Organic Carbon Storage and 14C Apparent Age of Upland and Riparian Soils in a Montane Subtropical Moist Forest of Southwestern China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Field Sampling and Laboratory Processing
2.3. Data Analyses
3. Results and Discussion
3.1. Soil Moisture, Texture and Carbon Density Fraction
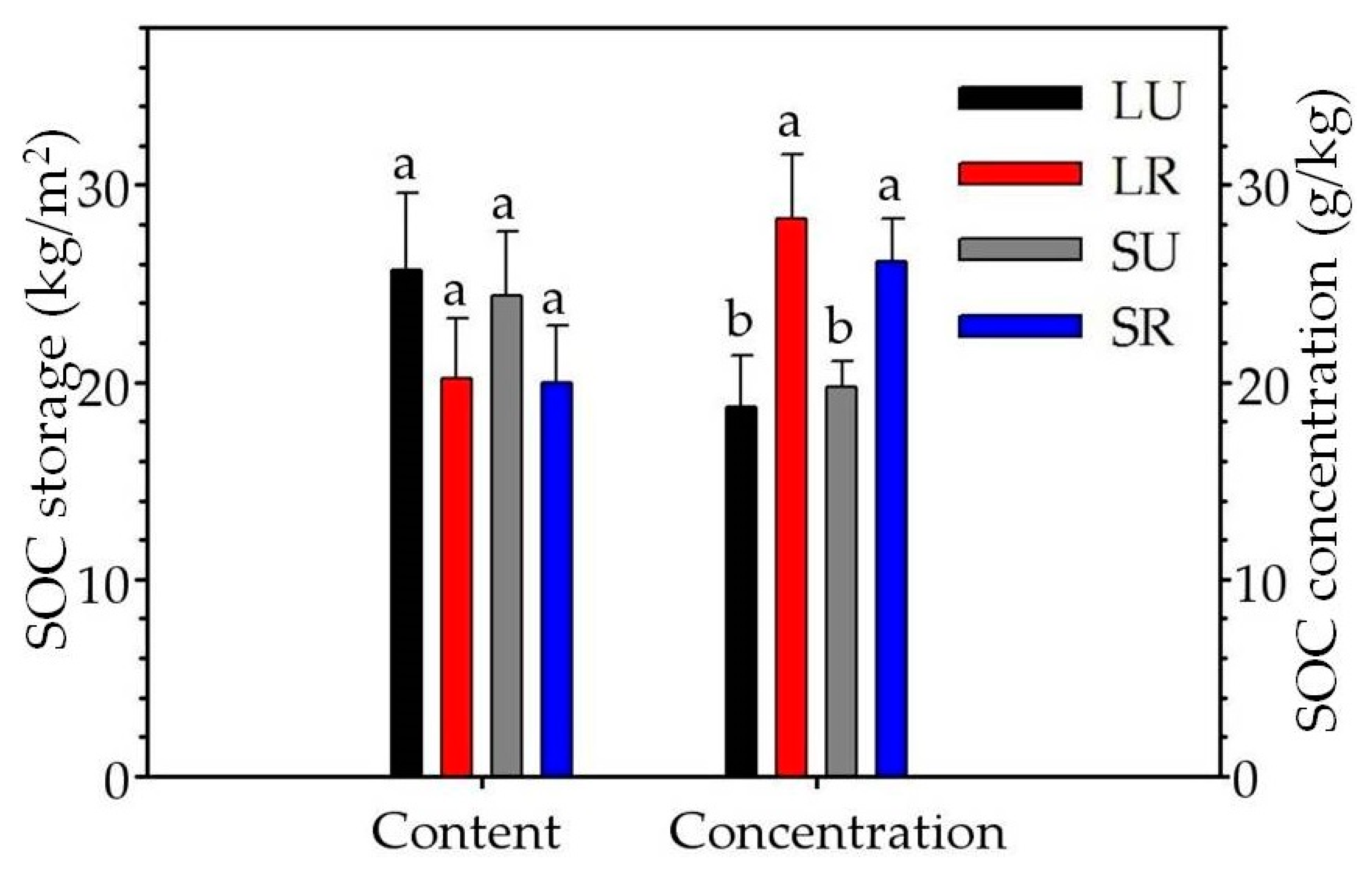
3.2. Vertical Variation and Storage of SOC
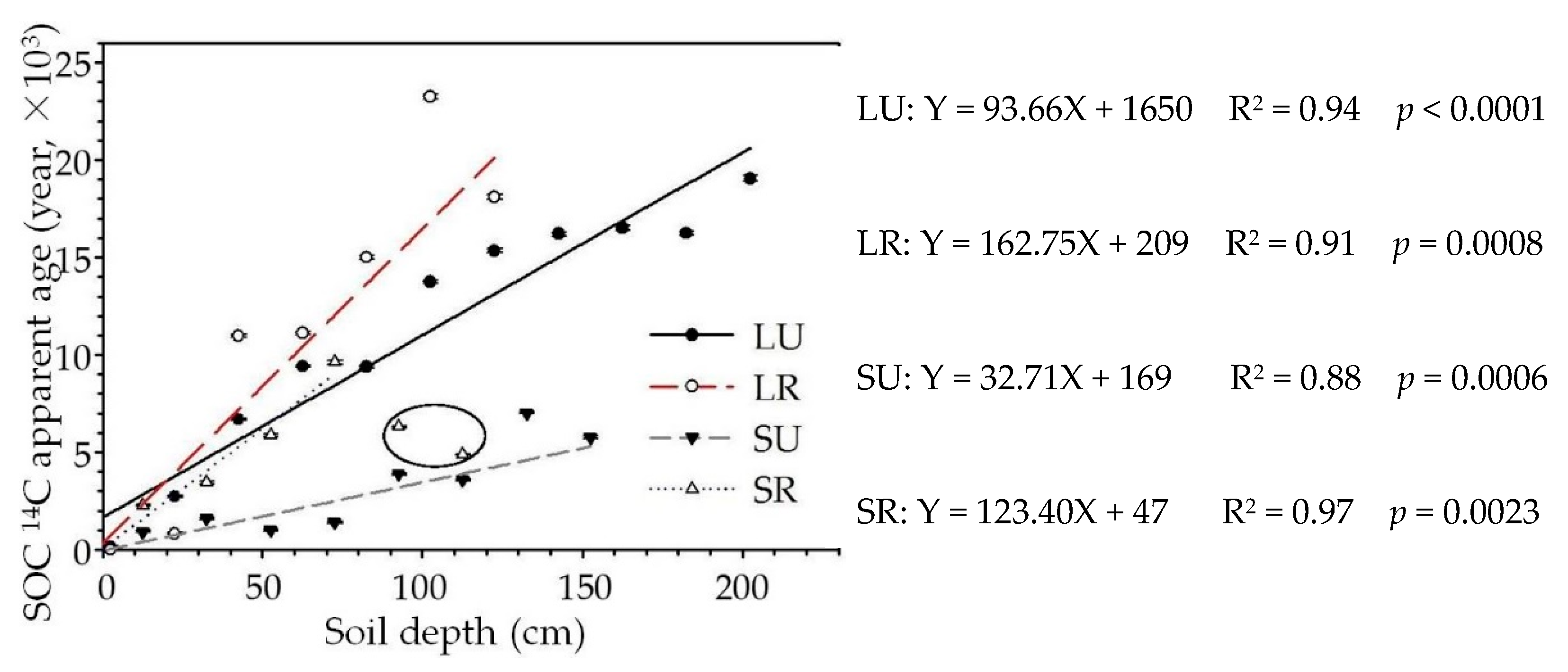
3.3. SOC 14C Apparent Age
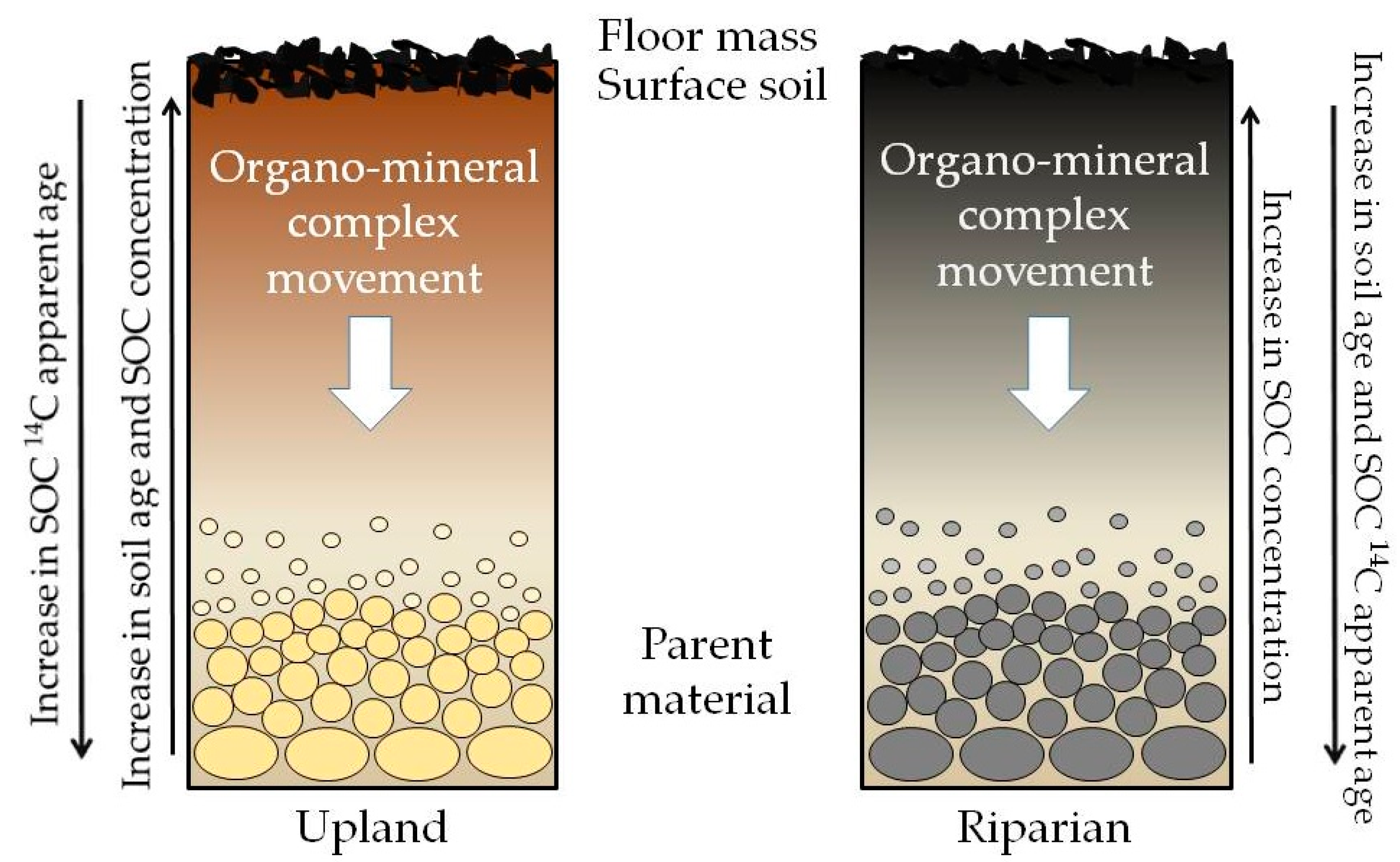
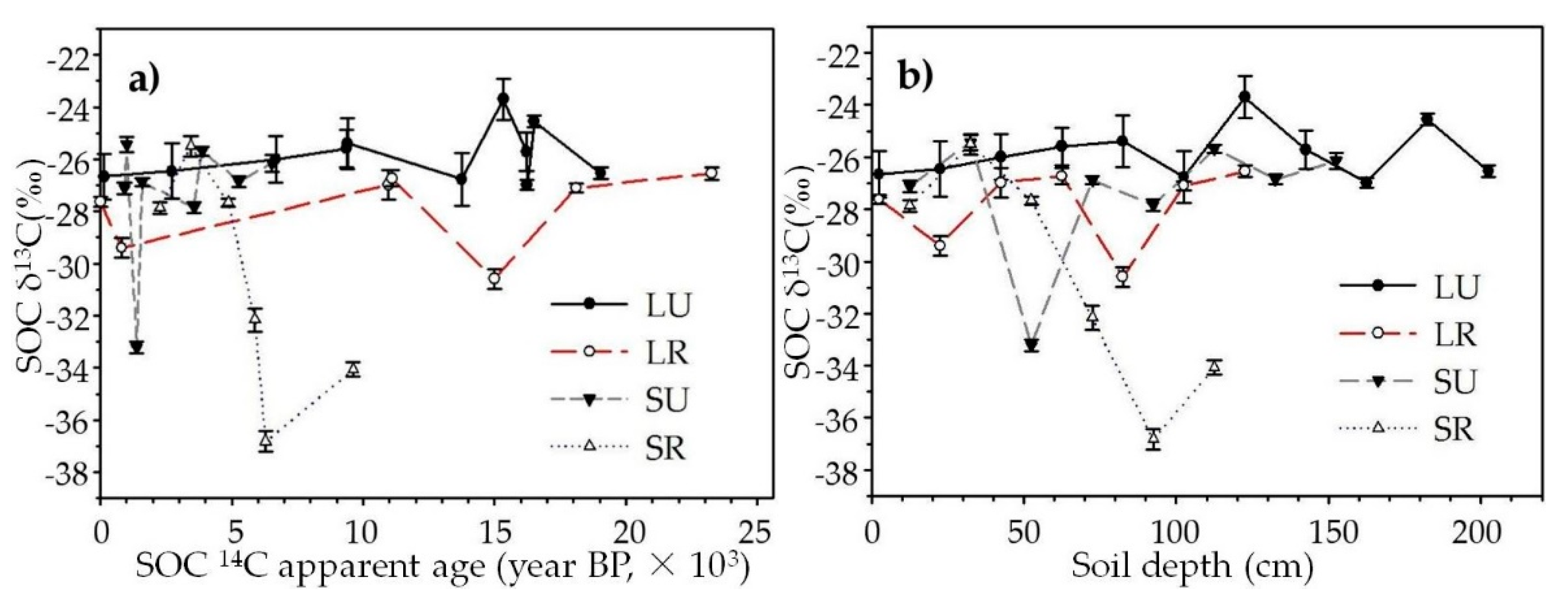
3.4. SOC δ13C Distribution
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Parton, W.J.; Scurlock, J.M.O.; Ojima, D.S.; Gilmanov, T.G.; Scholes, R.J.; Schimel, D.S.; Kirchner, T.; Menaut, J.-C.; Seastedt, T.; Garcia Moya, E.; et al. Observations and modeling of biomass and soil organic matter dynamics for the grassland biome worldwide. Glob. Biogeochem. Cycles 1993, 7, 785–809. [Google Scholar] [CrossRef]
- Paustian, K.; Parton, W.J.; Persson, J. Modeling soil organic matter in organic-amended and nitrogen-fertilized long-term plots. Soil Sci. Soc. Am. J. 1992, 56, 476–488. [Google Scholar] [CrossRef]
- Smith, P.; Smith, J.U.; Powlson, D.S.; McGill, W.B.; Arah, J.R.M.; Chertov, O.G.; Coleman, K.; Franko, U.; Frolking, S.; Jenkinson, D.S.; et al. A comparison of the performance of nine soil organic matter models using datasets from seven long-term experiments. Geoderma 1997, 81, 153–225. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kögel-Knabner, I.; Lehmann, J.; Manning, D.A.; et al. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. Methods Soil Anal. Part 3—Chem. Methods 1996, 5, 961–1010. [Google Scholar]
- Hohl, H.; Varma, A. Chapter 1: Soil-the living matrix. In Soil Heavy Metals; Sherameti, I., Varma, A., Eds.; Springer: Heidelberg, Germany; Dordrecht, The Netherlands; London, UK; New York, NY, USA, 2010; Volume 19, pp. 1–18. [Google Scholar]
- Cook, S.F. The nature of charcoal excavated at archaeological sites. Am. Antiq. 1964, 29, 514–517. [Google Scholar] [CrossRef]
- Nave, L.E.; Vance, E.D.; Swanston, C.W.; Curtis, P.S. Harvest impacts on soil carbon storage in temperate forests. For. Ecol. Manag. 2010, 259, 857–866. [Google Scholar] [CrossRef]
- Achat, D.L.; Fortin, M.F.; Landmann, G.; Ringeval, B.; Augusto, L. Forest soil carbon is threatened by intensive biomass harvesting. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef]
- Torn, M.S.; Trumbore, S.E.; Chadwick, O.A.; Vitousek, P.M.; Hendricks, D.M. Mineral control of soil organic carbon storage and turnover. Nature 1997, 389, 170–173. [Google Scholar] [CrossRef]
- Binkley, D.; Resh, S.C. Rapid changes in soils following Eucalyptus afforestation in Hawaii. Soil Sci. Soc. Am. J. 1999, 63, 222–225. [Google Scholar] [CrossRef]
- Scott, N.A.; Tate, K.R.; Ford-Robertson, J.; Giltrap, D.J.; Smith, C.T. Soil carbon storage in plantation forests and pastures: Land-use change implications. Tellus B Chem. Phys. Meteorol. 1999, 51B, 326–335. [Google Scholar] [CrossRef]
