Nitrogen Use Efficiency for Growth of Fagus crenata Seedlings Under Elevated Ozone and Different Soil Nutrient Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Design
2.2. Measurement of Plant Growth and Nitrogen Concentration
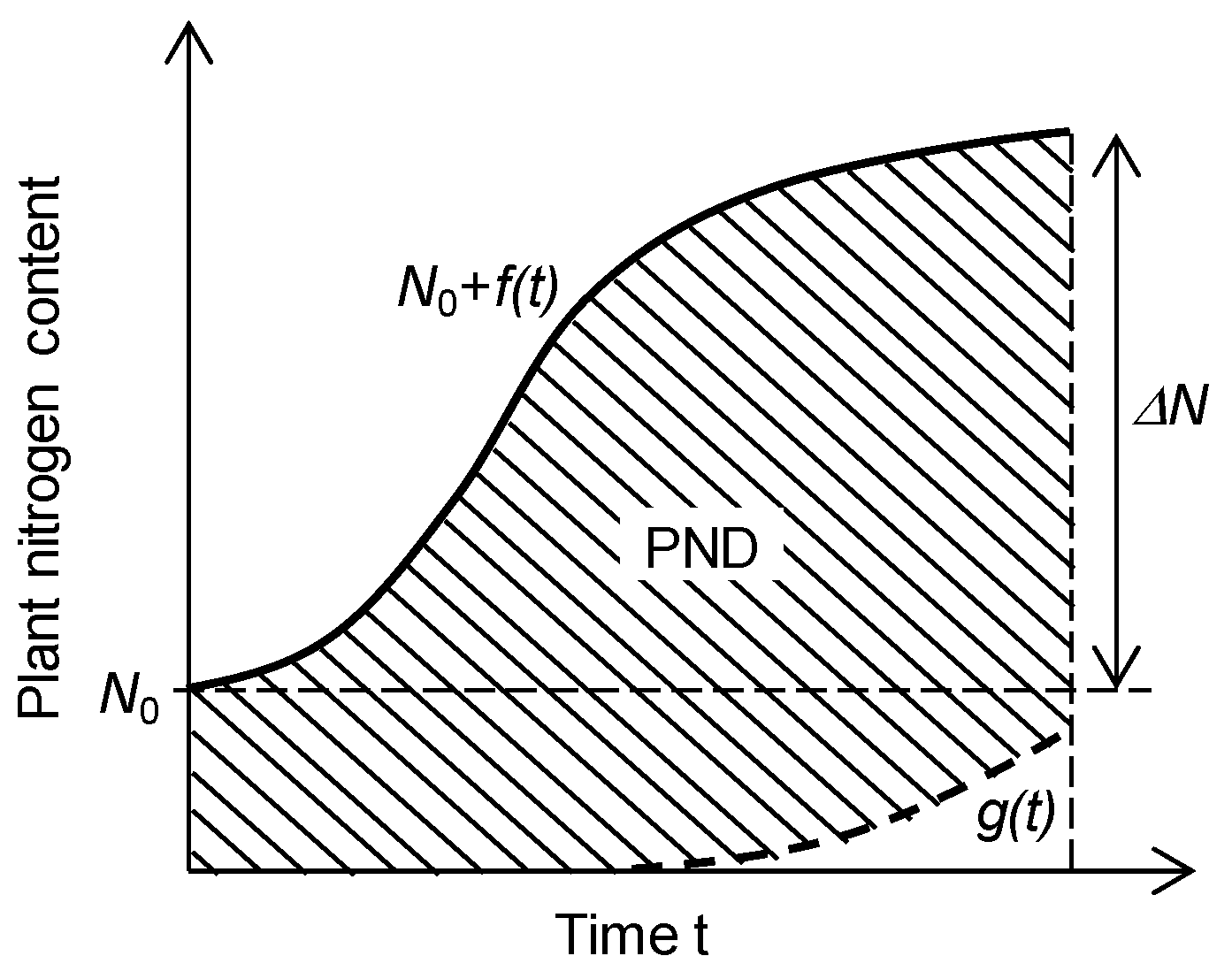
2.3. Determination of Nitrogen Use Efficiency and Its Components
2.4. Gas Exchange Measurement of Leaves
2.5. Statistical Analyses
3. Results
3.1. Nitrogen Use Efficiency and Its Components
3.2. Leaf Photosynthetic Traits
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| NUE | Nitrogen use efficiency |
| NP | Nitrogen productivity |
| MRT | Mean residence time of nitrogen |
| ΔW | Dry mass increment for a given period |
| ΔN | Amount of nitrogen uptake from the soil for a given period |
| N0 | Initial nitrogen content |
| PND | Plant nitrogen duration |
| Mean whole-plant nitrogen content during a given time period | |
| Mean whole-leaf nitrogen content during a given time period | |
| LNF | Leaf nitrogen fraction |
| LNP | Leaf nitrogen productivity |
| ∫Nloss | Time integral of nitrogen loss |
| ∫Nuptake | Time integral of nitrogen uptake |
| CF | Charcoal-filtered air |
| 1.0 × O3 | 1.0-fold ambient ozone concentration |
| 1.5 × O3 | 1.5-fold ambient ozone concentration |
| AOT40 | Accumulated exposure over a threshold of 40 nmol mol−1 |
| NF | Non-fertilised treatment |
| LF | Low-fertilised treatment |
| HF | High-fertilised treatment |
| Asat | Light-saturated net photosynthetic rate |
| Narea | Area-based nitrogen content in leaves |
| PNUE | Photosynthetic nitrogen use efficiency |
| D2H | Product of the square of the diameter and height |
| VPD | Vapour pressure deficit |
References
- Akimoto, H. Global air quality and pollution. Science 2003, 302, 1716–1719. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, D.S.; Dentener, F.J.; Schultz, M.G.; Ellingsen, K.; Van Noije, T.P.C.; Wild, O.; Zeng, G.; Amann, M.; Atherton, C.S.; Bell, N.; et al. Multi-model ensemble simulations of present-day and near-future tropospheric ozone. J. Geophys. Res. 2006, 111, D08301. [Google Scholar] [CrossRef]
- Paoletti, E. Ozone Impacts on Forests. In CAB Reviews: Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources No. 68; CABI: Wallingford, UK, 2007; pp. 1–13. [Google Scholar]
- Ohara, T.; Akimoto, H.; Kurokawa, J.; Horii, N.; Yamaji, K.; Yan, X.; Hatasaka, T. An Asian emission inventory of anthropogenic emission sources for the period 1980–2020. Atmos. Chem. Phys. 2007, 7, 4419–4444. [Google Scholar] [CrossRef]
- Naja, M.; Akimoto, H. Contribution of regional pollution and long-range transport to the Asia-Pacific region: Analysis of long-term ozonesonde data over Japan. J. Geophys. Res. 2004, 109, D21306. [Google Scholar] [CrossRef]
