Seasonal Pattern of Stem Diameter Growth of Qinghai Spruce in the Qilian Mountains, Northwestern China
Abstract
1. Introduction
2. Materials and Methods
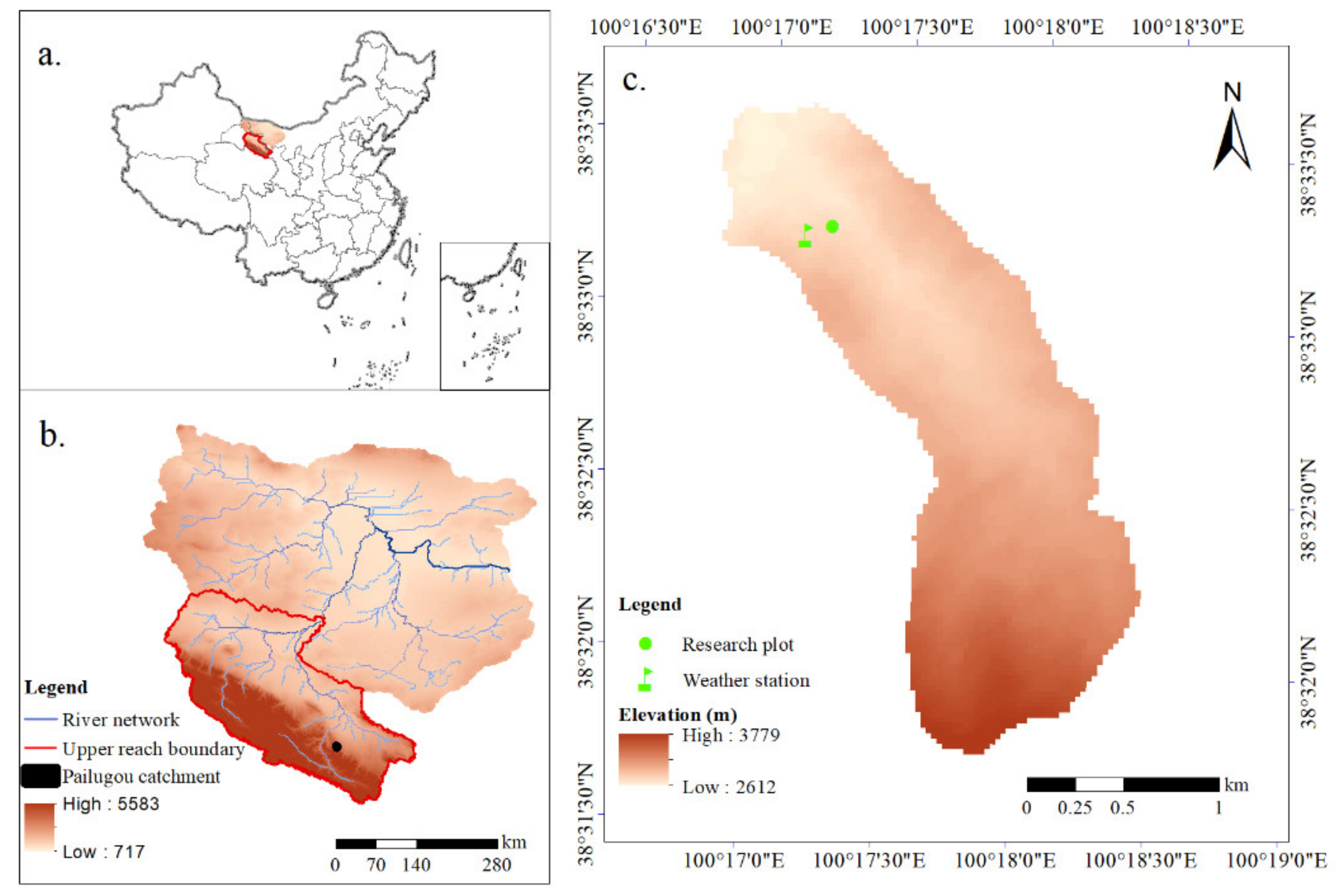
2.1. Study Area
2.2. Research Plot
2.3. Measurements of Stem Diameter Dynamics
2.4. Seasonal Growth Pattern Assessment
2.5. Weather and Soil Moisture Measurements
2.6. Data Analysis
3. Results
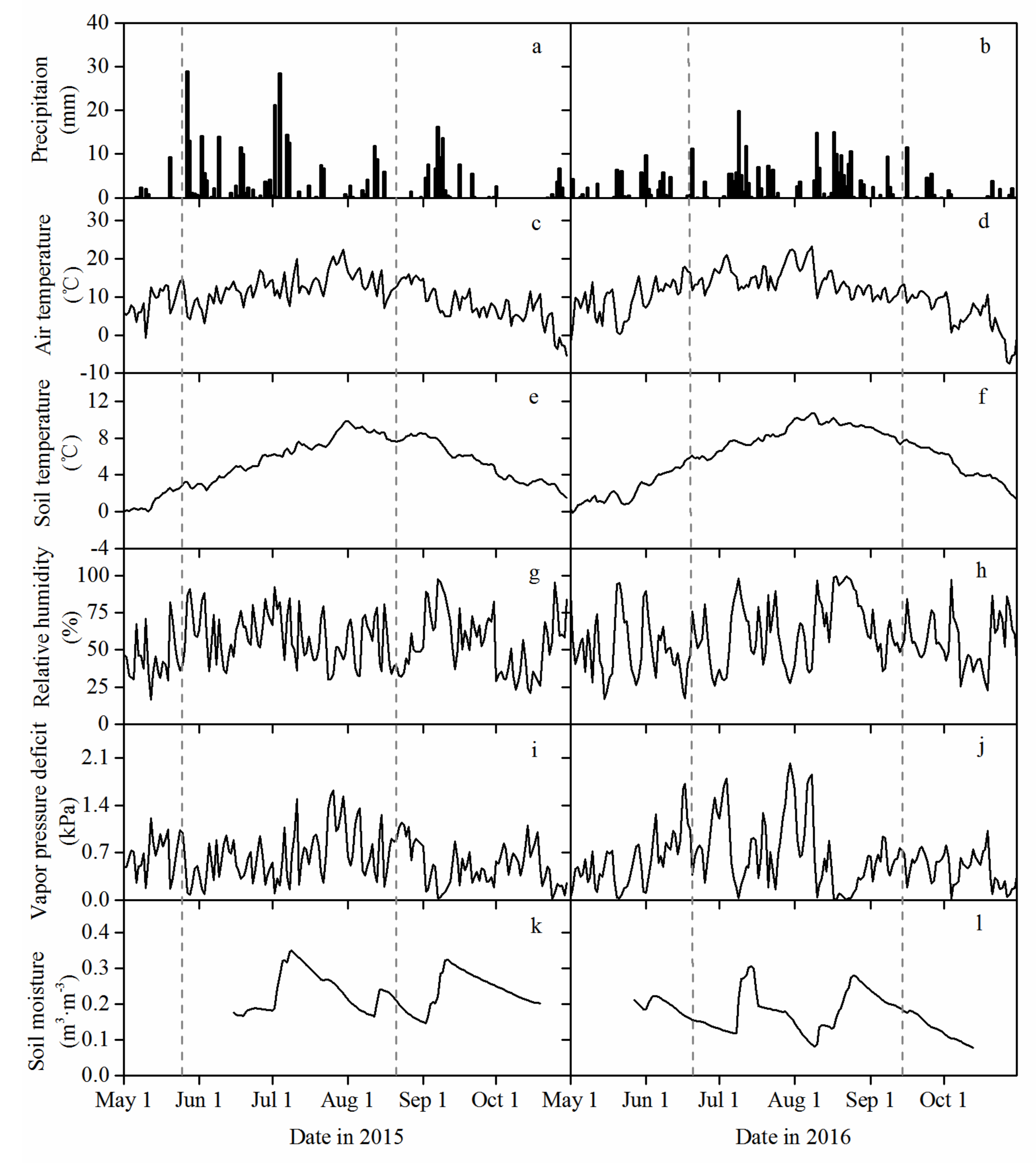
3.1. Environmental Characteristics during the Growing Season
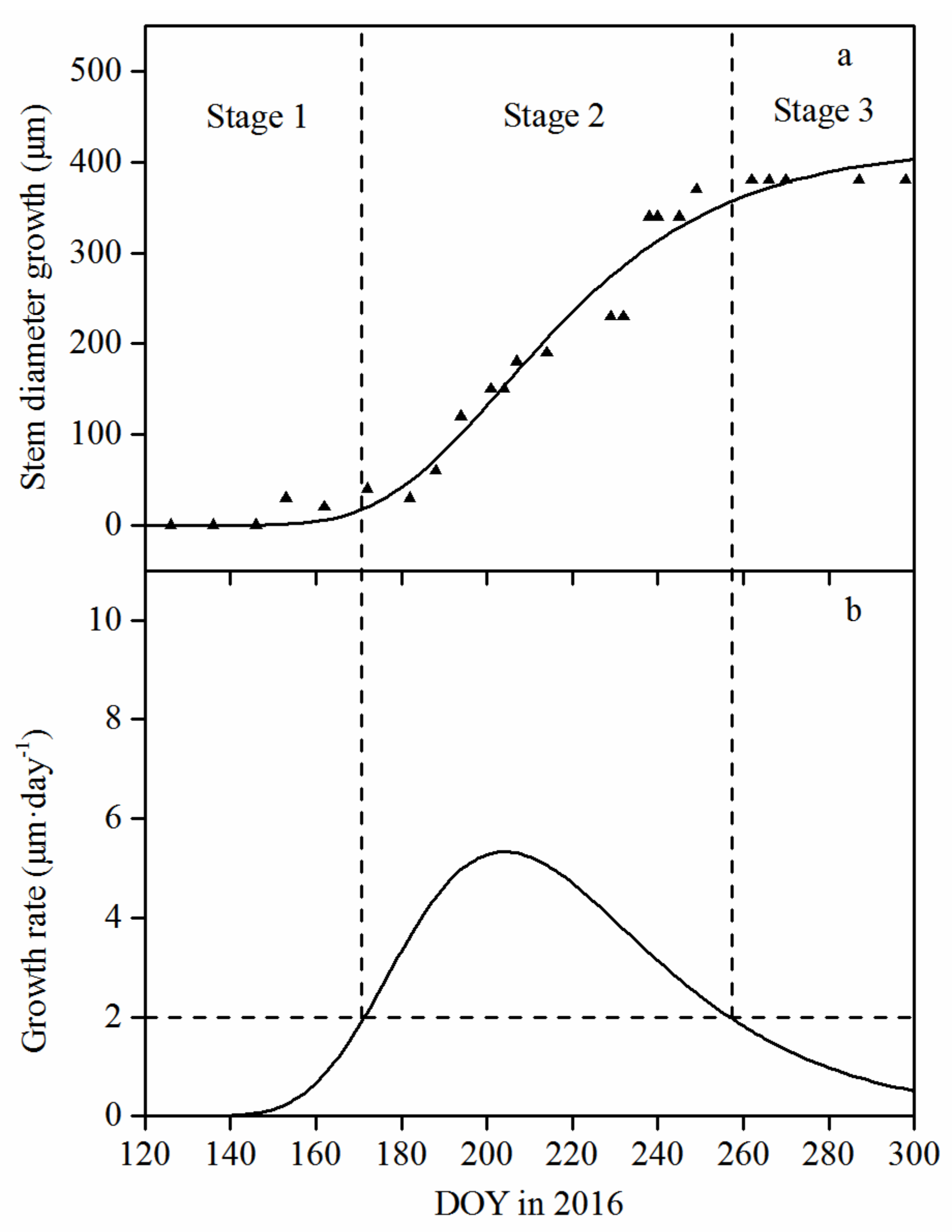
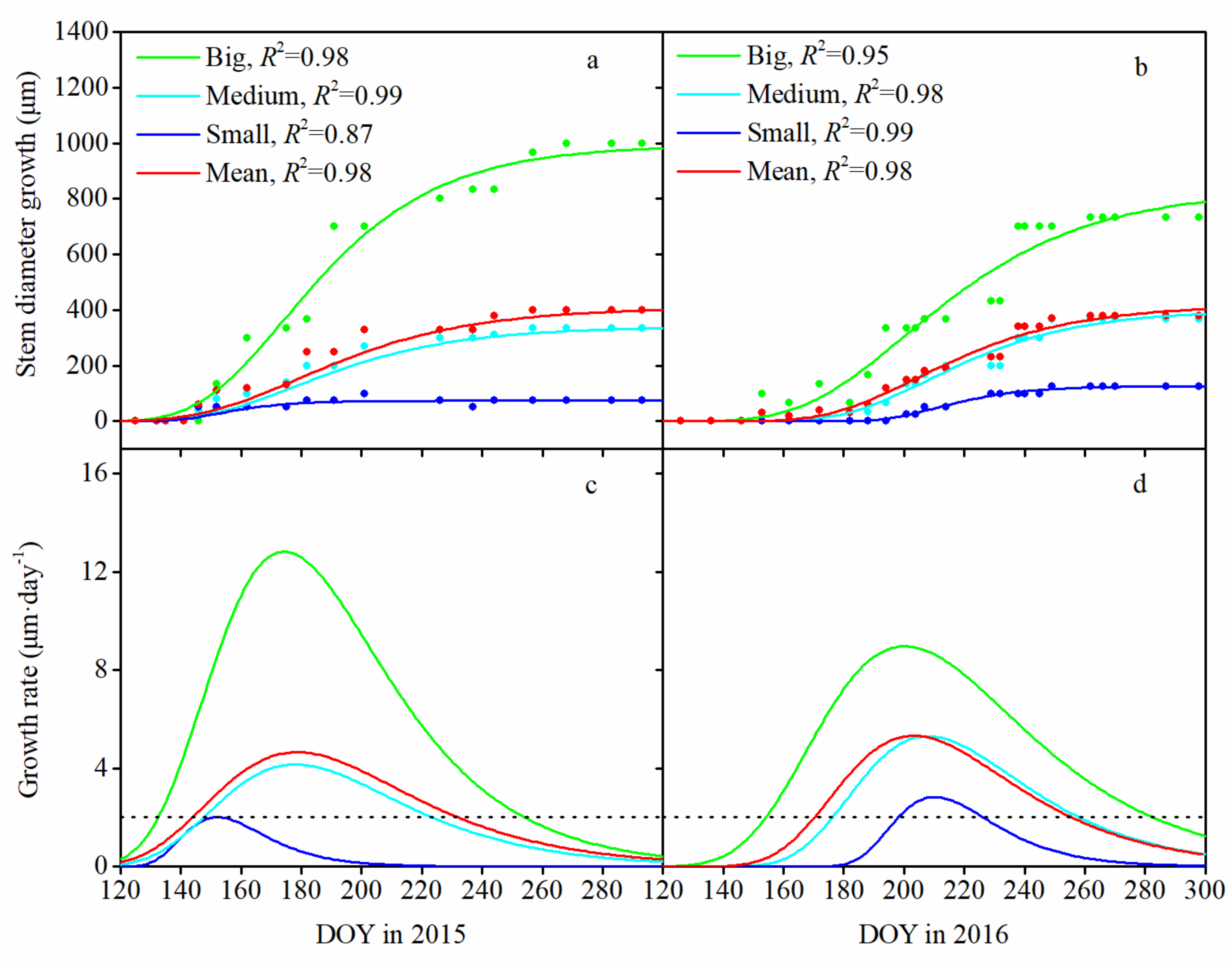
3.2. Seasonal Pattern of Stem Diameter Growth and Its Variation among Tree Sizes
3.3. Monthly Mean Rate of Stem Diameter Growth and Its Variation among Tree Sizes
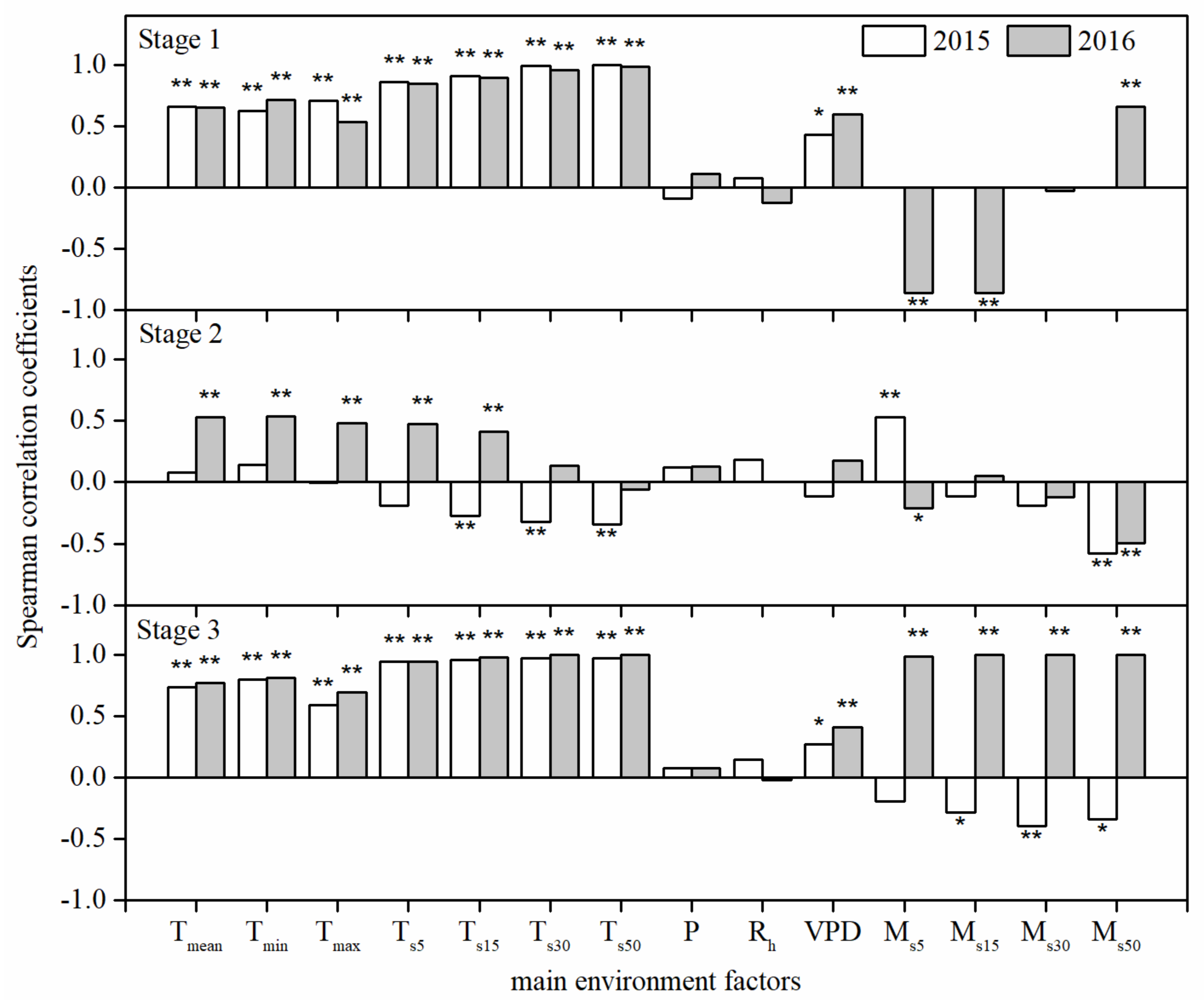
3.4. Effects of Environmental Factors on the Daily Mean Rate of Stem Diameter Growth
4. Discussion
4.1. Impact of Environmental Factors on Seasonal Diameter Growth
4.1.1. Temperature Controls Growth Initiation
4.1.2. Soil Moisture Controls Growth Cessation
4.1.3. Both Soil Moisture and Soil Temperature Control the Maximum Growth Rate
4.2. Seasonal Pattern of Stem Diameter Growth among Tree Sizes
4.3. Impact of Environmental Factors on Daily Mean Rate of Stem Diameter Growth
4.4. Implications for Forest Management
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Edenhofer, O., Pichs-Madruga, R., Sokona, Y., Farahani, E., Kadner, S., Seyboth, K., Adler, A., Baum, I., Brunner, S., Eickemeier, P., et al., Eds.; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Sheffield, J.; Andreadis, K.M.; Wood, E.F.; Lettenmaier, D.P. Global and continental drought in the second half of the twentieth century: Severity-area-duration analysis and temporal variability of large-scale events. J. Clim. 2009, 22, 1962–1981. [Google Scholar] [CrossRef]
