28. [Google Scholar] [CrossRef]
- Gao, H.; Li, Y.Z.; Tong, J.; Sun, J.Y. Microstructure and nanoindentation properties of mouthparts of Oriental Mole Cricket. Trans. Chin. Soc. Agric. Mach. 2011, 42, 224–227. [Google Scholar]
- Tong, J.; Xu, S.; Chen, D.; Li, M. Design of a bionic blade for vegetable chopper. J. Bionic. Eng. 2017, 14, 163–171. [Google Scholar] [CrossRef]
- Hillerton, J.E.; Reynolds, S.E.; Vincent, J.F.V. On the indentation hardness of insect cuticle. J. Exp. Biol. 1982, 96, 45–52. [Google Scholar]
- Cribb, B.W.; Stewart, A.; Huang, H.; Truss, R.; Noller, B.; Rasch, R.; Zalucki, M.P. Insect mandibles-comparative mechanical properties and links with metal incorporation. Naturwissenschaften 2008, 95, 17–23. [Google Scholar] [CrossRef]
- Edwards, A.J.; Fawke, J.D.; McClements, J.G.; Smith, S.A.; Wyeth, P. Correlation of zinc distribution and enhanced hardness in the mandibular cuticle of the leaf-cutting and Atta sexdens rubropilosa. Cell Biol. Int. 1993, 17, 697–698. [Google Scholar] [CrossRef]
- Fawke, J.D.; McClements, J.G.; Wyeth, P. Cuticularmetals: Quantification and mapping by complimentary techniques. Cell Biol. Int. 1997, 21, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Hillerton, J.E.; Vincent, J.F.V. The specific locations of zinc in insect mandibles. J. Exp. Biol. 1982, 101, 333–336. [Google Scholar]
- Hillerton, J.E.; Robertson, B.; Vincent, J.F.V. The presence of zinc of manganese as the predominant metal in the mandibles of adult, stored-product beetles. J. Stored Prod. Res. 1984, 20, 133–137. [Google Scholar] [CrossRef]
- Schofield, R.; Lefevre, H. High concentrations of zinc in the fangs and manganese in the teeth of spiders. J. Exp. Biol. 1989, 144, 577–581. [Google Scholar]
- Vincent, J.F.V.; Wegst, U.G.K. Design and mechanical properties of insect cuticle. Arthropod Struct. Dev. 2004, 33, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Guo, C.; Xu, S.; Li, X.; Han, C. Morphology and nanoindentation properties of mouthparts in Cyrtotrachelus longimanus (Coleoptera: Curculionidae). Microsc. Res. Tech. 2017, 80, 704–711. [Google Scholar] [CrossRef]
- Büsse, S.; Gorb, S.N. Material composition of the mouthpart cuticle in a damselfly larva (Insecta: Odonata) and its biomechanical significance. R. Soc. Open Sci. 2018, 5, 172117. [Google Scholar] [CrossRef]
- Casado-Sanz, M.M.; Silva-Castro, I.; Ponce-Herrero, L.; Martín-Ramos, P.; Martín-Gil, J.; Acuña-Rello, L. White-rot fungi control on Populus spp. Wood by pressure treatments with silver nanoparticles, chitosan oligomers and propolis. Forests 2019, 10, 885. [Google Scholar] [CrossRef]
- Kaur, P.; Thakur, R.; Choudhary, A. An in vitro study of the antifungal activity of silver/chitosan nanoformulations against important seed borne pathogens. Int. J. Sci. Technol. Res. 2012, 1, 83–86. [Google Scholar]
- Silva-Castro, I.; Martín-García, J.; Diez, J.J.; Flores-Pacheco, J.A.; Martín-Gil, J.; Martín-Ramos, P. Potential control of forest diseases by solutions of chitosan oligomers, propolis and nanosilver. Eur. J. Plant Pathol. 2017, 150, 401–411. [Google Scholar] [CrossRef]
- Kartal, S.N.; Green, F.; Clausen, C.A. Do the unique properties of nano-metals affect leachability or efficacy against fungi and termites? Int. Biodeterior. Biodegrad. 2009, 63, 490–495. [Google Scholar] [CrossRef]
- Matsunaga, H.; Kiguchi, M.; Evans, P.D. Microdistribution of copper-carbonate and iron oxide nanoparticles in treated wood. J. Nanopart. Res. 2008, 11, 1087–1098. [Google Scholar] [CrossRef]
- Marzbani, P.; Mohammadnia-afrouzi, Y. Investigation on leaching and decay resistance of wood treated with nano-titanium dioxide. Adv. Environ. Biol. 2014, 8, 974–979. [Google Scholar]
- Broomell, C.C.; Mattoni, M.A.; Zok, F.W.; Waite, J.H. Critical role of zinc in hardening of Nereis jaws. J. Exp. Biol. 2006, 209, 3219–3225. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Parameswaran, N. Mandibeln des Hausbockkäfers (Hylotrupes bajulus L.) und Fraßstruktur des Holzes im Rasterelektronenmikroskop. Zeitschrift für Angewandte Entomologie 1977, 84, 407–412. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez, R.D.; Basterra, L.-A.; Acuña, L.; Balmori, J.-A. Morphology and Material Composition of the Mouthparts of Stromatium unicolor Olivier 1795 (Coleoptera: Cerambycidae) for Bionic Application. Forests 2020, 11, 715. https://doi.org/10.3390/f11070715
Martínez RD, Basterra L-A, Acuña L, Balmori J-A. Morphology and Material Composition of the Mouthparts of Stromatium unicolor Olivier 1795 (Coleoptera: Cerambycidae) for Bionic Application. Forests. 2020; 11(7):715. https://doi.org/10.3390/f11070715
Chicago/Turabian StyleMartínez, Roberto D., Luis-Alfonso Basterra, Luis Acuña, and José-Antonio Balmori. 2020. "Morphology and Material Composition of the Mouthparts of Stromatium unicolor Olivier 1795 (Coleoptera: Cerambycidae) for Bionic Application" Forests 11, no. 7: 715. https://doi.org/10.3390/f11070715
APA StyleMartínez, R. D., Basterra, L.-A., Acuña, L., & Balmori, J.-A. (2020). Morphology and Material Composition of the Mouthparts of Stromatium unicolor Olivier 1795 (Coleoptera: Cerambycidae) for Bionic Application. Forests, 11(7), 715. https://doi.org/10.3390/f11070715





