Within-Site Variability of Liana Wood Anatomical Traits: A Case Study in Laussat, French Guiana
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Liana Sampling and Identification
2.3. Basic Specific Gravity Measurements
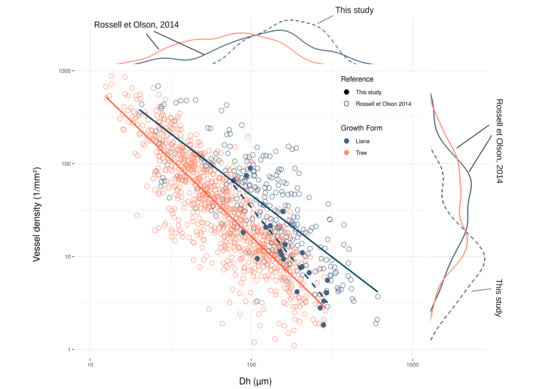
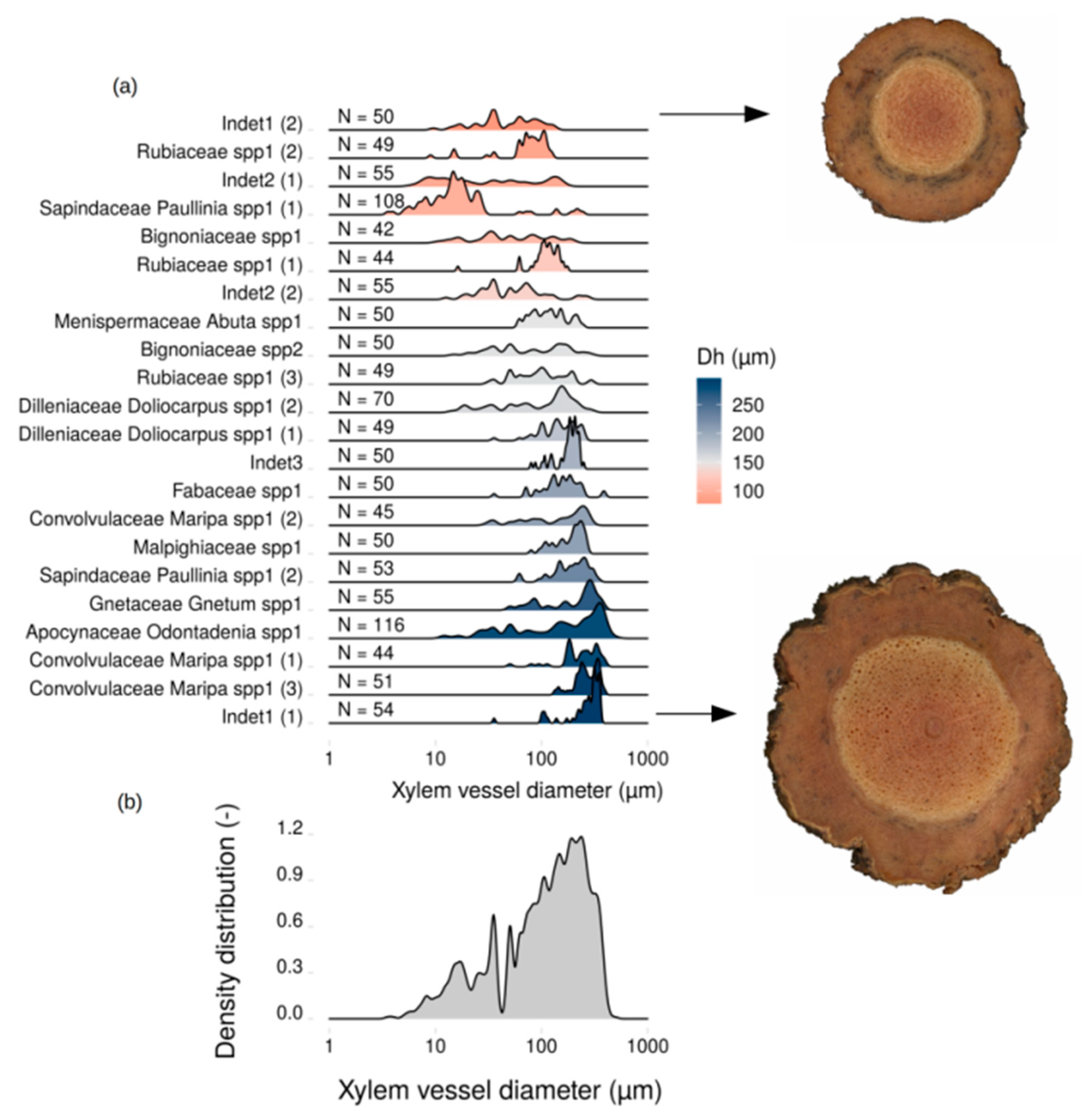
2.4. Vessel Diameter Distribution
2.5. Anatomical Traits
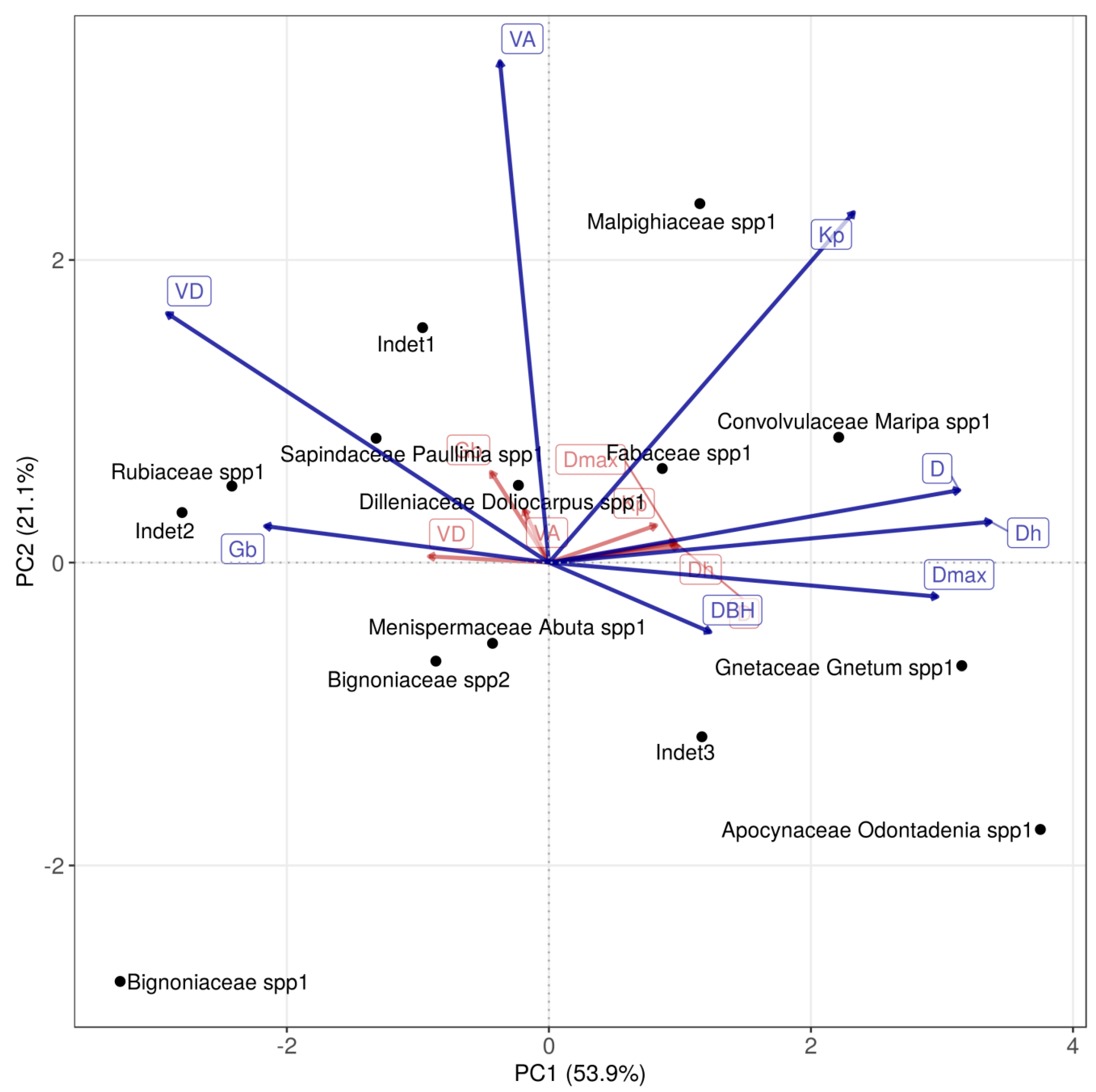
2.6. Analyses
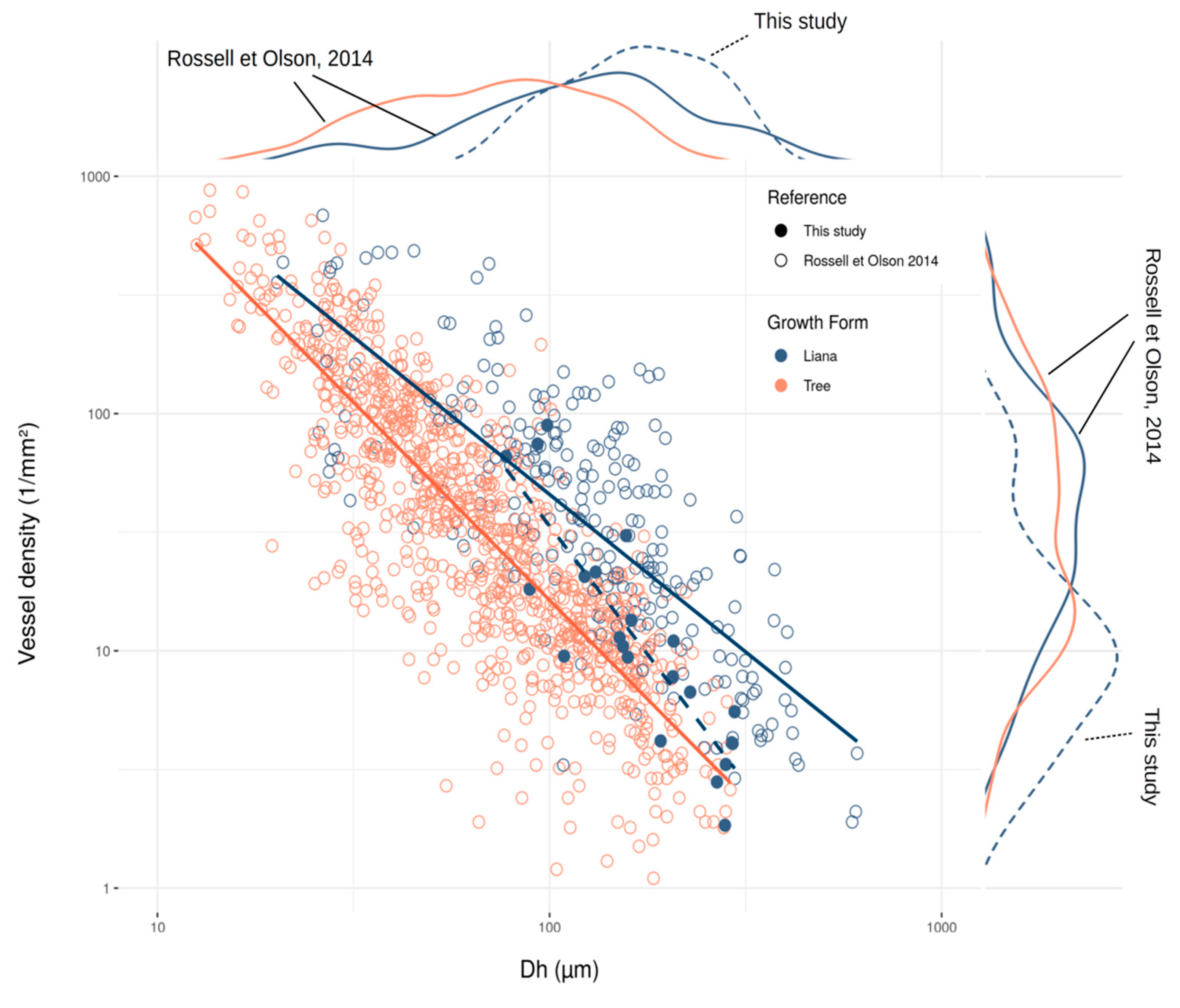
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Phillips, O.L. Increasing dominance of large lianas in Amazonian forests. Nature 2002, 418, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Schnitzer, S.A.; Bongers, F. Increasing liana abundance and biomass in tropical forests: Emerging patterns and putative mechanisms. Ecol. Lett. 2011, 14, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Schnitzer, S.A.; van der Heijden, G.M.F.; Powers, J.S. Reply to Verbeeck and Kearsley: Addressing the challenges of including lianas in global vegetation models. Proc. Natl. Acad. Sci. USA 2016, 113, E5–E6. [Google Scholar] [CrossRef] [PubMed]
- Verbeeck, H.; Kearsley, E. The importance of including lianas in global vegetation models. Proc. Natl. Acad. Sci. USA 2016, 113, E4. [Google Scholar] [CrossRef] [PubMed]
- Schnitzer, S. The ecology of lianas and their role in forests. Trends Ecol. Evol. 2002, 17, 223–230. [Google Scholar] [CrossRef]
- van der Heijden, G.M.; Schnitzer, S.A.; Powers, J.S.; Phillips, O.L. Liana impacts on carbon cycling, storage and sequestration in tropical forests. Biotropica 2013, 45, 682–692. [Google Scholar] [CrossRef]
- Ingwell, L.L.; Joseph Wright, S.; Becklund, K.K.; Hubbell, S.P.; Schnitzer, S.A. The impact of lianas on 10 years of tree growth and mortality on Barro Colorado Island. Panama J. Ecol. 2010, 98, 879–887. [Google Scholar] [CrossRef]
