Biogeochemical Processes of C and N in the Soil of Mangrove Forest Ecosystems
Abstract
1. Introduction
2. C Dynamics in Mangrove Forests
2.1. C Storage in Mangrove Forests
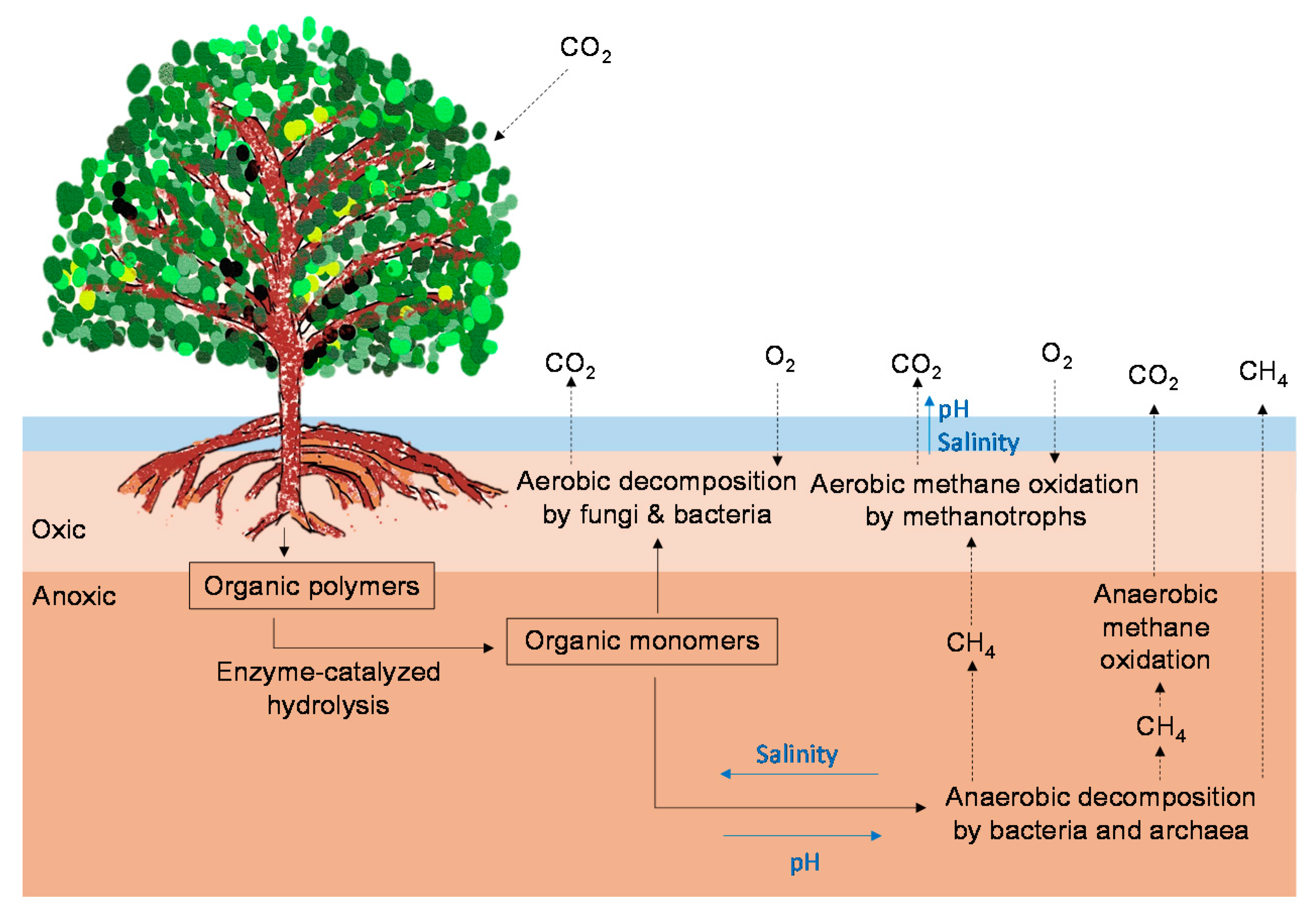
2.2. CO2 and CH4 Emissions in Mangrove Soils
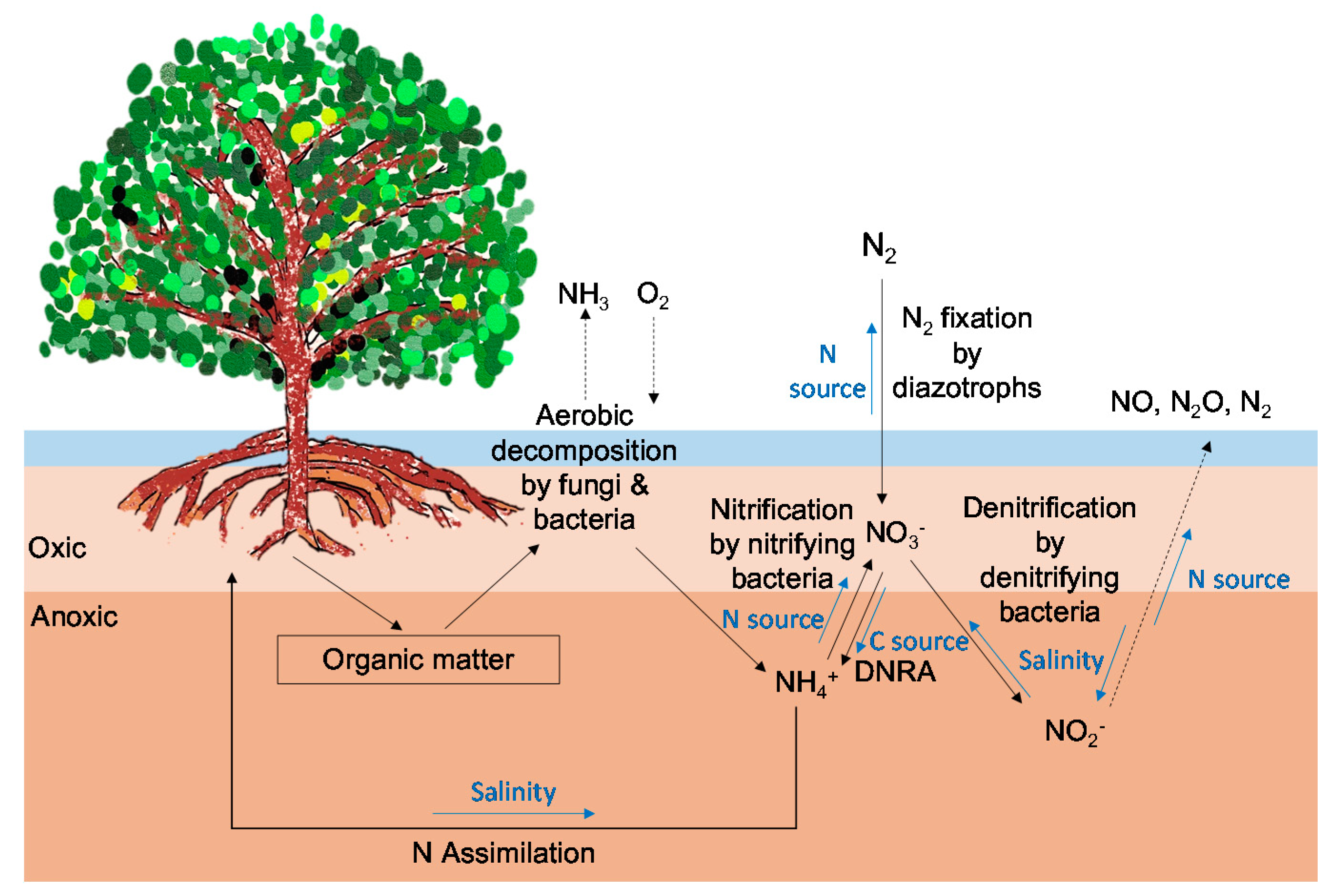
3. N Dynamics in Mangrove Forests
3.1. N Assimilation Rates of Mangrove Plants
3.2. N2 Fixation in Mangrove Soils
3.3. Dissimilatory Nitrate Reduction to Ammonium and Denitrification in Mangrove Soils
4. Mangrove C and N Dynamics under Climate Change
- ◆ Evaluating the impact of warming temperature on the compositions and distributions of C and N in mangrove forest litter and soil.
