Bird Assemblage Recovery in a Chronosequence of Tropical Dry Forests in Costa Rica
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Dry Forest Structural Characteristics and Forest Age
2.3. Acoustic Data Collection
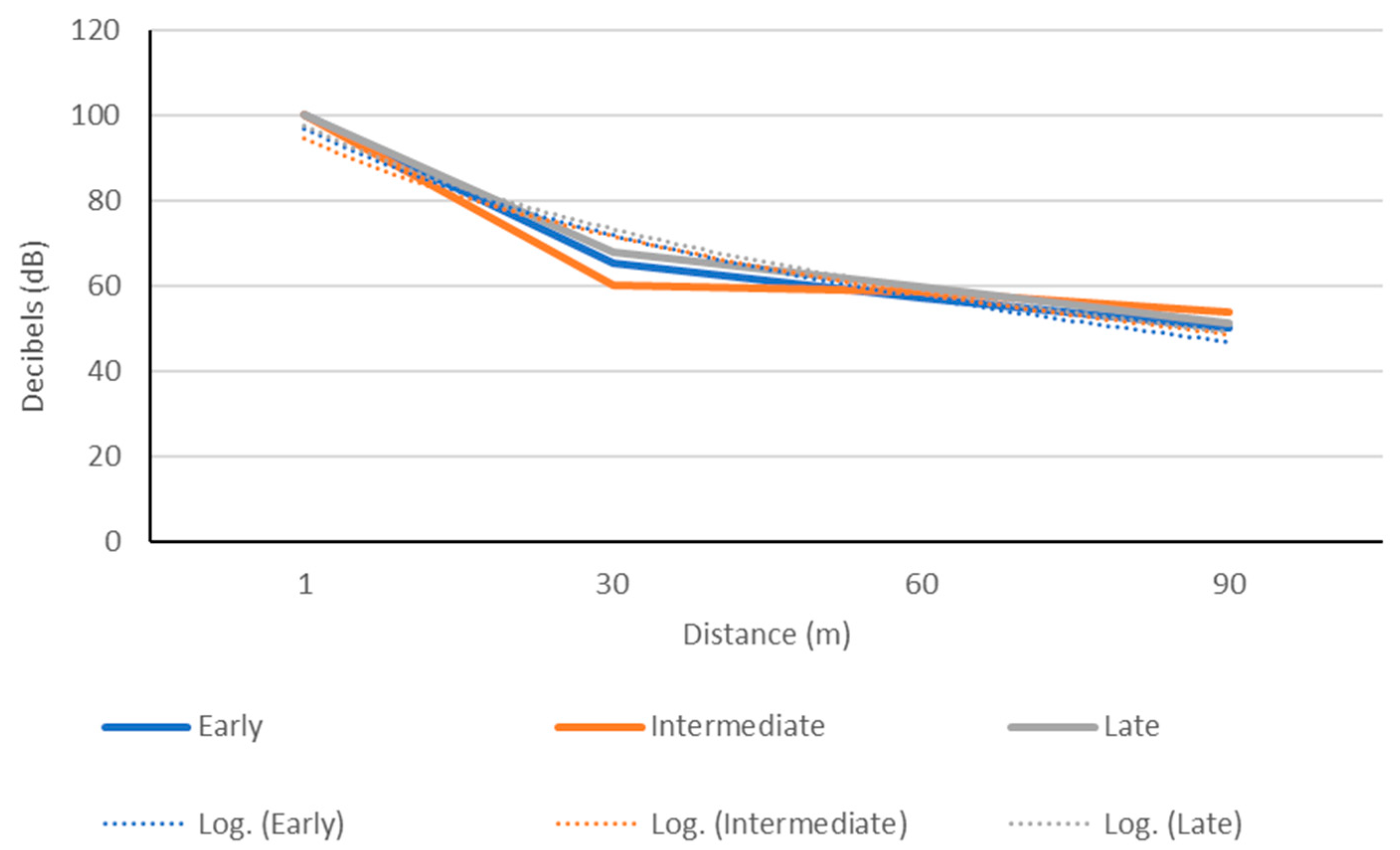
2.3.1. Acoustic Detection Extent
2.3.2. Bird Acoustic Data Collection and Analysis
2.4. Data Analysis
3. Results
3.1. Dry Forest Structural Characteristics and Forest Age
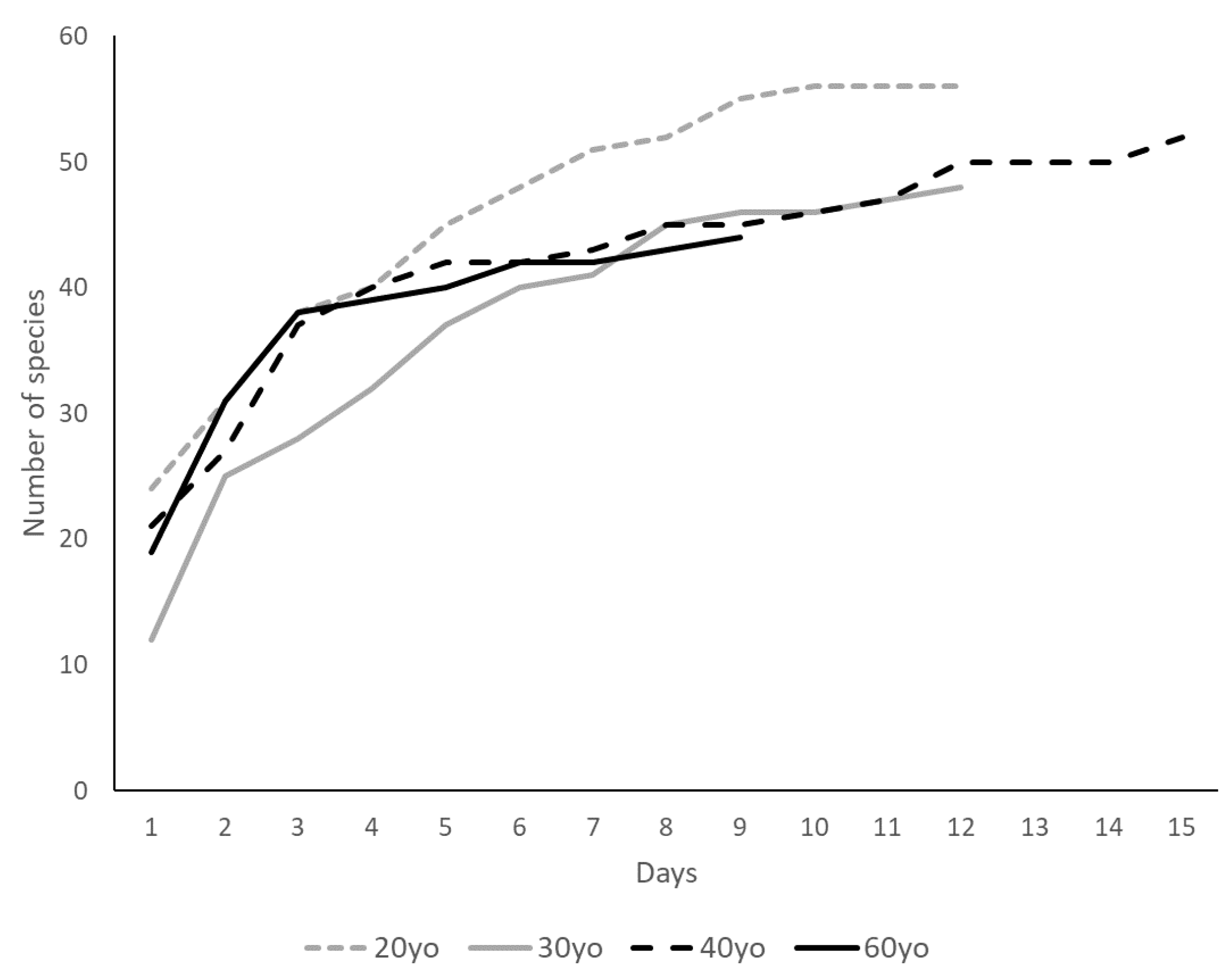
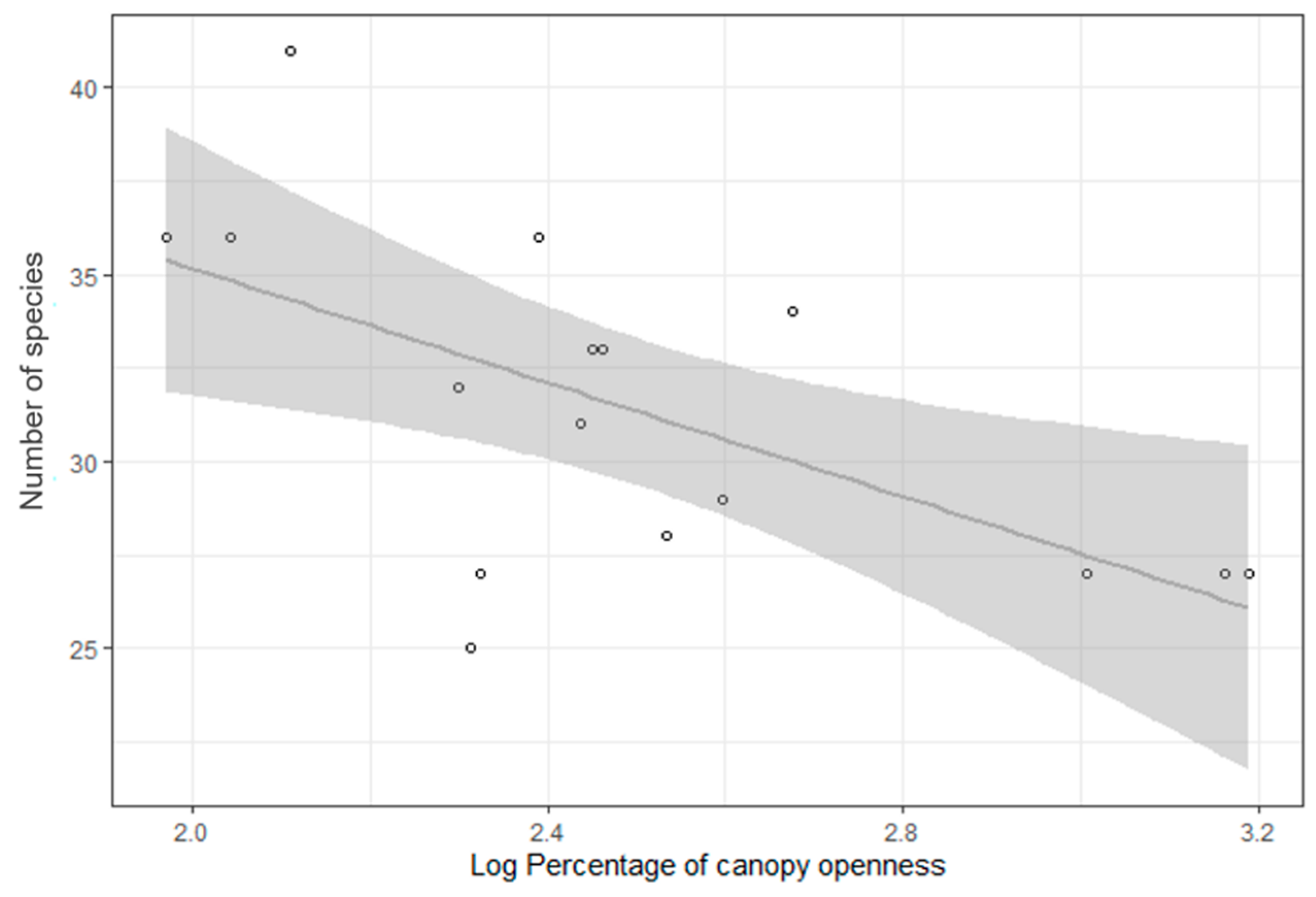
3.2. Bird Species Richness and Forest Age and Structural Characteristics
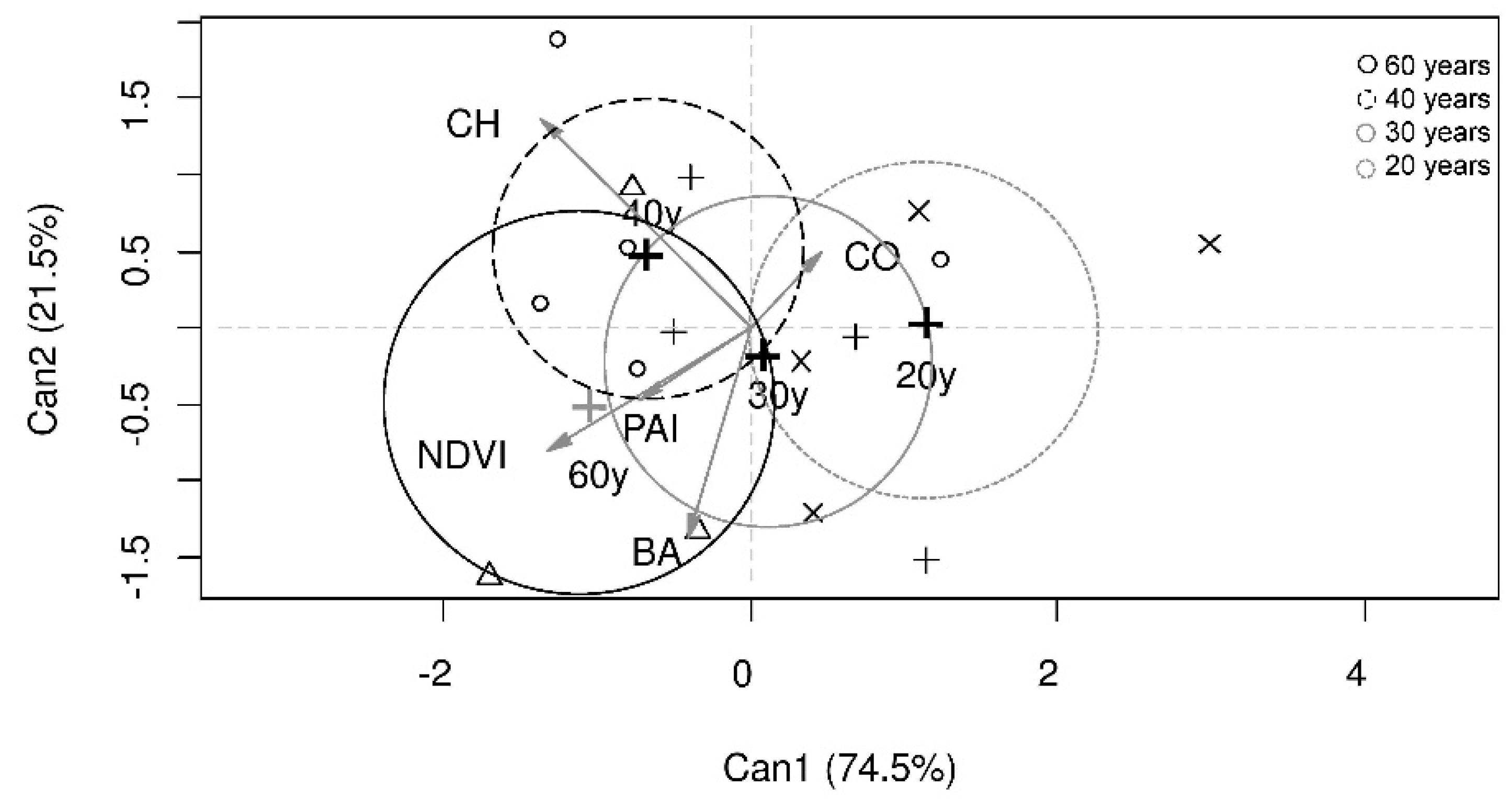
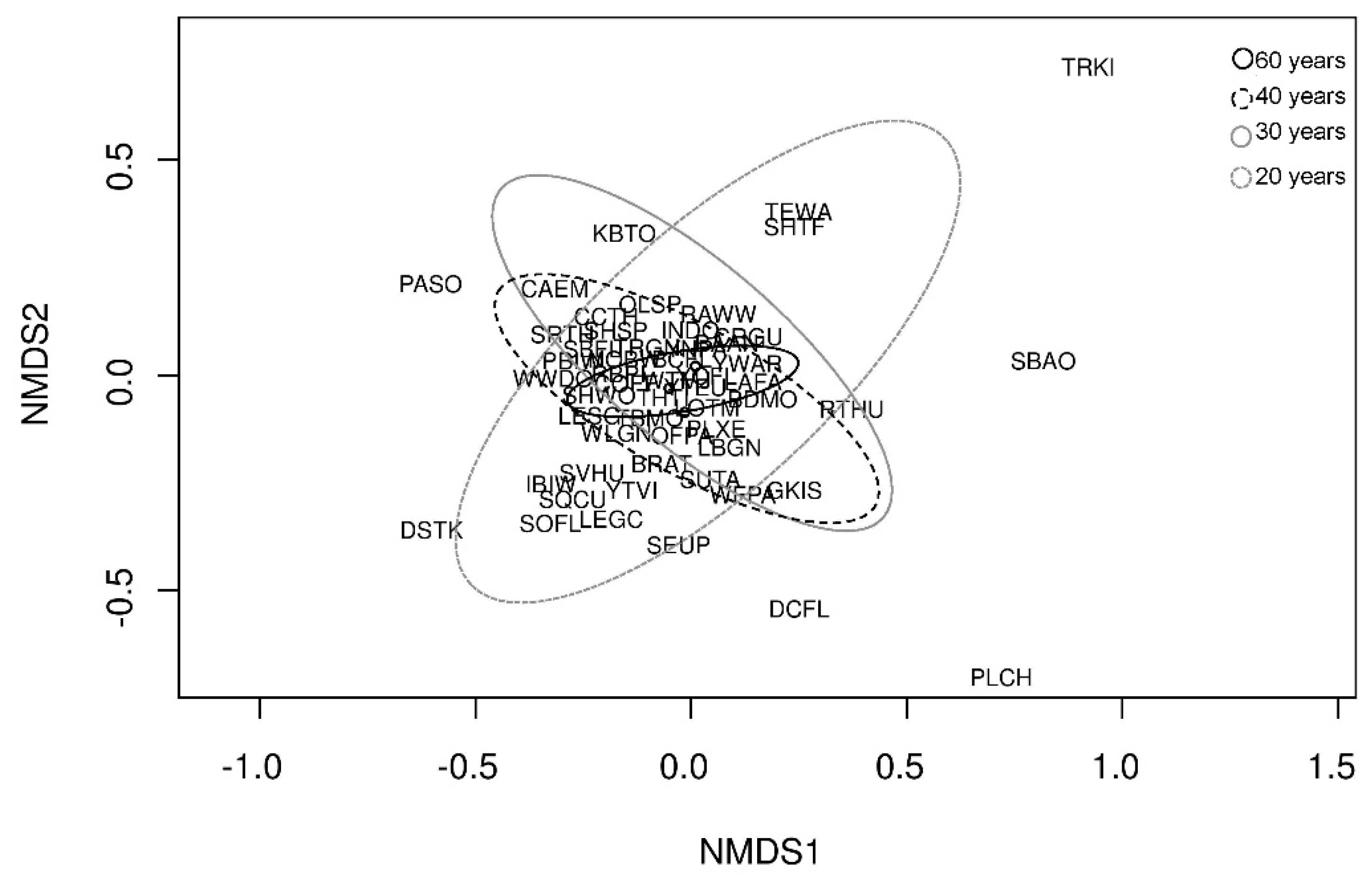
3.3. Bird Species Assemblage and Forest Age Class
4. Discussion
4.1. Dry Forest Structural Characteristics and Forest Age
4.2. Bird Species Richness and Forest Age and Structural Characteristics
4.3. Bird Assemblage, Forest Age and Forest Structural Characteristics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aide, T.M.; Clark, M.L.; Grau, H.R.; López-Carr, D.; Levy, M.A.; Redo, D.; Bonilla-Moheno, M.; Riner, G.; Andrade-Núñez, M.J.; Muñiz, M. Deforestation and Reforestation of Latin America and the Caribbean (2001–2010). Biotropica 2013, 45, 262–271. [Google Scholar] [CrossRef]
- Chazdon, R. Tropical forest recovery: Legacies of human impact and natural disturbances. Perspect. Plant Ecol. Evol. Syst. 2003, 6, 51–71. [Google Scholar] [CrossRef]