- van der Heijden, G.; Powers, J.S.; Schnitzer, S.A. Lianas reduce forest-level carbon accumulation and storage. Proc. Natl. Acad. Sci. USA 2015, 112, 13267–13271. [Google Scholar] [CrossRef]
- Baccini, A.; Walker, W.; Carvalho, L.; Farina, M.; Sulla-Menashe, D.; Houghton, R.A. Tropical forests are a net carbon source based on aboveground measurements of gain and loss. Science 2017, 358, 230–234. [Google Scholar] [CrossRef]
- Ewers, F.W.; Fisher, J.B.; Chiu, S.T. Water Transport in the Liana Bauhinia fassoglensis (Fabaceae). Plant. Physiol. 1989, 91, 1625–1631. [Google Scholar] [CrossRef]
- Caballé, G. Liana structure, function and selection: A comparative study of xylem cylinders of tropical rainforest species in Africa and America. Bot. J. Linn. Soc. 1993, 113, 41–60. [Google Scholar] [CrossRef]
- Chen, Y.J.; Schnitzer, S.A.; Zhang, Y.J.; Fan, Z.X.; Goldstein, G.; Tomlinson, K.W.; Cao, K.F. Physiological regulation and efficient xylem water transport regulate diurnal water and carbon balances of tropical lianas. Funct. Ecol. 2017, 31, 306–317. [Google Scholar] [CrossRef]
- Ewers, F.W.; Fisher, J.B.; Chiu, S.; Ewers, W.; Chi, S. A survey of vessel dimensions in stems of tropical lianas and other growth forms. Oecologia 1990, 84, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, E.C.D.; Martins, F.R.; Soares, A.A.; Oliveira, R.S.; Muniz, C.R.; Araújo, F.S. Hydraulic architecture of lianas in a semiarid climate: Efficiency or safety? Acta Bot. Bras. 2015, 29, 198–206. [Google Scholar] [CrossRef]
- Bastos, C.L.; Tamaio, N.; Angyalossy, V. Unravelling roots of lianas: A case study in Sapindaceae. Ann. Bot. 2016, 118, 733–746. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Cao, K.-F.; Schnitzer, S.A.; Fan, Z.-X.; Zhang, J.-L.; Bongers, F. Water-use advantage for lianas over trees in tropical seasonal forests. New Phytol. 2015, 205, 128–136. [Google Scholar] [CrossRef]
