The Possibility of Propolis Extract Application in Wood Protection
Abstract
1. Introduction
2. Materials and Methods
2.1. Wood Samples
2.2. Propolis Extract
2.3. Wood Treatment
2.4. Decay Resistance Test
2.5. Ergosterol Concentration Analysis
2.6. Fourier Transform Infrared Spectroscopy (FTIR)
2.7. Identification of Phenolic Compounds in Propolis Extract
2.8. Statistical Analysis
3. Results and Discussion
3.1. Decay Test
3.2. Ergosterol Concentration
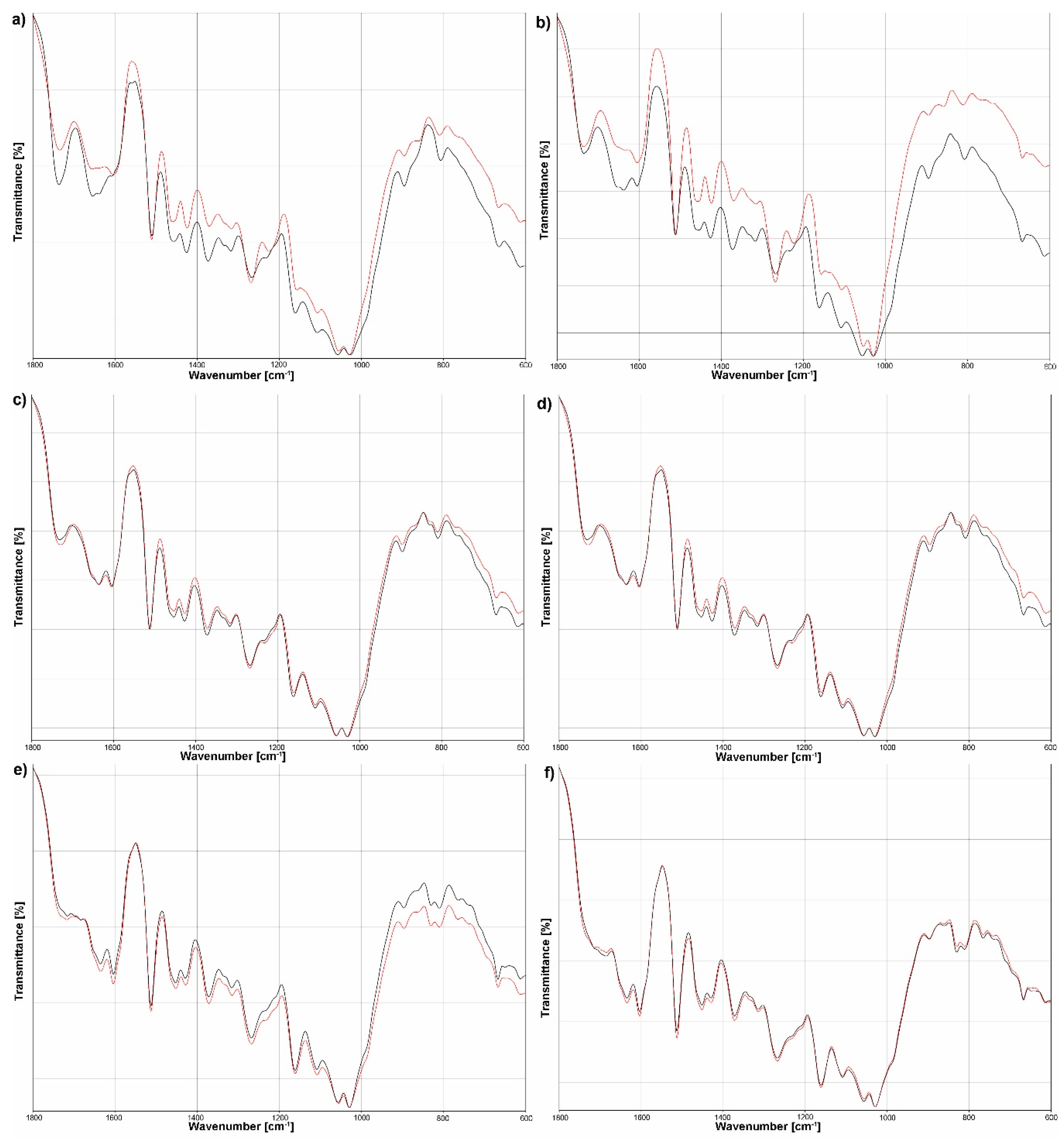
3.3. FTIR Analysis
3.4. Analysis of Propolis Extract Composition
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Panek, M.; Reinprecht, L.; Hulla, M. Ten essential oils for beech wood protection—Efficacy against wood-destroying fungi and moulds, and effect on wood discoloration. BioResources 2014, 9, 5588–5603. [Google Scholar] [CrossRef]
- Bahmani, M.; Schmidt, O. Plant essential oils for environment-friendly protection of wood objects against fungi. Maderas Cienc. Tecnol. 2018, 20, 325–332. [Google Scholar] [CrossRef]
- Singh, T.; Singh, A.P. A review on natural products as wood protectant. Wood Sci. Technol. 2012, 46, 851–870. [Google Scholar] [CrossRef]
- Yen, T.B.; Chang, S.T. Synergistic effects of cinnamaldehyde in combination with eugenol against wood decay fungi. Bioresour. Technol. 2008, 99, 232–236. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, Z.; Huang, Q.; Zhang, D. Antifungal activity of several essential oils and major components against wood-rot fungi. Ind. Crop Prod. 2017, 108, 278–285. [Google Scholar] [CrossRef]
- Hussain, I.; Singh, T.; Chittenden, C. Preparation of chitosan oligomers and characterization: Their antifungal activities and decay resistance. Holzforschung 2012, 66, 119–125. [Google Scholar] [CrossRef]
- Alfredsen, G.; Eikenes, M.; Militz, H.; Solheim, H. Screening of chitosan against wood-deteriorating fungi. Scand. J. For. Res. 2004, 19, 4–13. [Google Scholar] [CrossRef]
- Humar, M.; Lesar, B. Efficacy of linseed- and tung-oil-treated wood against wood-decay fungi and water uptake. Int. Biodeter. Biodegr. 2013, 85, 223–227. [Google Scholar] [CrossRef]
- Temiz, A.; Alfredsen, G.; Eikenes, M.; Terziev, N. Decay resistance of wood treated with boric acid and tall oil derivates. Bioresour. Technol. 2008, 99, 2102–2106. [Google Scholar] [CrossRef]
- Tomak, E.D.; Viitanen, H.; Yildiz, U.C.; Hughes, M. The combined effect of boron and oil heat treatment on the properties of beech and Scots pine wood. Part 2: Water absorption, compression strength, color changes and decay resistance. J. Mater. Sci. 2011, 46, 608–615. [Google Scholar] [CrossRef]
- Kwaśniewska-Sip, P.; Cofta, G.; Nowak, P.B. Resistance of fungal growth on Scots pine treated with caffeine. Int. Biodeter. Biodegr. 2018, 132, 178–184. [Google Scholar] [CrossRef]