- Yamaji, K.; Ohara, T.; Uno, I.; Kurokawa, J.; Pochanart, P.; Akimoto, H. Future prediction of surface ozone over east Asia using models-3 community multiscale air quality modeling system and regional emission inventory in Asia. J. Geophys. Res. 2008, 113, D08306. [Google Scholar] [CrossRef]
- Wittig, V.E.; Ainsworth, E.A.; Long, S.P. To what extent do current and projected increases in surface ozone affect photosynthesis and stomatal conductance of trees? A meta-analytic review of the last 3 decades of experiments. Plant Cell Environ. 2007, 30, 1150–1162. [Google Scholar] [CrossRef]
- Wittig, V.E.; Ainsworth, E.A.; Naidu, S.L.; Karnosky, D.F.; Long, S.P. Quantifying the impact of current and future tropospheric ozone on tree biomass, growth, physiology and biochemistry: A quantitative meta-analysis. Glob. Chang. Biol. 2009, 15, 396–424. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Watanabe, M.; Matsumura, H.; Kohno, Y.; Izuta, T. Experimental studies on the effects of ozone on growth and photosynthetic activity of Japanese forest tree species. Asian J. Atmos. Environ. 2011, 5, 65–87. [Google Scholar] [CrossRef]
- Watanabe, M.; Hoshika, Y.; Koike, T.; Izuta, T. Effects of ozone on Japanese trees. In Air Pollution Impacts on Plant in East Asia; Izuta, T., Ed.; Springer: Tokyo, Japan, 2017; pp. 73–100. [Google Scholar]
- Matyssek, R.; Sandermann, H. Impact of ozone on trees: An ecophysiological perspective. Prog. Bot. 2003, 64, 349–404. [Google Scholar]
- Sitch, S.; Cox, P.M.; Collins, W.J.; Huntingford, C. Indirect radiative forcing of climate change through ozone effects on the land-carbon sink. Nature 2007, 448, 791–794. [Google Scholar] [CrossRef]
- Magnani, F.; Mencuccini, M.; Borghetti, M.; Berbigier, P.; Berninger, F.; Delzon, S.; Grelle, A.; Hari, P.; Jarvis, P.G.; Kolari, P.; et al. The human footprint in the carbon cycle of temperate and boreal forests. Nature 2007, 447, 848–852. [Google Scholar] [CrossRef] [PubMed]
- Chapin, F.S., III; Matson, P.A.; Vitousek, P.M. Principles of Terrestrial Ecosystem Ecology, 2nd ed.; Springer: Berlin, Germany, 2012; pp. 1–529. [Google Scholar]
- Hirose, T. Relations between turnover rate, resource utility and structure of some plant populations: A study of the matter budgets. J. Fac. Sci. Univ. Tokyo 1975, 11, 355–407. [Google Scholar]
- Hirose, T. Nitrogen use efficiency revisited. Oecologia 2011, 166, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Ingestad, T. Nitrogen stress in birch seedlings. II. N, K, P, Ca and Mg nutrition. Physiol. Plant. 1979, 45, 149–157. [Google Scholar] [CrossRef]
- Berendse, F.; Aerts, R. Nitrogen-use-efficiency: A biologically meaningful definition? Funct. Ecol. 1987, 1, 293–296. [Google Scholar]
- Berendse, F. The effects of grazing on the outcome of competition between plant species with different nutrient requirements. Oikos 1985, 44, 35–39. [Google Scholar] [CrossRef]