- De Guzman, M.E.; Santiago, L.S.; Schnitzer, S.A.; Álvarez-Cansino, L. Trade-offs between water transport capacity and drought resistance in neotropical canopy liana and tree species. Tree Physiol. 2016. [Google Scholar] [CrossRef]
- Johnson, D.M.; Domec, J.C.; Woodruff, D.R.; McCulloh, K.A.; Meinzer, F.C. Contrasting hydraulic strategies in two tropical lianas and their host trees. Am. J. Bot. 2013, 100, 374–383. [Google Scholar] [CrossRef]
- Zhu, S.D.; Cao, K.F. Hydraulic Properties and Photosynthetic Rates in Co-occurring Lianas and Trees in a Seasonal Tropical Rainforest in Southwestern China. Plant. Ecol. 2009, 204, 295–304. [Google Scholar] [CrossRef]
- Angyalossy, V.; Angeles, G.; Pace, M.R.; Lima, A.C.; Dias-Leme, C.L.; Lohmann, L.G.; Madero-Vega, C. An overview of the anatomy, development and evolution of the vascular system of lianas. Plant Ecol. Divers. 2012, 5. [Google Scholar] [CrossRef]
- Schnitzer, S.A. A mechanistic explanation for global patterns of liana abundance and distribution. Am. Nat. 2005, 166, 262–276. [Google Scholar] [CrossRef]
- Jackson, P.C.; Cavelier, J.; Goldstein, G.; Meinzer, F.C.; Holbrook, N.M. Partitioning of water resources among plants of a lowland tropical forest. Oecologia 1995, 101, 197–203. [Google Scholar] [CrossRef]
- Zhu, S.D.; Cao, K.F. Contrasting cost-benefit strategy between lianas and trees in a tropical seasonal rain forest in southwestern China. Oecologia 2010, 163, 591–599. [Google Scholar] [CrossRef]
- Putz, F.E.; Mooney, H.A. (Eds.) The Biology of Vines; Cambridge University Press: Cambridge, UK, 1992. [Google Scholar]
