Altitude and Vegetation Affect Soil Organic Carbon, Basal Respiration and Microbial Biomass in Apennine Forest Soils
Abstract
1. Introduction
2. Materials and Methods
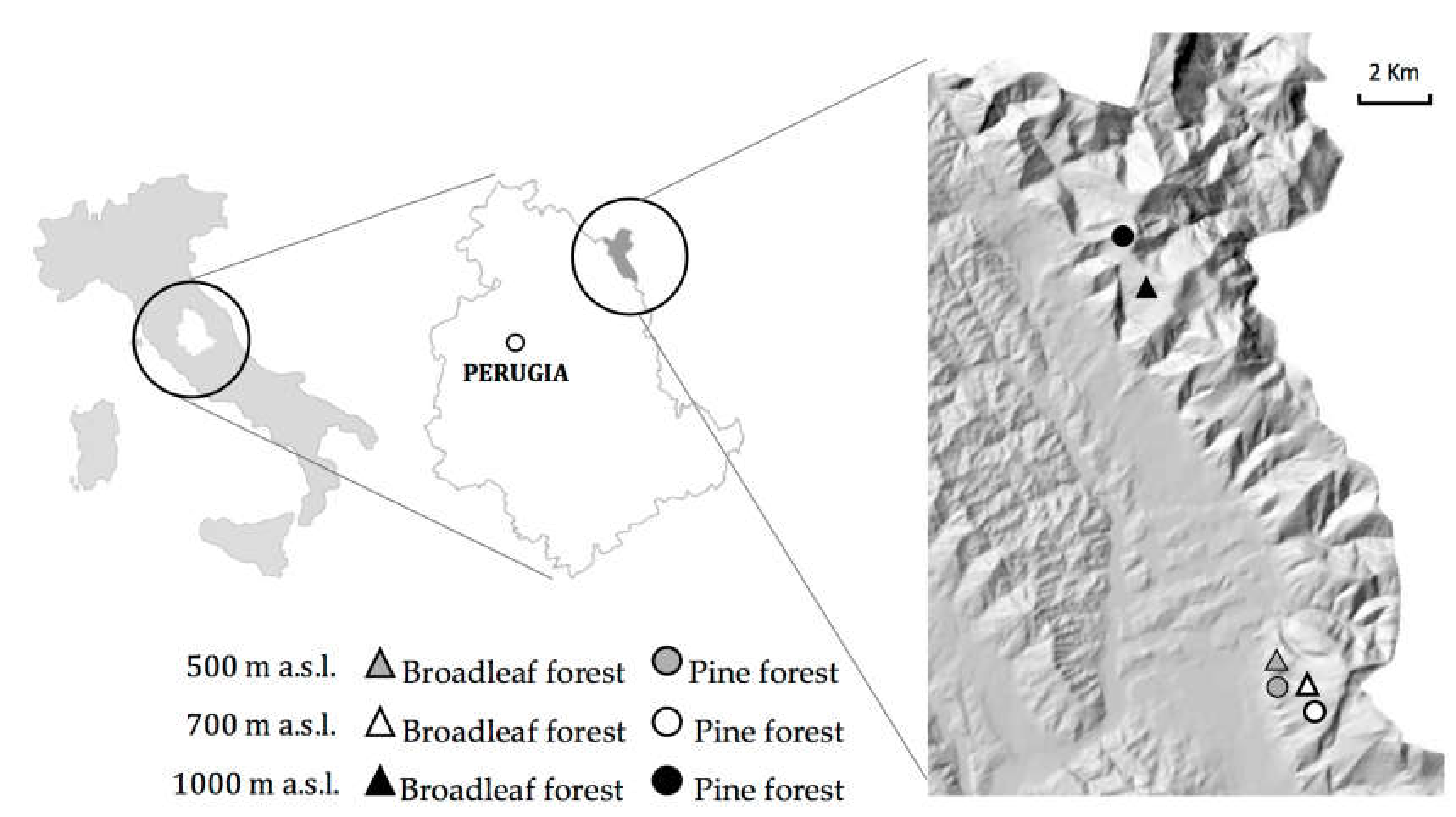
2.1. Study Sites
2.2. Soil Sampling
2.3. Chemical and Biochemical Analyses
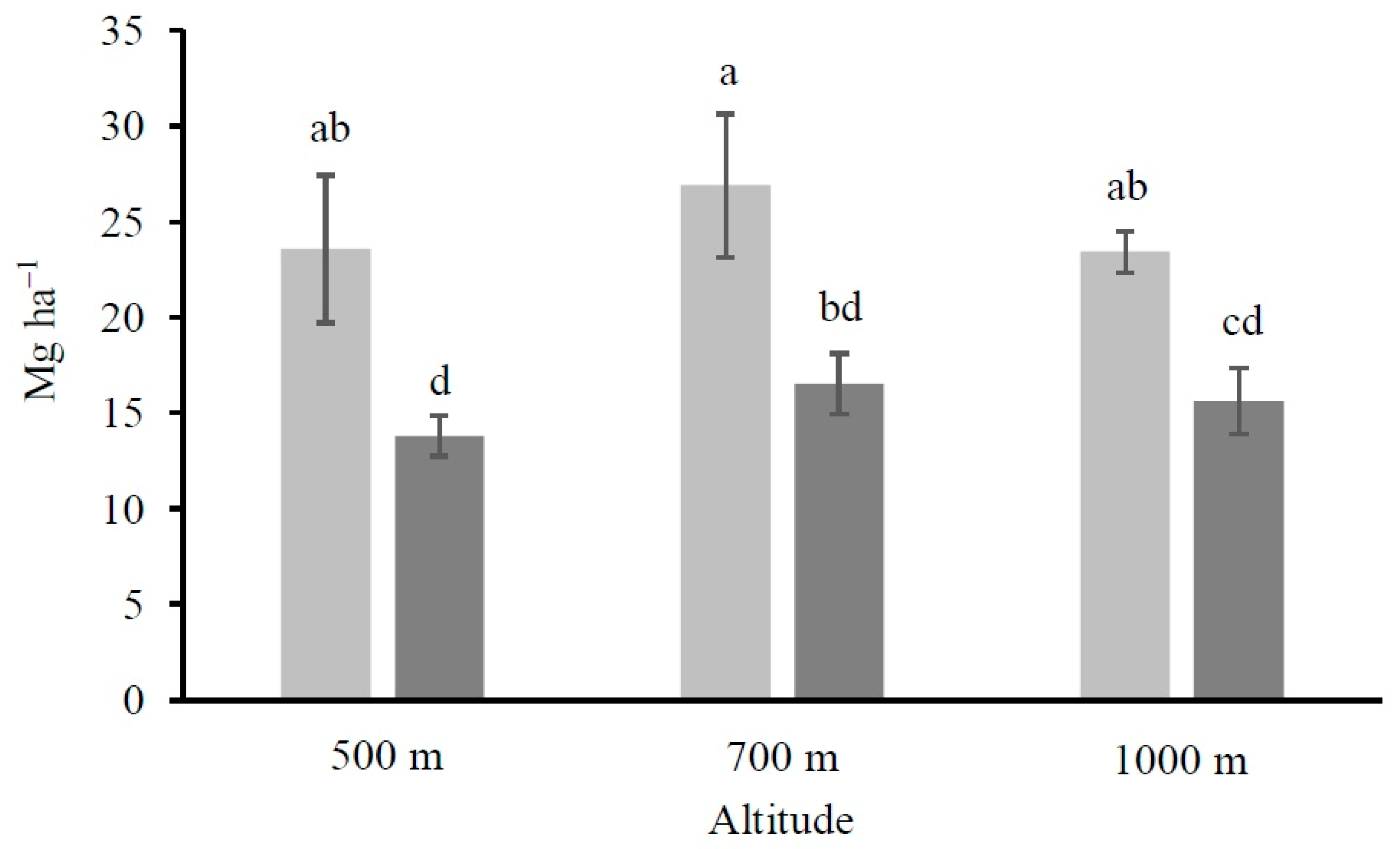
2.4. Calculation of the Organic Carbon Stocks
2.5. Statistical Analysis
3. Results
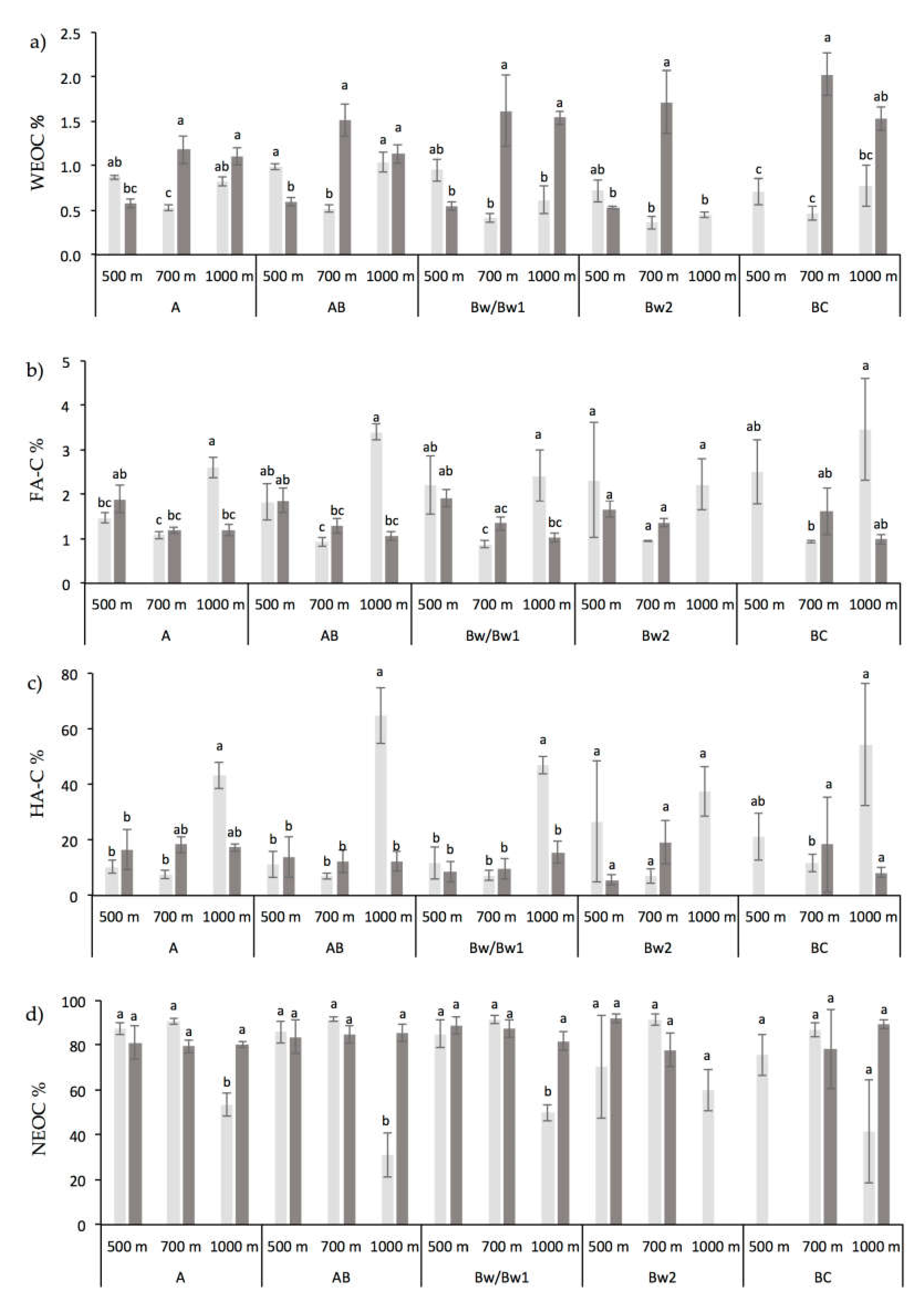
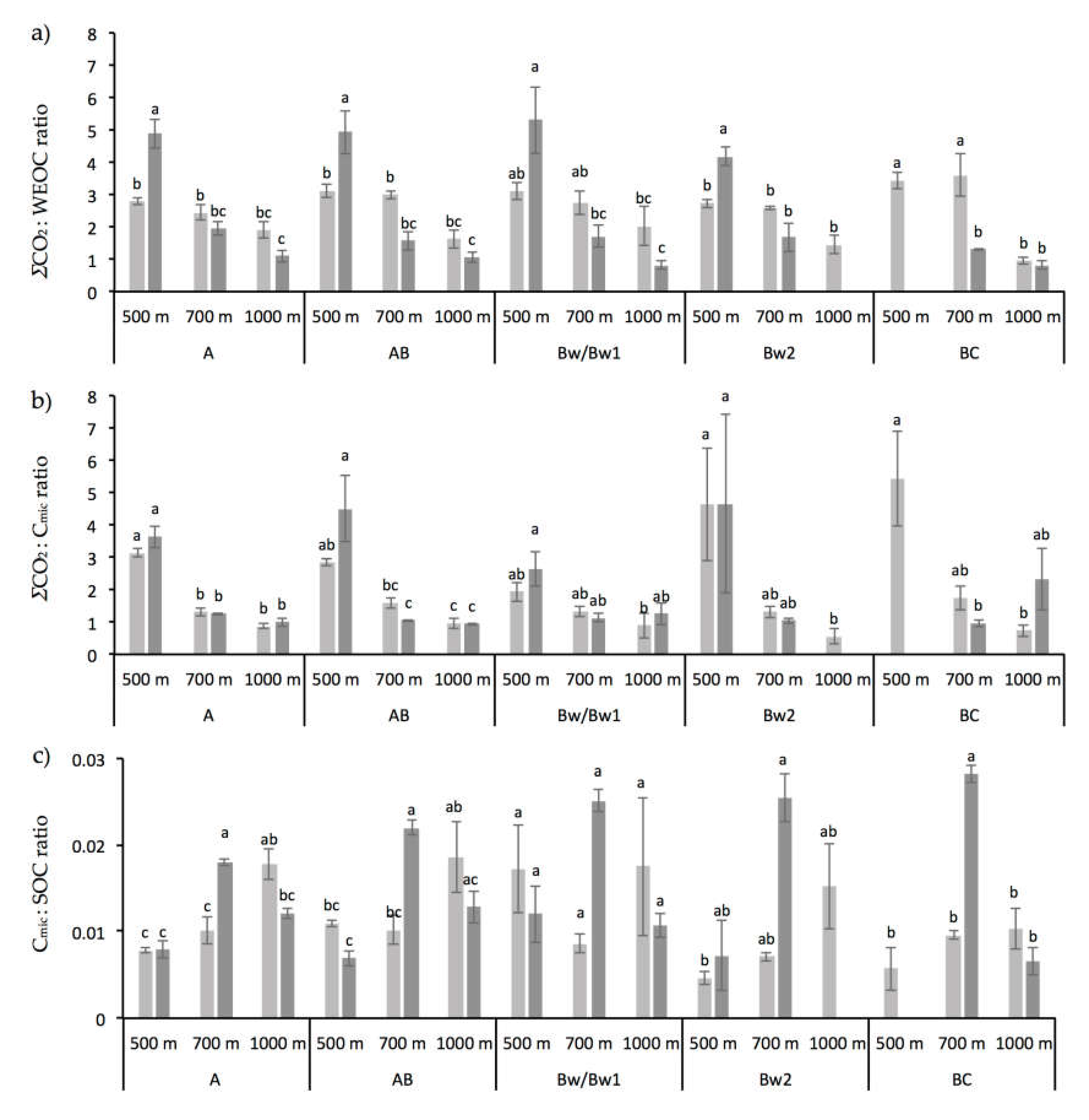
3.1. Chemical and Biochemical Soil Properties
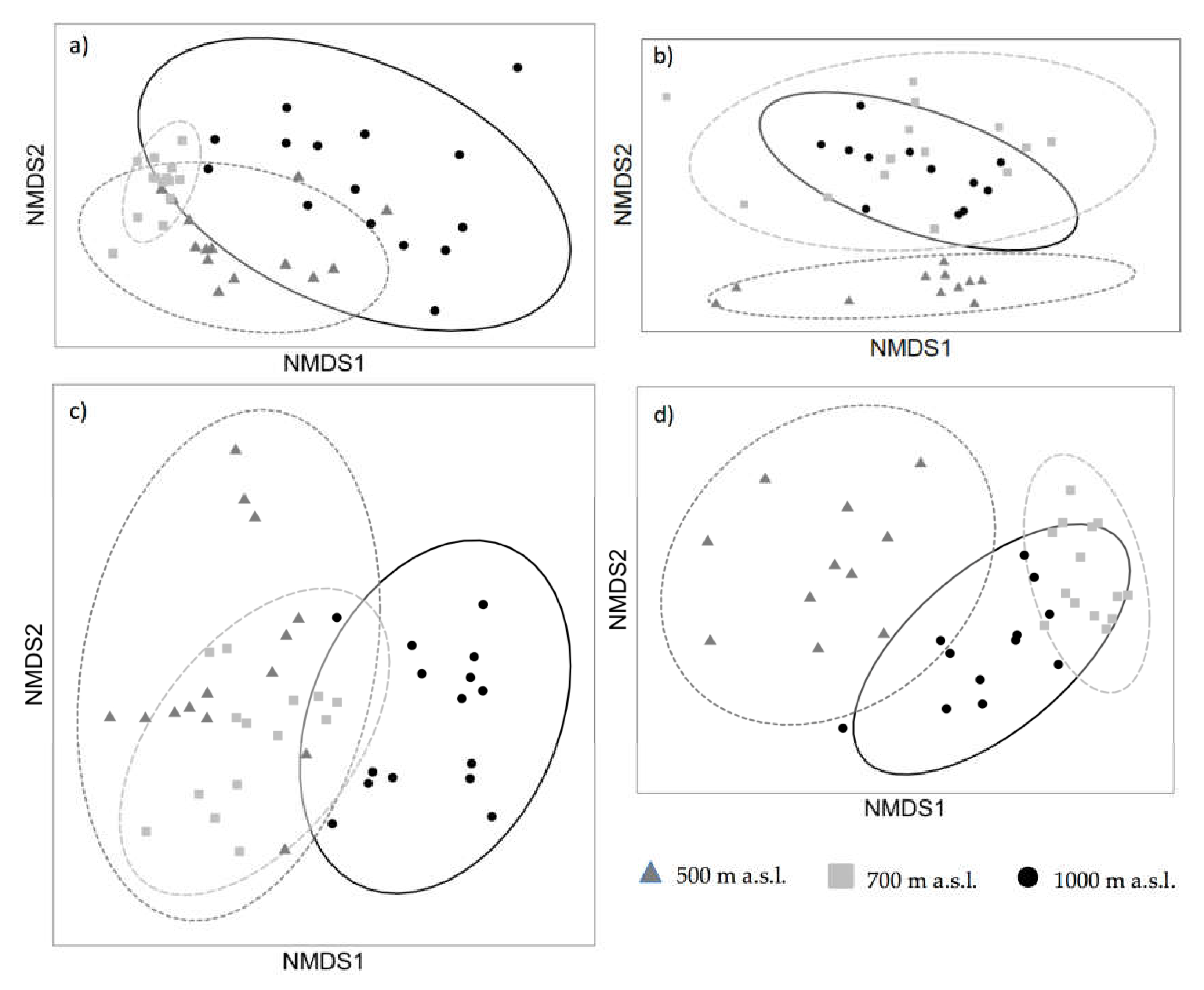
3.2. Non-Metric Multidimensional Scaling (NMDS) Analyses
4. Discussion
4.1. Altitude and Vegetation Effects on Soil Organic Carbon and Its Fractions Distribution
4.2. Altitude and Vegetation Effects on Biochemical Soil Properties
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Appendix A
| Horizon | Depth | Colour a | Structure b | Consistence c | Texture d | Roots e | Thickness | Boundary f | Rock Fragments g | Other Observations |
|---|---|---|---|---|---|---|---|---|---|---|
| cm | cm | |||||||||
| Altitude ≈500 m a.s.l. (43°16′57″ N, 12°45′39″ E); exposure: W; slope: ≈20%. Soil: loamy-skeletal, mixed, mesic, Typic Humustepts [30] Vegetation: Upper stratum: Quercus pubescens Willd.—Lower stratum: Fraxinus ornus L., Ostrya carpinifolia Scop.—Understory: Brachypodium rupestre (Host) Roem. & Schult, Lonicera caprifolium L, Cotynus cogygria Scop., Citisus sessilifolius L. 1753, Asparagus acutifolius L., Carex flacca Schreb. seedlings. | ||||||||||
| Oi | 5–2 | Quercus leafs with twigs and cupules | ||||||||
| Oe | 2–0 | |||||||||
| A | 0–12 | 5YR 4/4 | 2 f cr | s, ps | sl | 3mi,vf,f,m | 5–12 | cw | 40% Mgr | |
| AB | 12–24 | 5YR 4/6 | 2 f sbk | ss, ps | sl | 3mi,vf,f,m | 7–12 | cw | 50% Mgr | |
| Bw1 | 24–35 | 2.5YR4/6 | 2 fm sbk | so, po | sl | 3mi,vf,f,1m | 11 | cs | 50% Cgr, | |
| Bw2 | 35–48 | 2.5YR4/6 | 1 fm sbk | ss, ps | sl | 2vf,f,1m | 13 | cs | 60% VCgr | |
| BC | 48–61+ | 5YR4/8 | 1 fm sbk | ss, ps | sl | 1f,m | - | - | 60% VCgr | |
| Altitude ≈500 m a.s.l. (43°16′58″ N, 12°45′38″ E); exposure: W; slope: ≈20%. Soil: loamy-skeletal, mixed, mesic, Typic Humustepts [30] Vegetation: Upper stratum: Pinus nigra J.F. Arnold—Lower stratum: Fraxinus ornus L., Quercus pubescens L.—Understory: Brachypodium rupestre (Host) Roem. & Schult, Spartium junceum L., seedlings. | ||||||||||
| Oi | 5–2 | Pinus needles, with twigs and cones | ||||||||
| Oe | 2–0 | |||||||||
| A | 0–9 | 7,5YR5/4 | 2 f, m cr | ss, po | sl | 3mi,vf,f,2m | 8–12 | cw | 30% Mgr | |
| AB | 9–17 | 10YR4/3 | 2 f, m sbk | ss, ps | sl | 3vf,f,m | 7–10 | cw | 40% Mgr | |
| Bw1 | 17–26 | 7.5YR4/4 | 1 f, m sbk | ss, ps | sl | 2vf,f,m,v1c | 9 | cs | 40% Mgr | |
| Bw2 | 26–37 | 10YR3/4 | 1f, m sbk | so,ps | sl | 2f,1m | 8–12 | cw | 60% Mgr | |
| Cr | 37–46+ | Not described and not sampled | ||||||||
| Altitude ≈700 m a.s.l. (43°16′26″ N, 12°46′27″ E); exposure: W; slope: ≈35%. Soil: loamy-skeletal, mixed, mesic, Typic Humustepts [30] Vegetation: Upper stratum: Ostrya Carpinifolia Scop.—Lower stratum: Fraxinus ornus L., Quercus pubescens L., Acer opalus subsp. obtusatum (Waldst. & Kit. ex Willd.) Gams, Sorbus aria (L.) Crantz, Acer monspessulanum L.—Understory: Sesleria nitida Ten., Citisus sessilifolius L. 1753, Brachypodium rupestre (Host) Roem. & Schult | ||||||||||