- Johnson, D.W.; Curtis, P.S. Effects of forest management on soil C and N storage: Meta analysis. For. Ecol. Manag. 2001, 140, 227–238. [Google Scholar] [CrossRef]
- Eswaran, H.; Van Den Berg, E.; Reich, P. Organic carbon in soils of the world. Soil Sci. Soc. Am. J. 1993, 57, 192–194. [Google Scholar] [CrossRef]
- Pan, Y.D.; Birdsey, R.A.; Fang, J.Y.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Q.; Shen, C.D.; Sun, Y.M.; Peng, S.L.; Yi, W.X.; Li, Z.A.; Jiang, M.T. Spatial and temporal distribution of carbon isotopes in soil organic matter at the Dinghushan Biosphere Reserve, South China. Plant Soil 2005, 273, 115–128. [Google Scholar] [CrossRef]
- Townsend, A.R.; Asner, G.P.; Cleveland, C.C. The biogeochemical heterogeneity of tropical forests. Trends Ecol. Evol. 2008, 23, 424–431. [Google Scholar] [CrossRef]
- Chatterjee, N.; Nair, P.K.R.; Chakraborty, S.; Nair, V.D. Changes in soil carbon stocks across the forest-agroforest-agriculture/pasture continuum in various agroecological regions: A meta-analysis. Agric. Ecosyst. Environ. 2018, 266, 55–67. [Google Scholar] [CrossRef]
- De Stefano, A.; Jacobson, M.G. Soil carbon sequestration in agroforestry systems: A meta-analysis. Agrofor. Syst. 2018, 92, 285–299. [Google Scholar] [CrossRef]
- Guo, L.B.; Gifford, R.M. Soil carbon stocks and land use change: A meta analysis. Glob. Chang. Biol. 2002, 8, 345–360. [Google Scholar] [CrossRef]
- Bascomb, C.L. Distribution of pyrophosphate-extractable iron and organic carbon in soils of various groups. J. Soil Sci. 1968, 19, 251–268. [Google Scholar] [CrossRef]
- Jobbágy, E.G.; Jackson, R.B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- López-Rodríguez, S.R.; Blanco-Libreros, J.F. Illicit crops in tropical America: Deforestation, landslides, and the terrestrial carbon stocks. Ambio 2008, 37, 141–143. [Google Scholar] [CrossRef]
- Kavian, A.; Azmoodeh, A.; Solaimani, K. Deforestation effects on soil properties, runoff and erosion in northern Iran. Arab. J. Geosci. 2014, 7, 1941–1950. [Google Scholar] [CrossRef]
- Gandois, L.; Cobb, A.R.; Hei, I.C.; Lim, L.B.L.; Salim, K.A.; Harvey, C.F. Impact of deforestation on solid and dissolved organic matter characteristics of tropical peat forests: Implications for carbon release. Biogeochemistry 2013, 114, 183–199. [Google Scholar] [CrossRef]
- Burke, I.C.; Yonker, C.M.; Parton, W.J.; Cole, C.V.; Schimel, D.S.; Flach, K. Texture, climate, and cultivation effects on soil organic matter content in US grassland soils. Soil Sci. Soc. Am. J. 1989, 53, 800–805. [Google Scholar] [CrossRef]
- Fu, X.L.; Shao, M.G.; Wei, X.R.; Horton, R. Soil organic carbon and total nitrogen as affected by vegetation types in Northern Loess Plateau of China. Geoderma 2010, 155, 31–35. [Google Scholar] [CrossRef]
- Jenny, H. Chapter 2: Methods of presentation of soil data. In Factors of Soil Formation: A System of Quantitative Pedology; Dover Publications: Mineola, NY, USA, 1994; pp. 21–30. [Google Scholar]