- ◆ Determining the diel and diurnal cycles of C and N fluxes in tide-influenced coastal ecosystems.
- ◆ Discovering the compositions and activities of C- and N-related microorganisms associated with increases in nutrient load, salinity, and temperature in mangrove forest soils.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IPCC. AR4 Climate Change 2007: Synthesis Report; The Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2007. [Google Scholar]
- IPCC. Climate Change 2013: The Physical Science Basis; The Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2013. [Google Scholar]
- Bradford, M.A. A leaky sink. Nat. Clim. Chang. 2017, 7, 475–476. [Google Scholar] [CrossRef]
- Whiting, G.J.; Chanton, J.P. Greenhouse carbon balance of wetlands: Methane emission versus carbon sequestration. Tellus B 2001, 53, 521–528. [Google Scholar] [CrossRef]
- Gujer, W.; Zehnder, A.J.B. Conversion processes in anaerobic digestion. Water Sci. Technol. 1983, 15, 127–167. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Bernhardt, W.S. Biogeochemistry: An Analysis of Global Change; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Smith, P.H.; Mah, R.A. Kinetics of acetate metabolism during sludge digestion. Appl. Microbial. 1966, 14, 368–371. [Google Scholar] [CrossRef]
- Paul, E.A.; Clark, F.E. Soil Biology and Biochemistry; Academic Press: San Diego, CA, USA, 1996. [Google Scholar]
- Stein, L.Y. Surveying N2O-producting pathways in bacteria. Method. Enzymolog. 2011, 486, 131–152. [Google Scholar]
- Brix, H. Do macrophytes play a role in constructed treatment wetlands? Water Sci. Technol. 1997, 35, 11–17. [Google Scholar] [CrossRef]
- Etheridge, J.R.; Birgand, F.; Burchell, M.R., II. Quantifying nutrient and suspended solids fluxes in a constructed tidal marsh following rainfall: The value of capturing the rapid changes in flow and concentrations. Ecol. Eng. 2015, 78, 41–52. [Google Scholar] [CrossRef]
- Mumby, P.J.; Edwards, A.J.; Ernesto Arias-González, J.; Lindeman, K.C.; Blackwell, P.G.; Gall, A.; Gorczynska, M.I.; Harborne, A.R.; Pescod, C.L.; Renken, H.; et al. Mangroves enhance the biomass of coral reef fish communities in the Caribbean. Nature 2004, 427, 533. [Google Scholar] [CrossRef]
- Shiau, Y.J.; Dham, V.; Tian, G.; Chiu, C.Y. Factors influencing removal of sewage nitrogen through denitrification in mangrove soils. Wetlands 2016, 36, 621–630. [Google Scholar] [CrossRef]
- He, J.-Z.; Zhang, L. Advances in ammonia-oxidizing microorganisms and global nitrogen cycle. Shengtai Xuebao/Acta Ecol. Sin. 2009, 29, 406–415. [Google Scholar]
- Liu, N.; Wang, K.; Xie, Y.; Yang, G.; Duan, Y. Characteristics of the soil environment of Dongting Lake wetlands and its response to the converting farmland to lake project. Shengtai Xuebao/Acta Ecol. Sin. 2011, 31, 3758–3766. [Google Scholar]
- Steger, D.; Wentrup, C.; Braunegger, C.; Deevong, P.; Hofer, M.; Richter, A.; Baranyi, C.; Pester, M.; Wagner, M.; Loy, A. Microorganisms with novel dissimilatory (Bi) sulfite reductase genes are widespread and part of the core microbiota in low-sulfate peatlands. Appl. Environ. Microbiol. 2010, 77, 1231–1242. [Google Scholar] [CrossRef]
- Emmer, I.; von Unger, M.; Needelman, B.; Crooks, S.; Emmett-Mattox, S. A Manual for Using the VCS Methodology for Tidal Wetland and Seagrass Restoration VM0033; Restore America’s Estuaries: Arlington, VA, USA, 2015. [Google Scholar]
- Hamilton, S.E.; Casey, D. Creation of a high spatio-temporal resolution global database of continuous mangrove forest cover for the 21st century (CGMFC-21). Glob. Ecol. Biogeogr. 2016, 25, 729–738. [Google Scholar] [CrossRef]
- Pidgeon, E. Carbon Sequestration by Coastal Marine Habitats: Important Missing Sinks; IUCN: Gland, Switzerland, 2009; pp. 47–51. [Google Scholar]
- Giri, C.; Ochieng, E.; Tieszen, L.L.; Zhu, Z.; Singh, A.; Loveland, T.; Masek, J.; Duke, N. Status and distribution of mangrove forests of the world using earth observation satellite data. Glob. Ecol. Biogeogr. 2011, 20, 154–159. [Google Scholar] [CrossRef]
- Pandisamy, R.; Saxena, A.; Jayaraj, R.; Mohan, P.M.; Ravichandran, K.; Saravanan, S.; Vijayaraghavan, A. A review of the mangrove floristics of India. Taiwania 2016, 61, 224–242. [Google Scholar] [CrossRef]
- Duarte, C.M.; Losada, I.J.; Hendriks, I.E.; Mazarrasa, I.; Marbà, N. The role of coastal plant communities for climate change mitigation and adaptation. Nat. Clim. Chang. 2013, 3, 961–968. [Google Scholar] [CrossRef]
- Lovelock, C.E.; Adame, M.F.; Bennion, V.; Hayes, M.; O’Mara, J.; Reef, R.; Santini, N.S. Contemporary rates of carbon sequestration through vertical accretion of sediments in mangrove forests and saltmarshes of south east queensland, australia. Estuaries Coasts 2014, 37, 763–771. [Google Scholar] [CrossRef]
- Vo, Q.T.; Kuenzer, C.; Vo, Q.M.; Moder, F.; Oppelt, N. Review of valuation methods for mangrove ecosystem services. Ecol. Indic. 2012, 23, 431–446. [Google Scholar] [CrossRef]
- Costanza, R.; de Groot, R.; Sutton, P.; van der Ploeg, S.; Anderson, S.J.; Kubiszewski, I.; Farber, S.; Turner, R.K. Changes in the global value of ecosystem services. Glob. Environ. Chang. 2014, 26, 152–158. [Google Scholar] [CrossRef]