- Watanabe, M.; Hoshika, Y.; Inada, N.; Wang, X.; Mao, Q.; Koike, T. Photosynthetic traits of Siebold’s beech and oak saplings grown under free air ozone exposure in northern Japan. Environ. Pollut. 2013, 174, 50–56. [Google Scholar] [CrossRef]
- Kytöviita, M.; Thiec, D.; Dizengremel, P. Elevated CO2 and ozone reduce nitrogen acquisition by Pinus halepensis from its mycorrhizal symbiont. Physhiolosia Plant. 2001, 111, 305–312. [Google Scholar] [CrossRef]
- Häkiö, E.; Freiwald, V.; Silfver, T.; Beuker, E.; Holopainen, T.; Oksanen, E. Impacts of elevated ozone and nitrogen on growth and photosynthesis of European aspen (Populus tremula) and hybrid aspen (P. tremula × Populus tremuloides) clones. Can. J. For. Res. 2007, 37, 2326–2336. [Google Scholar] [CrossRef]
- Watanabe, M.; Yamaguchi, M.; Tabe, C.; Iwasaki, M.; Yamashita, R.; Funada, R.; Fukami, M.; Matsumura, H.; Kohno, Y.; Izuta, T. Influences of nitrogen load on the growth and photosynthetic responses of Quercus serrata seedlings to O3. Trees 2007, 21, 421–432. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Watanabe, M.; Iwasaki, M.; Tabe, C.; Matsumura, H.; Kohno, Y.; Izuta, T. Growth and photosynthetic responses of Fagus crenata seedlings to O3 under different nitrogen loads. Trees 2007, 21, 707–718. [Google Scholar] [CrossRef]
- Hoshika, Y.; Watanabe, M.; Inada, N.; Mao, Q.; Koike, T. Photosynthetic response of early and late leaves of white birch (Betula platyphylla var. japonica) grown under free-air ozone exposure. Environ. Pollut. 2013, 182, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Kinose, Y.; Fukamachi, Y.; Okabe, S.; Hiroshima, H.; Watanabe, M.; Izuta, T. Nutrient supply to soil offsets the ozone-induced growth reduction in Fagus crenata seedlings. Trees 2017, 31, 259–272. [Google Scholar] [CrossRef]
- Landolt, W.; Günthardt-Goerg, M.S.; Pfenninger, I.; Einig, W.; Hampp, R.; Maurer, S.; Matyssek, R. Effect of fertilization on ozone-induced changes in the metabolism of birch (Betula pendula) leaves. New Phytolosist 1997, 137, 389–397. [Google Scholar] [CrossRef]
- Kolb, T.E.; Matyssek, R. Limitations and perspectives about scaling ozone impacts in trees. Environ. Pollut. 2001, 115, 373–393. [Google Scholar] [CrossRef]
- Thomas, V.F.D.; Braun, S.; Flückiger, W. Effects of simultaneous ozone exposure and nitrogen loads on carbohydrate concentrations, biomass, growth, and nutrient concentrations of young beech trees (Fagus sylvatica). Environ. Pollut. 2005, 143, 341–354. [Google Scholar] [CrossRef]
- Watanabe, M.; Yamaguchi, M.; Iwasaki, M.; Matsuo, N.; Naba, J.; Tabe, C.; Matsumura, H.; Kohno, Y.; Izuta, T. Effects of ozone and/or nitrogen load on the growth of Larix kaempferi, Pinus densiflora and Cryptomeria japonica seedlings. J. Jpn. Soc. Atmos. Environ. 2006, 41, 320–334. [Google Scholar]
