Nitrogen Additions Retard Nutrient Release from Two Contrasting Foliar Litters in a Subtropical Forest, Southwest China
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Experimental Design
2.2. Litter Bag Experiment
2.3. Chemical Analysis
2.4. Calculations and Statistical Analysis
3. Results
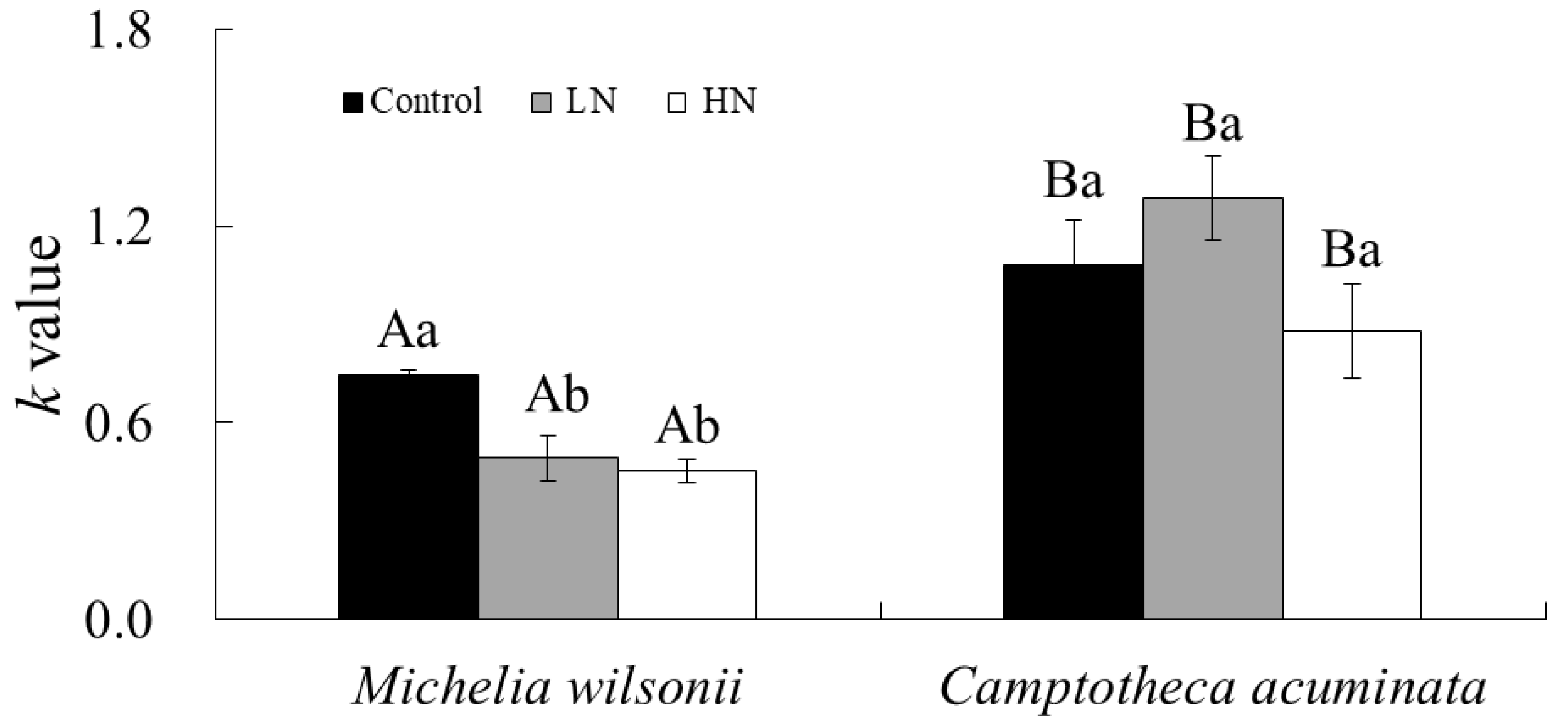
3.1. Decay Constant
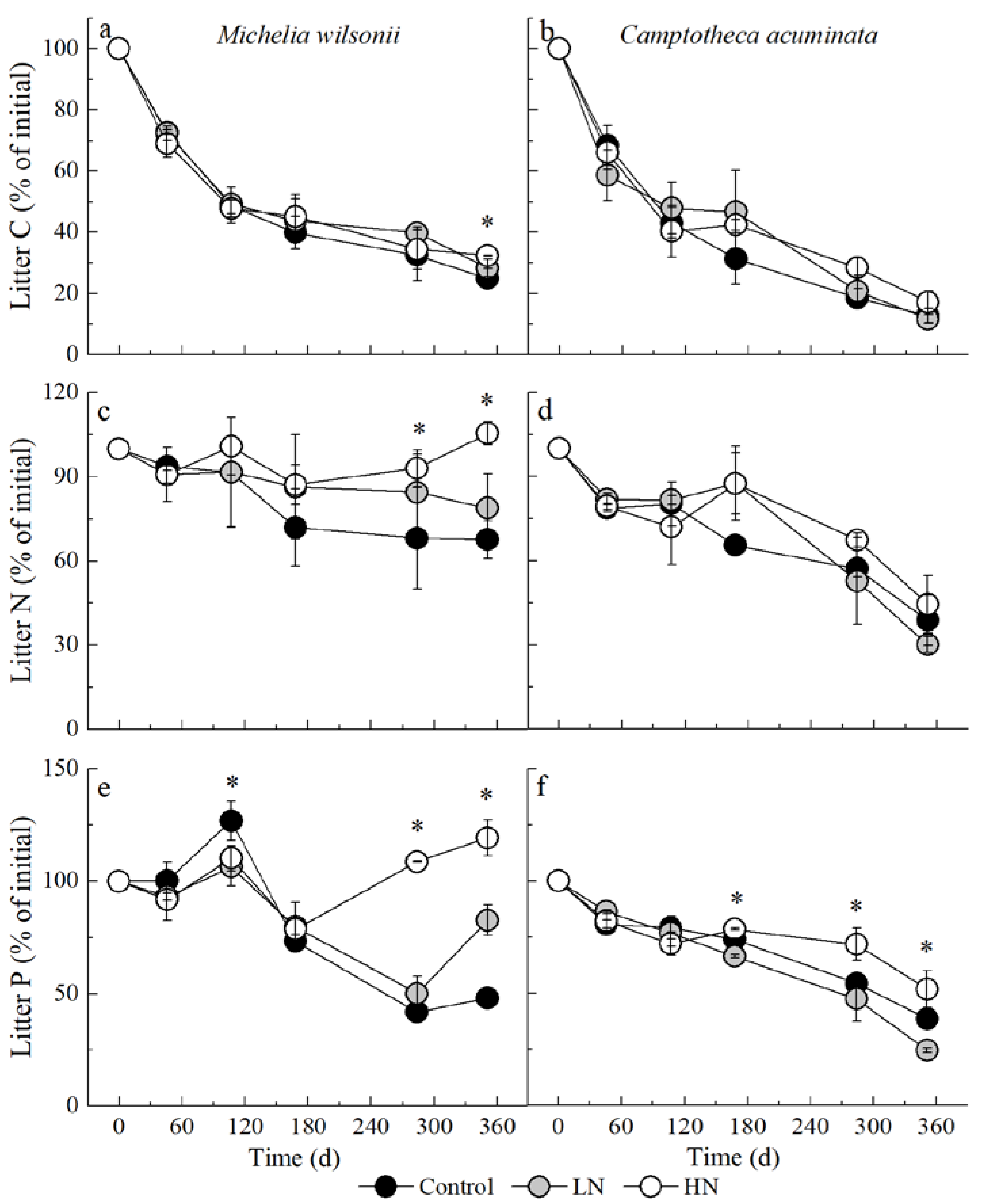
3.2. Litter C, N and P Remaining
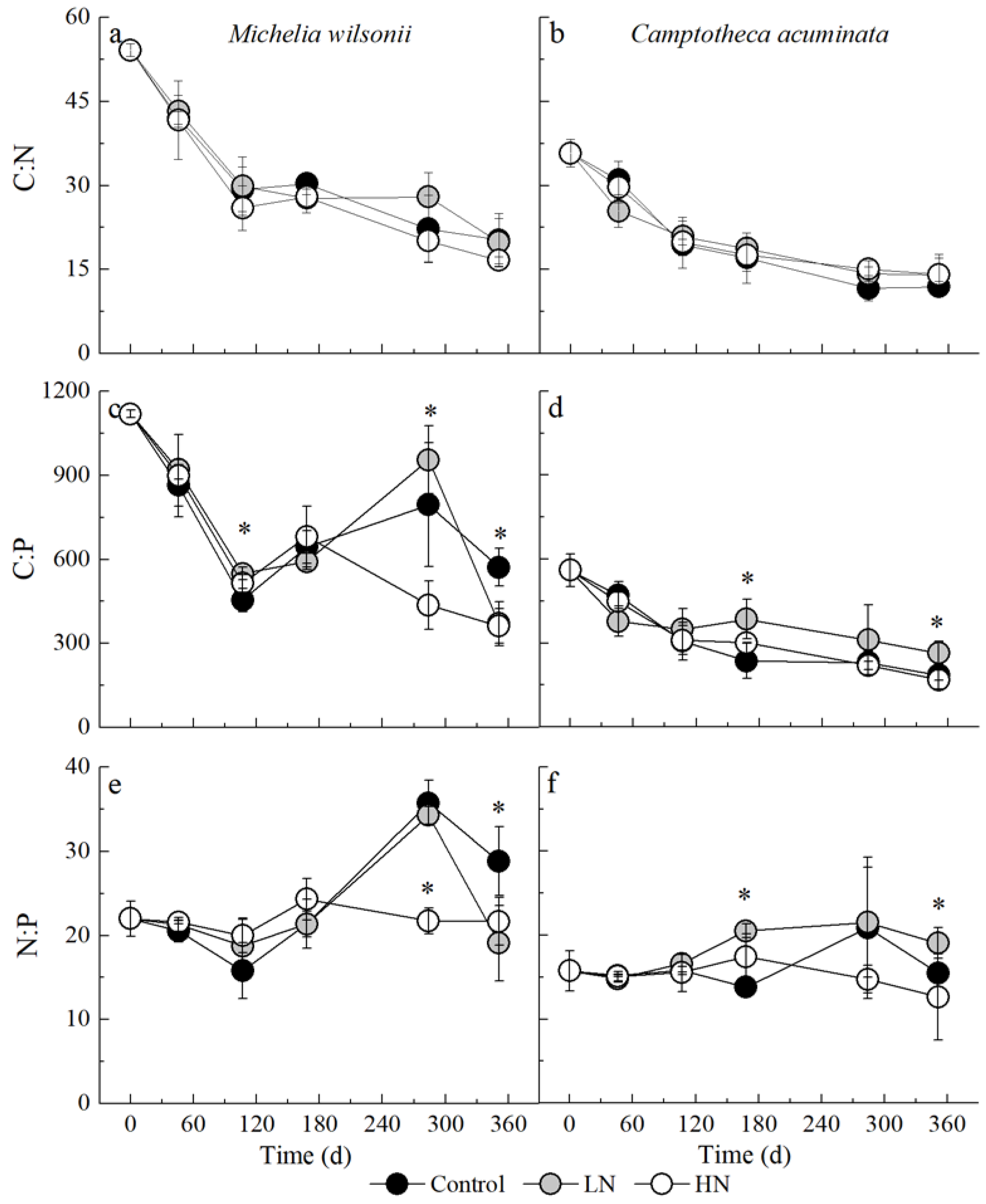
3.3. Stoichiometric Dynamic
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abelho, M. Litter traits and decomposer complexity set the stage for a global decomposition model. Funct. Ecol. 2016, 30, 674–675. [Google Scholar] [CrossRef]
- Zhang, D.Q.; Hui, D.F.; Luo, Y.Q.; Zhou, G.Y. Rates of litter decomposition in terrestrial ecosystems: Global patterns and controlling factors. J. Plant Ecol. 2008, 1, 85–93. [Google Scholar] [CrossRef]
- Matson, P.; Lohse, K.A.; Hall, S.J. The globalization of nitrogen deposition: Consequences for terrestrial ecosystems. Ambio A J. Hum. Environ. 2002, 31, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Knorr, M.; Frey, S.D.; Curtis, P.S. Nitrogen addition and litter decomposition: A meta-analysis. Ecology 2005, 86, 3252–3257. [Google Scholar] [CrossRef]
- Liu, X.J.; Duan, L.; Mo, J.M.; Du, E.Z.; Shen, J.L.; Lu, X.K.; Zhang, Y.; Zhou, X.B.; He, C.N.; Zhang, F.S. Nitrogen deposition and its ecological impact in China: An overview. Environ. Pollut. 2011, 159, 2251–2264. [Google Scholar] [CrossRef] [PubMed]
- Manning, P.; Saunders, M.; Bardgett, R.D.; Bonkowski, M.; Bradford, M.A.; Ellis, R.J.; Kandeler, E.; Marhan, S.; Tscherko, D. Direct and indirect effects of nitrogen deposition on litter decomposition. Soil Biol. Biochem. 2008, 40, 688–698. [Google Scholar] [CrossRef]