- Budija, F.; Humar, M.; Kricej, B.; Petric, M. Propolis for Wood Finishing; The International Research Group on Wood Protection: Bled, Slovenia, 2008; IRG/WP 08-30464. [Google Scholar]
- Akcay, C.; Birinci, E.; Birinci, C.; Kolayli, S. Durability of wood treated with propolis. BioResources 2020, 15, 1547–1562. [Google Scholar] [CrossRef]
- Toreti, V.C.; Sato, H.H.; Pastore, G.M.; Park, Y.K. Recent progress of propolis for its biological and chemical compositions and its botanical origin. Evid. Based Compl. Alt. 2013, 2013, 697390. [Google Scholar] [CrossRef]
- Castaldo, S.; Capasso, F. Propolis, an old remedy used in modern medicine. Fitoterapia 2002, 73, S1–S6. [Google Scholar] [CrossRef]
- Xu, X.; Pu, R.; Li, Y.; Wu, Z.; Li, C.; Miao, X.; Yang, W. Chemical composition of propolis from China and the United States and their antimicrobial activities against Penicillium notatum. Molecules 2019, 24, 3576. [Google Scholar] [CrossRef]
- Uzel, A.; Sorkun, K.; Oncag, O.; Cogulu, D.; Gencay, O.; Salih, B. Chemical compositions and antimicrobial activities of four different Anatolian propolis samples. Microbiol. Res. 2005, 160, 189–195. [Google Scholar] [CrossRef]
- Woźniak, M.; Mrówczyńska, L.; Waśkiewicz, A.; Rogoziński, T.; Ratajczak, I. The role of seasonality on the chemical composition, antioxidant activity and cytotoxicity of Polish propolis in human erythrocytes. Rev. Bras. Farmacogn. 2019, 29, 301–308. [Google Scholar] [CrossRef]
- Popova, M.; Giannopoulou, E.; Skalicka-Woźniak, K.; Graikou, K.; Widelski, J.; Bankova, V.; Kalofonos, H.; Sivolapenko, G.; Gaweł-Bęben, K.; Antosiewicz, B.; et al. Characterization and biological evaluation of propolis from Poland. Molecules 2017, 22, 1159. [Google Scholar] [CrossRef]
- Aguero, M.B.; Svetaz, L.; Baroni, V.; Lima, B.; Luna, L.; Zacchino, S.; Saavedra, P.; Wunderlin, D.; Feresin, G.E.; Tapia, A. Urban propolis from San Juan province (Argentina): Ethnopharmacological uses and antifungal activity against Candida and dermatophytes. Ind. Crop. Prod. 2014, 57, 166–173. [Google Scholar] [CrossRef]
- Salas, A.L.; Alberto, M.R.; Zampini, I.C.; Cuello, A.S.; Maldonado, L.; Rios, J.L.; Schmeda-Hirschmann, G.; Isla, M.I. Biological activities of polyphenols-enriched propolis from Argentina arid regions. Phytomedicine 2016, 23, 27–31. [Google Scholar] [CrossRef]
- Chen, Y.W.; Ye, S.R.; Ting, C.; Yu, Y.H. Antibacterial activity of propolins form Taiwanese green propolis. J. Food Drug Anal. 2018, 26, 761–768. [Google Scholar] [CrossRef]
- Kujumgiev, A.; Tsvetkova, I.; Serkedjieva, Y.; Bankova, V.; Christov, R.; Popov, S. Antibacterial, antifungal and antiviral activity of propolis of different geographic origin. J. Ethnopharmacol. 1999, 64, 235–240. [Google Scholar] [CrossRef]
