Variation in Stem Xylem Traits is Related to Differentiation of Upper Limits of Tree Species along an Elevational Gradient
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Elevational Transect and Choices of Species
2.3. Sampling and Trait Measurements
2.4. Climate Variables
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chabot, B.F.; Billings, W.D. Origins and Ecology of the Sierran Alpine Flora and Vegetation. Ecol. Monogr. 1972, 42, 163–199. [Google Scholar] [CrossRef]
- Kearney, M.R.; Porter, W. Mechanistic niche modelling: Combining physiological and spatial data to predict species’ ranges. Ecol. Lett. 2009, 12, 334–350. [Google Scholar] [CrossRef] [PubMed]
- Rueda, M.; Godoy, O.; Hawkins, B.A. Spatial and evolutionary parallelism between shade and drought tolerance explains the distributions of conifers in the conterminous United States. Glob. Ecol. Biogeogr. 2016, 26, 31–42. [Google Scholar] [CrossRef]
- Valladares, F.; Niinemets, Ü. Shade Tolerance, a Key Plant Feature of Complex Nature and Consequences. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 237–257. [Google Scholar] [CrossRef]
- Martínez-Tillería, K.; Loayza, A.P.; Sandquist, D.R.; Squeo, F. No evidence of a trade-off between drought and shade tolerance in seedlings of six coastal desert shrub species in north-central Chile. J. Veg. Sci. 2012, 23, 1051–1061. [Google Scholar] [CrossRef]
- Kunstler, G.; Falster, D.S.; Coomes, D.A.; Hui, F.; Kooyman, R.M.; Laughlin, D.C.; Poorter, L.; Vanderwel, M.; Vieilledent, G.; Wright, S.J.; et al. Plant functional traits have globally consistent effects on competition. Nature 2015, 529, 204–207. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Figueroa, J.A.; Cabrera, H.M.; Queirolo, C.; Hinojosa, L.F. Variability of water relations and photosynthesis in Eucryphia cordifolia Cav. (Cunoniaceae) over the range of its latitudinal and altitudinal distribution in Chile. Tree Physiol. 2010, 30, 574–585. [Google Scholar] [CrossRef][Green Version]
- Swaine, M.D. Rainfall and Soil Fertility as Factors Limiting Forest Species Distributions in Ghana. J. Ecol. 1996, 84, 419. [Google Scholar] [CrossRef]
- Engelbrecht, B.M.J.; Comita, L.; Condit, R.; Kursar, T.A.; Tyree, M.T.; Turner, B.L.; Hubbell, S.P. Drought sensitivity shapes species distribution patterns in tropical forests. Nature 2007, 447, 80–82. [Google Scholar] [CrossRef]
- Baltzer, J.L.; Davies, S.J.; Bunyavejchewin, S.; Noor, N.S.M. The role of desiccation tolerance in determining tree species distributions along the Malay–Thai Peninsula. Funct. Ecol. 2008, 22, 221–231. [Google Scholar] [CrossRef]
- Guisan, A.; Zimmermann, N.E. Predictive habitat distribution models in ecology. Ecol. Model. 2000, 135, 147–186. [Google Scholar] [CrossRef]
- Higgins, S.I.; O’Hara, R.B.; Römermann, C. A niche for biology in species distribution models. J. Biogeogr. 2012, 39, 2091–2095. [Google Scholar] [CrossRef]
- Svenning, J.-C.; Sandel, B. Disequilibrium vegetation dynamics under future climate change. Am. J. Bot. 2013, 100, 1266–1286. [Google Scholar] [CrossRef]
- Sakai, A.; Weiser, C.J. Freezing Resistance of Trees in North America with Reference to Tree Regions. Ecology 1973, 54, 118–126. [Google Scholar] [CrossRef]
- Normand, S.; Treier, U.A.; Randin, C.; Vittoz, P.; Guisan, A.; Svenning, J.-C. Importance of abiotic stress as a range-limit determinant for European plants: Insights from species responses to climatic gradients. Glob. Ecol. Biogeogr. 2009, 18, 437–449. [Google Scholar] [CrossRef]
- Mellert, K.H.; Fensterer, V.; Küchenhoff, H.; Reger, B.; Kolling, C.; Klemmt, H.J.; Ewald, J. Hypothesis-driven species distribution models for tree species in the Bavarian Alps. J. Veg. Sci. 2011, 22, 635–646. [Google Scholar] [CrossRef]