- Berg, B.; Matzner, E. Effect of N deposition on decomposition of plant litter and soil organic matter in forest systems. Environ. Rev. 1997, 5, 1–25. [Google Scholar] [CrossRef]
- Liu, P.; Sun, O.J.; Huang, J.; Li, L.; Han, X. Nonadditive effects of litter mixtures on decomposition and correlation with initial litter N and P concentrations in grassland plant species of northern China. Biol. Fertil. Soils. 2007, 44, 211–216. [Google Scholar] [CrossRef]
- Berg, B. Decomposition patterns for foliar litter—A theory for influencing factors. Soil Biol. Biochem. 2014, 78, 222–232. [Google Scholar] [CrossRef]
- Zhang, T.A.; Luo, Y.; Chen, H.Y.; Ruan, H. Responses of litter decomposition and nutrient release to N addition: A meta-analysis of terrestrial ecosystems. Appl. Soil Ecol. 2018, 128, 35–42. [Google Scholar] [CrossRef]
- Norris, M.D.; Avis, P.G.; Reich, P.B.; Hobbie, S.E. Positive feedbacks between decomposition and soil nitrogen availability along fertility gradients. Plant Soil 2013, 367, 347–361. [Google Scholar] [CrossRef]
- Downs, M.R.; Nadelhoffer, K.J.; Melillo, J.M.; Aber, J.D. Immobilization of a N-15-labeled nitrate addition by decomposing forest litter. Oecologia 1996, 105, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Brown, S.; Xue, J.; Fang, Y.; Li, Z. Response of litter decomposition to simulated N deposition in disturbed, rehabilitated and mature forests in subtropical China. Plant Soil 2006, 282, 135–151. [Google Scholar] [CrossRef]
- Zhou, S.X.; Huang, C.D.; Han, B.H.; Xiao, Y.X.; Tang, J.D.; Xiang, Y.B.; Luo, C. Simulated nitrogen deposition significantly suppresses the decomposition of forest litter in a natural evergreen broad-leaved forest in the rainy area of Western China. Plant Soil 2017, 420, 135–145. [Google Scholar] [CrossRef]