| Oi | 9–5 | Ostrya leafs with twigs and cupules | ||||||||
| Oe | 5–2 | |||||||||
| Oa | 2–0 | |||||||||
| A | 0–18 | 10YR3/2 | 2 f, m sbk | ss, ps | ls | 3vf,f,m v1c | 14–18 | cw | 35% Mgr, 25% Cgr | |
| AB | 18–29 | 10YR3/3 | 2 f, m sbk | ss, p | ls | 3vf,f 2m,1c | 8–12 | cw | 40% VCgr | |
| Bw1 | 29–50 | 7.5YR3/2 | 2 f, m sbk | ss, ps | sl | 3vf,f 2m | 18–22 | cw | 40% VCgr | |
| Bw2 | 50–62 | 7.5YR4/3 | 1 f, m sbk | s, p | sl | 3vf,f 2m | 9–14 | cw | 30% Mgr | |
| BC | 62–80+ | 7.5YR4/4 | 1 f sbk | s, p | sl | 3f, 2m | - | - | 60% VCgr | |
| Altitude ≈700 m a.s.l. (43°16′37″ N, 12°46′15″ E); exposure: W; slope: ≈25%. Soil: loamy-skeletal, mixed, mesic, Typic Humustepts [30] Vegetation: Upper stratum: Pinus nigra J.F. Arnold—Lower stratum: Fraxinus ornus L., Ostrya carpinifolia Scop., Sorbus aria (L.) Crantz—Understory: Brachypodium rupestre (Host) Roem. & Schult, Rubus ulmifolius Schott, 1818 | ||||||||||
| Oi | 5–2 | Pinus needles, with twigs and cones | ||||||||
| Oe | 2–0 | |||||||||
| A | 0–9 | 7.5YR3/2 | 2 f sbk | so, p | ls | 3mi,vf,f,1m | 5–10 | cw | 30% Mgr | |
| AB | 9–17 | 7.5YR4/2 | 2 f, m sbk | so, ps | scl | 3vf,f,1m | 6–9 | cw | 40% Mgr | |
| Bw1 | 17–30 | 5YR3/3 | 2 f, m,sbk | so, ps | scl | 3vf,f,1m,v1c | 16 | cs | 40% Cgr | |
| Bw2 | 30–46 | 5YR3/4 | 1 f, m sbk | so, ps | sl | 2vf,3f,1m,c | 12–18 | cw | 40% Cgr, 10% VCgr | |
| BC | 46–60+ | 5YR3/4 | 1 f, m sbk | so, ps | sl | 2f,m,1c | - | - | 55% Cgr, 10% VCgr | |
| Altitude ≈1000 m a.s.l. (43°23′36″ N, 12°42′31″ E); exposure: W; slope: ≈25%. Soil: loamy, mixed, mesic, Typic Humustepts [30] Vegetation: Upper stratum: Fagus sylvatica L.—Lower stratum: Acer campestre L., Quercus cerris L., Prunus avium L.—Understory: Aegopodium podagraria L., Arctium lappa L., 1753 | ||||||||||
| Oi | 5–1 | Fagus leafs with twigs, and cupules | ||||||||
| Oe | 1–0 | |||||||||
| A | 0–7 | 7.5YR3/2 | 2 f sbk | so, p | sl | 1vf, 2f | 4–8 | cw | 0% | |
| AB | 7–15 | 10YR4/2 | 2 f sbk | so, p | l | 1vf,f,m | 7–11 | cw | 0% | |
| Bw1 | 15–28 | 10YR4/3 | 2 f sbk | so, p | l | 2vf,f 1m | 9–14 | cw | 3% Fgr | |
| Bw2 | 28–42 | 10YR4/3 | 2 f, m sbk | ss, p | cl | 2vf,f 1m,c | 14 | cs | 10% Fgr | |
| BC | 42–55+ | 10YR4/2 | 1 f, m sbk | s, p | scl | 2f 1m | - | - | 20% Mgr | |
| Altitude ≈1000 m a.s.l. (43°22′53″ N, 12°42′48″ E); exposure: W; Slope: ≈30%. Soil: loamy-skeletal, mixed, mesic, Typic Humustepts [30] Vegetation: Upper stratum: Pinus nigra J.F. Arnold—Lower stratus: Acer opalus subsp. obtusatum (Waldst. & Kit. ex Willd.) Gams, Acer pseudoplatanus L., 1753, Sorbus aria (L.) Crantz, Quercus spp.—Understory: Brachypodium rupestre (Host) Roem. & Schult, Lonicera xylosteum L., Viola alba subsp. dehnhardtii (Ten.) W. Becker, Daphne laureola L. | ||||||||||
| Oi | 7–4 | Pinus needles, with twigs and cones | ||||||||
| Oe | 4–1 | |||||||||
| Oa | 1–0 | |||||||||
| A | 0–8 | 5YR3/2 | 3 f sbk | so, ps | ls | 3 vf,f | 7–9 | cw | 40% Cgr | |
| AB | 8–13 | 5YR3/3 | 3 f sbk | so, ps | sl | 3vf,f,m 1c | 5–9 | cw | 40% Cgr | |