- di Porcia E Brugnera, M.; Meunier, F.; Longo, M.; Krishna Moorthy, S.M.; De Deurwaerder, H.; Schnitzer, S.A.; Verbeeck, H. Modeling the impact of liana infestation on the demography and carbon cycle of tropical forests. Glob. Change Biol. 2019, 25, 3767–3780. [Google Scholar] [CrossRef]
- Smith-Martin, C.M.; Xu, X.; Medvigy, D.; Schnitzer, S.A.; Powers, J.S. Allometric scaling laws linking biomass and rooting depth vary across ontogeny and functional groups in tropical dry forest lianas and trees. New Phytol. 2019. [Google Scholar] [CrossRef]
- Wyka, T.P.; Oleksyn, J.; Karolewski, P.; Schnitzer, S.A. Phenotypic correlates of the lianescent growth form: A review. Ann. Bot. 2013, 112, 1667–1681. [Google Scholar] [CrossRef]
- Werden, L.K.; Waring, B.G.; Smith-Martin, C.M.; Powers, J.S. Tropical dry forest trees and lianas differ in leaf economic spectrum traits but have overlapping water-use strategies. Tree Physiol. 2017, 1–14. [Google Scholar] [CrossRef]
- Rosell, J.A.; Olson, M.E. Do lianas really have wide vessels? Vessel diameter–stem length scaling in non-self-supporting plants. Perspect. Plant. Ecol. Evol. Syst. 2014, 16, 288–295. [Google Scholar] [CrossRef]
- Gutierrez, M.; Miguel-Cha, R.S.; Terrazas, T. Xylem Conductivity and Anatomical Traits in Diverse Lianas and Small Tree Species from a Tropical Forest of Southwest Mexico. Int. J. Bot. 2009, 5, 279–286. [Google Scholar] [CrossRef]
- Baraloto, C.; Rabaud, S.; Molto, Q.; Blanc, L.; Fortunel, C.; Herault, B.; Fine, P.V. Disentangling stand and environmental correlates of aboveground biomass in Amazonian forests. Glob. Chang. Biol. 2011, 17, 2677–2688. [Google Scholar] [CrossRef]
- Schnitzer, S.A.; Dewalt, S.J.; Chave, J. Censusing and measuring lianas: A quantitative comparison of the common methods. Biotropica 2006, 38, 581–591. [Google Scholar] [CrossRef]