- Carreiro, M.; Sinsabaugh, R.; Repert, D.; Parkhurst, D. Microbial enzyme shifts explain litter decay responses to simulated nitrogen deposition. Ecology 2000, 81, 2359–2365. [Google Scholar] [CrossRef]
- Hobbie, S.E. Nitrogen effects on decomposition: A five-year experiment in eight temperate sites. Ecology 2008, 89, 2633–2644. [Google Scholar] [CrossRef]
- Reich, P.B.; Oleksyn, J.; Modrzynski, J.; Mrozinski, P.; Hobbie, S.E.; Eissenstat, D.M.; Chorover, J.; Chadwick, O.A.; Hale, C.M.; Tjoelker, M.G. Linking litter calcium, earthworms and soil properties: A common garden test with 14 tree species. Ecol. Lett. 2005, 8, 811–818. [Google Scholar] [CrossRef]
- Zhang, W.D.; Chao, L.; Yang, Q.P.; Wang, Q.K.; Fang, Y.T.; Wang, S.L. Litter quality mediated nitrogen effect on plant litter decomposition regardless of soil fauna presence. Ecology 2016, 97, 2834–2843. [Google Scholar] [CrossRef]
- Micks, P.; Downs, M.R.; Magill, A.H.; Nadelhoffer, K.J.; Aber, J.D. Decomposing litter as a sink for 15N-enriched additions to an oak forest and a red pine plantation. For. Ecol. Manag. 2004, 196, 71–87. [Google Scholar] [CrossRef]
- Zhu, X.M.; Chen, H.; Zhang, W.; Huang, J.; Fu, S.L.; Liu, Z.F.; Mo, J.M. Effects of nitrogen addition on litter decomposition and nutrient release in two tropical plantations with N2-fixing vs. non-N2-fixing tree species. Plant Soil 2016, 399, 61–74. [Google Scholar] [CrossRef]
- Parton, W.; Silver, W.L.; Burke, I.C.; Grassens, L.; Harmon, M.E.; Currie, W.S.; King, J.Y.; Adair, E.C.; Brandt, L.A.; Hart, S.C. Global-scale similarities in nitrogen release patterns during long-term decomposition. Science 2007, 315, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.R.; Jia, Y.L.; He, N.P.; Zhu, J.X.; Chen, Z.; Wang, Q.F.; Piao, S.L.; Liu, X.J.; He, H.L.; Guo, X.B.; et al. Stabilization of atmospheric nitrogen deposition in China over the past decade. Nat. Geosci. 2019, 12, 424–429. [Google Scholar] [CrossRef]