| Bw | 13–26 | 7.5YR3/2 | 2 f sbk | so, ps | sl | 2vf,f 1m,c | 13 | cs | 60% Cgr | |
| BC | 26–47+ | 7.5YR3/3 | 2 f sbk | so, ps | sl | 2vf,f,m | - | - | 90% VCgr | |
References
- Bispo, A.; Andersen, L.; Angers, D.A.; Bernoux, M.; Brossard, M.; Cécillon, L.; Comans, R.N.J.; Harmsen, J.; Jonassen, K.; Lamé, F.; et al. Accounting for carbon stocks in soils and measuring GHGs emission fluxes from soils: Do we have the necessary standards? Front. Environ. Sci. 2017, 5. [Google Scholar] [CrossRef]
- Dungait, J.A.J.; Hopkins, D.W.; Gregory, A.S.; Whitmore, A.P. Soil organic matter turnover is governed by accessibility not recalcitrance. Glob. Chang. Biol. 2012, 18, 1781–1796. [Google Scholar] [CrossRef]
- Xu, M.; Li, X.; Cai, X.; Gai, J.; Li, X.; Christie, P.; Zhang, J. Soil microbial community structure and activity along a montane elevational gradient on the Tibetan Plateau. Eur. J. Soil Biol. 2014, 64, 6–14. [Google Scholar] [CrossRef]
- Blume, E.; Bischoff, M.; Reichert, J.M.; Moorman, T.; Konopka, A.; Turco, R.F. Surface and subsurface microbial biomass, community structure and metabolic activity as a function of soil depth and season. Appl. Soil Ecol. 2002, 20, 171–181. [Google Scholar] [CrossRef]
- Chen, L.F.; He, Z.B.; Du, J.; Yang, J.J.; Zhu, X. Patterns and environmental controls of soil organic carbon and total nitrogen in alpine ecosystems of northwestern China. Catena 2016, 137, 37–43. [Google Scholar] [CrossRef]
- Buchan, G.D. Temperature effects in soil. In Encyclopedia of Earth Sciences Series; Springer: Berlin, Germany, 2011; Volume 4, pp. 891–895. [Google Scholar]
- Zhang, G.N.; Chen, Z.H.; Zhang, A.M.; Chen, L.J.; Wu, Z.J. Influence of climate warming and nitrogen deposition on soil phosphorus composition and phosphorus availability in a temperate grassland, China. J. Arid Land 2014, 6, 156–163. [Google Scholar] [CrossRef]
- Butler, S.M.; Melillo, J.M.; Johnson, J.E.; Mohan, J.; Steudler, P.A.; Lux, H.; Burrows, E.; Smith, R.M.; Vario, C.L.; Scott, L.; et al. Soil warming alters nitrogen cycling in a New England forest: Implications for ecosystem function and structure. Oecologia 2012, 168, 819–828. [Google Scholar] [CrossRef]
- Vincent, A.G.; Sundqvist, M.K.; Wardle, D.A.; Giesler, R. Bioavailable soil phosphorus decreases with increasing elevation in a subarctic tundra landscape. PLoS ONE 2014, 9, e92942. [Google Scholar] [CrossRef]
- Schindlbacher, A.; De Gonzalo, C.; Díaz-Pinés, E.; Gíorra, P.; Matthews, B.; Inclán, R.; Zechmeister-Boltenstern, S.; Rubio, A.; Jandl, R. Temperature sensitivity of forest soil organic matter decomposition along two elevation gradients. J. Geophys. Res. Biogeosci. 2010, 115. [Google Scholar] [CrossRef]
- Cardelli, V.; De Feudis, M.; Fornasier, F.; Massaccesi, L.; Cocco, S.; Agnelli, A.; Weindorf, D.C.; Corti, G. Changes of topsoil under Fagus sylvatica along a small latitudinal-altitudinal gradient. Geoderma 2019, 344, 164–178. [Google Scholar] [CrossRef]
- Kobler, J.; Zehetgruber, B.; Dirnböck, T.; Jandl, R.; Mirtl, M.; Schindlbacher, A. Effects of aspect and altitude on carbon cycling processes in a temperate mountain forest catchment. Landsc. Ecol. 2019, 34, 325–340. [Google Scholar] [CrossRef]