- Siefert, A.; Lesser, M.R.; Fridley, J.D. How do climate and dispersal traits limit ranges of tree species along latitudinal and elevational gradients? GloB. Ecol. Biogeogr. 2015, 24, 581–593. [Google Scholar] [CrossRef]
- Körner, C.; Paulsen, J. A world-wide study of high altitude treeline temperatures. J. Biogeogr. 2004, 31, 713–732. [Google Scholar] [CrossRef]
- Adler, P.B.; Salguero-Gómez, R.; Compagnoni, A.; Hsu, J.S.; Ray-Mukherjee, J.; Mbeau-Ache, C.; Franco, M. Functional traits explain variation in plant life history strategies. Proc. Natl. Acad. Sci. USA 2013, 111, 740–745. [Google Scholar] [CrossRef]
- Reich, P.B. The world-wide ‘fast-slow’ plant economics spectrum: A traits manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Salgado-Negret, B.; Canessa, R.; Valladares, F.; Armesto, J.J.; Pérez, F. Functional traits variation explains the distribution of Aextoxicon punctatum (Aextoxicaceae) in pronounced moisture gradients within fog-dependent forest fragments. Front. Plant Sci. 2015, 6, 511. [Google Scholar] [CrossRef] [PubMed]
- Saura, J.M.C.; Martínez-Vilalta, J.; Trabucco, A.; Spano, D.; Mereu, S. Specific leaf area and hydraulic traits explain niche segregation along an aridity gradient in Mediterranean woody species. Perspect. Plant Ecol. Evol. Syst. 2016, 21, 23–30. [Google Scholar] [CrossRef]
- Díaz, S.; Cabido, M. Vive la différence: Plant functional diversity matters to ecosystem processes. Trends Ecol. Evol. 2001, 16, 646–655. [Google Scholar] [CrossRef]
- McGill, B.; Enquist, B.J.; Weiher, E.; Westoby, M. Rebuilding community ecology from functional traits. Trends Ecol. Evol. 2006, 21, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Westoby, M.; Wright, I.J. Land-plant ecology on the basis of functional traits. Trends Ecol. Evol. 2006, 21, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-W.; Song, J.; Wang, M.; Liu, Y.-Y.; Li, N.; Zhang, Y.; Holbrook, N.M.; Hao, G.-Y. Divergences in hydraulic architecture form an important basis for niche differentiation between diploid and polyploid Betula species in NE China. Tree Physiol. 2017, 37, 604–616. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Hill, R.S. The importance of xylem constraints in the distribution of conifer species. New Phytol. 1999, 143, 365–372. [Google Scholar] [CrossRef]
- Cavender-Bares, J.; Holbrook, N.M. Hydraulic properties and freezing-induced cavitation in sympatric evergreen and deciduous oaks with contrasting habitats. Plant, Cell Environ. 2001, 24, 1243–1256. [Google Scholar] [CrossRef]
- Choat, B.; Sack, L.; Holbrook, N.M. Diversity of hydraulic traits in nine Cordia species growing in tropical forests with contrasting precipitation. New Phytol. 2007, 175, 686–698. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Bowman, D.J.M.S.; Nichols, S.; Delzon, S.; Burlett, R. Xylem function and growth rate interact to determine recovery rates after exposure to extreme water deficit. New Phytol. 2010, 188, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Markesteijn, L.; Poorter, L.; Paz, H.; Sack, L.; Bongers, F. Ecological differentiation in xylem cavitation resistance is associated with stem and leaf structural traits. Plant, Cell Environ. 2010, 34, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Bucci, S.J.; Scholz, F.G.; Campanello, P.I.; Montti, L.; Jiménez-Castillo, M.; Rockwell, F.A.; Manna, L.L.; Guerra, P.; Bernal, P.L.; Troncoso, O.; et al. Hydraulic differences along the water transport system of South American Nothofagus species: Do leaves protect the stem functionality? Tree Physiol. 2012, 32, 880–893. [Google Scholar] [CrossRef] [PubMed]
- Tyree, M.T.; Sperry, J.S. Vulnerability of xylem to cavitation and embolism. Annu. Rev. Plant Biol. 1989, 40, 19–36. [Google Scholar] [CrossRef]
- Zwieniecki, M.A.; Secchi, F. Threats to xylem hydraulic function of trees under ‘new climate normal’ conditions. Plant Cell Environ. 2014, 38, 1713–1724. [Google Scholar] [CrossRef]