- Percival, H.J.; Parfitt, R.L.; Scott, N.A. Factors controlling soil carbon levels in New Zealand grasslands is clay content important? Soil Sci. Soc. Am. J. 2000, 64, 1623–1630. [Google Scholar] [CrossRef]
- Chatterjee, N.; Nair, P.K.R.; Nair, V.D.; Bhattacharjee, A.; Filho, E.M.V.; Muschler, R.G.; Noponen, M.R.A. Do coffee agroforestry systems always improve soil carbon stocks deeper in the soil?—A case study from Turrialba, Costa Rica. Forests 2020, 11, 49. [Google Scholar] [CrossRef]
- Boutton, T.W. Stable carbon isotope ratios of organic matter and their use as indicators of vegetation and climate changes. In Mass Spectrometry of Soils; Boutton, T.W., Yamazaki, S., Eds.; Mercel Dekker: New York, NY, USA, 1996; pp. 47–82. [Google Scholar]
- Meyers, P.A.; Lallier-Vergès, E. Lacustrine sedimentary organic matter records of Late Quaternary paleoclimates. J. Paleolimnol. 1999, 21, 345–372. [Google Scholar] [CrossRef]
- Wang, G.; Feng, X.; Han, J.; Zhou, L.; Tan, W.; Su, F. Paleovegetation reconstruction using δ13C of soil organic matter. Biogeosciences 2008, 5, 1325–1337. [Google Scholar] [CrossRef]
- Shen, C.D.; Yi, W.X.; Sun, Y.M.; Xing, C.P.; Yang, Y.; Peng, S.L.; Li, Z.A. 14C apparent ages and δ13C distribution of forest soils in Dinghushan Natural Reserve. Quat. Sci 2000, 20, 335–344. [Google Scholar]
- Chen, Q.Q.; Shen, C.D.; Peng, S.L.; Sun, Y.M.; Yi, W.X.; Li, Z.A.; Jiang, M.T. Soil organic matter turnover in the subtropical mountainous region of South China. Soil Sci. 2002, 167, 401–415. [Google Scholar] [CrossRef]
- Ruan, H.H.; Li, Y.Q.; Zou, X.M. Soil communities and plant litter decomposition as influenced by forest debris: Variation across tropical riparian and upland sites. Pedobiologia 2005, 49, 529–538. [Google Scholar] [CrossRef]
- He, Y.J.; Trumbore, S.E.; Torn, M.S.; Harden, J.W.; Vaughn, L.J.S.; Allison, S.D.; Randerson, J.T. Radiocarbon constraints imply reduced carbon uptake by soils during the 21st century. Science 2016, 353, 1419–1424. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.G.; Li, Y.; Ye, X.H.; Chu, Y.; Wang, X.P. Profile storage of organic/inorganic carbon in soil: From forest to desert. Sci. Total Environ. 2010, 408, 1925–1931. [Google Scholar] [CrossRef]
- Song, Y.C. On the global position of the evergreen broad-leaved forests of China. In Vegetation Science in Forestry; Box, E.O., Peet, R.K., Masuzava, T., Yamada, I., Fujiwara, K., Maycock, P.F., Eds.; Kluwer Academic Publishers: Norwell, MA, USA, 1995; pp. 69–84. [Google Scholar]
- Song, Y.C.; Chen, X.Y.; Wang, X.H. Studies on evergreen broadleaved forests of China: A retrospect and prospect. J East China Norm. Univ. 2005, 1, 1–8. [Google Scholar]
- Wu, C.Z.; Hong, W.; Chen, H.; Lan, B.; Zheng, Q.R. Species diversity of subtropical evergreen broad-leaved forest in Wanmulin Mountain. J. Fujian Coll. For. 1996, 16, 33–37. [Google Scholar]
- Li, C.H. The distribution of evergreen broad-leaved forest in East Asia. Nat. Resour. 1997, 2, 37–46. [Google Scholar]