- Yu, G.R.; Chen, Z.; Piao, S.L.; Peng, C.H.; Ciais, P.; Wang, Q.F.; Li, X.R.; Zhu, X.J. High carbon dioxide uptake by subtropical forest ecosystems in the East Asian monsoon region. Proc. Natl. Acad. Sci. USA 2014, 111, 4910–4915. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhao, Y.H.; Liu, X.J.; Dore, A.J.; Zhang, L.; Liu, L.; Cheng, M.M. Atmospheric nitrogen deposition in the Yangtze River basin: Spatial pattern and source attribution. Environ. Pollut. 2018, 232, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.H.; Hu, H.L.; Chen, G.; Peng, Y.; Xiao, Y.L.; Hu, T.X.; Zhang, L.; Li, X.W.; Liu, L.; Tang, Y. Nitrogen addition significantly affects forest litter decomposition under high levels of ambient nitrogen deposition. PLoS ONE 2014, 9, e88752. [Google Scholar] [CrossRef]
- Chen, G.T.; Tu, L.H.; Peng, Y.; Hu, H.L.; Hu, T.X.; Xu, Z.F.; Liu, L.; Tang, Y. Effect of nitrogen additions on root morphology and chemistry in a subtropical bamboo forest. Plant Soil 2017, 412, 441–451. [Google Scholar] [CrossRef]
- Tu, L.H.; Hu, T.X.; Zhang, J.; Li, X.W.; Hu, H.L.; Liu, L.; Xiao, Y.L. Nitrogen addition stimulates different components of soil respiration in a subtropical bamboo ecosystem. Soil Biol. Biochem. 2013, 58, 255–264. [Google Scholar] [CrossRef]
- Liu, Q.; Zhuang, L.Y.; Ni, X.Y.; You, C.M.; Yang, W.Q.; Wu, F.Z.; Tan, B.; Yue, K.; Liu, Y.; Zhang, L.; et al. Nitrogen additions stimulate litter humification in a subtropical forest, southwestern China. Sci. Rep. 2018, 8, 17525. [Google Scholar] [CrossRef]
- Yang, K.J.; Yang, Y.L.; Xu, Z.F.; Wu, Q.G. Soil respiration in a subtropical forest of southwestern China: Components, patterns and controls. PLoS ONE 2018, 13, e0204341. [Google Scholar] [CrossRef]