- Li, J.B.; Cook, E.R.; D’arrigo, R.; Chen, F.H.; Gou, X.H. Moisture variability across China and Mongolia: 1951–2005. Clim. Dyn. 2009, 32, 1173–1186. [Google Scholar] [CrossRef]
- Piao, S.L.; Ciais, P.; Huang, Y.; Shen, Z.H.; Peng, S.S.; Li, J.S.; Zhou, L.P.; Liu, H.Y.; Ma, Y.C.; Ding, Y.H.; et al. The impacts of climate change on water resources and agriculture in China. Nature 2010, 467, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Andreu, L.; Gutierrez, E.; Macias, M.; Ribas, M.; Bosch, O.; Camarero, J.J. Climate increases regional tree-growth variability in Iberian pine forests. Glob. Chang. Biol. 2007, 13, 804–815. [Google Scholar] [CrossRef]
- Wang, B.; Yu, P.T.; Zhang, L.; Wang, Y.H.; Yu, P.T.; Wang, S.L. Differential trends of Qinghai spruce growth with elevation in Northwestern China during the recent warming hiatus. Forests 2019, 10, 712–726. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, H.; Yu, P.T.; Wang, Y.H.; Pan, S.F.; Wang, B.; Tian, H.Q. Divergent growth responses to warming between stand-grown and open-grown trees in a dryland montane forest in northwestern China. Forests 2019, 10, 1133–1149. [Google Scholar] [CrossRef]
- Deslauriers, A.; Anfodillo, T.; Rossi, S.; Carraro, V. Using simple causal modeling to understand how water and temperature affect daily stem radial variation in trees. Tree Physiol. 2007, 27, 1125–1136. [Google Scholar] [CrossRef]
- Tian, Q.; He, Z.; Xiao, S.; Peng, X.; Ding, A.; Lin, P. Response of stem radial growth of Qinghai spruce (Picea crassifolia) to environmental factors in the Qilian Mountains of China. Dendrochronologia 2017, 44, 76–83. [Google Scholar] [CrossRef]
- Wang, B.; Chen, T.; Xu, G.; Liu, X.; Wang, W.; Wu, G.; Zhang, Y. Alpine timberline population dynamics under climate change: A comparison between Qilian juniper and Qinghai spruce tree species in the middle Qilian Mountains of northeast Tibetan Plateau. Boreas 2016, 45, 411–422. [Google Scholar] [CrossRef]