- Huang, W.; Lin, C.L.; Wu, C.Z.; He, D.J.; Chen, K. Research on species diversity of evergreen broad-leaved shelter-forests in Jianxi River valley, Fujian. Chin. Biodivers. 1999, 7, 208–213. [Google Scholar]
- Ding, S.Y.; Song, Y.C. Research advances in vegetation dynamic of evergreen broad-leaved forest. Acta Ecol. Sin. 2004, 24, 1765–1775. [Google Scholar]
- Yan, S.J.; Hong, W.; Hong, T.; Wu, C. Changes of species diversity in gap gradient of mid-subtropical evergreen broad-leaved forest in Wanmulin Forest. J. Appl. Ecol. 2006, 17, 947–950. [Google Scholar]
- Zhang, S.B.; Zheng, Z. Quantity and distribution of standing dead trees in montane moist evergreen broad-leaved forest in Ailao Mountain, Yunnan. J. Northeast For. Univ. 2009, 37, 3–13. [Google Scholar]
- He, Y.T.; Li, G.C.; Cao, M.; Tang, Y. Tree species diversity of a secondary broadleaved forest on Ailao Mountain in Yunnan. J. Trop. Subtrop. Bot. 2003, 11, 104–108. [Google Scholar]
- Shi, J.P.; Zhao, C.J.; Zhu, H. Characteristics and species composition of main vegetation types on west slope of the Ailao Mountains in Yunnan. Chin. J. Appl. Environ. Biol. 2005, 11, 1–7. [Google Scholar]
- Li, X.S.; Liu, W.Y.; Chen, J.W.; Yuan, C.M. Seedling regeneration in primary moist evergreen broad-leaved forest and different type secondary vegetation in Ailao Mountains. Chin. J. Ecol. 2009, 28, 1921–1927. [Google Scholar]
- Zhang, K.Y.; Zhang, Y.P.; Liu, Y.H.; Li, Y.R. Vertical distribution characteristics of rainfall in the Ailao Mountain. Sci. Geogr. Sin. 1994, 2, 144–151. [Google Scholar]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef]
- Liu, W.Y.; Fox, J.E.D.; Xu, Z.F. Litterfall and nutrient dynamics in a montane moist evergreen broad-leaved forest in Ailao Mountains, SW China. Plant Ecol. 2003, 164, 157–170. [Google Scholar] [CrossRef]
- Yang, L.P.; Liu, W.Y.; Yang, G.P.; Ma, W.Z.; Li, D.W. Composition and carbon storage of woody debris in moist evergreen broad-leaved forest and its secondary forests in Ailao Mountains of Yunnan provinve. J. Appl. Ecol. 2007, 18, 2153–2159. [Google Scholar]
- Chai, Y.; Zhu, H.; Meng, G.T.; Shi, J.P.; Yang, G. Population structure and distribution pattern of dominant tree species in ancient tea tree community in Ailao Mountains of Yunnan province, China. For. Res. Beijing 2011, 24, 277–284. [Google Scholar]
- Li, Y.P.; Feng, Y.L. Differences in seed morphometric and germination traits of crofton weed (Eupatorium adenophorum) from different elevations. Weed Sci. 2009, 57, 26–30. [Google Scholar] [CrossRef]
- Zou, X.M.; Ruan, H.H.; Fu, Y.; Yang, X.D.; Sha, L.Q. Estimating soil labile organic carbon and potential turnover rates using a sequential fumigation–incubation procedure. Soil Biol. Biochem. 2005, 37, 1923–1928. [Google Scholar] [CrossRef]