- Gutiérrez-Girón, A.; Díaz-Pinés, E.; Rubio, A.; Gavilán, R.G. Both altitude and vegetation affect temperature sensitivity of soil organic matter decomposition in Mediterranean high mountain soils. Geoderma 2015, 237–238, 1–8. [Google Scholar] [CrossRef]
- Chang, R.; Wang, G.; Fei, R.; Yang, Y.; Luo, J.; Fan, J. Altitudinal Change in Distribution of Soil Carbon and Nitrogen in Tibetan Montane Forests. Soil Sci. Soc. Am. J. 2015, 79, 1455–1469. [Google Scholar] [CrossRef]
- Tsozué, D.; Nghonda, J.P.; Tematio, P.; Basga, S.D. Changes in soil properties and soil organic carbon stocks along an elevation gradient at Mount Bambouto, Central Africa. Catena 2019, 175, 251–262. [Google Scholar] [CrossRef]
- De Feudis, M.; Cardelli, V.; Massaccesi, L.; Trumbore, S.E.; Vittori Antisari, L.; Cocco, S.; Corti, G.; Agnelli, A. Small altitudinal change and rhizosphere affect the SOM light fractions but not the heavy fraction in European beech forest soil. Catena 2019, 181, 104091. [Google Scholar] [CrossRef]
- De Feudis, M.; Cardelli, V.; Massaccesi, L.; Lagomarsino, A.; Fornasier, F.; Westphalen, D.J.; Cocco, S.; Corti, G.; Agnelli, A. Influence of altitude on biochemical properties of European Beech (Fagus sylvatica L.) forest soils. Forests 2017, 8, 213. [Google Scholar] [CrossRef]
- De Feudis, M.; Cardelli, V.; Massaccesi, L.; Hofmann, D.; Berns, A.E.; Bol, R.; Cocco, S.; Corti, G.; Agnelli, A. Altitude affects the quality of the water-extractable organic matter (WEOM) from rhizosphere and bulk soil in European beech forests. Geoderma 2017, 302, 6–13. [Google Scholar] [CrossRef]
- Von Lützow, M.; Kögel-Knabner, I. Temperature sensitivity of soil organic matter decomposition-what do we know? Biol. Fertil. Soils 2009, 46, 1–15. [Google Scholar] [CrossRef]
- Paul, E.A. The nature and dynamics of soil organic matter: Plant inputs, microbial transformations, and organic matter stabilization. Soil Biol. Biochem. 2016, 98, 109–126. [Google Scholar] [CrossRef]
- Quideau, S.A.; Chadwick, O.A.; Benesi, A.; Graham, R.C.; Anderson, M.A. A direct link between forest vegetation type and soil organic matter composition. Geoderma 2001, 104, 41–60. [Google Scholar] [CrossRef]
- Ayres, E.; Steltzer, H.; Berg, S.; Wallenstein, M.D.; Simmons, B.L.; Wall, D.H. Tree species traits influence soil physical, chemical, and biological properties in high elevation forests. PLoS ONE 2009, 4. [Google Scholar] [CrossRef] [PubMed]
- Smolander, A.; Loponen, J.; Suominen, K.; Kitunen, V. Organic matter characteristics and C and N transformations in the humus layer under two tree species, Betula pendula and Picea abies. Soil Biol. Biochem. 2005, 37, 1309–1318. [Google Scholar] [CrossRef]
- Aponte, C.; García, L.V.; Marañón, T. Tree species effect on litter decomposition and nutrient release in Mediterranean oak Fforests changes over time. Ecosystems 2012, 15, 1204–1218. [Google Scholar] [CrossRef]
- Berger, T.W.; Duboc, O.; Djukic, I.; Tatzber, M.; Gerzabek, M.H.; Zehetner, F. Decomposition of beech (Fagus sylvatica) and pine (Pinus nigra) litter along an Alpine elevation gradient: Decay and nutrient release. Geoderma 2015, 251–252, 92–104. [Google Scholar] [CrossRef]