- Marzuoli, R.; Monga, R.; Finco, A.; Gerosa, G. Biomass and physiological responses of Quercus robur (L.) young trees during 2 years of treatments with different levels of ozone and nitrogen wet deposition. Trees 2016, 30, 1995–2000. [Google Scholar] [CrossRef]
- Marzuoli, R.; Monga, R.; Finco, A.; Chiesa, M.; Gerosa, G. Increased nitrogen wet deposition triggers negative effects of ozone on the biomass production of Carpinus betulus L. young trees. Environ. Exp. Bot. 2018, 152, 128–136. [Google Scholar] [CrossRef]
- Nakashizuka, T.; Iida, S. Composition, dynamics and disturbance regime of temperate deciduous forests in Monsoon Asia. Vegetatio 1995, 121, 23–30. [Google Scholar] [CrossRef]
- Nakashizuka, T. Story of Forest Trees and Japan; Tokai University Press: Hatano, Japan, 2004; pp. 1–236. (In Japanese) [Google Scholar]
- Terazawa, K.; Koyama, H. Applied Ecology for Restoration of Beech Forests; Bun-ichi Sogo Shuppan: Tokyo, Japan, 2008; pp. 1–310. (In Japanese) [Google Scholar]
- Kohno, Y.; Matsumura, H.; Ishii, T.; Izuta, T. Establishing critical levels of air pollutants for protecting East Asian vegetation—A challenge. In Plant Responses to Air Pollution and Global Change; Omasa, K., Nouchi, I., De Kok, L.J., Eds.; Springer: Tokyo, Japan, 2005; pp. 243–250. [Google Scholar]
- Kinose, Y.; Azuchi, F.; Uehara, Y.; Kanomata, T.; Kobayashi, A.; Yamaguchi, M.; Izuta, T. Modeling of stomatal conductance to estimate stomatal ozone uptake by Fagus crenata, Quercus serrata, Quercus mongolica var. crispula and Betula platyphylla. Environ. Pollut. 2014, 194, 235–245. [Google Scholar] [CrossRef] [PubMed]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. In World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015; pp. 1–192. Available online: http://www.fao.org/3/i3794en/I3794en.pdf (accessed on 29 November 2019).
- Kinose, Y. Photosynthetic and Growth Responses to Ozone of Fagus Crenata Seedlings Grown under Different Soil Nutrient Levels. Ph.D. Thesis, Tokyo University of Agriculture and Technology, Tokyo, Japan, 16 March 2017. [Google Scholar]
- Agathokleous, E.; Kitao, M.; Kinose, Y. A review study on O3 phytotoxicity metrics for setting critical levels in Asia. Asian J. Atmos. Environ. 2018, 12, 1–16. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; pp. 1–3520. Available online: http://www.R-project.org (accessed on 29 November 2019).
- Watanabe, M.; Okabe, S.; Kinose, Y.; Hiroshima, H.; Izuta, T. Effects of ozone on soil respiration rate of Siebold’s beech seedlings grown under different soil nutrient conditions. J. Agric. Meteorol. 2019, 75, 39–46. [Google Scholar] [CrossRef]
- Sugai, T.; Watanabe, T.; Kita, K.; Koike, T. Nitrogen loading increases the ozone sensitivity of larch seedlings with higher sensitivity to nitrogen loading. Sci. Total. Environ. 2019, 663, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Utriainen, J.; Holopainen, T. Nitrogen availability modifies the ozone responses of Scots pine seedlings exposed in an open-field system. Tree Physiol. 2001, 21, 1205–1213. [Google Scholar] [CrossRef]