- Wan, Y.F.; Yu, P.T.; Liu, X.D.; Wang, S.L.; Wang, Y.H.; Xiong, W. Variation in sap flow density among levels of tree dominance in Picea crassifolia in the Qilian Mountains. Acta Ecol. Sin. 2017, 37, 3106–3114. [Google Scholar] [CrossRef][Green Version]
- Zhao, Y.H.; Liu, X.D.; Li, G.; Wang, S.L.; Zhao, W.J.; Ma, J. Phenology of five shrub communities along an elevation gradient in the Qilian Mountains, China. Forests 2018, 9, 58. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, P.T.; Wang, Y.H.; Wang, S.L.; Liu, X.D. Biomass change of middle aged forest of Qinghai spruce along an altitudinal gradient on the north slope of Qilian Mountains. Sci. Silva. Sin. 2015, 51, 4–10. [Google Scholar] [CrossRef]
- Wang, B.; Yu, P.T.; Wang, S.L.; Wang, Y.H.; Liu, X.D.; Jin, M.; Zhang, X.L. Response of soil water storage to slope position of forestland in Qilian Mountains. Sci. Soil Water Conserv. 2016, 14, 101–108. [Google Scholar] [CrossRef]
- Wang, B. Study on eco-hydrological characteristics of typical shady slope Picea crassifolia forest in Qilian Mountains. Chin. Acad. For. 2017. [Google Scholar] [CrossRef]
- Duchesne, L.; Houle, D.; D’Orangeville, L. Influence of climate on seasonal patterns of stem increment of balsam fir in a boreal forest of Québec, Canada. Agric. Meteorol. 2012, 108–114. [Google Scholar] [CrossRef]
- Zeide, B. Analysis of growth equations. For. Sci. 1993, 39, 594–616. [Google Scholar] [CrossRef]
- Aznar, J.-C.; Richer-Laflèche, M.; Bégin, C.; Marion, J. Mining and smelting activities produce anomalies in tree-growth patterns (Murdochville, Québec). Water Air Soil Pollut. 2007, 186, 139–147. [Google Scholar] [CrossRef]
- Deslauriers, A.; Morin, H.; Urbinati, C. Daily weather re-sponse of balsam fir (Abies balsamea (L.) Mill.) stem radius increment from dendrometer analysis in the boreal forests of Qubec (Canada). Trees 2003, 17, 477–484. [Google Scholar] [CrossRef]