- Badalamenti, E.; Battipaglia, G.; Gristina, L.; Novara, A.; Ruhl, J.; Sala, G.; Sapienza, L.; Valentini, R.; La Mantia, T. Carbon stock increases up to old growth forest along a secondary succession in Mediterranean island ecosystems. PLoS ONE 2019, 14. [Google Scholar] [CrossRef]
- Wan, Q.; Zhu, G.; Guo, H.; Zhang, Y.; Pan, H.; Yong, L.; Ma, H. Influence of Vegetation Coverage and Climate Environment on Soil Organic Carbon in the Qilian Mountains. Sci. Rep. 2019, 9, 17623. [Google Scholar] [CrossRef]
- Regione Umbria. Parco del Monte Cucco—Aspetti Vegetazionali, Botanici e Forestali. Piani dei Parchi Regionali dell’Umbria. Available online: http://www.regione.umbria.it/documents/18/2512711/Cucco_vegetazione_ott_15.pdf/0fd79a30-6fea-4acd-9dac-5917c2a86a20 (accessed on 15 June 2020).
- Schoeneberger, P.J.; Wysocki, D.A.; Benham, E.C. Soil Survey Staff Field Book for Describing and Sampling Soils; Version 3.0; Natural Resources Conservation Service, National Soil Survey Center: Lincoln, NE, USA, 2012.
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA—Natural Resources Conservation Service: Washington, DC, USA, 2014.
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H.; Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis; American Society of Agronomy, Inc.: Madison, WI, USA; Soil Science Society of America, Inc.: Madison, WI, USA, 1996. [Google Scholar]
- Swift, R.S. Organic Matter Characterization. In Methods of Soil Analysis; American Society of Agronomy, Inc.: Madison, WI, USA; Soil Science Society of America, Inc.: Madison, WI, USA, 2018; pp. 1011–1069. [Google Scholar]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Bonarelli, G.; Principi, P.; Pilotti, C.; Scarsella, F.; Lipparini, T.; Moretti, A.; Selli, R. Carta Geologica d’Italia Alla Scala 1:100,000; Foglio 116; Servizio Geologico D’Italia: Gubbio, Italy, 1952. [Google Scholar]
- Jobbágy, E.; Jackson, R. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Massaccesi, L.; Bardgett, R.D.; Agnelli, A.; Ostle, N.; Wilby, A.; Orwin, K.H. Impact of plant species evenness, dominant species identity and spatial arrangement on the structure and functioning of soil microbial communities in a model grassland. Oecologia 2015, 177, 747–759. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Mommer, L.; De Vries, F.T. Going underground: Root traits as drivers of ecosystem processes. Trends Ecol. Evol. 2014, 29, 692–699. [Google Scholar] [CrossRef]
- Newcomb, C.J.; Qafoku, N.P.; Grate, J.W.; Bailey, V.L.; De Yoreo, J.J. Developing a molecular picture of soil organic matter-mineral interactions by quantifying organo-mineral binding. Nat. Commun. 2017, 8. [Google Scholar]
- Fissore, C.; Giardina, C.P.; Kolka, R.K.; Trettin, C.C.; King, G.M.; Jurgensen, M.F.; Barton, C.D.; Mcdowell, S.D. Temperature and vegetation effects on soil organic carbon quality along a forested mean annual temperature gradient in North America. Glob. Chang. Biol. 2008, 14, 193–205. [Google Scholar] [CrossRef]
- Eusterhues, K.; Rumpel, C.; Kleber, M.; Kögel-Knabner, I. Stabilisation of soil organic matter by interactions with minerals as revealed by mineral dissolution and oxidative degradation. Org. Geochem. 2003, 34, 1591–1600. [Google Scholar] [CrossRef]