- Socha, R.; Gałkowska, D.; Bugaj, M.; Juszczak, L. Phenolic composition and antioxidant activity of propolis from various regions of Poland. Nat. Prod. Res. 2015, 29, 416–422. [Google Scholar] [CrossRef]
- Kumazawa, S.; Hamasaka, T.; Nakayama, T. Antioxidant activity of propolis of various geographic origins. Food Chem. 2004, 84, 329–339. [Google Scholar] [CrossRef]
- Przybyłek, I.; Karpiński, T.M. Antibacterial properties of propolis. Molecules 2019, 24, 2047. [Google Scholar] [CrossRef]
- Yang, S.Z.; Peng, L.T.; Su, X.J.; Chen, F.; Cheng, Y.J.; Fan, G.; Pan, S.Y. Bioassay-guided isolation and identification of antifungal components from propolis against Penicillium italicum. Food Chem. 2011, 127, 210–215. [Google Scholar] [CrossRef]
- Garedew, A.; Schmolz, E.; Lamprecht, I. Microbiological and calorimetric investigations on the antimicrobial actions of different propolis extracts: An in vitro approach. Thermochim. Acta 2004, 422, 115–124. [Google Scholar] [CrossRef]
- Meneses, E.A.; Durango, D.I.; Garcia, C.M. Antifungal activity against postharvest fungi by extracts from Colombian propolis. Quim. Nova 2009, 32, 2011–2017. [Google Scholar] [CrossRef]
- Quiroga, E.N.; Sampietro, D.A.; Soberon, J.R.; Sgariglia, M.A.; Vattuone, M.A. Propolis from the northwest of Argentina as a source of antifungal principles. J. Appl. Microbiol. 2006, 101, 103–110. [Google Scholar] [CrossRef]
- Jones, D.; Howard, N.; Suttie, E. The Potential of Propolis and other Naturally Occurring Products for Preventing Biological Decay; The International Research Group On Wood Protection: Bled, Slovenia, 2011; IRG/WP 11-30575. [Google Scholar]
- Casado-Sanz, M.M.; Silva-Castro, I.; Ponce-Herrero, L.; Martin-Ramos, P.; Martun-Gil, J.; Acuna-Rello, L. White-rot fungi control on Populus spp. Wood by pressure treatments with silver nanoparticles, chitosan oligomers and propolis. Forests 2019, 10, 885. [Google Scholar] [CrossRef]
- Silva-Castro, I.; Casados-Sanz, M.; Alonso-Cortes, A.L.; Martin-Ramos, P.; Martin-Gil, J.; Acuna-Rello, L. Chitosan-based coatings to prevent the decay of Populus spp. wood caused by Trametes versicolor. Coatings 2018, 8, 415. [Google Scholar] [CrossRef]
- Ratajczak, I.; Woźniak, M.; Kwaśniewska-Sip, P.; Szentner, K.; Cofta, G.; Mazela, B. Chemical characterization of wood treated with a formulation based on propolis, caffeine and organosilanes. Eur. J. Wood Wood Prod. 2018, 76, 775–787. [Google Scholar] [CrossRef]
- Woźniak, M.; Ratajczak, I.; Lis, B.; Krystofiak, T. Hydrophobic properties of wood treated with propolis-silane formulations. Wood Res. Slovak. 2018, 63, 517–524. [Google Scholar]
- Woźniak, M.; Ratajczak, I.; Waśkiewicz, A.; Szentner, K.; Cofta, G.; Kwaśniewska-Sip, P. Investigation of the use of impregnating formulation with propolis extract and organosilanes in wood protection—Biological analyses. Ann. WULS SGGW For. Wood Technol. 2016, 96, 43–47. [Google Scholar]