- Kinose, Y.; Fukamachi, Y.; Okabe, S.; Hiroshima, H.; Watanabe, M.; Izuta, T. Photosynthetic responses to ozone of upper and lower canopy leaves of Fagus crenata Blume seedlings grown under different soil nutrient conditions. Environ. Pollut. 2017, 223, 213–222. [Google Scholar] [CrossRef]
- Kikuzawa, K. Leaf survival of woody plant in deciduous broad-leaved forests. 1. Tall trees. Can. J. Bot. 1983, 61, 2133–2139. [Google Scholar] [CrossRef]
- Tjoelker, M.G.; Luxmoore, R.J. Soil nitrogen and chronic ozone stress influence physiology, growth and nutrient status of Pinus taeda L. and Liriodendron tulipifera L. seedlings. New Phytol. 1991, 119, 69–81. [Google Scholar] [CrossRef]
- Reich, P.B. Effects of low concentrations of O3 on net photosynthesis, dark respiration, and chlorophyll contents in aging hybrid poplar leaves. Plant Physiol. 1983, 73, 291–296. [Google Scholar] [CrossRef]
- Zhu, B.; Izuta, T.; Watanabe, M. Nitrogen use efficiency of Quercus serrata seedlings under different soil nitrogen and phosphorus supplies. J. Agric. Meteorol. 2020, 76, 11–19. [Google Scholar]

| Concentration (nmol mol−1) | AOT40 (μmol mol−1 h) | |||
|---|---|---|---|---|
| 24 h | Daylight Hours | Daylight Hours | ||
| 2014 | CF | 8.1 (1.1) | 13.1 (1.2) | 0.1 (0.0) |
| 15 May–30 Nov. | 1.0 × O3 | 16.9 (0.7) | 22.6 (0.5) | 4.1 (0.0) |
| 1.5 × O3 | 24.4 (0.6) | 33.5 (0.7) | 14.4 (0.7) | |
| 2015 | CF | 8.1 (0.6) | 11.2 (1.7) | 0.1 (0.1) |
| 21 Apr.–26 Oct. | 1.0 × O3 | 23.9 (0.3) | 32.9 (0.4) | 8.9 (0.4) |
| 1.5 × O3 | 34.9 (0.2) | 49.3 (0.1) | 26.8 (0.0) | |
| Nutrient | Gas | ΔW | ΔN | LNF | LNP | N0 | PND | ∫Nloss | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (g) | (g N) | (g N g−1 N) | (g g−1 N day−1) | (g N) | (g N day) | (g N day) | |||||||||||||
| NF | CF | 131 | (4) | ab | 0.73 | (0.05) | 0.25 | (0.02) | 2.36 | (0.33) | 0.74 | (0.04) | 226 | (19) | 3.46 | (1.72) | |||
| 1.0 × O3 | 117 | (2) | bc | 0.71 | (0.17) | 0.24 | (0.03) | 2.42 | (0.13) | 0.62 | (0.16) | 204 | (8) | 3.37 | (0.60) | ||||
| 1.5 × O3 | 105 | (13) | c | 0.73 | (0.16) | 0.25 | (0.03) | 2.17 | (0.13) | 0.62 | (0.06) | 202 | (17) | 3.46 | (1.36) | ||||
| LF | CF | 130 | (4) | abc | 0.80 | (0.11) | 0.28 | (0.02) | 1.93 | (0.13) | 0.75 | (0.06) | 240 | (3) | 3.12 | (1.09) | |||
| 1.0 × O3 | 136 | (8) | ab | 0.93 | (0.27) | 0.27 | (0.01) | 2.13 | (0.35) | 0.67 | (0.20) | 237 | (22) | 4.94 | (1.90) | ||||
| 1.5 × O3 | 122 | (10) | abc | 0.88 | (0.04) | 0.25 | (0.02) | 2.08 | (0.10) | 0.69 | (0.15) | 232 | (36) | 6.31 | (0.52) | ||||
| HF | CF | 133 | (11) | ab | 1.16 | (0.10) | 0.25 | (0.04) | 1.90 | (0.38) | 0.75 | (0.09) | 284 | (21) | 4.28 | (1.32) | |||