- Zianis, D.; Mencuccini, M. Aboveground net primary productivity of a beech (Fagus moesiaca) forest: A case study of Naousa forest, northern Greece. Tree Physiol. 2005, 25, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, R.; Volařík, D.; Funda, T.; Fundová, I.; Kohutka, A.; Klapetek, V.; Martinková, M.; Anenkhonov, O.A.; Razuvaev, A. Pinus pumila growth at different altitudes in the Svyatoi Nos Peninsula (Russia). J. For. Sci. 2010, 56, 101–111. [Google Scholar] [CrossRef]
- Liu, X.Z.; Gao, C.C.; Su, Q.; Zhang, Y.; Song, Y. Altitudinal trends in δ13C value, stomatal density and nitrogen content of Pinus tabuliformis needles on the southern slope of the middle Qinling Mountains, China. J. Mt. Sci. 2016, 13, 1066–1077. [Google Scholar] [CrossRef]
- Kalbitz, K.; Kaiser, K. Contribution of dissolved organic matter to carbon storage in forest mineral soils. J. Plant. Nutr. Soil Sci. 2008, 171, 52–60. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, W.; He, X. Temporal dynamics of abiotic and biotic factors on leaf litter of three plant species in relation to decomposition rate along a subalpine elevation gradient. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Xu, G.; Chen, J.; Berninger, F.; Pumpanen, J.; Bai, J.; Yu, L.; Duan, B. Labile, recalcitrant, microbial carbon and nitrogen and the microbial community composition at two Abies faxoniana forest elevations under elevated temperatures. Soil Biol. Biochem. 2015, 91, 1–13. [Google Scholar] [CrossRef]
- Fernández Sanjurjo, M.J.; Corti, G.; Certini, G.; Ugolini, F.C. Pedogenesis induced by Genista aetnensis (Biv.) DC. on basaltic pyroclastic deposits at different altitudes, Mt. Etna, Italy. Geoderma 2003, 115, 223–243. [Google Scholar] [CrossRef]
- Paolo Di Lonardo, D.; De Boer, W.; Zweers, H.; van der Wal, A. Effect of the amount of organic trigger compounds, nitrogen and soil microbial biomass on the magnitude of priming of soil organic matter. PLoS ONE 2019, 14, e0216730. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.P.; Milcu, A.; Manning, P.; Niklaus, P.A.; Roscher, C.; Power, S.; Reich, P.B.; Scheu, S.; Tilman, D.; Ai, F.; et al. Plant diversity drives soil microbial biomass carbon in grasslands irrespective of global environmental change factors. Glob. Chang. Biol. 2015, 21, 4076–4085. [Google Scholar] [CrossRef] [PubMed]
- Fanin, N.; Hättenschwiler, S.; Fromin, N. Litter fingerprint on microbial biomass, activity, and community structure in the underlying soil. Plant. Soil 2014, 379, 79–91. [Google Scholar] [CrossRef]
- Li, W.; Yang, G.; Chen, H.; Tian, J.; Zhang, Y.; Zhu, Q.; Peng, C.; Yang, J. Soil available nitrogen, dissolved organic carbon and microbial biomass content along altitudinal gradient of the eastern slope of Gongga Mountain. Acta Ecol. Sin. 2013, 33, 266–271. [Google Scholar] [CrossRef]
- Djukic, I.; Zehetner, F.; Mentler, A.; Gerzabek, M.H. Microbial community composition and activity in different Alpine vegetation zones. Soil Biol. Biochem. 2010, 42, 155–161. [Google Scholar] [CrossRef]
- Kaiser, K.; Kalbitz, K. Cycling downwards—Dissolved organic matter in soils. Soil Biol. Biochem. 2012, 52, 29–32. [Google Scholar] [CrossRef]