- Thomas, N.; Lucas, R.; Bunting, P.; Hardy, A.; Rosenqvist, A.; Simard, M. Distribution and drivers of global mangrove forest change, 1996–2010. PLoS ONE 2017, 12, e0179302. [Google Scholar] [CrossRef]
- Chiu, C.Y.; Lee, S.C.; Juang, H.T.; Hur, M.T.; Hwang, Y.H. Nitrogen nutritional status and fate of applied N in mangrove soils. Bot. Bull. Acad. Sin. 1996, 37, 191–196. [Google Scholar]
- Jing, H.; Xia, X.; Liu, H.; Zhou, Z.; Wu, C.; Nagarajan, S. Anthropogenic impact on diazotrophic diversity in the mangrove rhizosphere revealed by nifH pyrosequencing. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef]
- Mcleod, E.; Chmura, G.L.; Bouillon, S.; Salm, R.; Björk, M.; Duarte, C.M.; Lovelock, C.E.; Schlesinger, W.H.; Silliman, B.R. A blueprint for blue carbon: Toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 2011, 9, 552–560. [Google Scholar] [CrossRef]
- Alongi, M.D. Impact of global change on nutrient dynamics in mangrove forests. Forests 2018, 9, 596. [Google Scholar] [CrossRef]
- Shiau, Y.-J.; Burchell, M.R.; Krauss, K.W.; Broome, S.W.; Birgand, F. Carbon storage potential in a recently created brackish marsh in eastern North Carolina, USA. Ecol. Eng. 2019, 127, 579–588. [Google Scholar] [CrossRef]
- Chmura, G.L.; Anisfeld, S.C.; Cahoon, D.R.; Lynch, J.C. Global carbon sequestration in tidal, saline wetland soils. Glob. Biogeochem. Cycles 2003, 17. [Google Scholar] [CrossRef]
- Kennedy, H.; Bjork, M. Seagrass Meadows; IUCN: Gland, Switzerland, 2009; pp. 23–30. [Google Scholar]
- Turner, R.E. Geographic variations in salt marsh macrophyte production: A review. Contrib. Mar. Sci. 1976, 20, 47–68. [Google Scholar]
- Simpson, L.T.; Osborne, T.Z.; Duckett, L.J.; Feller, I.C. Carbon storages along a climate induced coastal wetland gradient. Wetlands 2017, 37, 1023–1035. [Google Scholar] [CrossRef]
- Li, S.-B.; Chen, P.-H.; Huang, J.-S.; Hsueh, M.-L.; Hsieh, L.-Y.; Lee, C.-L.; Lin, H.-J. Factors regulating carbon sinks in mangrove ecosystems. Glob. Chang. Biol. 2018, 24, 4195–4210. [Google Scholar] [CrossRef]
- Murdiyarso, D.; Purbopuspito, J.; Kauffman, J.B.; Warren, M.W.; Sasmito, S.D.; Donato, D.C.; Manuri, S.; Krisnawati, H.; Taberima, S.; Kurnianto, S. The potential of Indonesian mangrove forests for global climate change mitigation. Nat. Clim. Chang. 2015, 5, 1089–1092. [Google Scholar] [CrossRef]
- Ewers Lewis, C.J.; Carnell, P.E.; Sanderman, J.; Baldock, J.A.; Macreadie, P.I. Variability and vulnerability of coastal ‘blue carbon’ stocks: A Case Study from Southeast Australia. Ecosystems 2018, 21, 263–279. [Google Scholar] [CrossRef]
- Kauffman, J.B.; Bernardino, A.F.; Ferreira, T.O.; Giovannoni, L.R.; Gomes, L.E.D.O.; Romero, D.J.; Jimenez, L.C.Z.; Ruiz, F. Carbon stocks of mangroves and salt marshes of the Amazon region, Brazil. Biol. Lett. 2018, 14, 20180208. [Google Scholar] [CrossRef] [PubMed]
- Nehren, U.; Wicaksono, P. Mapping soil carbon stocks in an oceanic mangrove ecosystem in Karimunjawa Islands, Indonesia. Estuar. Coast. Shelf Sci. 2018, 214, 185–193. [Google Scholar] [CrossRef]
- Nóbrega, G.N.; Ferreira, T.O.; Siqueira Neto, M.; Queiroz, H.M.; Artur, A.G.; Mendonça, E.D.S.; Silva, E.D.O.; Otero, X.L. Edaphic factors controlling summer (rainy season) greenhouse gas emissions (CO2 and CH4) from semiarid mangrove soils (NE-Brazil). Sci. Total Environ. 2016, 542, 685–693. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kögel-Knabner, I.; Lehmann, J.; Manning, D.A.C.; et al. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef]
- Kauffman, J.B.; Hughes, R.F.; Heider, C. Carbon pool and biomass dynamics associated with deforestation, land use, and agricultural abandonment in the neotropics. Ecol. Appl. 2009, 19, 1211–1222. [Google Scholar] [CrossRef]
- Donato, D.C.; Kauffman, J.B.; Murdiyarso, D.; Kurnianto, S.; Stidham, M.; Kanninen, M. Mangroves among the most carbon-rich forests in the tropics. Nat. Geosci. 2011, 4, 293–297. [Google Scholar] [CrossRef]
- Thompson, B.S.; Clubbe, C.P.; Primavera, J.H.; Curnick, D.; Koldewey, H.J. Locally assessing the economic viability of blue carbon: A case study from Panay Island, the Philippines. Ecosyst. Serv. 2014, 8, 128–140. [Google Scholar] [CrossRef]
- Kauffman, J.B.; Bhomia, R.K. Ecosystem carbon stocks of mangroves across broad environmental gradients in West-Central Africa: Global and regional comparisons. PLoS ONE 2017, 12, e0187749. [Google Scholar] [CrossRef]
- Elsey-Quirk, T.; Seliskar, D.M.; Sommerfield, C.K.; Gallagher, J.L. Salt marsh carbon pool distribution in a mid-atlantic lagoon, USA: Sea level rise implications. Wetlands 2011, 31, 87–99. [Google Scholar] [CrossRef]
- Radabaugh, K.R.; Moyer, R.P.; Chappel, A.R.; Powell, C.E.; Bociu, I.; Clark, B.C.; Smoak, J.M. Coastal blue carbon assessment of mangroves, Salt Marshes, and Salt Barrens in Tampa Bay, Florida, USA. Estuaries Coasts 2018, 41, 1496–1510. [Google Scholar] [CrossRef]
- Doughty, C.L.; Langley, J.A.; Walker, W.S.; Feller, I.C.; Schaub, R.; Chapman, S.K. Mangrove range expansion rapidly increases coastal wetland carbon storage. Estuaries Coasts 2016, 39, 385–396. [Google Scholar] [CrossRef]
- Comeaux, R.S.; Allison, M.A.; Bianchi, T.S. Mangrove expansion in the Gulf of Mexico with climate change: Implications for wetland health and resistance to rising sea levels. Estuar. Coast. Shelf Sci. 2012, 96, 81–95. [Google Scholar] [CrossRef]