- Cornwell, W.K.; Cornelissen, J.H.C.; Amatangelo, K.; Dorrepaal, E.; Eviner, V.T.; Godoy, O.; Hobbie, S.E.; Hoorens, B.; Kurokawa, H.; Pérez-Harguindeguy, N.; et al. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol. Lett. 2008, 11, 1065–1071. [Google Scholar] [CrossRef]
- Marklein, A.R.; Houlton, B.Z. Nitrogen inputs accelerate phosphorus cycling rates across a wide variety of terrestrial ecosystems. New Phytol. 2012, 193, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Aerts, R. Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: A triangular relationship. Oikos 1997, 79, 439–449. [Google Scholar] [CrossRef]
- Moore, T.R.; Trofymow, J.A.; Prescott, C.E.; Fyles, J.; Titus, B.D. Patterns of carbon, nitrogen and phosphorus dynamics in decomposing foliar litter in Canadian forests. Ecosystems 2006, 9, 46–62. [Google Scholar] [CrossRef]
- Tu, L.H.; Hu, H.L.; Hu, T.X.; Zhang, J.; Liu, L.; Li, R.H.; Dai, H.Z.; Luo, S.H. Decomposition of different litter fractions in a subtropical bamboo ecosystem as affected by experimental nitrogen deposition. Pedosphere 2011, 21, 685–695. [Google Scholar] [CrossRef]
- Kuperman, R.G. Litter decomposition and nutrient dynamics in oak–hickory forests along a historic gradient of nitrogen and sulfur deposition. Soil Biol. Biochem. 1999, 31, 237–244. [Google Scholar] [CrossRef]
- Zhou, G.; Zhang, J.; Qiu, X.; Wei, F.; Xu, X. Decomposing litter and associated microbial activity responses to nitrogen deposition in two subtropical forests containing nitrogen-fixing or non-nitrogen-fixing tree species. Sci. Rep. 2018, 8, 129334. [Google Scholar] [CrossRef]
- Zheng, Z.M.; Mamuti, M.; Liu, H.M.; Shu, Y.Q.; Hu, S.J.; Wang, X.H.; Li, B.B.; Lin, L.; Li, X. Effects of nutrient additions on litter decomposition regulated by phosphorus-induced changes in litter chemistry in a subtropical forest, China. For. Ecol. Manag. 2017, 400, 123–128. [Google Scholar] [CrossRef]