- Smolander, A.; Kitunen, V. Soil microbial activities and characteristics of dissolved organic C and N in relation to tree species. Soil Biol. Biochem. 2002, 34, 651–660. [Google Scholar] [CrossRef]
- Malik, A.A.; Puissant, J.; Buckeridge, K.M.; Goodall, T.; Jehmlich, N.; Chowdhury, S.; Gweon, H.S.; Peyton, J.M.; Mason, K.E.; van Agtmaal, M.; et al. Land use driven change in soil pH affects microbial carbon cycling processes. Nat. Commun. 2018, 9, 3591. [Google Scholar] [CrossRef]
- Motavalli, P.P.; Palm, C.A.; Parton, W.J.; Elliott, E.T.; Frey, S.D. Soil pH and organic C dynamics in tropical forest soils: Evidence from laboratory and simulation studies. Soil Biol. Biochem. 1995, 27, 1589–1599. [Google Scholar] [CrossRef]
- Cerri, C.C.; Jenkinson, D.S. Formation of Microbial Biomass During the Decomposition of 14C Labelled Ryegrass in Soil. J. Soil Sci. 1981, 32, 619–626. [Google Scholar] [CrossRef]
- Curtin, D.; Peterson, M.E.; Anderson, C.R. pH-dependence of organic matter solubility: Base type effects on dissolved organic C, N, P, and S in soils with contrasting mineralogy. Geoderma 2016, 271, 161–172. [Google Scholar] [CrossRef]
- Agnelli, A.; Massaccesi, L.; De Feudis, M.; Cocco, S.; Courchesne, F.; Corti, G. Holm oak (Quercus ilex L.) rhizosphere affects limestone-derived soil under a multi-centennial forest. Plant. Soil 2016, 400, 297–314. [Google Scholar] [CrossRef]
- Bölscher, T.; Wadsö, L.; Börjesson, G.; Herrmann, A.M. Differences in substrate use efficiency: Impacts of microbial community composition, land use management, and substrate complexity. Biol. Fertil. Soils 2016, 52, 547–559. [Google Scholar] [CrossRef]
| Horizon | Altitude (m a.s.l.) | pHH2O | SOC (g kg−1) | TN (g kg−1) | C:N ratio | ||||
|---|---|---|---|---|---|---|---|---|---|
| Broadleaf | Pine | Broadleaf | Pine | Broadleaf | Pine | Broadleaf | Pine | ||
| A | 500 | 7.19 (0.19) a | 7.44 (0.04) a | 74.8 (7.6) a | 39.7 (4.7) c | 4.47 (0.51) ab | 3.40 (0.24) bc | 16.8 (0.3) a | 11.6 (0.7) b |
| 700 | 7.45 (0.02) a | 7.42 (0.06) a | 108.7 (12.2) a | 46.2 (2.2) bc | 6.00 (0.32) a | 3.51 (0.41) bc | 18.0 (1.2) a | 13.4 (0.9) ab | |
| 1000 | 5.02 (0.09) b | 7.46 (0.03) a | 39.0 (2.1) c | 69.5 (6.5) ab | 2.25 (0.30) c | 6.70 (0.75) a | 18.1 (3.1) a | 10.4 (0.3) b | |
| AB | 500 | 7.63 (0.07) a | 7.60 (0.06) a | 44.8 (3.1) b | 28.5 (2.1) c | 3.67 (0.58) ab | 2.50 (0.18) bc | 12.6 (1.5) ab | 11.4 (0.1) ab |
| 700 | 7.70 (0.04) a | 7.55 (0.03) a | 75.5 (5.9) a | 34.1 (1.9) bc | 4.58 (0.24) a | 2.76 (0.16) b | 16.5 (1.6) a | 12.4 (0.7) ab | |
| 1000 | 5.05 (0.08) b | 7.58 (0.05) a | 19.8 (1.6) d | 47.0 (5.2) b | 1.66 (0.16) c | 4.81 (0.36) a | 12.1 (1.5) ab | 9.7 (0.3) b | |
| Bw1/Bw | 500 | 7.75 (0.06) a | 7.64 (0.03) a | 32.0 (8.3) bc | 25.6 (2.1) bc | 2.26 (0.29) b | 2.43 (0.09) b | 13.9 (2.4) ab | 10.5 (0.6) ab |
| 700 | 7.78 (0.05) a | 7.55 (0.06) a | 67.8 (7.0) a | 30.6 (2.2) bc | 3.63 (0.04) a | 2.40 (0.16) b | 18.2 (2.1) ab | 12.7 (0.1) ab | |
| 1000 | 5.31 (0.01) b | 7.62 (0.07) a | 20.4 (4.2) c | 41.8 (1.7) ab | 1.26 (0.05) c | 4.35 (0.23) a | 16.0 (2.9) ab | 9.6 (0.4) b | |
| Bw2 | 500 | 7.80 (0.06) a | 7.67 (0.04) a | 34.2 (6.2) b | 25.5 (3.4) bc | 1.95 (0.37) a | 2.34 (0.31) a | 18.8 (6.7) ac | 10.9 (0.0) bc |