- CEN. EN 113 Wood Preservatives—Test Methods for Determining the Protective Effectiveness against Wood Destroying Basidiomycetes—Determination of the Toxic Values; European Committee for Standardization: Bruxelles, Belgium, 1996. [Google Scholar]
- Woźniak, M.; Ratajczak, I.; Kwaśniewska, P.; Cofta, G.; Hołderna-Kędzia, E.; Kędzia, B.; Mazela, B. The activity of propolis extracts against selected moulds. Post. Fitoter. 2015, 16, 205–209. [Google Scholar]
- Woźniak, M.; Ratajczak, I.; Szentner, K.; Kwaśniewska, P.; Mazela, B. Propolis and organosilanes in wood protection. Part I: FTIR analysis and biological tests. Ann. WULS SGGW For. Wood Technol. 2015, 91, 218–224. [Google Scholar]
- Niemenmaa, O.; Galkin, S.; Hatakka, A. Ergosterol contents of some wood-rotting basidiomycete fungi grown in liquid and solid culture conditions. Int. Biodeter. Biodegr. 2008, 62, 125–134. [Google Scholar] [CrossRef]
- Perdoch, W.; Mazela, B.; Waśkiewicz, A. Antifungal Properties of Wood Treated with IPBC and Organosilicone Compounds against Coniophora Puteana; The International Research Group on Wood Protection: Bled, Slovenia, 2015; IRG/WP 15-30376. [Google Scholar]
- Pandey, K.K.; Pitman, A.J. FTIR studies of the changes in wood chemistry following decay by brown-rot and white-rot fungi. Int. Biodeterior. Biodegrad. 2003, 52, 151–160. [Google Scholar] [CrossRef]
- Irbe, I.; Andersone, I.; Andersons, B.; Noldt, G.; Dizhbite, T.; Kournosova, N.; Nuopponen, M.; Stewart, D. Characterisation of the initial degradation stage of Scots pine (Pinus sylvestris L.) sapwood after attack by brown-rot fungus Coniophora puteana. Biodegradation 2011, 22, 719–728. [Google Scholar] [CrossRef]
- Pandey, K.K.; Nagveni, H.C. Rapid characterization of rot and white rot degraded pine and rubber wood by FTIR spectroscopy. Holz als Roh und Werkst 2007, 65, 477–481. [Google Scholar] [CrossRef]
- Durmaz, S.; Ozgenc, O.; Boyaci, I.H.; Yildiz, U.C.; Erisir, E. Examination of the chemical changes in spruce wood degraded by brown-rot fungi using FT-IR and FT-Raman spectroscopy. Vib. Spectrosc. 2016, 85, 202–207. [Google Scholar] [CrossRef]
- Can, A.; Palanti, S.; Sivrikaya, H.; Hazer, B.; Stefani, F. Physical, biological and chemical characterization of wood treated with silver nanoparticles. Cellulose 2019, 26, 5075–5084. [Google Scholar] [CrossRef]
- Irbe, I.; Andersons, B.; Chirkova, J.; Kallavus, U.; Andersons, I.; Faix, O. On the changes of pinewood (Pinus sylvestris L.). Chemical composition and ultrastructure during the attack by brown-rot fungi Postia placenta and Coniophora puteana. Int. Biodeterior. Biodegrad. 2006, 57, 99–106. [Google Scholar] [CrossRef]