- Glass, S.V.; Zelinka, S.L. Moisture relations and physical properties of wood. In Wood Hanbook: Wood as an Engineering Material, Centennial ed; General technical report FPL, GTR-190; Department of Agriculture, Forest Service, Forest Products Laboratory: Madison, WI, USA, 2010; Chapter 4; pp. 4.1–4.19. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Sterck, F.J.; Zweifel, R.; Sass-Klaassen, U.; Chowdhury, Q. Persisting soil drought reduces leaf specific conductivity in Scots pine (Pinus sylvestris) and pubescent oak (Quercus pubescens). Tree Physiol. 2008, 28, 529–536. [Google Scholar] [CrossRef]
- Joanes, D.N.; Gill, C.A. Comparing Measures of Sample Skewness and Kurtosis. J. R. Stat. Soc. Ser. Stat. 1998, 47, 183–189. [Google Scholar] [CrossRef]
- Zanne, A.E.; Lopez-Gonzalez, G.; Coomes, D.A.; Ilic, J.; Jansen, S.; Lewis, S.L.; Chave, J. Wood Density Database. Dryad Digit. Repos. 2009. [Google Scholar] [CrossRef]
- Taseski, G.M.; Beloe, C.J.; Gallagher, R.V.; Chan, J.Y.; Dalrymple, R.L.; Cornwell, W.K. A global growth-form database for 143,616 vascular plant species. Ecology 2019, 100, e02614. [Google Scholar] [CrossRef]
- Poorter, L.; McDonald, I.; Alarcón, A.; Fichtler, E.; Licona, J.C.; Peña-Claros, M.; Sass-Klaassen, U. The importance of wood traits and hydraulic conductance for the performance and life history strategies of 42 rainforest tree species. New Phytol. 2010, 185, 481–492. [Google Scholar] [CrossRef]
- Hietz, P.; Rosner, S.; Hietz-Seifert, U.; Wright, S.J. Wood traits related to size and life history of trees in a Panamanian rainforest. New Phytol. 2017, 213, 170–180. [Google Scholar] [CrossRef]
- Ewers, F.W. Xylem’ Structure and Water Conduction in Conifer Trees, Dicot Trees, and Llanas. IAWA J. 1985, 6, 309–317. [Google Scholar] [CrossRef]
- Wheeler, E.A.; Baas, P.; Rodgers, S. Variations in Dieot Wood Anatomy: A Global Analysis Based on the Insidewood Database. IAWA J. 2007, 28, 229–258. [Google Scholar] [CrossRef]