- Pinhassi, J.; Sala, M.M.; Havskum, H.; Peters, F.; Guadayol, Ò.; Malits, A.; Marrasé, C. Changes in bacterioplankton composition under different phytoplankton regimens. Appl. Environ. Microbiol. 2004, 70, 6753. [Google Scholar] [CrossRef]
- Gomes, N.C.M.; Cleary, D.F.R.; Pinto, F.N.; Egas, C.; Almeida, A.; Cunha, A.; Mendonca-Hagler, L.C.S.; Smalla, K. Taking root: Enduring effect of rhizosphere bacterial colonization in mangroves. PLoS ONE 2010, 5. [Google Scholar] [CrossRef]
- Lyimo, T.J.; Pol, A.; Jetten, M.S.M.; Op den Camp, H.J.M. Diversity of methanogenic archaea in a mangrove sediment and isolation of a new Methanococcoides strain. FEMS Microbiol. Lett. 2009, 291, 247–253. [Google Scholar] [CrossRef]
- Weston, N.B.; Dixon, R.E.; Joye, S.B. Ramifications of increased salinity in tidal freshwater sediments: Geochemistry and microbial pathways of organic matter mineralization. J. Geophys. Res. Biogeosci. 2006, 111. [Google Scholar] [CrossRef]
- Chambers, L.; Reddy, K.; Osborne, T. Short-term response of carbon cycling to salinity pulses in a freshwater wetland. Soil Sci. Soc. Am. J. 2011, 75, 2000–2007. [Google Scholar] [CrossRef]
- Shiau, Y.J.; Burchell, M.R.; Krauss, K.W.; Birgand, F.; Broome, S.W. Greenhouse gas emissions from a created brackish marsh in eastern North Carolina. Wetlands 2016, 36, 1009–1024. [Google Scholar] [CrossRef]
- Lu, W.; Xiao, J.; Liu, F.; Zhang, Y.; Liu, C.A.; Lin, G. Contrasting ecosystem CO2 fluxes of inland and coastal wetlands: A meta-analysis of eddy covariance data. Glob. Chang. Biol. 2017, 23, 1180–1198. [Google Scholar] [CrossRef]
- Castillo, J.A.A.; Apan, A.A.; Maraseni, T.N.; Salmo, S.G. Soil greenhouse gas fluxes in tropical mangrove forests and in land uses on deforested mangrove lands. CATENA 2017, 159, 60–69. [Google Scholar] [CrossRef]
- Hien, H.T.; Marchand, C.; Aimé, J.; Cuc, N.T.K. Seasonal variability of CO2 emissions from sediments in planted mangroves (Northern Viet Nam). Estuar. Coast. Shelf Sci. 2018, 213, 28–39. [Google Scholar] [CrossRef]
- Jacotot, A.; Marchand, C.; Allenbach, M. Tidal variability of CO2 and CH4 emissions from the water column within a Rhizophora mangrove forest (New Caledonia). Sci. Total Environ. 2018, 631–632, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Rosentreter, J.A.; Maher, D.T.; Erler, D.V.; Murray, R.; Eyre, B.D. Seasonal and temporal CO2 dynamics in three tropical mangrove creeks—A revision of global mangrove CO2 emissions. Geochim. Cosmochim. Acta 2018, 222, 729–745. [Google Scholar] [CrossRef]
- Krauss, K.W.; Whitbeck, J.L. Soil greenhouse gas fluxes during wetland forest retreat along the Lower Savannah River, Georgia (USA). Wetlands 2012, 32, 73–81. [Google Scholar] [CrossRef]
- Vepraskas, M.J.; Craft, C.B. Wetland Soils: Genesis, Hydrology, Landscapes, and Classification, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Chang, T.-C.; Yang, S.-S. Methane emission from wetlands in Taiwan. Atmos. Environ. 2003, 37, 4551–4558. [Google Scholar] [CrossRef]
- Alongi, D.M.; Pfitzner, J.; Trott, L.A.; Tirendi, F.; Dixon, P.; Klumpp, D.W. Rapid sediment accumulation and microbial mineralization in forests of the mangrove Kandelia candel in the Jiulongjiang Estuary, China. Estuar. Coast. Shelf Sci. 2005, 63, 605–618. [Google Scholar] [CrossRef]
- Krithika, K.; Purvaja, R.; Ramesh, R. Fluxes of methane and nitrous oxide from an Indian mangrove. Curr. Sci. 2008, 94, 218–224. [Google Scholar]
- Lovelock, C.E. Soil respiration and belowground carbon allocation in mangrove forests. Ecosystems 2008, 11, 342–354. [Google Scholar] [CrossRef]
- Chen, G.C.; Tam, N.F.Y.; Ye, Y. Spatial and seasonal variations of atmospheric N2O and CO2 fluxes from a subtropical mangrove swamp and their relationships with soil characteristics. Soil Biol. Biochem. 2012, 48, 175–181. [Google Scholar] [CrossRef]
- Livesley, S.J.; Andrusiak, S.M. Temperate mangrove and salt marsh sediments are a small methane and nitrous oxide source but important carbon store. Estuar. Coast. Shelf Sci. 2012, 97, 19–27. [Google Scholar] [CrossRef]
- Jin, L.; Lu, C.-Y.; Ye, Y.; Ye, G.-F. Soil Respiration in a subtropical mangrove wetland in the Jiulong River Estuary, China. Pedosphere 2013, 23, 678–685. [Google Scholar] [CrossRef]
- Leopold, A.; Marchand, C.; Deborde, J.; Chaduteau, C.; Allenbach, M. Influence of mangrove zonation on CO2 fluxes at the sediment-air interface (New Caledonia). Geoderma 2013, 202–203, 62–70. [Google Scholar] [CrossRef]
- Konnerup, D.; Betancourt-Portela, J.M.; Villamil, C.; Parra, J.P. Nitrous oxide and methane emissions from the restored mangrove ecosystem of the Ciénaga Grande de Santa Marta, Colombia. Estuar. Coast. Shelf Sci. 2014, 140, 43–51. [Google Scholar] [CrossRef]
- Chen, G.; Chen, B.; Dan, Y.; Tam, N.; Ye, Y.; Shunyang, C. Soil greenhouse gas emissions reduce the contribution of mangrove plants to the atmospheric cooling effect. Environ. Res. Lett. 2016, 11, 124019. [Google Scholar] [CrossRef]
- Wang, H.; Liao, G.; D’Souza, M.; Yu, X.; Yang, J.; Yang, X.; Zheng, T. Temporal and spatial variations of greenhouse gas fluxes from a tidal mangrove wetland in Southeast China. Environ. Sci. Pollut. Res. 2016, 23, 1873–1885. [Google Scholar] [CrossRef]