- Choat, B.; Jansen, S.; Brodribb, T.J.; Cochard, H.; Delzon, S.; Bhaskar, R.; Bucci, S.J.; Feild, T.S.; Gleason, S.M.; Hacke, U.; et al. Global convergence in the vulnerability of forests to drought. Nature 2012, 491, 752–755. [Google Scholar] [CrossRef]
- Barigah, T.S.; Charrier, O.; Douris, M.; Bonhomme, M.; Herbette, S.; Ameglio, T.; Fichot, R.; Brignolas, F.; Cochard, H. Water stress-induced xylem hydraulic failure is a causal factor of tree mortality in beech and poplar. Ann. Bot. 2013, 112, 1431–1437. [Google Scholar] [CrossRef]
- Tyree, M.T.; Zimmermann, M.H.; Tyree, P.M.T. Xylem Structure and the Ascent of Sap; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2002; pp. 27–48. [Google Scholar]
- Fu, P.-L.; Jiang, Y.-J.; Wang, A.-Y.; Brodribb, T.J.; Zhang, J.-L.; Zhu, S.-D.; Cao, K.-F. Stem hydraulic traits and leaf water-stress tolerance are co-ordinated with the leaf phenology of angiosperm trees in an Asian tropical dry karst forest. Ann. Bot. 2012, 110, 189–199. [Google Scholar] [CrossRef]
- Qian, H.; Hao, Z.; Zhang, J. Phylogenetic structure and phylogenetic diversity of angiosperm assemblages in forests along an elevational gradient in Changbaishan, China. J. Plant Ecol. 2014, 7, 154–165. [Google Scholar] [CrossRef]
- Cobb, A.R.; Choat, B.; Holbrook, N.M. Dynamics of freeze-thaw embolism in Smilax rotundifolia (Smilacaceae). Am. J. Bot. 2007, 94, 640–649. [Google Scholar] [CrossRef]
- Körner, C.; Basler, D.; Hoch, G.; Kollas, C.; Lenz, A.; Randin, C.F.; Vitasse, Y.; Zimmermann, N.E. Where, why and how? Explaining the low temperature range limits of temperate tree species. J. Ecol. 2016, 104, 1076–1088. [Google Scholar] [CrossRef]
- Tyree, M.T.; Davis, S.D.; Cochard, H. Biophysical Perspectives of Xylem Evolution: Is there a Tradeoff of Hydraulic Efficiency for Vulnerability to Dysfunction? IAWA J. 1994, 15, 335–360. [Google Scholar] [CrossRef]
- Davis, S.D.; Sperry, J.S.; Hacke, U. The relationship between xylem conduit diameter and cavitation caused by freezing. Am. J. Bot. 1999, 86, 1367–1372. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Jiang, P.; Wei, J.; Shao, H. Nutrients and biomass spatial patterns in alpine tundra ecosystem on Changbai Mountains, Northeast China. Coll. Surf. B Biointerf. 2007, 60, 250–257. [Google Scholar] [CrossRef]
- Dai, L.; Qi, L.; Wang, Q.-W.; Su, D.; Yu, D.; Wang, Y.; Ye, Y.; Jiang, S.; Zhao, W. Changes in forest structure and composition on Changbai Mountain in Northeast China. Ann. For. Sci. 2011, 68, 889–897. [Google Scholar] [CrossRef]
- Liu, Q.-J. Structure and dynamics of the subalpine coniferous forest on Changbai mountain, China. Plant Ecol. 1997, 132, 97–105. [Google Scholar] [CrossRef]
- Xu, Z.; Yu, G.; Zhang, X.; Ge, J.; He, N.; Wang, Q.; Wang, D. The variations in soil microbial communities, enzyme activities and their relationships with soil organic matter decomposition along the northern slope of Changbai Mountain. Appl. Soil Ecol. 2015, 86, 19–29. [Google Scholar] [CrossRef]
- Wang, D.; He, N.; Wang, Q.; Lü, Y.; Wang, Q.; Xu, Z.; Zhu, J. Effects of Temperature and Moisture on Soil Organic Matter Decomposition Along Elevation Gradients on the Changbai Mountains, Northeast China. Pedosphere 2016, 26, 399–407. [Google Scholar] [CrossRef]
- Vilagrosa, A.; Hernández, E.I.; Luis, V.C.; Cochard, H.; Pausas, J.G. Physiological differences explain the co-existence of different regeneration strategies in Mediterranean ecosystems. New Phytol. 2013, 201, 1277–1288. [Google Scholar] [CrossRef]
- Niu, C.-Y.; Meinzer, F.; Hao, G.-Y. Divergence in strategies for coping with winter embolism among co-occurring temperate tree species: The role of positive xylem pressure, wood type and tree stature. Funct. Ecol. 2017, 31, 1550–1560. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: www.R-project.org (accessed on 1 August 2019).