- Crouzeilles, R.; Curran, M.; Ferreira, M.S.; Lindenmayer, D.; Grelle, C.E.V.; Benayas, J.M.R. A global meta-analysis on the ecological drivers of forest restoration success. Nat. Commun. 2016, 7, 11666. [Google Scholar] [CrossRef] [PubMed]
- Boukili, V.K.; Chazdon, R. Environmental filtering, local site factors and landscape context drive changes in functional trait composition during tropical forest succession. Perspect. Plant Ecol. Evol. Syst. 2017, 24, 37–47. [Google Scholar] [CrossRef]
- Finegan, B. Pattern and process in neotropical secondary rain forests: The first 100 years of succession. Trends Ecol. Evol. 1996, 11, 119–124. [Google Scholar] [CrossRef]
- Aide, T.M.; Zimmerman, J.K.; Rosario, M.; Marcano, H. Forest Recovery in Abandoned Cattle Pastures Along an Elevational Gradient in Northeastern Puerto Rico. Biotropica 1996, 28, 537. [Google Scholar] [CrossRef]
- Clark, D.B. Abolishing virginity. J. Trop. Ecol. 1996, 12, 735–739. [Google Scholar] [CrossRef]
- Guariguata, M.R.; Chazdon, R.; Denslow, J.S.; Dupuy, J.M.; Anderson, L. Structure and floristics of secondary and old-growth forest stands in lowland Costa Rica. Plant Ecol. 1997, 132, 107–120. [Google Scholar] [CrossRef]
- Montgomery, R.A.; Chazdon, R.L. Forest structure, canopy architecture, and light transmittance in tropical wet forests. Ecology 2001, 82, 2707–2718. [Google Scholar] [CrossRef]
- Wiens, J.A. The Ecology of Bird Communities. Ecol. Bird Communities 1989, 1, 340. [Google Scholar]
- Acevedo-Charry, O.; Aide, T.M. Recovery of amphibian, reptile, bird, and mammal diversity during secondary forest succession in the tropics. Oikos 2019, 128, 1065–1078. [Google Scholar] [CrossRef]