- Rossi, S.; Dealauriers, A.; Morin, H. Application of the Gompertz equation for the study of xylem cell development. Dendrochronologia 2003, 21, 33–39. [Google Scholar] [CrossRef]
- Rossi, S.; Deslauriers, A.; Anfodillo, T.; Morin, H.; Saracino, A.; Motta, R.; Borghetti, M. Conifers in cold environments synchronize maximum growth rate of tree-ring formation with day length. New Phytol. 2006, 170, 301–310. [Google Scholar] [CrossRef]
- Murray, F.W. On the computation of saturation vapor pressure. J. Appl. Meteorol. 1967, 6, 203–204. [Google Scholar] [CrossRef]
- Li, X.Q.; Liu, X.D.; Wang, L.; Yu, P.T.; Niu, Y.; Wang, S.L.; Wan, Y.F. Diameter structure and its effect on radial growth of Picea crassifolia forest in the Qilian Mountains. Arid Zone Res. 2017, 34, 1117–1123. [Google Scholar] [CrossRef]
- Deslauriers, A.; Morin, H. Intra-annual tracheid production in balsam fir stems and the effect of meteorological variables. Trees 2005, 19, 402–408. [Google Scholar] [CrossRef]
- Deslauriers, A.; Rossi, S.; Anfodillo, T.; Saracino, A. Cambial phenology, wood formation and temperature thresholds in two contrasting years at high altitude in southern Italy. Tree Physiol. 2008, 28, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Lenz, A.; Hoch, G.; Körner, C. Early season temperature controls cambial activity and total tree ring width at the alpine treeline? Plant. Ecol. Divers. 2013, 6, 365–375. [Google Scholar] [CrossRef]
- Moser, L.; Fonti, P.; Buntgen, U.; Esper, J.; Luterbacher, J.; Franzen, J.; Frank, D. Timing and duration of European larch growing season along altitudinal gradients in the Swiss Alps. Tree Physiol. 2010, 30, 225–233. [Google Scholar] [CrossRef]
- Rossi, S.; Morin, H.; Deslauriers, A.; Plourde, P.Y. Predicting xylem phenology in black spruce under climate warming. Glob. Chang. Biol. 2011, 17, 614–625. [Google Scholar] [CrossRef]