| 700 | 7.88 (0.04) a | 7.58 (0.05) a | 66.8 (4.5) a | 24.8 (1.6) bc | 3.75 (0.69) a | 2.32 (0.22) a | 18.2 (2.1) ab | 10.8 (0.4) c | |
| 1000 | 5.84 (0.19) b | 19.2 (1.4) c | 0.86 (0.06) b | 22.6 (2.1) a | |||||
| BC | 500 | 7.93 (0.07) a | 26.4 (3.0) bc | 1.47 (0.33) bc | 20.6 (6.5) a | ||||
| 700 | 7.86 (0.04) a | 7.57 (0.07) a | 49.6 (5.4) a | 23.3 (1.3) bc | 3.67 (0.25) a | 2.53 (0.65) ab | 13.6 (1.6) a | 9.7 (2.0) a | |
| 1000 | 6.20 (0.22) b | 7.76 (0.07) a | 12.0 (3.5) c | 38.4 (3.7) ab | 0.68 (0.04) c | 4.76 (0.81) a | 18.0 (5.9) a | 8.4 (0.9) a | |
| Altitude (m a.s.l.) | C (g kg−1) | N (g kg−1) | C:N ratio | |||
|---|---|---|---|---|---|---|
| Broadleaf | Pine | Broadleaf | Pine | Broadleaf | Pine | |
| 500 | 436.50 (1.76) b | 500.23 (0.99) a | 13.08 (0.14) b | 7.26 (0.08) e | 33.38 (0.50) d | 68.91 (0.82) a |
| 700 | 430.34 (10.17) b | 490.01 (2.22) a | 16.41 (0.16) a | 9.38 (0.09) d | 26.23 (0.39) e | 52.23 (0.31) b |
| 1000 | 360.40 (12.90) b | 495.89 (2.68) a | 17.37 (0.40) a | 12.31 (0.20) c | 20.75 (0.66) f | 40.28 (0.82) c |
| Horizon | Altitude (m a.s.l.) | Cmic (mg kg−1) | ΣCO2-C (mg kg−1) | ||
|---|---|---|---|---|---|
| Broadleaf | Pine | Broadleaf | Pine | ||
| A | 500 | 579 (37) c | 308 (31) d | 1819 (179) a | 1111 (124) bc |
| 700 | 1059 (65) a | 831(39) ab | 1385 (134) ab | 1037 (36) bc | |
| 1000 | 688 (35) bc | 836 (49) ab | 607 (81) d | 826 (73) c | |
| AB | 500 | 488 (19) ab | 199 (38) c | 1387 (93) a | 825 (58) bc |
| 700 | 750 (93) a | 752 (66) a | 1169 (49) ab | 788 (79) c | |
| 1000 | 363 (77) bc | 597 (86) ab | 349 (105) d | 550 (62) cd | |
| Bw1/Bw | 500 | 474 (82) ab | 310 (86) b | 875 (62) a | 726 (103) a |
| 700 | 568 (58) ab | 766 (48) a | 754 (107) a | 849 (68) a | |
| 1000 | 290 (68) b | 449 (72) b | 210 (33) b | 530 (90) a | |
| Bw2 | 500 | 162 (53) b | 169 (79) b | 657 (33) a | 570 (102) a |
| 700 | 470 (0) ab | 625 (51) a | 621 (87) a | 644 (14) a | |
| 1000 | 287 (82) b | 125 (23) b | |||
| BC | 500 | 134 (42) c | 612 (12) ab | ||
| 700 | 470 (37) b | 655 (16) a | 788 (114) a | 630 (54) ab | |
| 1000 | 127 (54) c | 262 (76) b | 76 (14) c | 466 (54) b | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massaccesi, L.; De Feudis, M.; Leccese, A.; Agnelli, A. Altitude and Vegetation Affect Soil Organic Carbon, Basal Respiration and Microbial Biomass in Apennine Forest Soils. Forests 2020, 11, 710. https://doi.org/10.3390/f11060710
Massaccesi L, De Feudis M, Leccese A, Agnelli A. Altitude and Vegetation Affect Soil Organic Carbon, Basal Respiration and Microbial Biomass in Apennine Forest Soils. Forests. 2020; 11(6):710. https://doi.org/10.3390/f11060710
Chicago/Turabian StyleMassaccesi, Luisa, Mauro De Feudis, Angelo Leccese, and Alberto Agnelli. 2020. "Altitude and Vegetation Affect Soil Organic Carbon, Basal Respiration and Microbial Biomass in Apennine Forest Soils" Forests 11, no. 6: 710. https://doi.org/10.3390/f11060710
APA StyleMassaccesi, L., De Feudis, M., Leccese, A., & Agnelli, A. (2020). Altitude and Vegetation Affect Soil Organic Carbon, Basal Respiration and Microbial Biomass in Apennine Forest Soils. Forests, 11(6), 710. https://doi.org/10.3390/f11060710