- Chan, O.C.; Yang, X.D.; Fu, Y.; Feng, Z.L.; Sha, L.Q.; Casper, P.; Zou, X.M. 16S rRNA gene analyses of bacterial community structures in the soils of evergreen broad-leaved forests in south-west China. Fems Microbiol. Ecol. 2006, 58, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.Y.; Fox, J.E.; Xu, Z.F. Nutrient fluxes in bulk precipitation, throughfall and stemflow in montane subtropical moist forest on Ailao Mountains in Yunnan, south-west China. J. Trop. Ecol. 2002, 18, 527–548. [Google Scholar] [CrossRef]
- Feng, W.T.; Zou, X.M.; Schaefer, D. Above-and belowground carbon inputs affect seasonal variations of soil microbial biomass in a subtropical monsoon forest of southwest China. Soil Biol. Biochem. 2009, 41, 978–983. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhang, S.B.; Yang, G.P.; Tang, Y.; Baskin, J.; Baskin, C.; Yang, L.Y. Abundance and distribution of cavity trees in an old-growth subtropical montane evergreen broad-leaved forest. Can. J. For. Res. 2009, 39, 2234–2245. [Google Scholar] [CrossRef]
- Srivastava, A.; Verkouteren, R.M. Metrology for stable isotope reference materials: 13C/12C and 18O/16O isotope ratio value assignment of pure carbon dioxide gas samples on the Vienna PeeDee Belemnite-CO2 scale using dual-inlet mass spectrometry. Anal. Bioanal. Chem. 2018, 410, 4153–4163. [Google Scholar] [CrossRef]
- Mook, W.G.; Van Der Plicht, J. Reporting 14C activities and concentrations. Radiocarbon 1999, 41, 227–239. [Google Scholar] [CrossRef]
- Fernandez-Illescas, C.P.; Porporato, A.; Laio, F.; Rodriguez-Iturbe, I. The ecohydrological role of soil texture in a water-limited ecosystem. Water Resour. Res. 2001, 37, 2863–2872. [Google Scholar] [CrossRef]
- Alvarez, R.; Russo, M.E.; Prystupa, P.; Scheiner, J.D.; Blotta, L. Soil carbon pools under conventional and no-tillage systems in the Argentine Rolling Pampa. Agron. J. 1998, 90, 138–143. [Google Scholar] [CrossRef]
- Kalembasa, S.J.; Jenkinson, D.S. A comparative study of titrimetric and gravimetric methods for the determination of organic carbon in soil. J. Sci. Food Agric. 1973, 24, 1085–1090. [Google Scholar] [CrossRef]
- O’Neill, M.E.; Mathews, K. Theory & methods: A weighted least squares approach to Levene’s test of homogeneity of variance. Aust. New Zealand J. Stat. 2000, 42, 81–100. [Google Scholar]
- Razali, N.M.; Wah, Y.B. Power comparisons of Shapiro-Wilk, Kolmogorov-Smirnov, Lilliefors and Anderson-Darling tests. J. Stat. Modeling Anal. 2011, 2, 21–33. [Google Scholar]
- Puth, M.T.; Neuhäuser, M.; Ruxton, G.D. Effective use of Pearson’s product–moment correlation coefficient. Anim. Behav. 2014, 93, 183–189. [Google Scholar] [CrossRef]
- Mage, S.M.; Porder, S. Parent material and topography determine soil phosphorus status in the Luquillo Mountains of Puerto Rico. Ecosystems 2013, 16, 284–294. [Google Scholar] [CrossRef]
- Parton, W.J.; Schimel, D.S.; Cole, C.V.; Ojima, D.S. Analysis of factors controlling soil organic matter levels in Great Plains Grasslands. Soil Sci. Soc. Am. J. 1987, 51, 1173–1179. [Google Scholar] [CrossRef]
- Sollins, P.; Homann, P.; Caldwell, B.A. Stabilization and destabilization of soil organic matter: Mechanisms and controls. Geoderma 1996, 74, 65–105. [Google Scholar] [CrossRef]