- Guthery, F.S.; Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. J. Wildl. Manag. 2003, 67, 655. [Google Scholar] [CrossRef]
- Nakagawa, S.; Schielzeth, H. A general and simple method for obtaining R2 from generalized linear mixed?effects models. Methods Ecol. Evol. 2012, 4, 133–142. [Google Scholar] [CrossRef]
- Fisher, J.B.; Goldstein, G.; Jones, T.J.; Cordell, S. Wood vessel diameter is related to elevation and genotype in the Hawaiian tree Metrosideros polymorpha (Myrtaceae). Am. J. Bot. 2007, 94, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, J.S.; Pockman, W. Freezing regime and trade-offs with water transport efficiency generate variation in xylem structure across diploid populations of Larrea sp. (Zygophyllaceae). Am. J. Bot. 2014, 101, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Feild, T.S.; Brodribb, T.; Brodribb, T.J. Stem water transport and freeze-thaw xylem embolism in conifers and angiosperms in a Tasmanian treeline heath. Oecologia 2001, 127, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Cavender-Bares, J.; Cortes, P.; Rambal, S.; Joffre, R.; Miles, B.; Rocheteau, A. Summer and winter sensitivity of leaves and xylem to minimum freezing temperatures: A comparison of co-occurring Mediterranean oaks that differ in leaf lifespan. New Phytol. 2005, 168, 597–612. [Google Scholar] [CrossRef]