- Şekercioğlu-Çağan, H.; Daily, G.C.; Ehrlich, P.R. Ecosystem consequences of bird declines. Proc. Natl. Acad. Sci. USA 2004, 101, 18042–18047. [Google Scholar] [CrossRef] [PubMed]
- Şekercioğlu-Çağan, H.; Ehrlich, P.R.; Daily, G.C.; Aygen, D.; Goehring, D.; Sandí, R.F.; Şekercioḡlu, Çaḡan H. Disappearance of insectivorous birds from tropical forest fragments. Proc. Natl. Acad. Sci. USA 2002, 99, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Thompson, F.R., III; Probst, J.R.; Raphael, M.G. Impacts of silviculture: Overview and management. In Ecology and Management of Neotropical Migratory Birds: A Synthesis and Review of Critical Issues; Martin, T.E., Finch, D.M., Eds.; Oxford University Press: New York, NY, USA, 1995; pp. 201–219. [Google Scholar]
- McClanahan, T.R.; Wolfe, R. Accelerating Forest Succession in a Fragmented Landscape: The Role of Birds and Perches. Conserv. Boil. 1993, 7, 279–288. [Google Scholar] [CrossRef]
- Barlow, J.; Gardner, T.A.; Araujo, I.S.; Avila-Pires, T.C.; Bonaldo, A.B.; Costa, J.E.; Esposito, M.C.; Ferreira, L.V.; Hawes, J.; Hernández, M.I.M.; et al. Quantifying the biodiversity value of tropical primary, secondary, and plantation forests. Proc. Natl. Acad. Sci. USA 2007, 104, 18555–18560. [Google Scholar] [CrossRef]
- Michel, N.L.; Robinson, W.D.; Sherry, T.W. Liana-bird relationships: A review. In Ecology of Lianas; Wiley: Hoboken, NJ, USA, 2014; pp. 362–397. [Google Scholar]