| 1.0 × O3 | 138 | (12) | ab | 1.20 | (0.13) | 0.26 | (0.02) | 1.93 | (0.13) | 0.66 | (0.11) | 272 | (7) | 4.80 | (4.15) | ||||
| 1.5 × O3 | 143 | (10) | a | 1.25 | (0.11) | 0.26 | (0.02) | 1.96 | (0.17) | 0.69 | (0.08) | 288 | (20) | 4.79 | (2.73) | ||||
| Nutrient pooled | |||||||||||||||||||
| CF | 131 | (7) | 0.89 | (0.23) | 0.26 | (0.03) | 2.06 | (0.29) | 0.75 | (0.09) | 250 | (29) | 3.62 | (1.25) | |||||
| 1.0 × O3 | 130 | (12) | 0.95 | (0.26) | 0.26 | (0.02) | 2.16 | (0.29) | 0.65 | (0.13) | 238 | (36) | 4.37 | (1.35) | |||||
| 1.5 × O3 | 124 | (19) | 0.95 | (0.25) | 0.25 | (0.02) | 2.07 | (0.24) | 0.66 | (0.09) | 241 | (41) | 4.85 | (2.85) | |||||
| Gas pooled | |||||||||||||||||||
| NF | 118 | (13) | a | 0.72 | (0.08) | b | 0.24 | (0.02) | 2.32 | (0.28) | a | 0.66 | (0.08) | 211 | (18) | c | 3.43 | (1.21) | |
| LF | 129 | (10) | ab | 0.87 | (0.18) | b | 0.27 | (0.02) | 2.04 | (0.22) | b | 0.70 | (0.14) | 236 | (13) | b | 4.79 | (2.69) | |
| HF | 138 | (10) | b | 1.20 | (0.11) | a | 0.26 | (0.02) | 1.93 | (0.12) | b | 0.70 | (0.10) | 281 | (24) | a | 4.63 | (1.57) | |
| ANOVA | Ozone (O3) | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |||||||||||
| Nutrient (N) | ** | *** | 0.058 | * | n.s. | *** | n.s. | ||||||||||||
| O3 × N | * | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | ||||||||||||
| Nutrient | Gas | May | July | September | |||||
|---|---|---|---|---|---|---|---|---|---|
| Upper canopy leaves | |||||||||
| NF | CF | 10.4 | (0.7) | bcd | 9.7 | (0.6) | 7.1 | (0.5) | bc |
| 1.0 × O3 | 10.1 | (0.8) | cde | 9.8 | (0.9) | 6.7 | (0.7) | bcde | |
| 1.5 × O3 | 11.0 | (0.3) | abc | 9.0 | (0.4) | 6.1 | (0.2) | cdef | |
| LF | CF | 12.1 | (0.3) | b | 11.3 | (0.6) | 7.7 | (0.8) | ab |
| 1.0 × O3 | 11.3 | (0.5) | abc | 10.2 | (0.2) | 7.4 | (0.3) | bc | |
| 1.5 × O3 | 11.3 | (0.8) | abc | 9.6 | (1.6) | 6.0 | (0.3) | cdef | |
| HF | CF | 12.3 | (0.7) | a | 10.8 | (1.1) | 9.0 | (1.0) | a |
| 1.0 × O3 | 11.5 | (0.2) | ab | 9.5 | (0.8) | 7.3 | (0.4) | bc | |
| 1.5 × O3 | 11.1 | (0.2) | abc | 9.6 | (0.2) | 7.3 | (0.3) | bc | |
| Lower canopy leaves | |||||||||
| NF | CF | 8.6 | (0.6) | f | 7.1 | (0.4) | 5.3 | (0.7) | ef |
| 1.0 × O3 | 8.5 | (0.1) | f | 6.8 | (0.6) | 5.4 | (0.0) | def | |
| 1.5 × O3 | 9.2 | (0.4) | def | 6.6 | (0.9) | 5.0 | (0.4) | f | |
| LF | CF | 8.5 | (0.2) | f | 8.1 | (0.4) | 6.8 | (0.1) | bcd |
| 1.0 × O3 | 8.3 | (0.2) | f | 8.1 | (0.9) | 6.1 | (0.4) | cdef | |
| 1.5 × O3 | 8.9 | (0.2) | ef | 7.8 | (0.5) | 5.1 | (0.2) | f | |
| HF | CF | 8.6 | (0.3) | f | 8.5 | (1.1) | 7.2 | (0.3) | bc |
| 1.0 × O3 | 8.7 | (0.6) | ef | 8.0 | (0.4) | 6.4 | (0.4) | bcdef | |
| 1.5 × O3 | 8.6 | (0.1) | f | 8.0 | (0.8) | 5.1 | (0.6) | f | |
| Nutrient pooled | |||||||||
| CF | 9.5 | (2.9) | 8.8 | (2.7) | 6.8 | (2.0) | a | ||
| 1.0 × O3 | 9.2 | (2.6) | 8.3 | (2.4) | 6.2 | (1.7) | a | ||
