Intra-Annual Radial Growth of Pinus kesiya var. langbianensis Is Mainly Controlled by Moisture Availability in the Ailao Mountains, Southwestern China
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Site
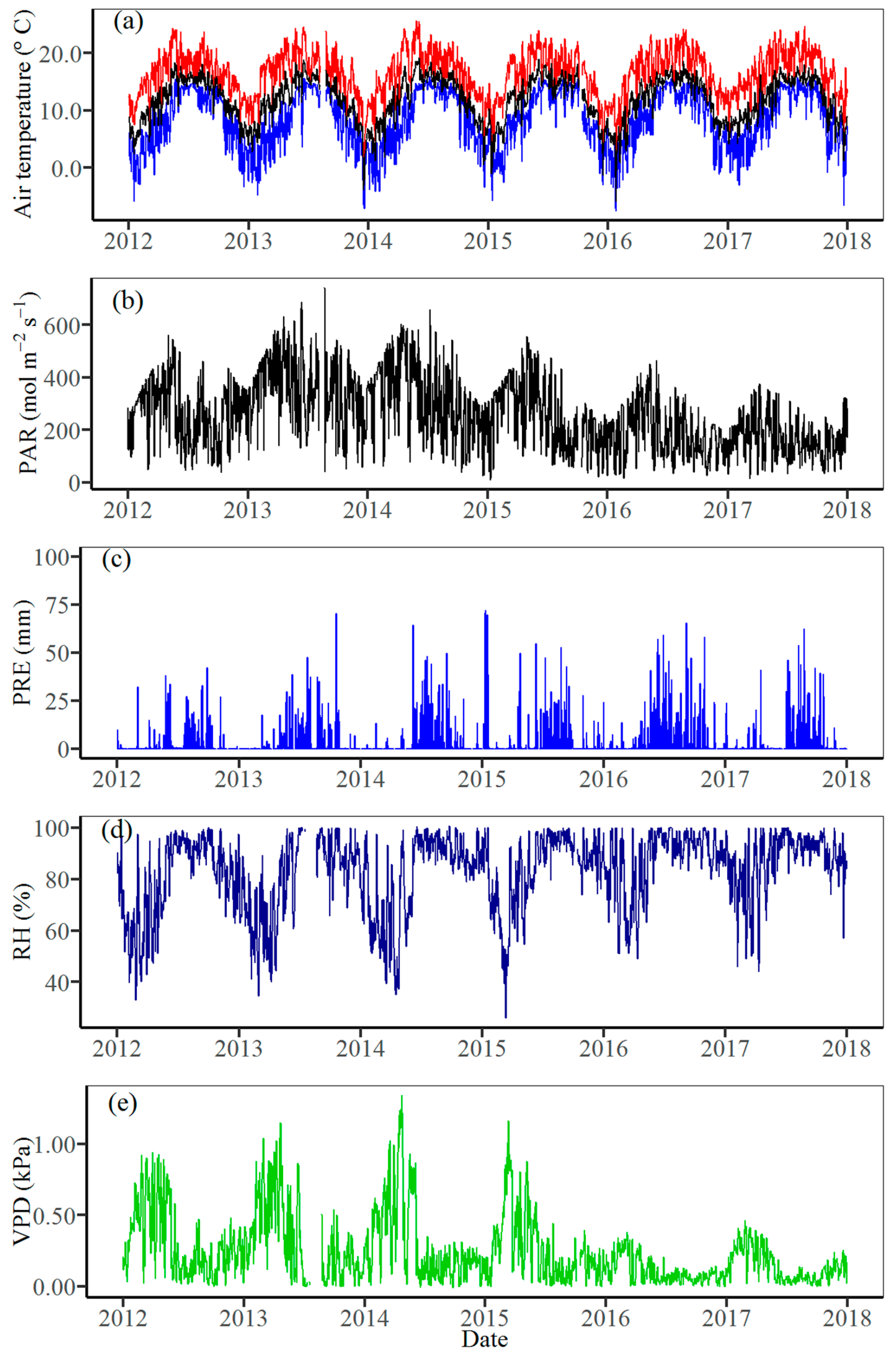
2.2. Meteorological Data
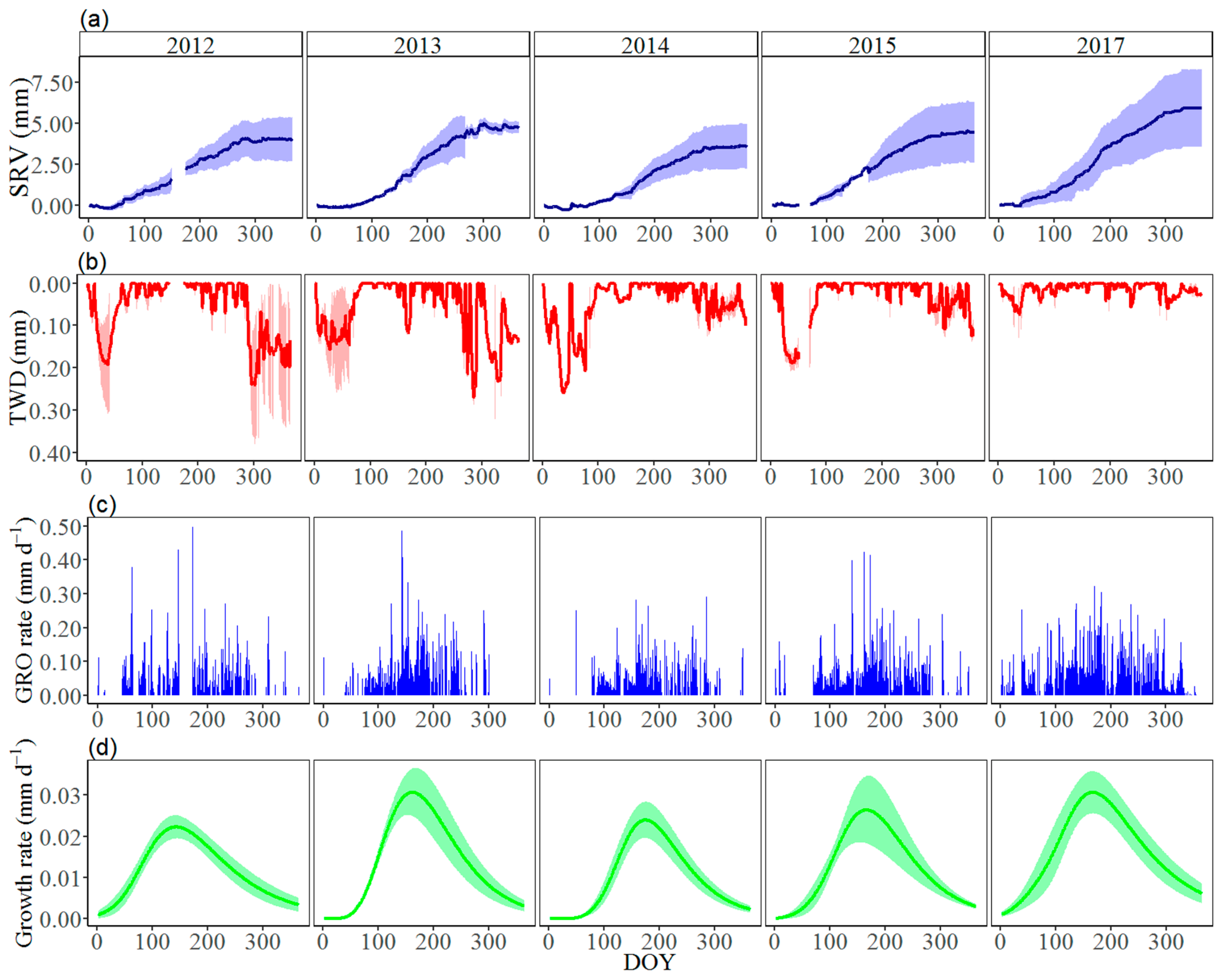
2.3. Dendrometer Measurements