- Vivanco, L.; Austin, A.T. Nitrogen addition stimulates forest litter decomposition and disrupts species interactions in Patagonia, Argentina. Glob. Chang. Biol. 2011, 17, 1963–1974. [Google Scholar] [CrossRef]
- Chen, H.; Dong, S.; Liu, L.; Ma, C.; Zhang, T.; Zhu, X.; Mo, J. Effects of experimental nitrogen and phosphorus addition on litter decomposition in an old-growth tropical forest. PLoS ONE 2013, 8, e84101. [Google Scholar] [CrossRef]
- Gong, J.; Zhu, C.; Yang, L.; Yang, B.; Wang, B.; Baoyin, T.T.; Liu, M.; Zhang, Z.; Shi, J. Effects of nitrogen addition on above-and belowground litter decomposition and nutrient dynamics in the litter-soil continuum in the temperate steppe of Inner Mongolia, China. J. Arid Environ. 2020, 172, 104036. [Google Scholar] [CrossRef]
- Yuan, Z.; Chen, H.Y. Negative effects of fertilization on plant nutrient resorption. Ecology 2015, 96, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Cotrufo, M.F.; Ineson, P.; Roberts, J.D. Decomposition of birch leaf litters with varying C-to-N ratios. Soil Biol. Biochem. 1995, 27, 1219–1221. [Google Scholar] [CrossRef]
- Finn, D.; Page, K.; Catton, K.; Strounina, E.; Kienzle, M.; Robertson, F.; Armstrong, R.; Dalal, R. Effect of added nitrogen on plant litter decomposition depends on initial soil carbon and nitrogen stoichiometry. Soil Biol. Biochem. 2015, 91, 160–168. [Google Scholar] [CrossRef]
- Manzoni, S.; Trofymow, J.A.; Jackson, R.B.; Porporato, A. Stoichiometric controls on carbon, nitrogen, and phosphorus dynamics in decomposing litter. Ecol. Monogr. 2010, 80, 89–106. [Google Scholar] [CrossRef]
| C Remaining | N Remaining | P Remaining | C:N | C:P | N:P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | F | p | F | p | |
| S | 45.90 | <0.001 | 52.22 | <0.001 | 68.98 | <0.001 | 173.58 | < 0.001 | 257.68 | <0.001 | 68.21 | <0.001 |