- Muscolo, A.; Bagnato, S.; Sidari, M.; Mercurio, R. A review of the roles of forest canopy gaps. J. For. Res. 2014, 25, 725–736. [Google Scholar] [CrossRef]
- Fetcher, N.; Oberbauer, S.F.; Strain, B.R. Vegetation effects on microclimate in lowland tropical forest in Costa Rica. Int. J. Biometeorol. 1985, 29, 145–155. [Google Scholar] [CrossRef]
- Laurance, W.F.; Bierregaard, R.O. Tropical Forest Remnants: Ecology, Management, and Conservation of Fragmented Communitie; University of Chicago Press: Chicago, IL, USA, 1997; p. 616. [Google Scholar]
- Kato, M.; Inoue, T.; Hamid, A.A.; Nagamitsu, T.; Merdek, M.B.; Nona, A.R.; Itino, T.; Yamane, S.; Yumoto, T. Seasonality and vertical structure of ligh-attracted insect communities in a dipterocarp forest in Sarawak. Popul. Ecol. 1995, 37, 59–79. [Google Scholar] [CrossRef]
- Andrade, G.I.; Rubio-Torgler, H. Sustainable Use of the Tropical Rain Forest: Evidence from the Avifauna in a Shifting?Cultivation Habitat Mosaic in the Colombian Amazon. Conserv. Boil. 1994, 8, 545–554. [Google Scholar] [CrossRef]
- Bu, W.; Zang, R.; Ding, Y. Functional diversity increases with species diversity along successional gradient in a secondary tropical lowland rainforest. Trop. Ecol. 2014, 55, 393–401. [Google Scholar]
- Santamaria-Rivero, W.; Leyequien, E.; Hernandez-Stefanoni, J.L.; Wood, P. Influence of landscape structure and forest age on the richness and abundance of different bird feeding guilds and forest-dependent birds in a seasonal dry tropical forest of Yucatan, Mexico. Trop. Ecol. 2016, 57, 313–332. [Google Scholar]