- Rossi, S.; Deslauriers, A.; Anfodillo, T.; Carraro, V. Evidence of threshold temperatures for xylogenesis in conifers at high altitudes. Oecologia 2007, 152, 1–12. [Google Scholar] [CrossRef]
- Rossi, S.; Deslauriers, A.; Griçar, J.; Seo, J.W.; Rathgeber, C.B.K.; Anfodillo, T.; Morin, H.; Levanic, T.; Oven, P.; Jalkanen, R. Critical temperatures for xylogenesis in conifers of cold climates. Glob. Ecol. Biogeogr. 2008, 17, 696–707. [Google Scholar] [CrossRef]
- Tardif, J.; Flannigan, M.; Bergeron, Y. An analysis of the daily radial activity of 7 boreal tree species: Northwestern Quebec. Env. Monit. Assess. 2001, 67, 141–160. [Google Scholar] [CrossRef]
- Gruber, A.; Zimmermann, J.; Wieser, G.; Oberhuber, W. Effects of climate variables on intra-annual stem radial increment in Pinus cembra (L.) along the alpine treeline ecotone. Ann. Sci. 2009, 66, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Körner, C. Alpine plant life: Functional plant ecology of high mountain ecosystems. SpringerBerl. Heidelb. 2003. [Google Scholar] [CrossRef]
- Ren, L.; Wang, S.L.; Yu, P.T.; Wang, Y.H.; Zhang, X.P.; Wang, B.; Liu, X.D.; Jin, M. Seasonal change and numerical simulation of the frozen soil under two types of vegetation in Qilian Mountains. For. Res. 2016, 29, 596–602. [Google Scholar] [CrossRef]
- Levanič, T.; Gričar, J.; Gagen, M.; Jalkanen, R.; Loader, N.J.; McCarroll, D.; Oven, P.; Robertson, I. The climate sensitivity of Norway spruce (Picea abies (L.) Karst) in the southeastern European Alps. Trees 2008, 23, 169–180. [Google Scholar] [CrossRef]
- Oberhuber, W.; Gruber, A.; Kofler, W.; Swidrak, I. Radial stem growth in response to microclimate and soil moisture in a drought-prone mixed coniferous forest at an inner Alpine site. Eur. J. Res. 2014, 133, 467–479. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, B.; Dong, M.; Huang, Y.; Wang, M.; Wang, B. Response of daily stem radial growth of Platycladus orientalis to environmental factors in a semi-arid area of north China. Trees 2015, 29, 87–96. [Google Scholar] [CrossRef]