| 1.5 × O3 | 9.5 | (2.6) | 8.0 | (2.3) | 5.4 | (1.6) | b | ||
| Gas pooled | |||||||||
| NF | 9.6 | (1.1) | 8.2 | (1.5) | 5.9 | (0.9) | b | ||
| LF | 10.1 | (1.6) | 9.2 | (1.5) | 6.5 | (1.0) | ab | ||
| HF | 10.1 | (1.6) | 9.1 | (1.2) | 7.0 | (1.3) | a | ||
| Leaf position | |||||||||
| Upper | 11.2 | (0.8) | 9.9 | (1.0) | 7.2 | (1.0) | |||
| Lower | 8.6 | (0.4) | 7.7 | (0.9) | 5.8 | (0.9) | |||
| ANOVA | |||||||||
| O3 | n.s. | * | *** | ||||||
| Nutrient (N) | ** | *** | *** | ||||||
| Leaf position (LP) | *** | *** | *** | ||||||
| O3 × N | * | n.s. | * | ||||||
| O3 × LP | * | n.s. | n.s. | ||||||
| N × LP | *** | n.s. | n.s. | ||||||
| O3 × N × LP | n.s. | n.s. | n.s. | ||||||
| Nutrient | Gas | May | July | September | |||
|---|---|---|---|---|---|---|---|
| Upper canopy leaves | |||||||
| NF | CF | 1.5 | (0.2) | 1.7 | (0.3) | 1.5 | (0.1) |
| 1.0 × O3 | 1.5 | (0.2) | 1.6 | (0.1) | 1.4 | (0.1) | |
| 1.5 × O3 | 1.7 | (0.2) | 1.9 | (0.1) | 1.5 | (0.1) | |
| LF | CF | 1.8 | (0.2) | 1.8 | (0.1) | 1.5 | (0.2) |
| 1.0 × O3 | 1.6 | (0.2) | 1.8 | (0.3) | 1.5 | (0.1) | |
| 1.5 × O3 | 1.7 | (0.1) | 1.8 | (0.2) | 1.5 | (0.1) | |
| HF | CF | 1.7 | (0.1) | 1.9 | (0.1) | 1.7 | (0.1) |
| 1.0 × O3 | 1.8 | (0.2) | 1.8 | (0.1) | 1.6 | (0.1) | |
| 1.5 × O3 | 1.8 | (0.0) | 1.9 | (0.0) | 1.6 | (0.0) | |
| Lower canopy leaves | |||||||
| NF | CF | 0.9 | (0.1) | 1.0 | (0.1) | 1.0 | (0.1) |
| 1.0 × O3 | 0.9 | (0.0) | 1.1 | (0.1) | 1.0 | (0.1) | |
| 1.5 × O3 | 1.1 | (0.0) | 1.3 | (0.1) | 1.0 | (0.1) | |
| LF | CF | 1.1 | (0.0) | 1.1 | (0.1) | 1.1 | (0.1) |
| 1.0 × O3 | 1.0 | (0.2) | 1.1 | (0.1) | 1.0 | (0.1) | |
| 1.5 × O3 | 1.1 | (0.1) | 1.3 | (0.1) | 1.1 | (0.1) | |
| HF | CF | 1.1 | (0.1) | 1.3 | (0.2) | 1.2 | (0.0) |
| 1.0 × O3 | 1.1 | (0.0) | 1.3 | (0.1) | 1.2 | (0.0) | |
| 1.5 × O3 | 1.1 | (0.0) | 1.3 | (0.0) | 1.1 | (0.1) | |
| Nutrient pooled | |||||||
| CF | 1.3 | (0.5) | 1.4 | (0.5) | 1.3 | (0.4) | |
| 1.0 × O3 | 1.2 | (0.5) | 1.4 | (0.5) | 1.2 | (0.4) | |
| 1.5 × O3 | 1.3 | (0.5) | 1.5 | (0.5) | 1.2 | (0.4) | |
| Gas pooled | |||||||
| NF | 1.3 | (0.3) | 1.4 | (0.3) | 1.2 | (0.3) | |
| LF | 1.4 | (0.4) | 1.5 | (0.3) | 1.3 | (0.3) | |
| HF | 1.4 | (0.3) | 1.6 | (0.3) | 1.4 | (0.3) | |
| Leaf position | |||||||
| Upper | 1.7 | (0.2) | 1.8 | (0.2) | 1.5 | (0.1) | |
| Lower | 1.0 | (0.1) | 1.2 | (0.1) | 1.1 | (0.1) | |
| ANOVA | |||||||
| O3 | n.s. | n.s. | n.s. | ||||
| Nutrient (N) | ** | * | *** | ||||
| Leaf position (LP) | *** | *** | *** | ||||
| O3 × N | n.s. | n.s. | n.s. | ||||
| O3 × LP | n.s. | n.s. | n.s. | ||||
| N × LP | n.s. | n.s. | n.s. | ||||
| O3 × N × LP | n.s. | n.s. | n.s. | ||||
| Nutrient | Gas | May | July | September | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Upper canopy leaves | ||||||||||
| NF | CF | 97 | (8) | bcd | 85 | (23) | 71 | (4) | ||
| 1.0 × O3 | 94 | (5) | cd | 83 | (13) | 67 | (4) | |||