- Kalacska, M.; Sanchez-Azofeifa, G.; Calvo-Alvarado, J.C.; Quesada, M.; Rivard, B.; Janzen, D.; Sánchez-Azofeifa, G.A. Species composition, similarity, and diversity in three successional stages of a seasonally dry tropical forest. For. Ecol. Manag. 2004, 200, 227–247. [Google Scholar] [CrossRef]
- Janzen, D.H. Management of Habitat Fragments in a Tropical Dry Forest: Growth. Ann. Mo. Bot. Gard. 1988, 75, 105. [Google Scholar] [CrossRef]
- Hilje, B.; Calvo-Alvarado, J.C.; Jiménez-Rodríguez, C.D.; Sánchez-Azofeifa, G.A. Tree Species Composition, Breeding Systems, and Pollination and Dispersal Syndromes in Three Forest Successional Stages in a Tropical Dry Forest in Mesoamerica. Trop. Conserv. Sci. 2015, 8, 76–94. [Google Scholar] [CrossRef]
- Janzen, D.H. Costa Rican Natural History; University of Chicago Press: Chicago, IL, USA, 1983; p. 823. [Google Scholar]
- Sánchez-Azofeifa, G.A.; Vega-Araya, M.; Campos-Vargas, C.; Durán, S.M.; Gianoli, T.; Portillo-Quintero, C.; Sharp, I.; D’Souza, N. Can terrestrial laser scanners (TLSs) and hemispherical photographs predict tropical dry forest succession with liana abundance? Biogeosciences 2017, 14, 977–988. [Google Scholar] [CrossRef]
- Phillips, O.; Miller, J.S. Global Patterns of Plant Diversity: Alwyn H. Gentry’s Forest Transect Data Set; Missouri Botanical Press: St. Louis, MO, USA, 2002; p. 319. [Google Scholar]
- Ornl Daac. Modis Collection 6 Land Products Global Subsetting and Visualization Tool. Available online: https://daac.ornl.gov/LAND_VAL/guides/MODIS_Global_C6.html/ (accessed on 25 October 2018).