- Medana, C.; Carbone, F.; Aigotti, R.; Appendino, G.; Baiocchi, C. Selective analysis of phenolic compounds in propolis by HPLC-MS/MS. Phytochem. Anal. 2008, 19, 32–39. [Google Scholar] [CrossRef]
- Pellati, F.; Orlandini, G.; Pinetti, D.; Benvenuti, S. HPLC-DAD and HPLC-ESI-MS/MS methods for metabolite profiling of propolis extracts. J. Pharmaceut. Biomed. 2011, 55, 934–948. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Konteles, S.J.; Troullidou, E.; Mourtzinos, I.; Karathanos, V.T. Chemical composition, antioxidant activity and antimicrobial properties of propolis extracts from Greece and Cyprus. Food Chem. 2009, 116, 452–461. [Google Scholar] [CrossRef]

| Propolis Extract Concentration (%) | Retention (kg/m3) | Mass Loss (%) |
|---|---|---|
| 0 | - | 48.8 a ± 1.1 |
| 3.0 | 19.0 ± 0.3 | 31.6 b ± 1.6 |
| 7.5 | 48.0 ± 0.5 | 5.9 c ± 0.7 |
| 12.0 | 80.3 ± 0.3 | 3.3 d ± 0.4 |
| 18.9 | 129.2 ± 0.4 | 2.3 d ± 0.3 |
| 30.0 | 216.6 ± 0.5 | 2.7 d ± 0.5 |
| Propolis Extract Concentration (%) | Ergosterol Concentration (µg/g) | Ergosterol Reduction (%) |
|---|---|---|
| 0 | 180.00 a ± 5.58 | - |
| 3.0 | 118.66 b ± 6.49 | 34 |
| 7.5 | 100.93 c ± 1.12 | 44 |
| 12.0 | 85.68 d ± 2.71 | 52 |
| 18.9 | 57.92 e ± 2.63 | 68 |
| 30.0 | 28.96 f ± 1.03 | 84 |
| Phenolic Compound | Concentration (mg/g of Extract) |
|---|---|
| Apigenin | 11.98 ± 0.49 |
| Chrysin | 23.33 ± 0.69 |
| Galangin | 28.96 ± 0.58 |
| Kaempferol | 16.33 ± 0.84 |
| Quercetin | 3.23 ± 0.25 |
| Myricetin | 0.80 ± 0.08 |
| Naringenin | 1.14 ± 0.18 |
| Pinobanksin | 3.85 ± 0.26 |
| Pinocembrin | 45.68 ± 0.78 |
| Rutin | 0.37 ± 0.02 |
| Caffeic acid | 3.08 ± 0.43 |
| Coumaric acid | 10.34 ± 0.66 |
| Ferulic acid | 2.74 ± 0.28 |
| Cinnamic acid | 6.54 ± 0.61 |
| Chlorogenic acid | 0.26 ± 0.05 |
| Vanillic acid | 0.10 ± 0.04 |
| Hydroxybenzoic acid | 0.34 ± 0.70 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woźniak, M.; Kwaśniewska-Sip, P.; Waśkiewicz, A.; Cofta, G.; Ratajczak, I. The Possibility of Propolis Extract Application in Wood Protection. Forests 2020, 11, 465. https://doi.org/10.3390/f11040465
Woźniak M, Kwaśniewska-Sip P, Waśkiewicz A, Cofta G, Ratajczak I. The Possibility of Propolis Extract Application in Wood Protection. Forests. 2020; 11(4):465. https://doi.org/10.3390/f11040465
Chicago/Turabian StyleWoźniak, Magdalena, Patrycja Kwaśniewska-Sip, Agnieszka Waśkiewicz, Grzegorz Cofta, and Izabela Ratajczak. 2020. "The Possibility of Propolis Extract Application in Wood Protection" Forests 11, no. 4: 465. https://doi.org/10.3390/f11040465
APA StyleWoźniak, M., Kwaśniewska-Sip, P., Waśkiewicz, A., Cofta, G., & Ratajczak, I. (2020). The Possibility of Propolis Extract Application in Wood Protection. Forests, 11(4), 465. https://doi.org/10.3390/f11040465