- Bird, M.; Kracht, O.; Derrien, D.; Zhou, Y.P. The effect of soil texture and roots on the stable carbon isotope composition of soil organic carbon. Soil Res. 2003, 41, 77–94. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Kleber, M.; Fendorf, S. Are oxygen limitations under recognized regulators of organic carbon turnover in upland soils? Biogeochemistry 2016, 127, 157–171. [Google Scholar] [CrossRef]
- Cambardella, C.C. Carbon cycle in soils formation and decomposition. In Encyclopedia of Soil in the Environment; Elsevier Ltd.: Amsterdam, The Netherlands, 2005; pp. 170–175. [Google Scholar]
- McCauley, A.; Jones, C.; Jacobsen, J. Soil pH and organic matter. Nutr. Manag. Modul. 2009, 8, 1–12. [Google Scholar]
- Ricker, M.C.; Stolt, M.H.; Zavada, M.S. Comparison of soil organic carbon dynamics in forested riparian wetlands and adjacent uplands. Soil Sci. Soc. Am. J. 2014, 78, 1817–1827. [Google Scholar] [CrossRef]
- Hill, A.R.; Devito, K.J.; Campagnolo, S.; Sanmugadas, K. Subsurface denitrification in a forest riparianzone: Interactions between hydrology and supplies ofnitrate and organic carbon. Biogeochemistry 2000, 51, 193–223. [Google Scholar] [CrossRef]
- Ricker, M.C.; Stolt, M.H.; Donohue, S.W.; Blazejewski, G.A.; Zavada, M.S. Soil organic carbon pools in riparian landscapes of southern New England. Soil Sci. Soc. Am. J. 2013, 77, 1070–1079. [Google Scholar] [CrossRef]
- Luke, S.H.; Luckai, N.J.; Burke, J.M.; Prepas, E.E. Riparian areas in the Canadian boreal forest and linkages with water quality in streams. Environ. Rev. 2007, 15, 79–97. [Google Scholar] [CrossRef]
- Lin, H. Linking principles of soil formation and flow regimes. J. Hydrol. 2010, 393, 3–19. [Google Scholar] [CrossRef]
- Li, L.H.; Zhang, Y.P.; Tan, Z.H.; Song, Q.H.; Luo, Y.Y. Variation patterns of solar radiation above subtropical evergreen broadleaved forest and open area in Ailao Mountains. Chin. J. Ecol. 2011, 30, 1435–1440. [Google Scholar]
- Ding, P.; Shen, C.D.; Wang, N.; Yi, W.X.; Ding, X.F.; Fu, D.P.; Liu, K.X.; Zhou, L.P. Turnover rate of soil organic matter and origin of soil 14CO2 in deep soil from a subtropical forest in Dinghushan Biosphere Reserve, South China. Radiocarbon 2010, 52, 1422–1434. [Google Scholar] [CrossRef]
- Silver, W.L.; Lugo, A.E.; Keller, M. Soil oxygen availability and biogeochemistry along rainfall and topographic gradients in upland wet tropical forest soils. Biogeochemistry 1999, 44, 301–328. [Google Scholar] [CrossRef]
- Dwivedi, D.; Tang, J.Y.; Bouskill, N.; Georgiou, K.; Chacon, S.S.; Riley, W.J. Abiotic and biotic controls on soil organo–mineral interactions: Developing model structures to analyze why soil organic matter persists. Rev. Mineral. Geochem. 2019, 85, 329–348. [Google Scholar] [CrossRef]
- Keiluweit, M.; Bougoure, J.J.; Nico, P.S.; Pett-Ridge, J.; Weber, P.K.; Kleber, M. Mineral protection of soil carbon counteracted by root exudates. Nat. Clim. Chang. 2015, 5, 588–595. [Google Scholar] [CrossRef]