| 1.5 × O3 | 92 | (12) | cd | 68 | (2) | 57 | (3) | |||
| LF | CF | 97 | (9) | bcd | 87 | (7) | 72 | (13) | ||
| 1.0 × O3 | 98 | (9) | bcd | 82 | (12) | 72 | (3) | |||
| 1.5 × O3 | 96 | (9) | cd | 78 | (21) | 58 | (7) | |||
| HF | CF | 102 | (7) | bcd | 82 | (8) | 75 | (14) | ||
| 1.0 × O3 | 92 | (9) | cd | 74 | (8) | 65 | (6) | |||
| 1.5 × O3 | 88 | (1) | d | 72 | (2) | 65 | (4) | |||
| Lower canopy leaves | ||||||||||
| NF | CF | 138 | (16) | a | 100 | (3) | 78 | (13) | ||
| 1.0 × O3 | 126 | (3) | ab | 84 | (4) | 78 | (7) | |||
| 1.5 × O3 | 122 | (2) | abc | 73 | (14) | 68 | (9) | |||
| LF | CF | 110 | (6) | abcd | 100 | (5) | 89 | (10) | ||
| 1.0 × O3 | 121 | (20) | abc | 103 | (12) | 91 | (5) | |||
| 1.5 × O3 | 118 | (11) | abc | 84 | (10) | 66 | (5) | |||
| HF | CF | 107 | (14) | bcd | 93 | (0) | 85 | (7) | ||
| 1.0 × O3 | 118 | (3) | abcd | 86 | (4) | 78 | (6) | |||
| 1.5 × O3 | 108 | (4) | bcd | 88 | (5) | 64 | (7) | |||
| Nutrient pooled | ||||||||||
| CF | 103 | (30) | 86 | (24) | a | 74 | (21) | a | ||
| 1.0 × O3 | 102 | (30) | 81 | (23) | ab | 71 | (20) | a | ||
| 1.5 × O3 | 98 | (28) | 73 | (21) | b | 60 | (16) | b | ||
| Gas pooled | ||||||||||
| NF | 112 | (20) | 82 | (15) | 70 | (10) | ||||
| LF | 107 | (14) | 89 | (14) | 74 | (14) | ||||
| HF | 102 | (12) | 82 | (9) | 72 | (10) | ||||
| Leaf position | ||||||||||
| Upper | 95 | (8) | 79 | (12) | 67 | (9) | ||||
| Lower | 119 | (13) | 90 | (11) | 78 | (11) | ||||
| ANOVA | ||||||||||
| O3 | n.s. | ** | *** | |||||||
| Nutrient (N) | * | n.s. | n.s. | |||||||
| Leaf position (LP) | *** | *** | *** | |||||||
| O3 × N | n.s. | n.s. | n.s. | |||||||
| O3 × LP | n.s. | n.s. | n.s. | |||||||
| N × LP | * | n.s. | n.s. | |||||||
| O3 × N × LP | n.s. | n.s. | n.s. | |||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, M.; Hiroshima, H.; Kinose, Y.; Okabe, S.; Izuta, T. Nitrogen Use Efficiency for Growth of Fagus crenata Seedlings Under Elevated Ozone and Different Soil Nutrient Conditions. Forests 2020, 11, 371. https://doi.org/10.3390/f11040371
Watanabe M, Hiroshima H, Kinose Y, Okabe S, Izuta T. Nitrogen Use Efficiency for Growth of Fagus crenata Seedlings Under Elevated Ozone and Different Soil Nutrient Conditions. Forests. 2020; 11(4):371. https://doi.org/10.3390/f11040371
Chicago/Turabian StyleWatanabe, Makoto, Hiroka Hiroshima, Yoshiyuki Kinose, Shigeaki Okabe, and Takeshi Izuta. 2020. "Nitrogen Use Efficiency for Growth of Fagus crenata Seedlings Under Elevated Ozone and Different Soil Nutrient Conditions" Forests 11, no. 4: 371. https://doi.org/10.3390/f11040371
APA StyleWatanabe, M., Hiroshima, H., Kinose, Y., Okabe, S., & Izuta, T. (2020). Nitrogen Use Efficiency for Growth of Fagus crenata Seedlings Under Elevated Ozone and Different Soil Nutrient Conditions. Forests, 11(4), 371. https://doi.org/10.3390/f11040371