- Allen, D.; Dalal, R.C.; Rennenberg, H.; Schmidt, S. Seasonal variation in nitrous oxide and methane emissions from subtropical estuary and coastal mangrove sediments. Aust. Plant Biol. 2011, 13, 126–133. [Google Scholar] [CrossRef]
- Chen, G.C.; Tam, N.F.Y.; Wong, Y.S.; Ye, Y. Effect of wastewater discharge on greenhouse gas fluxes from mangrove soils. Atmos. Environ. 2011, 45, 1110–1115. [Google Scholar] [CrossRef]
- Zheng, X.; Guo, J.; Song, W.; Feng, J.; Lin, G. Methane Emission from mangrove wetland soils is marginal but can be stimulated significantly by anthropogenic activities. Forests 2018, 9, 738. [Google Scholar] [CrossRef]
- Chen, G.C.; Ulumuddin, Y.I.; Pramudji, S.; Chen, S.Y.; Chen, B.; Ye, Y.; Ou, D.Y.; Ma, Z.Y.; Huang, H.; Wang, J.K. Rich soil carbon and nitrogen but low atmospheric greenhouse gas fluxes from North Sulawesi mangrove swamps in Indonesia. Sci. Total Environ. 2014, 487, 91–96. [Google Scholar] [CrossRef]
- Tariq, A.; Vu, Q.D.; Jensen, L.S.; de Tourdonnet, S.; Sander, B.O.; Wassmann, R.; Van Mai, T.; de Neergaard, A. Mitigating CH4 and N2O emissions from intensive rice production systems in northern Vietnam: Efficiency of drainage patterns in combination with rice residue incorporation. Agric. Ecosyst. Environ. 2017, 249, 101–111. [Google Scholar] [CrossRef]
- Liu, J.; Xu, H.; Jiang, Y.; Zhang, K.; Hu, Y.; Zeng, Z. Methane emissions and microbial communities as influenced by dual cropping of Azolla along with Early Rice. Sci. Rep. 2017, 7, 40635. [Google Scholar] [CrossRef] [PubMed]
- Stadmark, J.; Leonardson, L. Emissions of greenhouse gases from ponds constructed for nitrogen removal. Ecol. Eng. 2005, 25, 542–551. [Google Scholar] [CrossRef]
- Bridgham, S.D.; Megonigal, J.P.; Keller, J.K.; Bliss, N.B.; Trettin, C. The carbon balance of North American wetlands. Wetlands 2006, 26, 889–916. [Google Scholar] [CrossRef]
- Chauhan, R.; Datta, A.; Ramanathan, A.L.; Adhya, T.K. Factors influencing spatio-temporal variation of methane and nitrous oxide emission from a tropical mangrove of eastern coast of India. Atmos. Environ. 2015, 107, 95–106. [Google Scholar] [CrossRef]
- Bartlett, K.B.; Bartlett, D.S.; Harriss, R.C.; Sebacher, D.I. Methane emissions along a salt-marsh salinity gradient. Biogeochemistry 1987, 4, 183–202. [Google Scholar] [CrossRef]
- Liu, H.; Wu, X.; Li, Z.; Wang, Q.; Liu, D.; Liu, G. Responses of soil methanogens, methanotrophs, and methane fluxes to land-use conversion and fertilization in a hilly red soil region of southern China. Environ. Sci. Pollut. Res. 2017, 24, 8731–8743. [Google Scholar] [CrossRef]
- He, Y.; Guan, W.; Xue, D.; Liu, L.; Peng, C.; Liao, B.; Hu, J.; Zhu, Q.A.; Yang, Y.; Wang, X.; et al. Comparison of methane emissions among invasive and native mangrove species in Dongzhaigang, Hainan Island. Sci. Total Environ. 2019, 697, 133945. [Google Scholar] [CrossRef]
- Arai, H.; Yoshioka, R.; Hanazawa, S.; Minh, V.Q.; Tuan, V.Q.; Tinh, T.K.; Phu, T.Q.; Jha, C.S.; Rodda, S.R.; Dadhwal, V.K.; et al. Function of the methanogenic community in mangrove soils as influenced by the chemical properties of the hydrosphere. Soil Sci. Plant Nutr. 2016, 62, 150–163. [Google Scholar] [CrossRef]
- Sowers, K.R.; Baron, S.F.; Ferry, J.G. Methanosarcina acetivorans sp. nov., an acetotrophic methane-producing bacterium isolated from marine sediments. Appl. Environ. Microbial. 1984, 47, 971–978. [Google Scholar] [CrossRef]
- Lyimo, T.J.; Pol, A.; Op den Camp, H.J.; Harhangi, H.R.; Vogels, G.D. Methanosarcina semesiae sp. nov., a dimethylsulfide-utilizing methanogen from mangrove sediment. Int. J. Syst. Evol. Microbiol. 2000, 50, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Coyne, M. Soil Microbiology: An Exploratory Approach; Delmar Publishers: New York, NY, USA, 1999. [Google Scholar]
- Roslev, P.; King, G.M. Regulation of methane oxidation in a freshwater wetland by water table changes and anoxia. FEMS Microbiol. Ecol. 1996, 19, 105–115. [Google Scholar] [CrossRef]
- Le Mer, J.; Roger, P. Production, oxidation, emission and consumption of methane by soils: A review. Eur. J. Soil Biol. 2001, 37, 25–50. [Google Scholar] [CrossRef]
- Megonigal, J.P.; Schlesinger, W.H. Methane-limited methanotrophy in tidal freshwater swamps. Glob. Biogeochem. Cycles 2002, 16. [Google Scholar] [CrossRef]
- Cai, Y.; Zheng, Y.; Bodelier, P.L.E.; Conrad, R.; Jia, Z. Conventional methanotrophs are responsible for atmospheric methane oxidation in paddy soils. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef]
- Pandit, P.S.; Ranade, D.R.; Dhakephalkar, P.K.; Rahalkar, M.C. A pmoA-based study reveals dominance of yet uncultured Type I methanotrophs in rhizospheres of an organically fertilized rice field in India. 3 Biotech 2016, 6, 135. [Google Scholar] [CrossRef]
- Oswald, K.; Graf, J.S.; Littmann, S.; Tienken, D.; Brand, A.; Wehrli, B.; Albertsen, M.; Daims, H.; Wagner, M.; Kuypers, M.M.M.; et al. Crenothrix are major methane consumers in stratified lakes. ISME J. 2017, 11, 2124–2140. [Google Scholar] [CrossRef]
- Shiau, Y.J.; Cai, Y.F.; Lin, Y.T.; Jia, Z.; Chiu, C.Y. Community structure of active aerobic methanotrophs in Red Mangrove (Kandelia obovata) soils under different frequency of tides. Microb. Ecol. 2017. [Google Scholar] [CrossRef]