2.4. Data Analysis
3. Results
3.1. Climate Variability
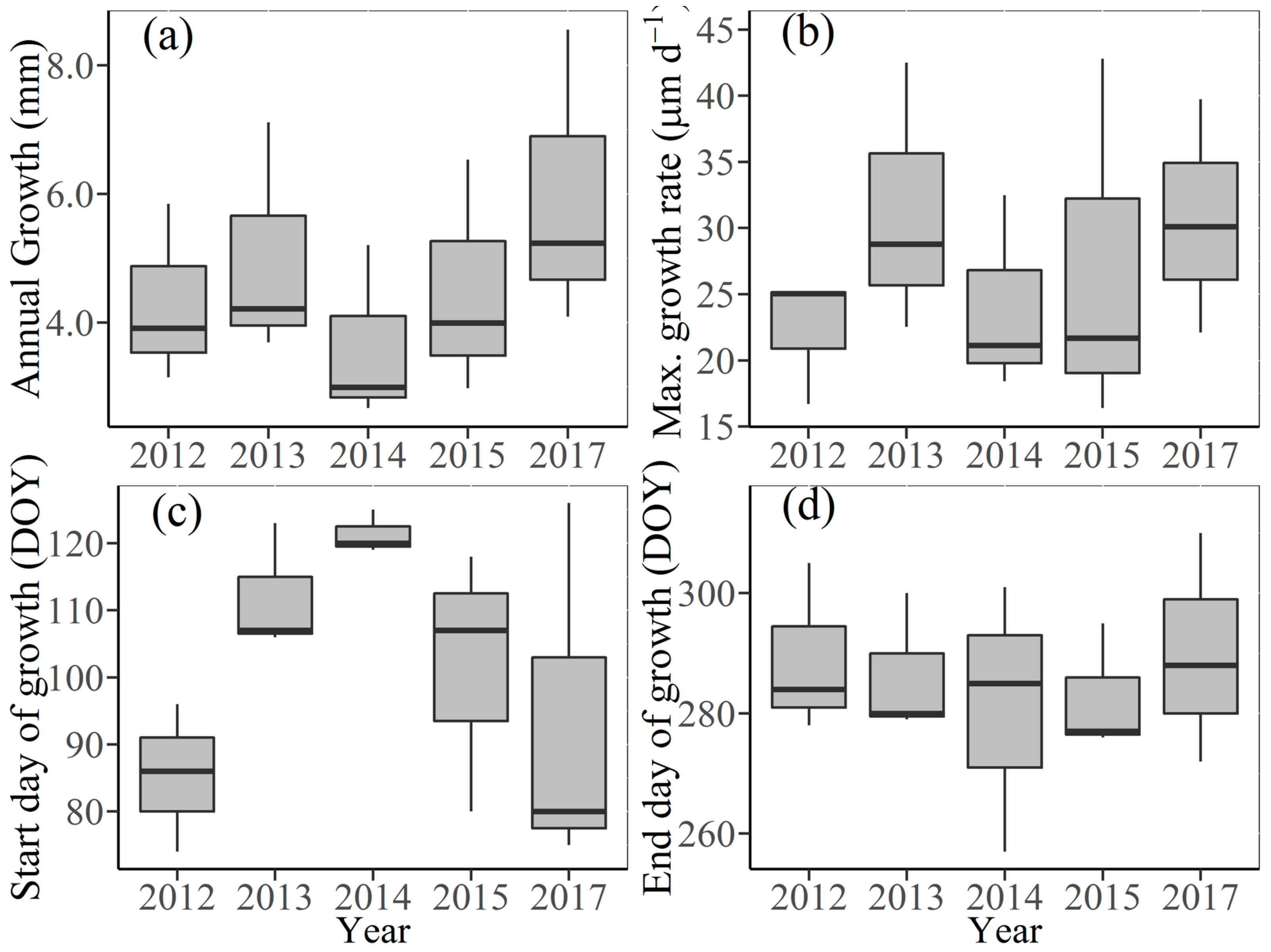
3.2. Seasonal Stem Radius Variations
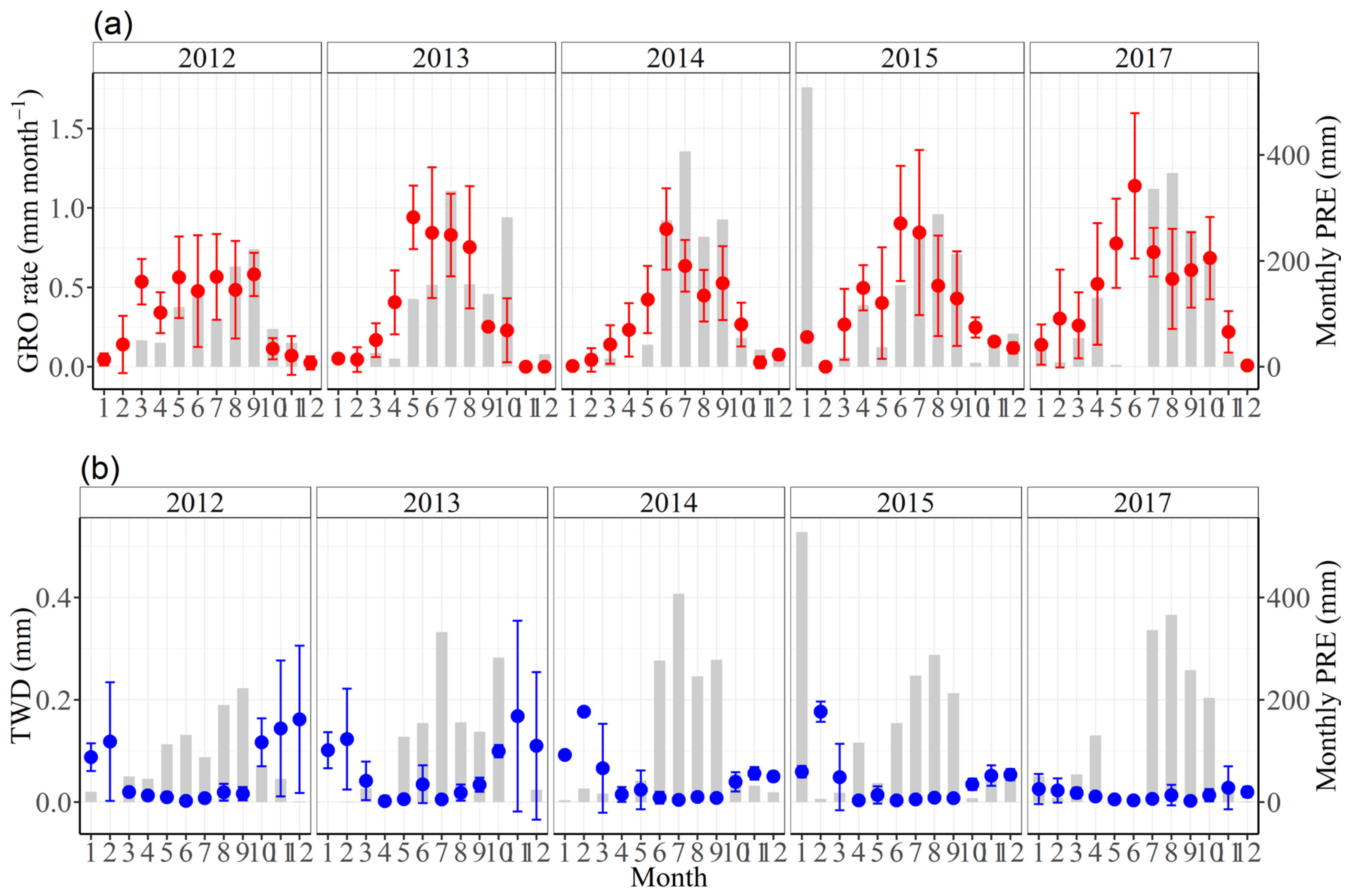