- Schreiber, S.; Hacke, U.; Hamann, A. Variation of xylem vessel diameters across a climate gradient: Insight from a reciprocal transplant experiment with a widespread boreal tree. Funct. Ecol. 2015, 29, 1392–1401. [Google Scholar] [CrossRef]
- Christman, M.A.; Sperry, J.; Smith, D. Rare pits, large vessels and extreme vulnerability to cavitation in a ring-porous tree species. New Phytol. 2011, 193, 713–720. [Google Scholar] [CrossRef]
- Hacke, U.; Sperry, J.S. Functional and ecological xylem anatomy. Perspect. Plant Ecol. Evol. Syst. 2001, 4, 97–115. [Google Scholar] [CrossRef]
- Sperry, J.S.; Nichols, K.L.; Sullivan, J.E.M.; Eastlack, S.E. Xylem Embolism in Ring-Porous, Diffuse-Porous, and Coniferous Trees of Northern Utah and Interior Alaska. Ecol. 1994, 75, 1736–1752. [Google Scholar] [CrossRef]
- Sperry, J.S.; Sullivan, J.E.M.; Zhu, G.L.; Boyer, J.S. Xylem Embolism in Response to Freeze-Thaw Cycles and Water Stress in Ring-Porous, Diffuse-Porous, and Conifer Species. Plant Physiol. 1992, 100, 605–613. [Google Scholar] [CrossRef]
- Jacobsen, A.; Pratt, R.B. Vulnerability to cavitation of central California Arctostaphylos (Ericaceae): A new analysis. Oecologia 2012, 171, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, F.; Quezada, I.; Saldaña, A. Variation in traits related to water transport in Nothofagus dombeyi helps to explain its latitudinal distribution limit in the Chilean Andes. Plant Ecol. Divers. 2018, 11, 307–317. [Google Scholar] [CrossRef]