- Shiau, Y.J.; Cai, Y.F.; Jia, Z.J.; Chen, C.L.; Chiu, C.Y. Phylogenetically distinct methanotrophs modulate methane oxidation in rice paddies across Taiwan. Soil Biol. Biochem. 2018, 124, 59–69. [Google Scholar] [CrossRef]
- He, R.; Wooller, M.J.; Pohlman, J.W.; Catranis, C.; Quensen, J.; Tiedje, J.M.; Leigh, M.B. Identification of functionally active aerobic methanotrophs in sediments from an arctic lake using stable isotope probing. Environ. Microbiol. 2012, 14, 1403–1419. [Google Scholar] [CrossRef]
- Dumont, M.G.; Luke, C.; Deng, Y.; Frenzel, P. Classification of pmoA amplicon pyrosequences using BLAST and the lowest common ancestor method in MEGAN. Front. Microbiol. 2014, 5. [Google Scholar] [CrossRef]
- Zheng, Y.; Jia, Z. The application of biomarker genes for DNA/RNA-stable isotope probing of active methanotrophs responsible for aerobic methane oxidation in six paddy soils. Acta Pedol. Sin. 2016, 53, 490–501. [Google Scholar] [CrossRef]
- Lüke, C.; Frenzel, P. Potential of pmoA amplicon pyrosequencing for methanotroph diversity studies. Appl. Environ. Microbiol. 2011, 77, 6305. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.; Mo, Y.L.; Lee, H.J.; Sauheitl, L.; Jia, Z.J.; Horn, M.A. Effect of salt stress on aerobic methane oxidation and associated methanotrophs; a microcosm study of a natural community from a non-saline environment. Soil Biol. Biochem. 2018, 125, 210–214. [Google Scholar] [CrossRef]
- Bowman, J.P. Methylobacter. In Bergey’s Manual of Systematics of Archaea and Bacteria; Whitman, W.B., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015. [Google Scholar]
- Bowman, J.P. Methylomonas. In Bergey’s Manual of Systematics of Archaea and Bacteria; Whitman, W.B., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015. [Google Scholar]
- Feller, I.C.; McKee, K.L.; Whigham, D.F.; O’Neill, J.P. Nitrogen vs. phosphorus limitation across an ecotonal gradient in a mangrove forest. Biogeochemistry 2003, 62, 145–175. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Aber, J.D.; Howarth, R.W.; Likens, G.E.; Matson, P.A.; Schindler, D.W.; Schlesinger, W.H.; Tilman, D. Human alteration of the global nitrogen cycle: Sources and consequences. Ecol. Appl. 1997, 7, 737–750. [Google Scholar] [CrossRef]
- De-Leon-Herrera, R.; Flores-Verdugo, F.; Flores-de-Santiago, F.; Gonzalez-Farias, F. Nutrient removal in a closed silvofishery system using three mangrove species (Avicennia germinans, Laguncularia racemosa, and Rhizophora mangle). Mar. Pollut. Bull. 2015, 91, 243–248. [Google Scholar] [CrossRef]
- Reef, R.; Feller, I.C.; Lovelock, C.E. Nutrition of mangroves. Tree Physiol. 2010, 30, 1148–1160. [Google Scholar] [CrossRef]
- Datta, R.; Datta, B.K. Desiccation induced nitrate and ammonium uptake in the red alga Catenella repens (Rhodophyta, Gigartinales). Indian J. Geo Mar. Sci. 1999, 28, 458–460. [Google Scholar]
- Shiau, Y.J.; Lin, M.F.; Tan, C.C.; Tian, G.; Chiu, C.Y. Assessing N2 fixation in estuarine mangrove soils. Estuar. Coast. Shelf Sci. 2017, 189, 84–89. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B.; Mittra, B. Effects of salt on growth, ion accumulation, photosynthesis and leaf anatomy of the mangrove, Bruguiera parviflora. Trees Struct. Funct. 2004, 18, 167–174. [Google Scholar] [CrossRef]
- Khan, M.A.; Aziz, I. Salinity tolerance in some mangrove species from Pakistan. Wetlands Ecol. Manag. 2001, 9, 229–233. [Google Scholar] [CrossRef]
- Holguin, G.; Vazquez, P.; Bashan, Y. The role of sediment microorganisms in the productivity, conservation, and rehabilitation of mangrove ecosystems: An overview. Biol. Fertil. Soils 2001, 33, 265–278. [Google Scholar] [CrossRef]
- Kyaruzi, J.J.; Kyewalyanga, M.; Muruke, M. Cyanobacteria composition and impact of seasonality on their in situ nitrogen fixation rate in a mangrove ecosystem adjacent to Zanzibar Town. Western Indian Ocean J. Mar. Sci. 2003, 2, 35–44. [Google Scholar] [CrossRef]
- Alongi, D.M.; Sasekumar, A.; Chong, V.C.; Pfitzner, J.; Trott, L.A.; Tirendi, F.; Dixon, P.; Brunskill, G.J. Sediment accumulation and organic material flux in a managed mangrove ecosystem: Estimates of land–ocean-atmosphere exchange in peninsular Malaysia. Mar. Geol. 2004, 208, 383–402. [Google Scholar] [CrossRef]
- Romero, I.C.; Jacobson, M.; Fuhrman, J.A.; Fogel, M.; Capone, D.G. Long-term nitrogen and phosphorus fertilization effects on N-2 fixation rates and nifH gene community patterns in mangrove sediments. Mar. Ecol. Evolut. Perspect. 2012, 33, 117–127. [Google Scholar] [CrossRef]
- Welsh, D.T.; Bourgues, S.; de Wit, R.; Herbert, R.A. Seasonal variations in nitrogen-fixation (acetylene reduction) and sulphate-reduction rates in the rhizosphere of Zostera noltii: Nitrogen fixation by sulphate reducing bacteria. Mar. Biol. 1996, 125, 619–628. [Google Scholar] [CrossRef]
- Šantrůčková, H.; Rejmánková, E.; Pivničková, B.; Snyder, J.M. Nutrient enrichment in tropical wetlands: Shifts from autotrophic to heterotrophic nitrogen fixation. Biogeochemistry 2010, 101, 295–310. [Google Scholar] [CrossRef]
- Tyler, A.C.; Mastronicola, T.A.; McGlathery, K.J. Nitrogen fixation and nitrogen limitation of primary production along a natural marsh chronosequence. Oecologia 2003, 136, 431–438. [Google Scholar] [CrossRef]