- Zhu, S.-D.; Chen, Y.-J.; Fu, P.-L.; Cao, K.-F. Different hydraulic traits of woody plants from tropical forests with contrasting soil water availability. Tree Physiol. 2017, 37, 1469–1477. [Google Scholar] [CrossRef] [PubMed]


| Family | Genus | DBH (mm) | Gb (-) | Vessel N (-) | Median Di (min–max) (µm) | D (±SD) (µm) | Dh (µm) | VD (mm−2) | VA (%) | Kp (kg m−1 s−1 MPa−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| Apocynaceae | Odontadenia spp1 | 52 | 0.38 | 116 | 177 (12–494) | 194 ± 132 | 280 | 1.8 | 8 | 277.54 |
| Bignoniaceae | spp1 | 15 | 0.54 | 42 | 50 (11–192) | 67 ± 51 | 109 | 9.5 | 5.2 | 32.69 |
| spp2 | 19 | 0.53 | 50 | 80 (15–292) | 100 ± 71 | 154 | 10.4 | 12.3 | 143.71 | |
| 17 ± 2.8 | 0.54 ± 0.01 | 46 ± 6 | 65 (11–292) | 83 ± 23 | 131 ± 32 | 10 ± 0.7 | 8.8 ± 5 | 88.2 ± 78.5 | ||
| Convolvulaceae | Maripa spp1 (1) | 52 | 0.42 | 44 | 248 (50–393) | 247 ± 85 | 282 | 3.3 | 17.8 | 510.76 |
| Maripa spp1 (2) | 41 | 0.55 | 45 | 171 (30–292) | 162 ± 84 | 206 | 7.8 | 20.3 | 344.4 | |
| Maripa spp1 (3) | 41 | 0.5 | 51 | 265 (134–394) | 271 ± 65 | 293 | 4.1 | 24.8 | 734.17 | |
| 44.7 ± 6.4 | 0.49 ± 0.07 | 47 ± 4 | 248 (30–394) | 227 ± 57 | 260 ± 47 | 5.1 ± 2.4 | 20.9 ± 3.6 | 529.78 ± 195.58 | ||
| Dilleniaceae | Doliocarpus spp1 (1) | 22 | 0.48 | 49 | 144 (36–251) | 154 ± 55 | 178 | 9.4 | 19.6 | 229.65 |
| Doliocarpus spp1 (2) | 12 | 0.47 | 70 | 126 (14–296) | 116 ± 71 | 162 | 13.4 | 19.5 | 225.45 | |
| 17 ± 7.1 | 0.47 ± 0.01 | 60 ± 15 | 135 (14–296) | 135 ± 27 | 170 ± 11 | 11.4 ± 2.9 | 19.5 ± 0.1 | 227.55 ± 2.97 | ||
| Fabaceae | spp1 | 30 | 0.61 | 50 | 158 (36–393) | 164 ± 68 | 206 | 7.7 | 19.1 | 342.57 |
| Gnetaceae | Gnetum spp1 | 53 | 0.33 | 55 | 252 (50–379) | 218 ± 102 | 267 | 2.8 | 12.7 | 350.19 |
| Malpighiaceae | spp1 | 29.5 | 0.34 | 50 | 200 (80–272) | 189 ± 53 | 207 | 11 | 33.2 | 495.55 |