- Mayr, S.; Wolfschwenger, M.; Bauer, H. Winter-drought induced embolism in Norway spruce (Picea abies) at the Alpine timberline. Physiol. Plant. 2002, 115, 74–80. [Google Scholar] [CrossRef]
- Granda, E.; Scoffoni, C.; Rubio-Casal, A.E.; Sack, L.; Valladares, F. Leaf and stem physiological responses to summer and winter extremes of woody species across temperate ecosystems. Oikos 2014, 123, 1281–1290. [Google Scholar] [CrossRef]
- Li, M.; Wu, Z.; Qin, L.; Meng, X. Extracting vegetation phenology metrics in Changbai Mountains using an improved logistic model. Chin. Geogr. Sci. 2011, 21, 304–311. [Google Scholar] [CrossRef]
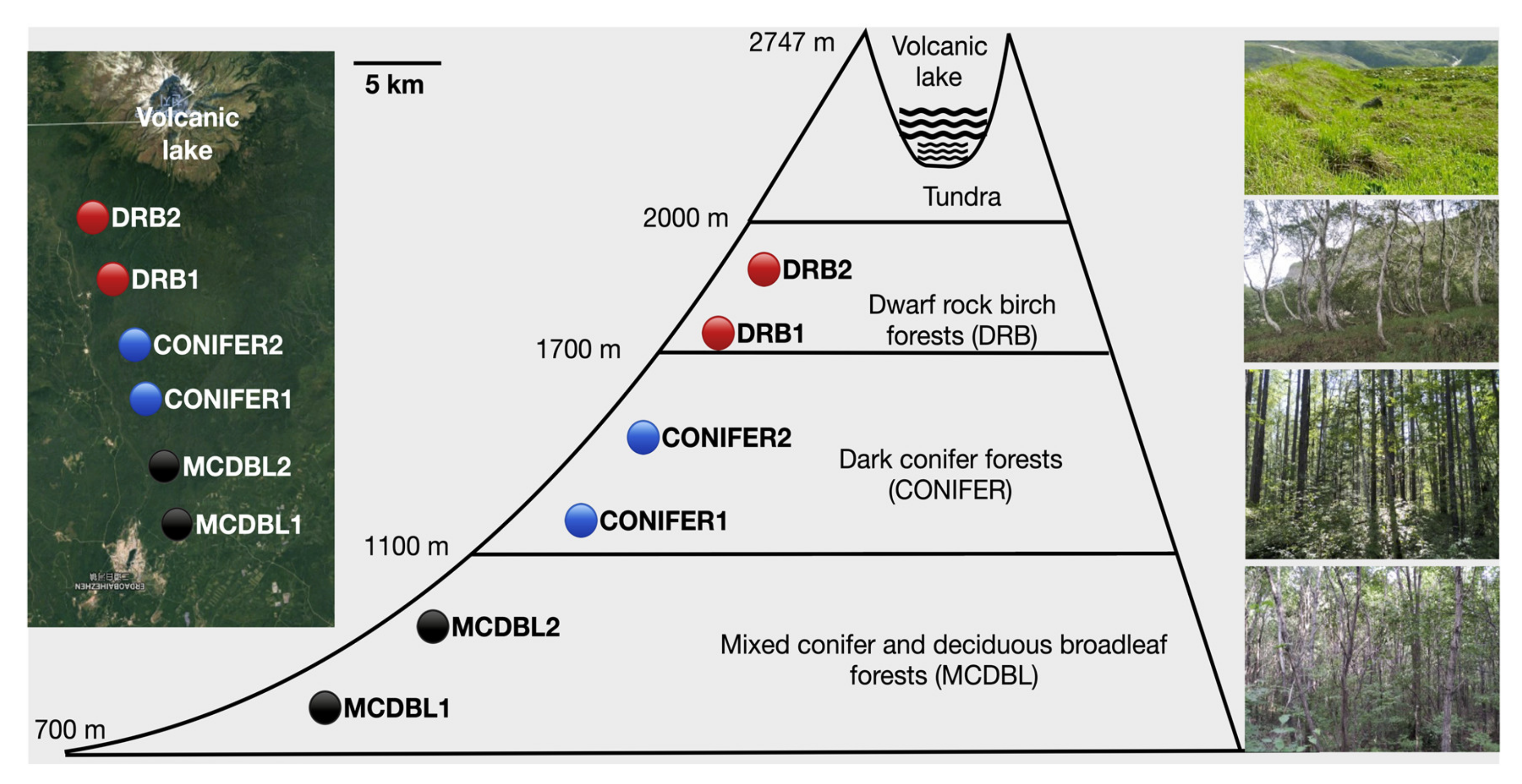
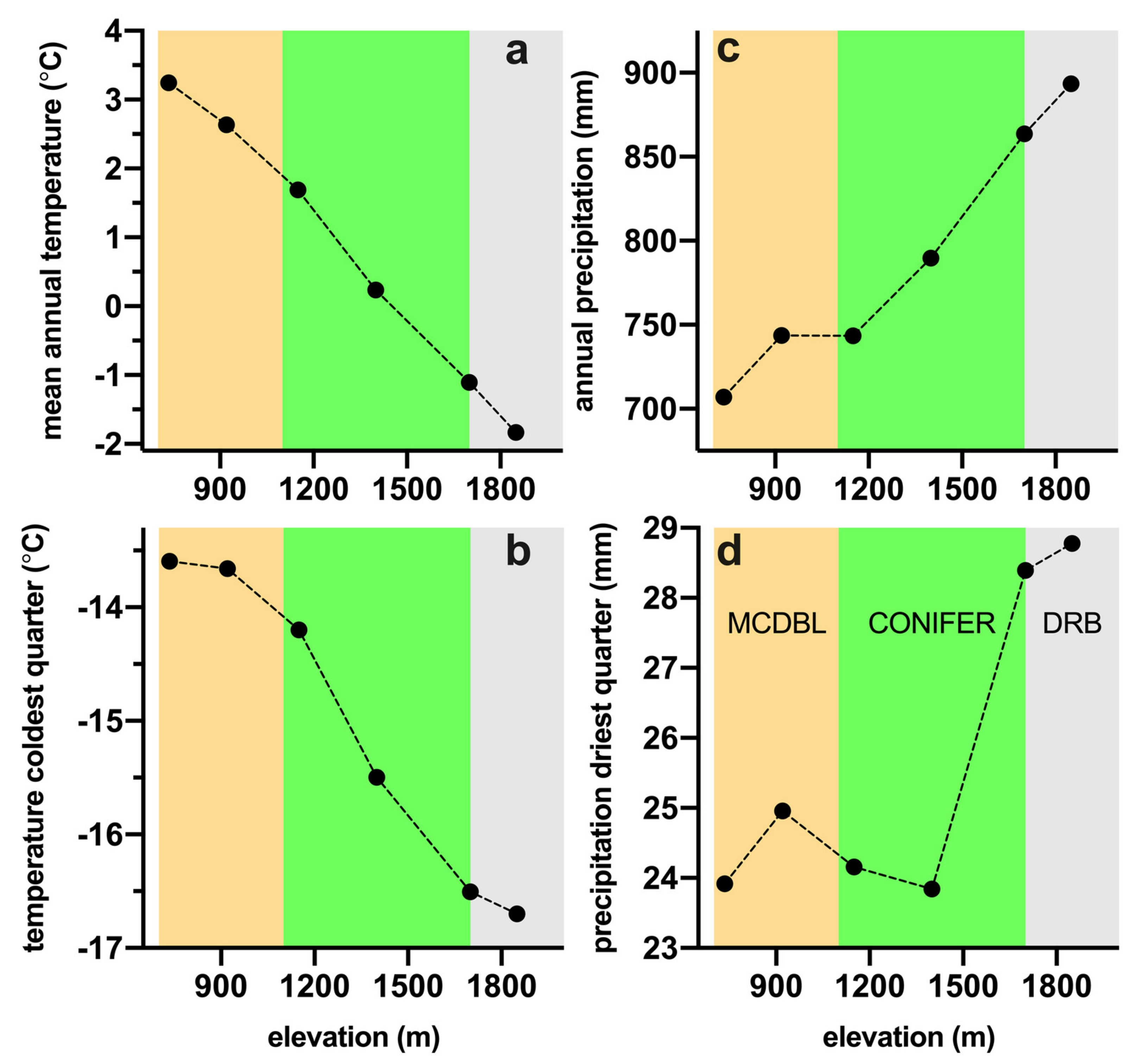

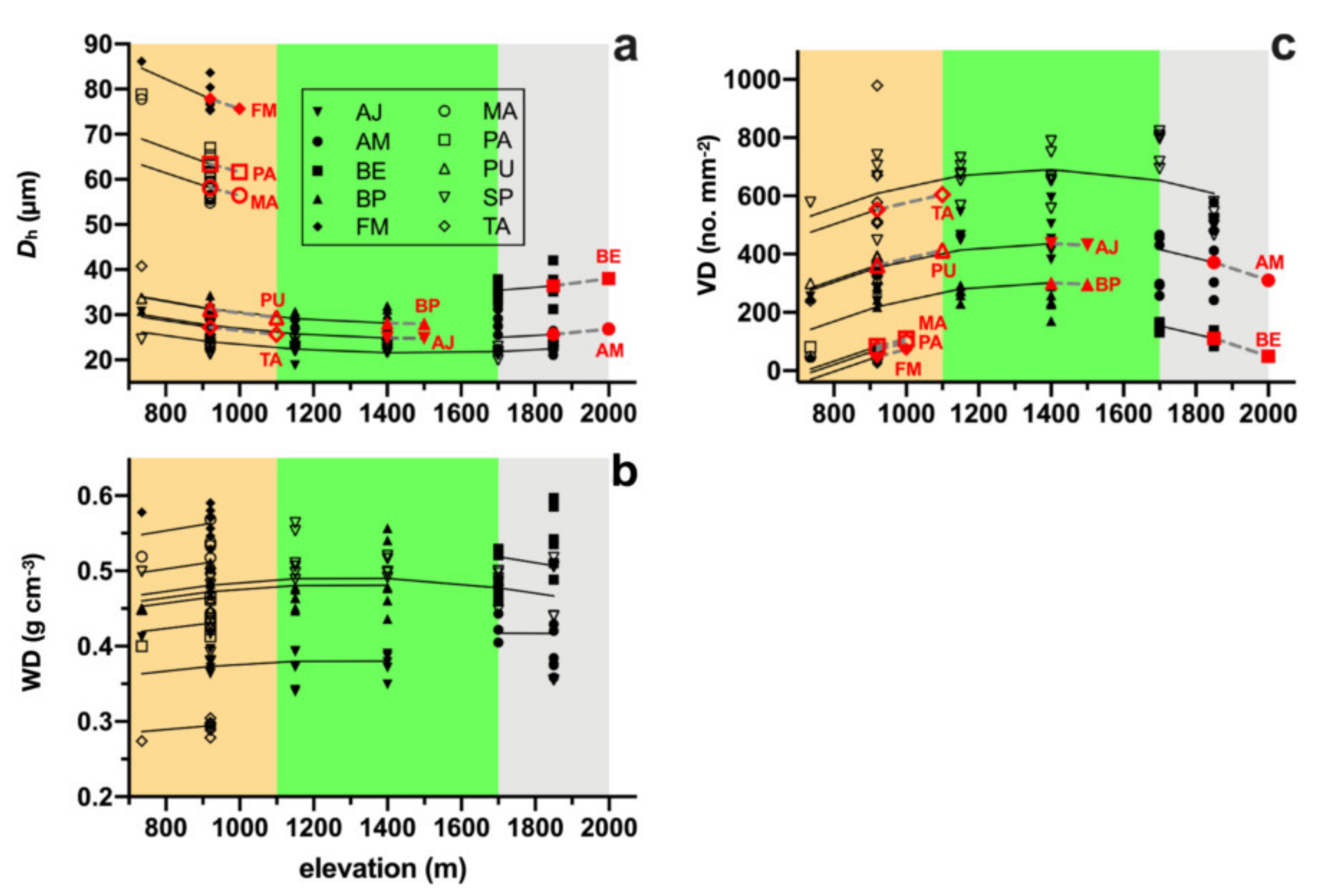
| Species | Species Code | Family | Upper Limit (m) | Wood Type |
|---|---|---|---|---|
| Alnus japonica Sieb. et. Zucc. | AJ | Betulaceae | 1500 | D |
| Alnus mandshurica (Callier ex C. K. Schneider) Hand. | AM | Betulaceae | 2000 | D |
| Betula ermanii Cham. | BE | Betulaceae | 2000 | D |
| Betula platyphylla Suk. | BP | Betulaceae | 1500 | D |
| Fraxinus mandschurica Rupr. | FM | Oleaceae | 1000 | R |
| Maackia amurensis Rupr. et Maxim. | MA | Leguminosae | 1000 | R |
| Phellodendron amurense Rupr. | PA | Rutaceae | 1000 | R |
| Populus ussuriensis Kom. | PU | Salicaceae | 1100 | D |
| Sorbus pohuashanensis (Hance) Hedl. | SP | Rosaceae | 1850 | D |
| Tilia amurensis Rupr. | TA | Tiliaceae | 1100 | D |