- Ford, K.R.; Breckheimer, I.K.; Franklin, J.F.; Freund, J.A.; Kroiss, S.J.; Larson, A.J.; Theobald, E.J.; HilleRisLambers, J. Competition alters tree growth responses to climate at individual and stand scales. Can. J. Res. 2017, 47, 53–62. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.J.; Wang, Y.Q.; Zhang, S.H.; Zhao, X.L. Effects of social position and competition on tree transpiration of a natural mixed forest in Chongqing, China. Trees 2019, 33. [Google Scholar] [CrossRef]
- Liang, E.; Dawadi, B.; Pederson, N.; Eckstein, D. Is the growth of birch at the upper timberline in the Himalayas limited by moisture or by temperature? Ecology 2014, 95, 2453–2465. [Google Scholar] [CrossRef]
- Dong, M.Y.; Jiang, Y.; Yang, H.C.; Wang, M.C.; Zhang, W.T.; Guo, Y.Y. Dynamics of stem radial growth of Picea meyeri during the growing season at the treeline of Luya Mountain, China. Chin. J. Plant. Ecol. 2012, 236, 956–964. [Google Scholar] [CrossRef]
- Ren, P.; Rossi, S.; Camarero, J.J.; Ellison, A.M.; Liang, E.; Peñuelas, J. Critical temperature and precipitation thresholds for the onset of xylogenesis of Juniperus przewalskii in a semi-arid area of the north-eastern Tibetan Plateau. Ann. Bot. 2017. [Google Scholar] [CrossRef]
- Way, D.A.; Sage, R.F. Elevated growth temperatures reduce the carbon gain of black spruce (Picea mariana (Mill.) BSP). Glob. Chang. Biol. 2008, 14, 624–636. [Google Scholar] [CrossRef]
- Zweifel, R.; Zimmermann, L.; Zeugin, F.; Newbery, D.M. Intra-annual radial growth and water relations of trees: Implications towards a growth mechanism. J. Exp. Bot. 2006, 57, 1445–1459. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Z. Cambial phenology and intra-annual radial growth dynamics of conifers over the Qilian Mountains. Lanzhou Univ. 2018. [Google Scholar] [CrossRef]