| Menispermaceae | Abuta spp1 | 95 | 0.57 | 50 | 116 (62–245) | 125 ± 49 | 151 | 11.4 | 16 | 144.15 |
| Rubiaceae | spp1 (1) | 29 | 0.58 | 44 | 113 (16–175) | 113 ± 30 | 123 | 20.6 | 22.2 | 114.79 |
| spp1 (2) | 37 | 0.58 | 49 | 80 (9–124) | 78 ± 29 | 89 | 18.2 | 9.8 | 27.88 | |
| spp1 (3) | 35 | 0.58 | 49 | 101 (32–296) | 111 ± 62 | 157 | 30.6 | 38.6 | 451.79 | |
| 33.7 ± 4.2 | 0.58 ± 0 | 47 ± 3 | 101 (9–296) | 101 ± 20 | 123 ± 34 | 23.1 ± 6.6 | 23.6 ± 14.5 | 198.15 ± 223.91 | ||
| Sapindaceae | Paullinia spp1 (1) | 28 | 0.58 | 108 | 15 (4–245) | 26 ± 43 | 99 | 89.3 | 17.5 | 206.67 |
| Paullinia spp1 (2) | 26 | 0.58 | 53 | 205 (62–344) | 199 ± 70 | 228 | 6.7 | 23.3 | 446.41 | |
| 27 ± 1.4 | 0.58 ± 0 | 80 ± 39 | 110 (4–344) | 112 ± 122 | 163 ± 92 | 48 ± 58.4 | 20.4 ± 4 | 326.54 ± 169.52 | ||
| Indet1 | (1) | 26 | 0.45 | 54 | 300 (36–355) | 271± 80 | 296 | 5.4 | 34.7 | 1041.38 |
| (2) | 16 | 0.47 | 50 | 50 (10–134) | 56 ± 32 | 77 | 66 | 21.4 | 58.27 | |
| 21 ± 7.1 | 0.46 ± 0.02 | 52 ± 3 | 175 (10–355) | 163 ± 152 | 187 ± 154 | 35.8 ± 42.8 | 28.1 ± 9.4 | 549.82 ± 695.16 | ||
| Indet2 | (1) | 33 | 0.49 | 55 | 29 (6–156) | 50 ± 48 | 93 | 74.4 | 28.1 | 137.39 |
| (2) | 37 | 0.58 | 55 | 50 (13–276) | 67 ± 57 | 131 | 21.5 | 13.1 | 155.28 | |
| 35 ± 2.8 | 0.53 ± 0.06 | 55 | 40 (6–276) | 59 ± 12 | 112 ± 27 | 48 ± 37.4 | 20.6 ± 10.6 | 146.34 ± 12.65 | ||
| Indet3 | 30 | 0.26 | 50 | 189 (80–250) | 181 ± 40 | 192 | 4.2 | 11.3 | 138.66 | |
| Entire dataset | mean ± sd | 34.5 ± 17.8 | 0.49 ± 0.10 | 56 ± 19 | 142 ± 82 | 144 ± 72 | 180 ± 71 | 19.5 ± 24.5 | 19.5 ± 8.7 | 300.43 ± 244.14 |
| min–max | 12 - 95 | 0.26–0.61 | 42–116 | 4–494 | 26–271 | 77–296 | 1.8–89.3 | 5.2–38.6 | 27.88–1041.38 |
| Gb (-) | D (µm) | Dh (µm) | VD (mm−2) | VA (%) | Kp (kg m−1 s−1 MPa−1) | ||