- Sanderman, J.; Amundson, R. A comparative study of dissolved organic carbon transport and stabilization in California forest and grassland soils. Biogeochemistry 2008, 89, 309–327. [Google Scholar] [CrossRef]
- Michalzik, B.; Tipping, E.; Mulder, J.; Gallardo Lancho, J.F.; Matzner, E.; Bryant, C.L.; Clarke, N.; Lofts, S.; Vicente Esteban, M.A. Modelling the production and transport of dissolved organic carbon in forest soils. Biogeochemistry 2003, 66, 241–264. [Google Scholar] [CrossRef]
- Miltner, A.; Bombach, P.; Schmidt-Brücken, B.; Kästner, M. SOM genesis: Microbial biomass as a significant source. Biogeochemistry 2012, 111, 41–55. [Google Scholar] [CrossRef]
- Certano, A.K.; Fernandez, C.W.; Heckman, K.A.; Kennedy, P.G. The afterlife effects of fungal morphology: Contrasting decomposition rates between diffuse and rhizomorphic necromass. Soil Biol. Biochem. 2018, 126, 76–81. [Google Scholar] [CrossRef]
- Willis, K.J.; Rudner, E.; Sümegi, P. The full-glacial forests of central and southeastern Europe. Quat. Res. 2000, 53, 203–213. [Google Scholar] [CrossRef]
- Wang, X.; van der Kaars, S.; Kershaw, P.; Bird, M.; Jansen, F. A record of fire, vegetation and climate through the last three glacial cycles from Lombok Ridge core G6-4, eastern Indian Ocean, Indonesia. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1999, 147, 241–256. [Google Scholar] [CrossRef]
- Burbridge, R.E.; Mayle, F.E.; Killeen, T.J. Fifty-thousand-year vegetation and climate history of Noel Kempff Mercado National park, Bolivian Amazon. Quat. Res. 2004, 61, 215–230. [Google Scholar] [CrossRef]
- Huang, W.; Liu, X.B.; González, G.; Zou, X.M. Late Holocene fire history and charcoal decay in subtropical dry forests of Puerto Rico. Fire Ecol. 2019, 15, 14. [Google Scholar] [CrossRef]
- Geist, H.J.; Lambin, E.F. What drives tropical deforestation. Lucc Rep. Ser. 2001, 4, 116. [Google Scholar]
- Kaimowitz, D.; Angelsen, A. Section 2: Types of economic deforestation models. In Economic Models of Tropical Deforestation: A Review; Cifor: Bogor, Indonesia, 1998; pp. 7–14. [Google Scholar]
- Landry, J.S.; Matthews, H.D. Non-deforestation fire vs. fossil fuel combustion: The source of CO2 emissions affects the global carbon cycle and climate responses. Biogeosciences (BG) 2016, 13, 2137–2149. [Google Scholar] [CrossRef]
- Houghton, R.A. Tropical deforestation and atmospheric carbon dioxide. In Tropical Forests and Climate; Springer: Dordrecht, The Netherlands, 1991; Volume 19, pp. 99–118. [Google Scholar]
- Ascough, P.L.; Cook, G.T.; Church, M.J.; Dunbar, E.; Einarsson, Á.; McGovern, T.H.; Dugmore, A.J.; Perdikaris, S.; Hastie, H.; Friðriksson, A. Temporal and spatial variations in freshwater 14C reservoir effects: Lake Mývatn, northern Iceland. Radiocarbon 2010, 52, 1098–1112. [Google Scholar] [CrossRef]
- Saggar, S.; McIntosh, P.D.; Hedley, C.B.; Knicker, H. Changes in soil microbial biomass, metabolic quotient, and organic matter turnover under Hieracium (H. pilosella L.). Biol. Fertil. Soils 1999, 30, 232–238. [Google Scholar] [CrossRef]