- Park, C.E.; Jeong, S.J.; Joshi, M.; Osborn, T.J.; Ho, C.H.; Piao, S.; Chen, D.; Liu, J.; Yang, H.; Park, H.; et al. Keeping global warming within 1.5 °C constrains emergence of aridification. Nat. Clim. Chang. 2018, 8, 70–74. [Google Scholar] [CrossRef]
| Tree Size Classes | Number of Trees | Average Diameter at Breast Height (DBH) (cm) | Average Tree Height (m) | Average Canopy Width (m) | Average Canopy Thickness (m) | Percent of Total Trees (%) |
|---|---|---|---|---|---|---|
| Big | 6 | 27.2 ± 4.9 | 12.9 ± 2.2 | 5.47 ± 0.66 | 9.62 ± 1.70 | 7.14 |
| Medium | 36 | 15.0 ± 1.7 | 11.2 ± 1.1 | 3.30 ± 0.51 | 6.03 ± 2.23 | 42.86 |
| Small | 22 | 9.8 ± 2.1 | 8.1 ± 1.8 | 2.82 ± 0.43 | 4.65 ± 1.55 | 26.19 |
| Seedling | 20 | 3.6 ± 0.7 | 2.5 ± 0.5 | 1.97 ± 0.43 | 1.26 ± 0.52 | 23.81 |
| Tree Size Classes | Tree No. | DBH (cm) | Height (m) | Canopy Width (m) | Canopy Thickness (m) |
|---|---|---|---|---|---|
| Big | 1 | 31.94 | 16.3 | 6.0 | 12.1 |
| 2 | 31.16 | 14.2 | 4.9 | 11.0 | |
| 3 | 28.85 | 13.3 | 6.2 | 8.5 | |
| Medium | 4 | 16.63 | 11.6 | 4.1 | 7.0 |
| 5 | 16.24 | 13.1 | 3.2 | 8.1 | |
| 6 | 15.02 | 10.8 | 3.5 | 4.4 | |
| Small | 7 | 11.68 | 10.3 | 3.1 | 5.2 |
| 8 | 10.87 | 8.2 | 3.5 | 5.0 | |
| 9 | 7.08 | 6.4 | 1.9 | 5.3 | |
| 10 | 4.39 | 2.8 | 2.2 | 1.1 |
| Year | Tree Size Classes | Cumulative Stem Growth (μm) | Timing of Growth Initiation (DOY) | Timing of Growth Cessation (DOY) | Growing Season Duration (days) | Maximum Growth Rate (μm·day−1) | Day of Maximum Growth (DOY) |
|---|---|---|---|---|---|---|---|
| 2015 | Mean | 400.0 | 145 | 233 | 89 | 4.658 | 179 |
| Big | 1000.0 | 134 | 254 | 121 | 12.824 | 175 | |
| Medium | 333.3 | 148 | 224 | 77 | 4.160 | 178 | |
| Small | 75.0 | 149 | 158 | 10 | 2.008 | 153 | |
| 2016 | Mean | 380.0 | 172 | 258 | 87 | 5.327 | 204 |
| Big | 733.3 | 155 | 283 | 129 | 8.967 | 201 | |
| Medium | 366.7 | 177 | 258 | 82 | 5.305 | 208 | |