- Wildlife Acoustics. Songscope: Bioacoustics Software, Version 4.0; Wildlife Acoustics Inc.: Maynard, MA, USA, 2011; p. 44. [Google Scholar]
- Brackenbury, J.H. Power capabilities of the avian sound-producing system. J. Exp. Biol. 1979, 78, 163–166. [Google Scholar]
- Nicholls, J.A.; Goldizen, A. Habitat type and density influence vocal signal design in satin bowerbirds. J. Anim. Ecol. 2006, 75, 549–558. [Google Scholar] [CrossRef]
- Swearingen, M.E.; White, M.J.; Guertin, P.J.; Albert, D.G.; Tunick, A. Influence of a forest edge on acoustical propagation: Experimental results. J. Acoust. Soc. Am. 2013, 133, 2566–2575. [Google Scholar] [CrossRef]
- Center for Conservation Bioacoustics. Raven Pro: Interactive Sound Analysis Software (Version 1.5) [Computer software]. The Cornell Lab of Ornithology. Available online: http://ravensoundsoftware.com/ (accessed on 11 May 2018).
- Berg, R.E. The Physics of Sound. Am. J. Phys. 1982, 50, 953. [Google Scholar] [CrossRef]
- Barrantes, G.; Ocampo, D.; Ramírez-Fernández, J.D.; Fuchs, E.J. Effect of fragmentation on the Costa Rican dry forest avifauna. Peer J. 2016, 4, 2422. [Google Scholar] [CrossRef]
- Ruiz-Gutiérrez, V.; Zipkin, E.F.; Dhondt, A.A. Occupancy dynamics in a tropical bird community: Unexpectedly high forest use by birds classified as non-forest species. J. Appl. Ecol. 2010, 47, 621–630. [Google Scholar] [CrossRef]
- Furnas, B.J.; Callas, R.L. Using automated recorders and occupancy models to monitor common forest birds across a large geographic region. J. Wildl. Manag. 2015, 79, 325–337. [Google Scholar] [CrossRef]
- Hilje, B.; Stack, S.; Sánchez-Azofeifa, G.A. Lianas Abundance is Positively Related with the Avian Acoustic Community in Tropical Dry Forests. Forests 2017, 8, 311. [Google Scholar] [CrossRef]
- Izenman, A.J. Modern Multivariate Statistical Techniques; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2008; p. 758. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S-PLUS; Springer: New York, NY, USA, 2013; p. 447. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H.; et al. Package ‘vegan’. Community Ecol. Pack. 2013, 2, 1–295. [Google Scholar]
- Core-Team. R. A Language and Environment for Statistical Computing; Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67. [Google Scholar] [CrossRef]
- Burham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference, a Practical Information-Theoretic Approach; Springer: New York, NY, USA, 2002; p. 488. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Multimodel inference: Understanding AIC and BIC in model selection. Sociol. Methods Res. 2004, 33, 261–304. [Google Scholar] [CrossRef]
- Richards, S.A. Testing Ecological Theoty Using the Information-Theoretic Approach: Examples and Cautionary Results. Ecology 2005, 86, 2805–2814. [Google Scholar] [CrossRef]
- Symonds, M.R.E.; Moussalli, A. A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behav. Ecol. Sociobiol. 2010, 65, 13–21. [Google Scholar] [CrossRef]
- Stiles, F.G.; Skutch, A.F. Guide to the Birds of Costa Rica; Cornell University Press: Ithaca, NY, USA, 1989; p. 511. [Google Scholar]
- Li, W.; Cao, S.; Campos-Vargas, C.; Sánchez-Azofeifa, G.A. Identifying tropical dry forests extent and succession via the use of machine learning techniques. Int. J. Appl. Earth Obs. Geoinf. 2017, 63, 196–205. [Google Scholar] [CrossRef]
- Lopezaraiza-Mikel, M.; Alvarez-Añorve, M.; Avila-Cabadilla, L.; Marten-Rodriguez, S.; Calvo-Alvarado, J.C.; Espírito-Santo, M.M.D.; Fernandes, G.; Sánchez-Azofeifa, G.A.; Aguilar-Aguilar, M.; Balvino-Olvera, F.; et al. Phenological Patterns of Tropical Dry Forests along Latitudinal and Successional Gradients in the Neotropics. In Tropical Dry Forests in the Americas; Informa UK Limited: London, UK, 2013; pp. 101–128. [Google Scholar]
- Levey, D.J. Tropical Wet Forest Treefall Gaps and Distributions of Understory Birds and Plants. Ecology 1988, 69, 1076–1089. [Google Scholar] [CrossRef]