- Postgate, J.R. The Fundamentals of Nitrogen Fixation; CUP Archive: Cambridge, UK, 1982. [Google Scholar]
- Oremland, R.S.; Capone, D.G. Use of specific inhibitors in biogeochemistry and microbial ecology. In Advances in Microbial Ecology; Marshall, K.C., Ed.; Plenum Press: New York, NY, USA, 1988; Volume 10, pp. 285–383. [Google Scholar]
- Riederer-Henderson, M.-A.; Wilson, P.W. Nitrogen fixation by sulphate-reducing bacteria. J. Gen. Microbiol. 1970, 61, 27–31. [Google Scholar] [CrossRef]
- Taylor, B.F.; Oremland, R.S. Depletion of adenosine triphosphate in Desulfovibrio by oxyanions of group VI elements. Curr. Microbiol. 1979, 3, 101–103. [Google Scholar] [CrossRef]
- Bertics, V.J.; Sohm, J.A.; Treude, T.; Chow, C.-E.T.; Capone, D.G.; Fuhrman, J.A.; Ziebis, W. Burrowing deeper into benthic nitrogen cycling: The impact of bioturbation on nitrogen fixation coupled to sulfate reduction. Mar. Ecol. Prog. Ser. 2010, 409, 1–15. [Google Scholar] [CrossRef]
- Flores-Mireles, A.; Winans, S.; Holguin, G. Molecular characterization of diazotrophic and denitrifying bacteria associated with mangrove roots. Appl. Environ. Microbial. 2007, 73, 7308–7321. [Google Scholar] [CrossRef] [PubMed]
- Currin, C.A.; Joye, S.B.; Paerl, H.W. Diel Rates of N2-fixation and denitrification in a transplanted Spartina alterniflora marsh: Implications for N-flux dynamics. Estuar. Coast. Shelf Sci. 1996, 42, 597–616. [Google Scholar] [CrossRef]
- Fernandes, S.O.; Michotey, V.D.; Guasco, S.; Bonin, P.C.; Loka Bharathi, P.A. Denitrification prevails over anammox in tropical mangrove sediments (Goa, India). Mar. Environ. Res. 2012, 74, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Tiedje, J.M. cology of denitrification and dissimilatory nitrate reduction to ammonium. In Biology of Anaerobic Microorganisms; Zehnder, A.J., Ed.; John Wiley and Sons Inc.: New York, NY, USA, 1988; pp. 179–244. [Google Scholar]
- Fazzolari, É.; Nicolardot, B.; Germon, J.C. Simultaneous effects of increasing levels of glucose and oxygen partial pressures on denitrification and dissimilatory nitrate reduction to ammonium in repacked soil cores. Eur. J. Soil Biol. 1998, 34, 47–52. [Google Scholar] [CrossRef]
- Yin, S.X.; Chen, D.; Chen, L.M.; Edis, R. Dissimilatory nitrate reduction to ammonium and responsible microorganisms in two Chinese and Australian paddy soils. Soil Biol. Biochem. 2002, 34, 1131–1137. [Google Scholar] [CrossRef]
- Bonin, P.; Omnes, P.; Chalamet, A. The influence of nitrate and carbon inputs on the end products of bacterial nitrate dissimilation in marine sediment. Toxicol. Environ. Chem. 1999, 73, 67–79. [Google Scholar] [CrossRef]
- Balk, M.; Laverman, A.M.; Keuskamp, J.A.; Laanbroek, H.J. Nitrate ammonification in mangrove soils: A hidden source of nitrite? Front. Microbial. 2015, 6, 166. [Google Scholar] [CrossRef]
- Fernandes, S.O.; Gonsalves, M.-J.; Michotey, V.D.; Bonin, P.C.; LokaBharathi, P.A. Denitrification activity is closely linked to the total ambient Fe concentration in mangrove sediments of Goa, India. Estuar. Coast. Shelf Sci. 2013, 131, 64–74. [Google Scholar] [CrossRef]
- Giblin, A.E.; Tobias, C.R.; Song, B.; Weston, N.; Banta, G.T.; Rivera-Monroy, V.H. The importance of dissimilatory nitrate reduction to ammonium (DNRA) in the nitrogen cycle of coastal ecosystems. Oceanography 2013, 26, 124–131. [Google Scholar] [CrossRef]
- Cao, Y.; Green, P.G.; Holden, P.A. Microbial community composition and denitrifying enzyme activities in salt marsh sediments. Appl. Environ. Microbiol. 2008, 74, 7585–7595. [Google Scholar] [CrossRef] [PubMed]
- Marton, J.M.; Herbert, E.R.; Craft, C.B. Effects of salinity on denitrification and greenhouse gas production from laboratory-incubated tidal forest soils. Wetlands 2012, 32, 347–357. [Google Scholar] [CrossRef]
- Fernandes, S.O.; Bonin, P.C.; Michotey, V.D.; Garcia, N.; LokaBharathi, P.A. Nitrogen-limited mangrove ecosystems conserve N through dissimilatory nitrate reduction to ammonium. Sci. Rep. 2012, 2, 419. [Google Scholar] [CrossRef] [PubMed]
- Weng, B.; Xie, X.; Yang, J.; Liu, J.; Lu, H.; Yan, C. Research on the nitrogen cycle in rhizosphere of Kandelia obovata under ammonium and nitrate addition. Mar. Pollut. Bull. 2013, 76, 227–240. [Google Scholar] [CrossRef]
- Maier, R.M.; Pepper, I.L.; Gerba, C.P. Environmental Microbiology; Academic Press: San Diego, CA, USA, 2000. [Google Scholar]
- Huang, C.-M.; Yuan, C.-S.; Yang, W.-B.; Yang, L. Temporal variations of greenhouse gas emissions and carbon sequestration and stock from a tidal constructed mangrove wetland. Mar. Pollut. Bull. 2019, 149, 110568. [Google Scholar] [CrossRef]
- Chen, W.-B.; Liu, W.-C.; Hsu, M.-H. Modeling assessment of a saltwater intrusion and a transport time scale response to sea-level rise in a tidal estuary. Environ. Fluid Mech. 2015, 15, 491–514. [Google Scholar] [CrossRef]
- Yang, Z.; Song, W.; Zhao, Y.; Zhou, J.; Wang, Z.; Luo, Y.; Li, Y.; Lin, G. Differential responses of litter decomposition to regional excessive nitrogen input and global warming between two mangrove species. Estuar. Coast. Shelf Sci. 2018, 214, 141–148. [Google Scholar] [CrossRef]