| N | 2.34 | 0.139 | 4.69 | 0.031 | 9.04 | 0.004 | 1.12 | 0.359 | 4.13 | 0.043 | 3.29 | 0.073 |
| T | 182.18 | <0.001 | 32.81 | <0.001 | 39.36 | <0.001 | 81.73 | < 0.001 | 55.80 | <0.001 | 8.63 | 0.004 |
| S × T | 3.78 | 0.027 | 24.65 | <0.001 | 35.38 | <0.001 | 3.25 | 0.039 | 15.38 | <0.001 | 3.32 | 0.062 |
| N × T | 2.13 | 0.090 | 6.86 | 0.006 | 11.92 | 0.001 | 0.77 | 0.589 | 6.49 | <0.001 | 4.18 | 0.030 |
| N × S | 0.43 | 0.659 | 0.95 | 0.415 | 1.21 | 0.333 | 2.82 | 0.099 | 0.24 | 0.210 | 0.20 | 0.237 |
| N × S × T | 1.37 | 0.266 | 5.51 | 0.013 | 14.36 | <0.001 | 0.83 | 0.542 | 5.92 | <0.001 | 8.52 | 0.003 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuang, L.; Liu, Q.; Liang, Z.; You, C.; Tan, B.; Zhang, L.; Yin, R.; Yang, K.; Bol, R.; Xu, Z. Nitrogen Additions Retard Nutrient Release from Two Contrasting Foliar Litters in a Subtropical Forest, Southwest China. Forests 2020, 11, 377. https://doi.org/10.3390/f11040377
Zhuang L, Liu Q, Liang Z, You C, Tan B, Zhang L, Yin R, Yang K, Bol R, Xu Z. Nitrogen Additions Retard Nutrient Release from Two Contrasting Foliar Litters in a Subtropical Forest, Southwest China. Forests. 2020; 11(4):377. https://doi.org/10.3390/f11040377
Chicago/Turabian StyleZhuang, Liyan, Qun Liu, Ziyi Liang, Chengming You, Bo Tan, Li Zhang, Rui Yin, Kaijun Yang, Roland Bol, and Zhenfeng Xu. 2020. "Nitrogen Additions Retard Nutrient Release from Two Contrasting Foliar Litters in a Subtropical Forest, Southwest China" Forests 11, no. 4: 377. https://doi.org/10.3390/f11040377
APA StyleZhuang, L., Liu, Q., Liang, Z., You, C., Tan, B., Zhang, L., Yin, R., Yang, K., Bol, R., & Xu, Z. (2020). Nitrogen Additions Retard Nutrient Release from Two Contrasting Foliar Litters in a Subtropical Forest, Southwest China. Forests, 11(4), 377. https://doi.org/10.3390/f11040377