- Sánchez, N.V.; Vargas-Castro, L.E.; Avalos, G.; Paniagua, F. Effect of prey availability on the abundance of White-breasted Wood-Wrens, insectivorous birds of tropical lowland forests. J. Field Ornithol. 2014, 85, 347–354. [Google Scholar] [CrossRef]
- Neves, F.D.S.; Silva, J.; Marques, T.; Mota-Souza, J.; Madeira, B.; Espírito-Santo, M.D.; Fernandes, G. Spatiotemporal Dynamics of Insects in a Brazilian Tropical Dry Forest. In Tropical Dry Forests in the Americas; Informa UK Limited: London, UK, 2013; pp. 221–235. [Google Scholar]
- Martin, P.H.; Sherman, R.E.; Fahey, T.J. Forty years of tropical forest recovery from agriculture: Structure and floristics of secondary and old-growth riparian forests in the Dominican Republic. Biotropica 2004, 36, 297–317. [Google Scholar]
- Letcher, S.G.; Chazdon, R. Rapid Recovery of Biomass, Species Richness, and Species Composition in a Forest Chronosequence in Northeastern Costa Rica. Biotropica 2009, 41, 608–617. [Google Scholar] [CrossRef]
- Dunn, R.R. Recovery of Faunal Communities during Tropical Forest Regeneration. Conserv. Boil. 2004, 18, 302–309. [Google Scholar] [CrossRef]

| Age Class | BA | PAI | NDVI | CH | LR | CO |
|---|---|---|---|---|---|---|
| 20 years | 16.75 (13.19) | 2.26 (0.31) | 0.83 (0.03) | 15.38 (3.85) | 0.03 (0.04) | 14.11 (4.91) |
| 30 years | 22.40 (11.09) | 2.38 (0.71) | 0.84 (0.02) | 18.98 (5.78) | 0.17 (0.13) | 13.08 (7.55) |
| 40 years | 16.62 (8.07) | 2.47 (0.77) | 0.85 (0.02) | 24.86 (6.97) | 0.18 (0.13) | 13.12 (6.19) |
| 60 years | 25.00 (9.40) | 2.71 (0.48) | 0.88 (0.07) | 20.43 (7.85) | 0.07 (0.04) | 11.16 (1.33) |
| Species | Latin Name | Species Code | 20 | 30 | 40 | 60 years | Red List |
|---|---|---|---|---|---|---|---|
| Banded Wren | Thryothorus pleurostictus | BANW | × | × | × | × | LC |
| Barred Antshrike | Thamnophilus doliatus | BAAN | × | × | × | × | LC |
| Black-headed Trogon | Trogon melanocephalus | BHTR | × | × | × | × | LC |
| Boat-billed Flycatcher | Megarynchus pitangua | BOBF | × | × | × | × | LC |
| Bright-rumped Attila | Attila spadiceus | BRAT | × | × | × | LC | |
| Brown-crested Flycatcher | Myiarchus tyrannulus | BCFL | × | × | × | × | LC |
| Canivet’s Emerald | Chlorostilbon canivetii | CAEM | × | × | LC | ||
| Clay-colored Thrush | Turdus grayi | CCTH | × | × | × | LC | |
| Collared Forest-Falcon | Micrastur semitorquatus | COFF | × | × | LC | ||
| Crested Guan | Penelope purpurascens | CRGU | × | × | × | LC | |
| Double-striped Thick-knee | Burhinus bistriatus | DSTK | × | LC | |||
| Dusky-capped Flycatcher | Myiarchus tuberculifer | DCFL | × | × | × | LC | |
| Elegant Trogon | Trogon elegans | ELTR | × | × | × | × | LC |
| Ferruginous Pygmy-Owl | Glaucidium brasilianum | FEPO | × | LC | |||
| Great Curassow | Crax rubra | GRCU | × | VU | |||
| Great Kiskadee | Pitangus sulphuratus | GKIS | × | × | LC | ||
| Inca Dove | Columbina inca | INDO | × | × | LC | ||
| Ivory-billed Woodcreeper | Xiphorhynchus flavigaster | IBIW | × | × | LC | ||
| Keel-billed Toucan | Ramphastos sulfuratus | KBTO | × | LC | |||
| Laughing Falcon | Herpetotheres cachinnans | LAFA | × | × | × | × | LC |
| Lesser Greenlet | Hylophilus decurtatus | LESG | × | × | × | LC | |
| Lesser Ground-Cuckoo | Morococcyx erythropygus | LEGC | × | × | LC | ||
| Lesson’s Motmot | Momotus lessonii | LEMO | × | × | × | × | LC |
| Long-billed Gnatwren | Ramphocaenus melanurus | LBGN | × | × | × | × | LC |
| Long-tailed Manakin | Chiroxiphia linearis | LOTM | × | × | × | × | LC |
| Northern Barred-Woodcreeper | Dendrocolaptes sanctithomae | NOBW | × | × | × | × | LC |
| Olive Sparrow | Arremonops rufivirgatus | OLSP | × | × | × | × | LC |
| Orange-fronted Parakeet | Aratinga canicularis | OFPA | × | × | × | × | LC |
| Pacific Screech-Owl | Megascops cooperi | PASO | × | × | LC | ||
| Pale-billed Woodpecker | Campephilus guatemalensis | PBIW | × | × | × | LC | |
| Plain Chachalaca | Ortalis vetula | PLCH | × | LC | |||
| Plain Xenops | Xenops minutus | PLXE | × | × | LC | ||
| Red-billed Pigeon | Patagioenas flavirostris | RBPI | × | × | × | × | LC |