| Forest Type | Site | Species | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Code | Elevation | FM | MA | PA | PU | TA | SP | BP | BE | AJ | AM | |
| MCDBL | MCDBL1 | 735 | + | + | + | + | + | + | + | + | ||
| MCDBL2 | 920 | + | + | + | + | + | + | + | + | |||
| CONIFER | CONIFER1 | 1150 | + | + | + | |||||||
| CONIFER2 | 1400 | + | + | + | ||||||||
| DRB | DRB1 | 1700 | + | + | + | |||||||
| DRB2 | 1850 | + | + | + | ||||||||
| Species | Dh | VD | WD | |||
|---|---|---|---|---|---|---|
| Mean | CV | Mean | CV | Mean | CV | |
| FM | 79.23 | 0.05 | 29.68 | 0.24 | 0.56 | 0.04 |
| MA | 59.42 | 0.14 | 54.54 | 0.09 | 0.51 | 0.08 |
| PA | 64.69 | 0.11 | 66.6 | 0.13 | 0.43 | 0.05 |
| PU | 31.57 | 0.04 | 352.44 | 0.1 | 0.46 | 0.07 |
| TA | 27.94 | 0.22 | 550.03 | 0.43 | 0.29 | 0.04 |
| SP | 22.58 | 0.05 | 644.29 | 0.17 | 0.48 | 0.09 |
| BP | 29.78 | 0.06 | 260.19 | 0.14 | 0.48 | 0.06 |
| BE | 35.99 | 0.08 | 129.68 | 0.2 | 0.52 | 0.09 |
| AJ | 26.42 | 0.12 | 395.09 | 0.27 | 0.38 | 0.05 |
| AM | 25.43 | 0.12 | 395.16 | 0.28 | 0.42 | 0.1 |
| Total | 37.87 | 0.53 | 327.7 | 0.67 | 0.45 | 0.16 |
| Trait/Model | LL | k | ΔAICc | wAICc | Rm |
|---|---|---|---|---|---|
| (a) hydraulically weighted vessel diameter | |||||
| ~ elev + elev2 + 1|spp | 208.5 | 5 | - | 0.997 | 1.6 |
| ~ elev + 1|spp | 195.6 | 4 | 11.7 | 0.003 | 1.4 |
| ~ 1|spp | 199.2 | 3 | 22.8 | <0.001 | - |
| (b) vessel density | |||||