|---|---|---|---|---|---|---|---|
| This study | mean ± sd | 0.48 ± 0.11 | 145 ± 54 | 184 ± 56 | 16.4 ± 16.0 | 17.9 ± 7.7 | 278.8 ± 161.3 |
| (14 liana species) | min–max | 0.26–0.61 | 59–227 | 109–280 | 1.8–48.0 | 5.2–33.2 | 32.7–549.8.1 |
| Poorter et al. [39] | mean ± sd | 0.55 ± 0.13 | 206 ± 120 | 178 ± 108 | 26.2 ± 52.9 | 11.1 ± 5.4 | 186.1 ± 251.9 |
| (42 tree species) | min–max | 0.28–0.77 | 40–490 | 34–462 | 0.5–269.5 | 2.7–23.4 | 1.2–1298.9 |
| Hietz et al. [40] | mean ± sd | 0.57 ± 0.14 | 120 ± 52 | 19.5 ± 28.5 | 8.4 ± 4.5 | 55.8 ± 72.7 | |
| (325 tree species) | min–max | 0.17–0.87 | 29–310 | 1.0–274.0 | 1.1–30.5 | 2.7–601 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meunier, F.; Krishna Moorthy, S.M.; De Deurwaerder, H.P.T.; Kreus, R.; Van den Bulcke, J.; Lehnebach, R.; Verbeeck, H. Within-Site Variability of Liana Wood Anatomical Traits: A Case Study in Laussat, French Guiana. Forests 2020, 11, 523. https://doi.org/10.3390/f11050523
Meunier F, Krishna Moorthy SM, De Deurwaerder HPT, Kreus R, Van den Bulcke J, Lehnebach R, Verbeeck H. Within-Site Variability of Liana Wood Anatomical Traits: A Case Study in Laussat, French Guiana. Forests. 2020; 11(5):523. https://doi.org/10.3390/f11050523
Chicago/Turabian StyleMeunier, Félicien, Sruthi M. Krishna Moorthy, Hannes P. T. De Deurwaerder, Robin Kreus, Jan Van den Bulcke, Romain Lehnebach, and Hans Verbeeck. 2020. "Within-Site Variability of Liana Wood Anatomical Traits: A Case Study in Laussat, French Guiana" Forests 11, no. 5: 523. https://doi.org/10.3390/f11050523
APA StyleMeunier, F., Krishna Moorthy, S. M., De Deurwaerder, H. P. T., Kreus, R., Van den Bulcke, J., Lehnebach, R., & Verbeeck, H. (2020). Within-Site Variability of Liana Wood Anatomical Traits: A Case Study in Laussat, French Guiana. Forests, 11(5), 523. https://doi.org/10.3390/f11050523