| Ruby-throated Hummingbird | Archilochus colubris | RTHU | × | × | × | LC | |
| Rufous-and-white Wren | Thryothorus rufalbus | RAWW | × | × | × | × | LC |
| Rufous-capped Warbler | Basileuterus rufifrons | RCWA | × | × | × | × | LC |
| Rufous-naped Wren | Campylorhynchus rufinucha | RNAW | × | × | × | × | LC |
| Scrub Euphonia | Euphonia affinis | SEUP | × | × | × | LC | |
| Slate-headed Tody-Flycatcher | Poecilotriccus sylvia | SHTF | × | × | LC | ||
| Social Flycatcher | Myiozetetes similis | SOFL | × | × | LC | ||
| Squirrel Cuckoo | Piaya cayana | SQCU | × | × | × | LC | |
| Steely-vented Hummingbird | Amazilia saucerrottei | SVHU | × | × | LC | ||
| Streak-backed Oriole | Icterus pustulatus | SBAO | × | × | LC | ||
| Streak-headed Woodcreeper | Lepidocolaptes souleyetii | SHWO | × | × | × | × | LC |
| Stripe-headed Sparrow | Peucaea ruficauda | SHSP | × | × | × | LC | |
| Stripe-throated Hermit | Phaethornis striigularis | SRTH | × | × | × | × | LC |
| Sulphur-bellied Flycatcher | Myiodynastes luteiventris | SBFL | × | × | × | LC | |
| Summer Tanager | Piranga rubra | SUTA | × | × | × | × | LC |
| Tennessee Warbler | Oreothlypis peregrina | TEWA | × | × | × | LC | |
| Thicket Tinamou | Crypturellus cinnamomeus | THTI | × | × | × | × | LC |
| Tropical Gnatcatcher | Polioptila plumbea | TRGN | × | × | × | × | LC |
| Tropical Kingbird | Tyrannus melancholicus | TRKI | × | LC | |||
| Turquoise-browed Motmot | Eumomota superciliosa | TBMO | × | × | × | × | LC |
| White-fronted Parrot | Amazona albifrons | WFPA | × | × | × | × | LC |
| White-lored Gnatcatcher | Polioptila albiloris | WLGN | × | × | × | × | LC |
| White-throated Magpie-Jay | Calocitta formosa | WTMJ | × | × | × | × | LC |
| White-tipped Dove | Leptotila verreauxi | WTDO | × | × | × | × | LC |
| White-winged Dove | Zenaida asiatica | WWDO | × | × | × | × | LC |
| Yellow Warbler | Dendroica petechia | YWAR | × | × | × | × | LC |
| Yellow-green Vireo | Vireo flavoviridis | YGVI | × | × | × | × | LC |
| Yellow-naped Parrot | Amazona auropalliata | YNPA | × | × | × | × | EN |
| Yellow-olive Flycatcher | Tolmomyias sulphurescens | YOFL | × | × | × | × | LC |
| Yellow-throated Euphonia | Euphonia hirundinacea | YTEU | × | × | × | × | LC |
| Yellow-throated Vireo | Vireo flavifrons | YTVI | × | × | × | × | LC |
| Models | k | AICc | ΔAICc | w |
|---|---|---|---|---|
| species~CO | 2 | 95.203 | 0.000 | 0.498 |
| species~1 (null) | 1 | 98.316 | 3.113 | 0.105 |
| species~LAI | 2 | 98.883 | 3.680 | 0.079 |
| species~area | 2 | 98.930 | 3.727 | 0.077 |
| species~CO + BA | 3 | 99.780 | 4.577 | 0.050 |
| species~BA | 2 | 100.183 | 4.980 | 0.041 |
| species~CO + LAI | 3 | 100.220 | 5.017 | 0.041 |
| species~NDVIm | 2 | 100.273 | 5.070 | 0.039 |
| species~CH | 2 | 100.283 | 5.080 | 0.039 |
| species~NDVIm + CO | 3 | 100.550 | 5.347 | 0.034 |
| species~liana | 2 | 100.933 | 5.730 | 0.028 |
| species~CO*BA | 3 | 100.940 | 5.737 | 0.028 |
| species~CO*LAI | 3 | 102.080 | 6.877 | 0.016 |
| species~CO + LAI + BA + CH + NDVIm + liana | 7 | 119.090 | 23.887 | 0.000 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hilje, B.; Sánchez, N.V.; Bayne, E.M.; Sánchez-Azofeifa, A. Bird Assemblage Recovery in a Chronosequence of Tropical Dry Forests in Costa Rica. Forests 2020, 11, 629. https://doi.org/10.3390/f11060629
Hilje B, Sánchez NV, Bayne EM, Sánchez-Azofeifa A. Bird Assemblage Recovery in a Chronosequence of Tropical Dry Forests in Costa Rica. Forests. 2020; 11(6):629. https://doi.org/10.3390/f11060629
Chicago/Turabian StyleHilje, Branko, Natalie V. Sánchez, Erin M. Bayne, and Arturo Sánchez-Azofeifa. 2020. "Bird Assemblage Recovery in a Chronosequence of Tropical Dry Forests in Costa Rica" Forests 11, no. 6: 629. https://doi.org/10.3390/f11060629
APA StyleHilje, B., Sánchez, N. V., Bayne, E. M., & Sánchez-Azofeifa, A. (2020). Bird Assemblage Recovery in a Chronosequence of Tropical Dry Forests in Costa Rica. Forests, 11(6), 629. https://doi.org/10.3390/f11060629