- Lu, W.; Chen, L.; Wang, W.; Fung-Yee Tam, N.; Lin, G. Effects of sea level rise on mangrove Avicennia population growth, colonization and establishment: Evidence from a field survey and greenhouse manipulation experiment. Acta Oecol. 2013, 49, 83–91. [Google Scholar] [CrossRef]
- Krauss, K.W.; McKee, K.L.; Lovelock, C.E.; Cahoon, D.R.; Saintilan, N.; Reef, R.; Chen, L. How mangrove forests adjust to rising sea level. New Phytol. 2014, 202, 19–34. [Google Scholar] [CrossRef]
- Reef, R.; Slot, M.; Motro, U.; Motro, M.; Motro, Y.; Adame, M.F.; Garcia, M.; Aranda, J.; Lovelock, C.E.; Winter, K. The effects of CO2 and nutrient fertilisation on the growth and temperature response of the mangrove Avicennia germinans. Photosynth. Res. 2016, 129, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Hughes, L. Climate change and Australia: Trends, projections and impacts. Austral Ecol. 2003, 28, 423–443. [Google Scholar] [CrossRef]
- Osland, M.; Feher, L.; López-Portillo, J.; Day, R.; Suman, D.; Guzmán Menéndez, J.; Rivera-Monroy, V. Mangrove forests in a rapidly changing world: Global change impacts and conservation opportunities along the Gulf of Mexico coast. Estuar. Coast. Shelf Sci. 2018, 214. [Google Scholar] [CrossRef]
- Gabler, C.; Osland, M.; Grace, J.; Stagg, C.; Day, R.; Hartley, S.; Enwright, N.; From, A.; McCoy, M.; McLeod, J. Macroclimatic change expected to transform coastal wetland ecosystems this century. Nat. Clim. Chang. 2017, 7. [Google Scholar] [CrossRef]
- Feng, X.; Simpson, A.; Wilson, K.; Williams, D.; Simpson, M. Increased cuticular carbon sequestration and lignin oxidation in response to soil warming. Nat. Geosci. 2008, 1, 836–839. [Google Scholar] [CrossRef]
| Study | Site | Ecosystem | Average Soil C Stock (Mg C ha−1) |
|---|---|---|---|
| [43] | Mexico | Mangrove | 622 |
| [44] | Global | Mangrove | 650 |
| [45] | Philippines | Mangrove | 442 |
| [37] | Indonesia | Mangrove | 572 |
| Malaysia | Mangrove | 1059 | |
| [35] | FL, USA | Mangrove | 307 |
| [46] | Global | Mangrove | 749 |
| [38] | Australia | Mangrove | 66 |
| Tidal marsh | 87 | ||
| Seagrass | 24 | ||
| [39] | Brazil | Mangrove | 341 |
| Salt marsh | 257 | ||
| [47] | MD, USA | Salt marsh (S. patens) | 24 |
| Salt marsh (S. alterniflora) | 22 | ||
| [48] | FL, USA | Salt marsh | 72 |
| Study | Ecosystem | Salinity | CO2 Efflux (mg C m−2 h−1) | CH4 Efflux (mg C m−2 h−1) | N2O Efflux (mg N m−2 h−1) | Global Warming Potential (GWP) (mg CO2-eq m−2 h−1) |
|---|---|---|---|---|---|---|
| [64] | Mangrove (Taiwan) | 0.14 | ||||
| [65] | Mangrove (China) | 31–74 | ||||
| [66] | Mangrove (India) | 0.018–0.034 | ||||
| [67] | Mangrove (Australia) | −11–128 | ||||
| [68] | Mangrove (Hong Kong) | 15–21 | 10–1,374 | 0.032–0.534 | ||
| [69] | Mangrove (Australia) | 17–25 * | 36.9–59.0 | 0–0.06 | 0–0.05 | 136–245 |
| [70] | Mangrove (China) | 16–267 | ||||
| [71] | Mangrove (New Caledonia) | 36–44 | ||||
| [72] | Mangrove (Colombia) | 2.7–23.4 | 0–23.68 | 0.009–0.375 | ||
| [58] | Mangrove (Philippines) | 16.8–79.3 | 108–151 | 0.06–0.12 | 0–0.084 | 396–604 |
| [73] | Mangrove (China) | 12–14 | −9–140 | 0–4.02 | 0–0.016 | −33–889 |
| [74] | Mangrove (China) | 10–21 | 0–55 | 0.35–23.09 | 0–0.017 | 32–2,326 |
| [59] | Mangrove (Vietnam) | 7–16 | Wet season: 112 Dry season: 25 | |||
| [60] | Mangrove (New Caledonia) | 40.2 | 0.22 | |||
| [61] | Mangrove (Australia) | 28 | ||||
| [75] | Mangrove (Australia) | 9–35 * | 0.04–1.18 | 0.004–0.13 | ||
| [76] | Mangrove (China) | 8.4–14.8 | 0.63–4.12 | |||
| [77] | Mangrove (China) | 12–26 | 11–114 | 0–0.17 | ||
| [78] | Mangrove (Indonesia) | 25–34 | −16.8–46.6 | −0.003–0.007 | −0.17–0.37 | −139–344 |
| [56] | Brackish salt marsh (NC, USA) | 22.5 | −45–88 | −0.17–0.23 | −0.046–0.048 | −202–366 |
| [62] | Tidal freshwater wetland (GA, USA) | 0.4–2.1 | 15–59 | 0.04–0.24 | −0.009–0.012 | 54–244 |
| [79] | Rice paddies (Vietnam) | 0–75 | 0–0.132 | |||
| [80] | Rice paddies (China) | 0–630 | ||||
| [81] | Ponds (Sweden) | 0.75–40.50 |
| Location | Dominant Plant | Soil N2-Fixation Potential (μmol N m−2 h−1) | Reference |
|---|---|---|---|
| Mangrove (Tanzania) | - | 21.6–26.4 | [115] * |
| Mangrove (Malaysia) | Rhizophora apiculata | 0–125 | [116] |
| Mangrove (Belize) | Rhizophora mangle | 128 | [117] * |
| Mangrove (Taiwan) | Tall Kandelia obovata Dwarf Kandelia obovata | 6.96 1.56 | [111] * |
| Salt marsh (Arcachon, Fr) | Zostera noltii | 72–152 | [118] * |
| Salt marsh (Belize) | Eleocharis spp. | 20–24 | [119] * |
| Natural salt marsh (VA, USA) | Spartina alterniflora | 72–420 | [120] * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiau, Y.-J.; Chiu, C.-Y. Biogeochemical Processes of C and N in the Soil of Mangrove Forest Ecosystems. Forests 2020, 11, 492. https://doi.org/10.3390/f11050492
Shiau Y-J, Chiu C-Y. Biogeochemical Processes of C and N in the Soil of Mangrove Forest Ecosystems. Forests. 2020; 11(5):492. https://doi.org/10.3390/f11050492
Chicago/Turabian StyleShiau, Yo-Jin, and Chih-Yu Chiu. 2020. "Biogeochemical Processes of C and N in the Soil of Mangrove Forest Ecosystems" Forests 11, no. 5: 492. https://doi.org/10.3390/f11050492
APA StyleShiau, Y.-J., & Chiu, C.-Y. (2020). Biogeochemical Processes of C and N in the Soil of Mangrove Forest Ecosystems. Forests, 11(5), 492. https://doi.org/10.3390/f11050492