| Small | 125.0 | 199 | 226 | 28 | 2.828 | 212 |
| Year | Tree Size Classes | May | June | July | August | September |
|---|---|---|---|---|---|---|
| 2015 | Mean | 1.94 ± 2.26 | 2.33 ± 4.98 | 5.16 ± 7.75 | 1.29 ± 4.29 | 2.33 ± 3.53 |
| Big | 4.30 ± 1.86 | 7.78 ± 2.36 | 12.37 ± 3.42 | 2.69 ± 1.14 | 5.56 ± 2.36 | |
| Medium | 1.29 ± 1.11 | 3.33 ± 1.24 | 4.19 ± 1.59 | 1.00 ± 0.69 | 1.11 ± 0.92 | |
| Small | 1.61 ± 0.41 | 1.08 ± 0.54 | 1.21 ± 1.14 | −1.21 ± 0.93 | 0.83 ± 0.00 | |
| 2016 | Mean | 0.97 ± 1.56 | 0.67 ± 1.41 | 4.52 ± 5.52 | 4.84 ± 5.54 | 1.33 ± 2.33 |
| Big | 3.23 ± 0.00 | 1.11 ± 0.23 | 7.53 ± 2.28 | 10.75 ± 2.28 | 1.11 ± 0.92 | |
| Medium | 0.00 ± 0.00 | 1.11 ± 0.32 | 5.38 ± 2.28 | 3.23 ± 2.28 | 2.22 ± 1.85 | |
| Small | 0.00 ± 0.00 | 0.00 ± 0.00 | 1.61 ± 0.00 | 1.61 ± 0.86 | 0.83 ± 0.67 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, Y.; Yu, P.; Li, X.; Wang, Y.; Wang, B.; Yu, Y.; Zhang, L.; Liu, X.; Wang, S. Seasonal Pattern of Stem Diameter Growth of Qinghai Spruce in the Qilian Mountains, Northwestern China. Forests 2020, 11, 494. https://doi.org/10.3390/f11050494
Wan Y, Yu P, Li X, Wang Y, Wang B, Yu Y, Zhang L, Liu X, Wang S. Seasonal Pattern of Stem Diameter Growth of Qinghai Spruce in the Qilian Mountains, Northwestern China. Forests. 2020; 11(5):494. https://doi.org/10.3390/f11050494
Chicago/Turabian StyleWan, Yanfang, Pengtao Yu, Xiaoqing Li, Yanhui Wang, Bin Wang, Yipeng Yu, Lei Zhang, Xiande Liu, and Shunli Wang. 2020. "Seasonal Pattern of Stem Diameter Growth of Qinghai Spruce in the Qilian Mountains, Northwestern China" Forests 11, no. 5: 494. https://doi.org/10.3390/f11050494
APA StyleWan, Y., Yu, P., Li, X., Wang, Y., Wang, B., Yu, Y., Zhang, L., Liu, X., & Wang, S. (2020). Seasonal Pattern of Stem Diameter Growth of Qinghai Spruce in the Qilian Mountains, Northwestern China. Forests, 11(5), 494. https://doi.org/10.3390/f11050494