| ~ elev + elev2 + 1|spp | −119.1 | 5 | - | 0.998 | 3.2 |
| ~ elev + 1|spp | −135.4 | 4 | 13.0 | 0.001 | 1.3 |
| ~ 1|spp | −129.5 | 3 | 14.3 | <0.001 | - |
| (c) sapwood density | |||||
| ~ 1|spp | 229.0 | 3 | - | 0.444 | - |
| ~ elev + elev2 + 1|spp | 226.6 | 5 | 0.3 | 0.392 | 0.6 |
| ~ elev + 1|spp | 218.8 | 4 | 2.0 | 0.164 | <0.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, D.; Wang, A.-Y.; Zhang, J.-L.; Bradshaw, C.J.A.; Hao, G.-Y. Variation in Stem Xylem Traits is Related to Differentiation of Upper Limits of Tree Species along an Elevational Gradient. Forests 2020, 11, 349. https://doi.org/10.3390/f11030349
Yang D, Wang A-Y, Zhang J-L, Bradshaw CJA, Hao G-Y. Variation in Stem Xylem Traits is Related to Differentiation of Upper Limits of Tree Species along an Elevational Gradient. Forests. 2020; 11(3):349. https://doi.org/10.3390/f11030349
Chicago/Turabian StyleYang, Da, Ai-Ying Wang, Jiao-Lin Zhang, Corey J. A. Bradshaw, and Guang-You Hao. 2020. "Variation in Stem Xylem Traits is Related to Differentiation of Upper Limits of Tree Species along an Elevational Gradient" Forests 11, no. 3: 349. https://doi.org/10.3390/f11030349
APA StyleYang, D., Wang, A.-Y., Zhang, J.-L., Bradshaw, C. J. A., & Hao, G.-Y. (2020). Variation in Stem Xylem Traits is Related to Differentiation of Upper Limits of Tree Species along an Elevational Gradient. Forests, 11(3), 349. https://doi.org/10.3390/f11030349