- Li, X.S.; Liu, W.Y.; Tang, C.Q. The role of the soil seed and seedling bank in the regeneration of diverse plant communities in the subtropical Ailao Mountains, Southwest China. Ecol. Res. 2010, 25, 1171–1182. [Google Scholar] [CrossRef]
- Bottrell, S.; Hipkins, E.V.; Lane, J.M.; Zegos, R.A.; Banks, D.; Frengstad, B.S. Carbon-13 in groundwater from English and Norwegian crystalline rock aquifers: A tool for deducing the origin of alkalinity? Sustain. Water Resour. Manag. 2019, 5, 267–287. [Google Scholar] [CrossRef]
- Ehleringer, J.R.; Buchmann, N.; Flanagan, L.B. Carbon isotope ratios in belowground carbon cycle processes. Ecol. Appl. 2000, 10, 412–422. [Google Scholar] [CrossRef]
- Cerling, T.E.; Solomon, D.K.; Quade, J.; Bowman, J.R. On the isotopic composition of carbon in soil carbon dioxide. Geochim. Cosmochim. Acta 1991, 55, 3403–3405. [Google Scholar] [CrossRef]
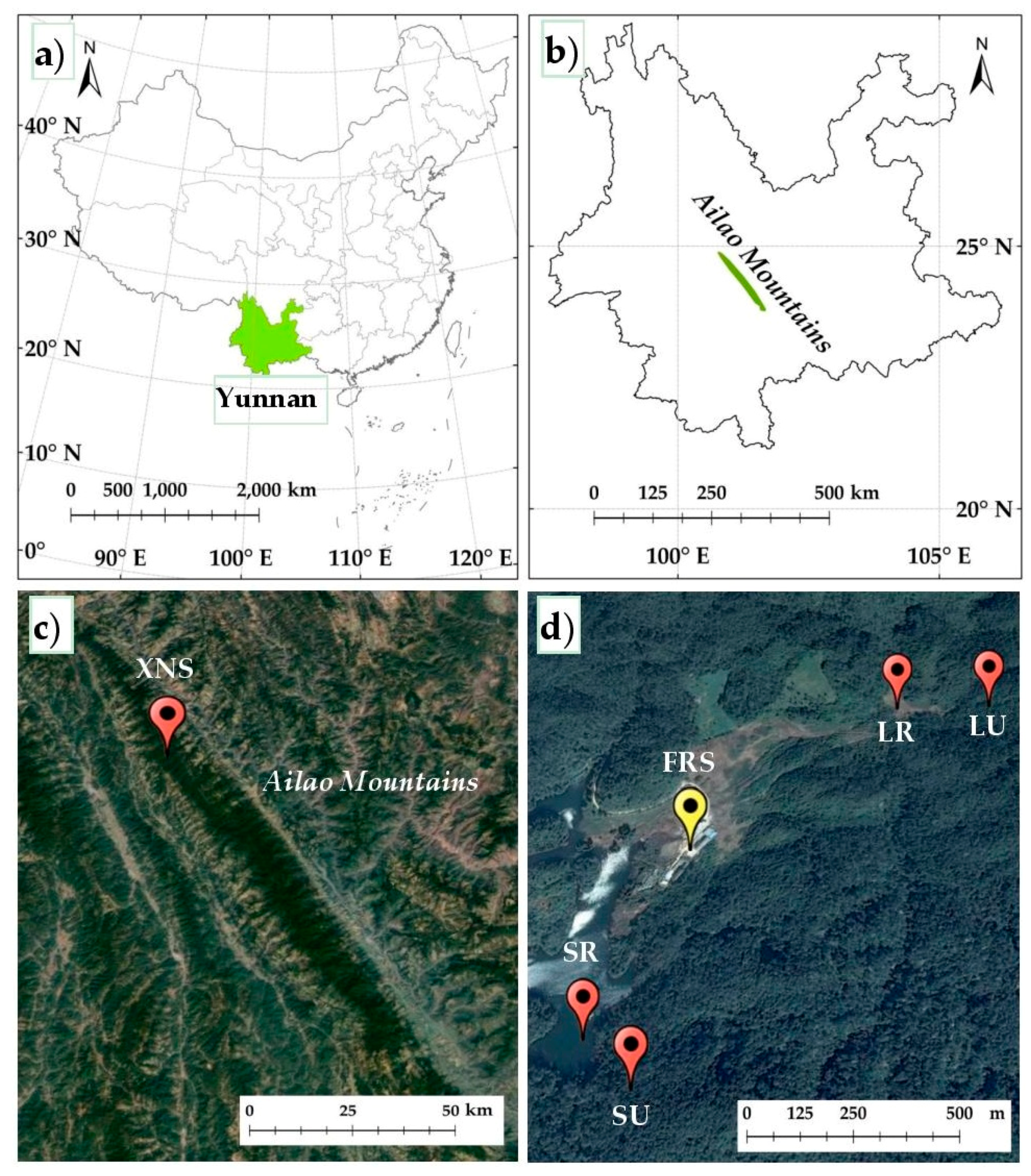

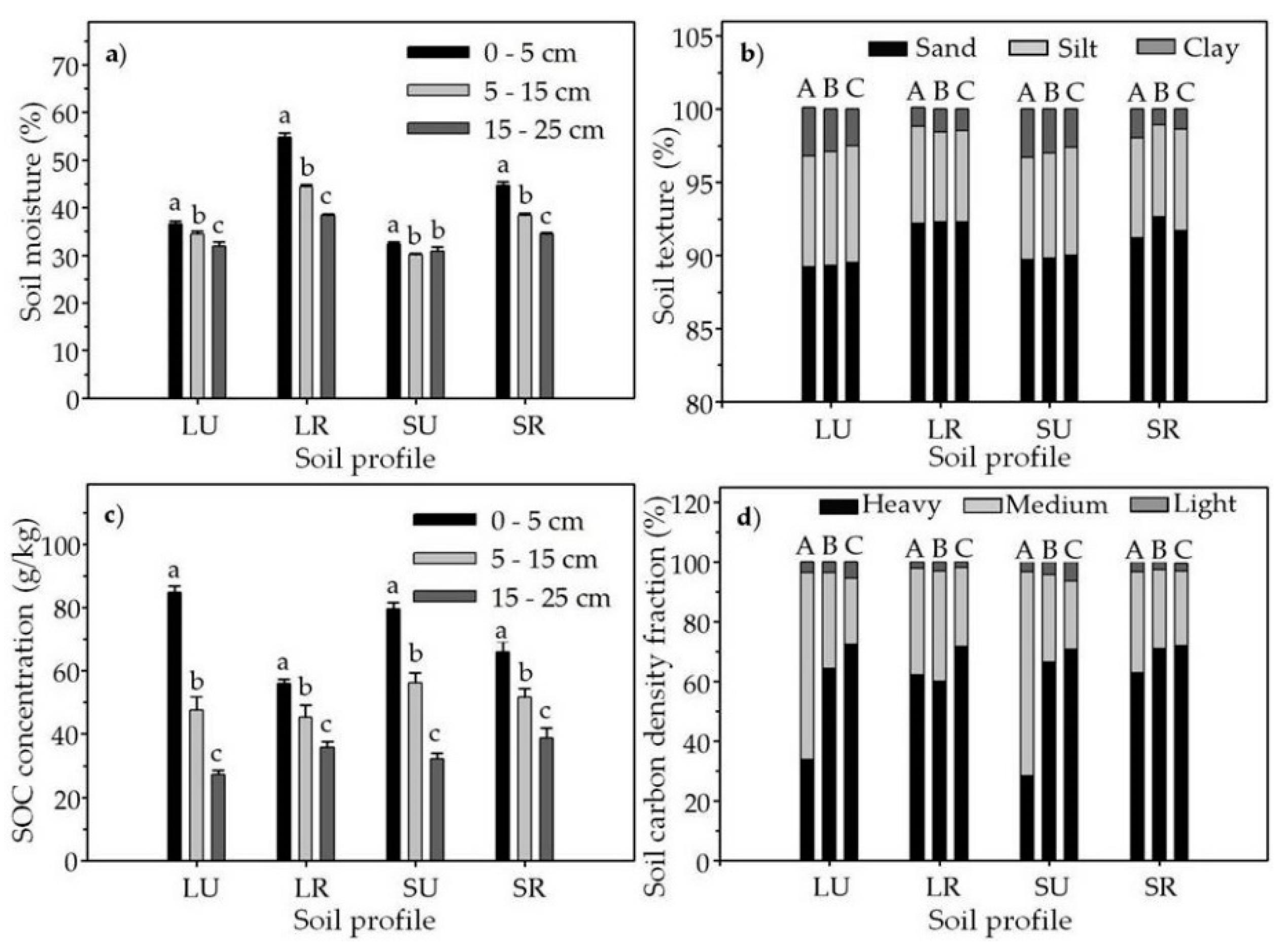
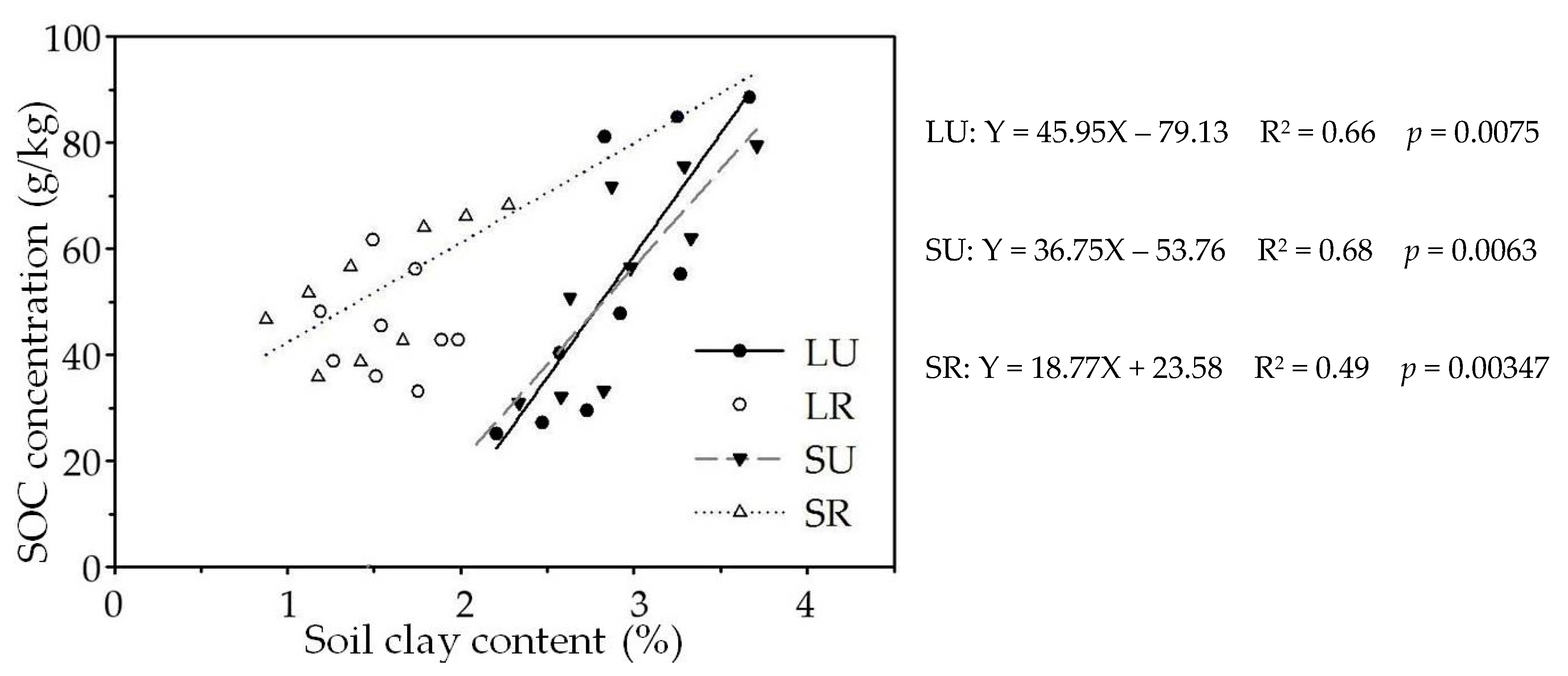
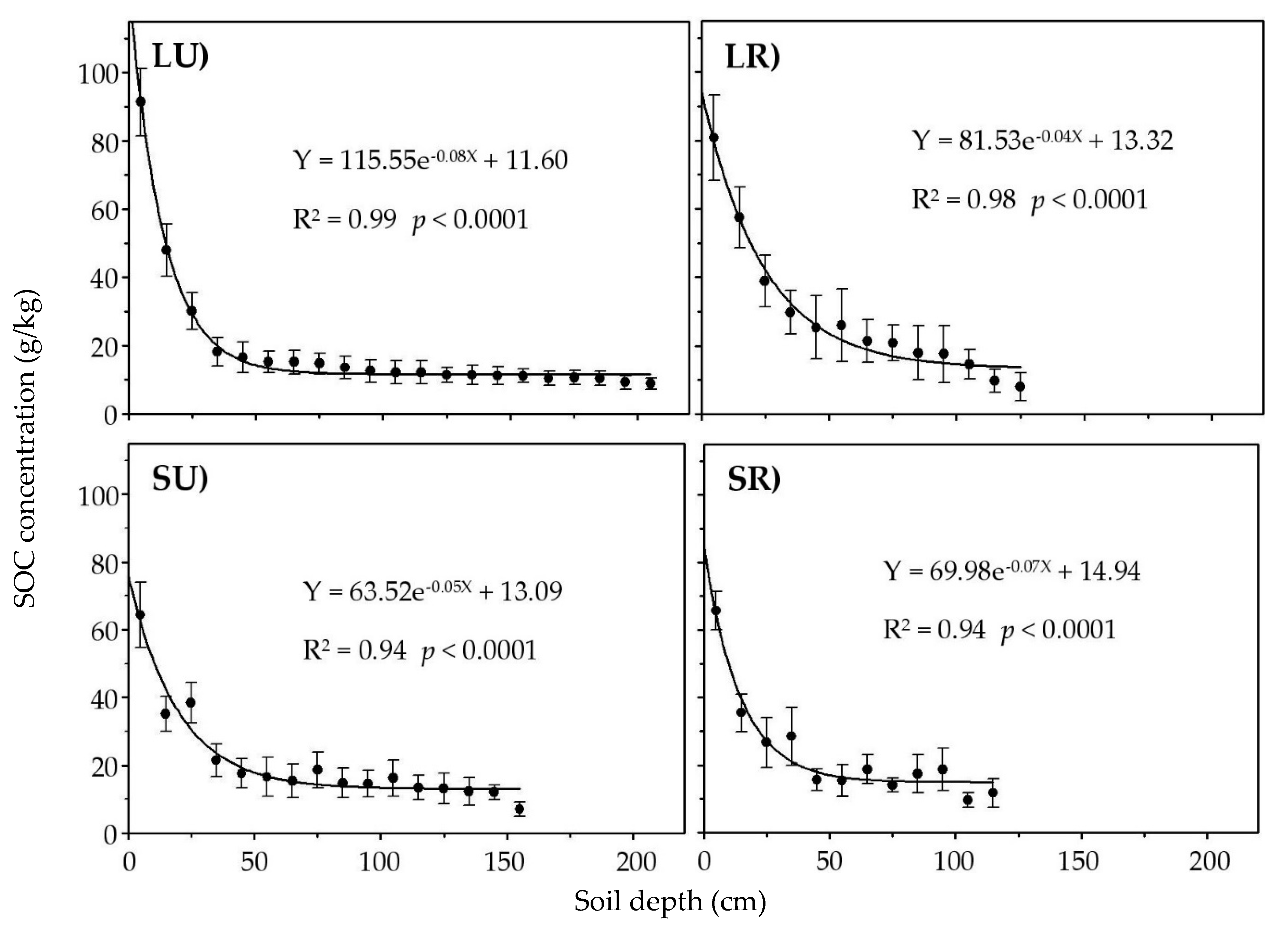
| Soil Profile | Coordinates | Elevation (m a.s.l.) | Aspect | Soil Depth (m) | Soil Type | Vegetation Type |
|---|---|---|---|---|---|---|
| LU | 24°32′47.19″ N 101°01′51.48″ E | 2500 | Southwest | 2.05 | Residual | Primary forest |
| LR | 24°32′47.83″ N 101°01′49.13″ E | 2496 | East | 1.55 | Alluvial | Perennial shrubby meadow |
| SU | 24°32′29.99″ N 101°01′39.48″ E | 2496 | North | 1.20 | Residual | Primary forest |
| SR | 24°32′30.77″ N 101°01′37.23″ E | 2475 | North | 1.15 | Alluvial | Perennial shrubby meadow |
| Soil Profile | Slope a | Intercept b | ||||||
|---|---|---|---|---|---|---|---|---|
| LU | LR | SU | SR | LU | LR | SU | SR | |
| LU | ––– | ––– | ||||||
| LR | 0.025 | ––– | 0.012 | ––– | ||||
| SU | <0.000 | <0.000 | ––– | 0.006 | 0.649 | ––– | ||
| SR | 0.008 | 0.708 | 0.001 | ––– | 0.010 | 0.499 | 0.046 | ––– |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Zou, X.; Cao, M.; Luo, T. Organic Carbon Storage and 14C Apparent Age of Upland and Riparian Soils in a Montane Subtropical Moist Forest of Southwestern China. Forests 2020, 11, 645. https://doi.org/10.3390/f11060645
Liu X, Zou X, Cao M, Luo T. Organic Carbon Storage and 14C Apparent Age of Upland and Riparian Soils in a Montane Subtropical Moist Forest of Southwestern China. Forests. 2020; 11(6):645. https://doi.org/10.3390/f11060645
Chicago/Turabian StyleLiu, Xianbin, Xiaoming Zou, Min Cao, and Tushou Luo. 2020. "Organic Carbon Storage and 14C Apparent Age of Upland and Riparian Soils in a Montane Subtropical Moist Forest of Southwestern China" Forests 11, no. 6: 645. https://doi.org/10.3390/f11060645
APA StyleLiu, X., Zou, X., Cao, M., & Luo, T. (2020). Organic Carbon Storage and 14C Apparent Age of Upland and Riparian Soils in a Montane Subtropical Moist Forest of Southwestern China. Forests, 11(6), 645. https://doi.org/10.3390/f11060645