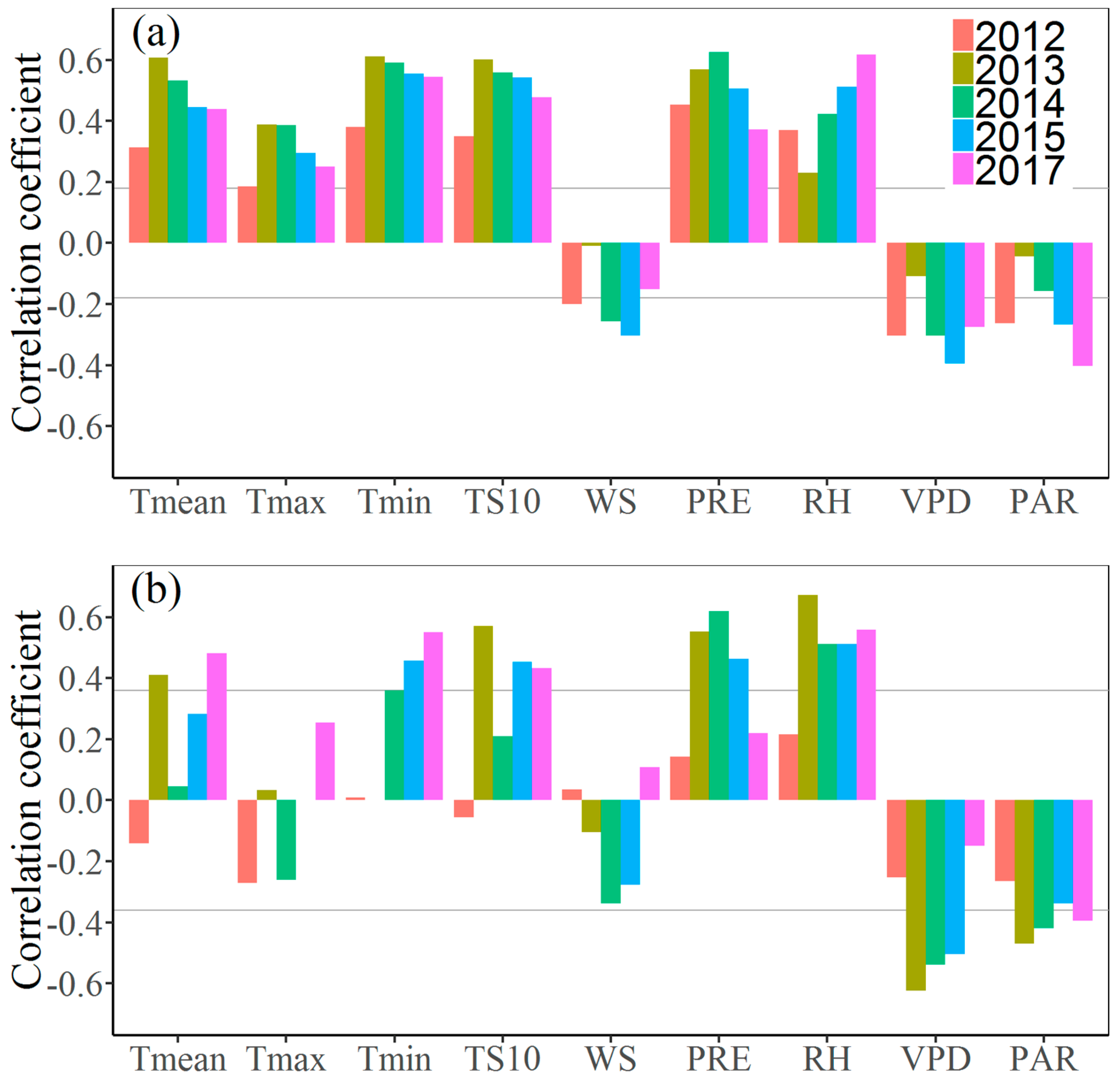
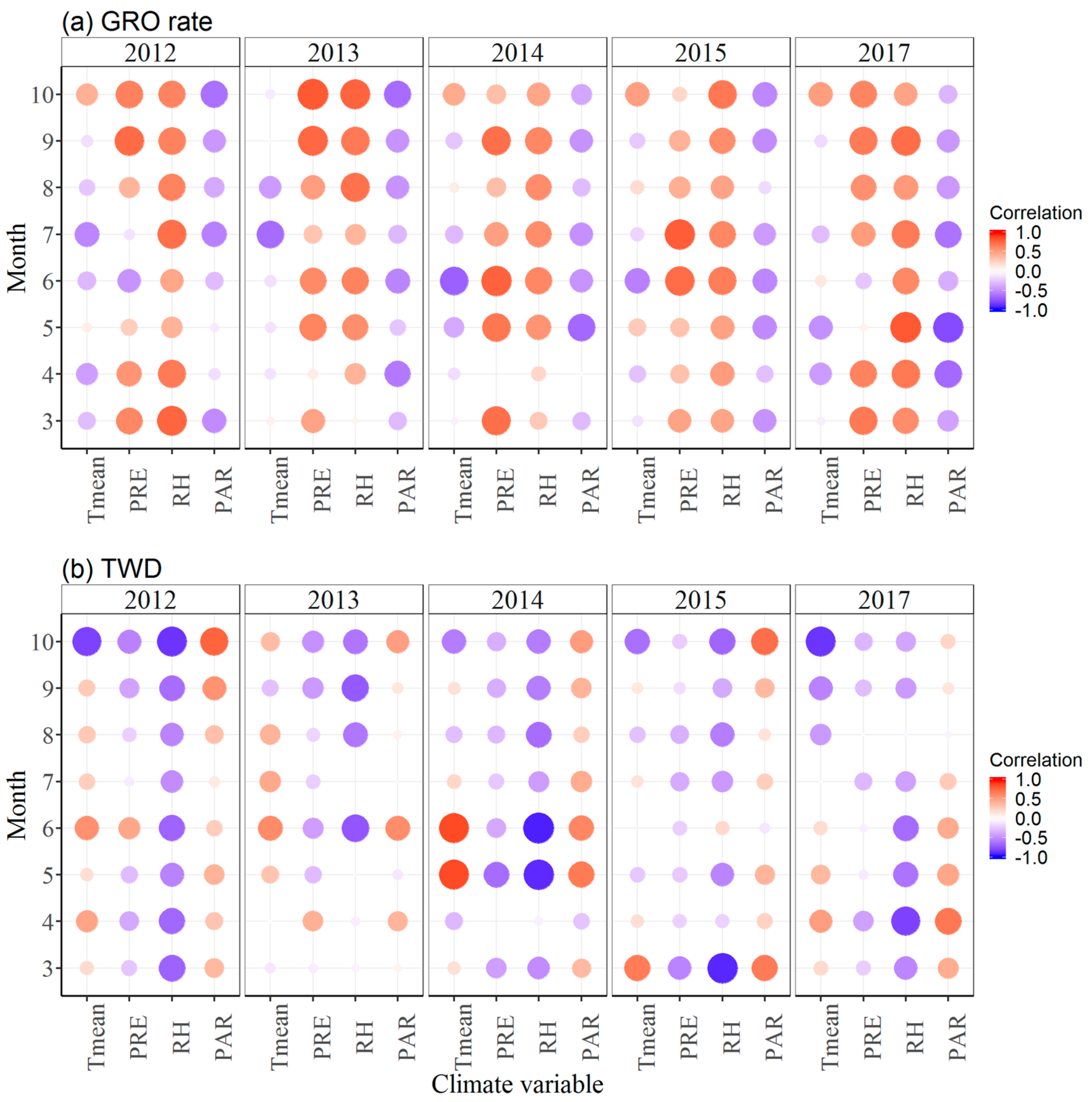
3.3. Effects of Climate Factors on Stem Growth
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tee mortality reveals emerging climate change risks for forest. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Boisvenue, C.; Running, S.W. Impacts of climate change on natural forest productivity–evidence since the middle of the 20th century. Glob. Chang. Biol. 2006, 12, 862–882. [Google Scholar] [CrossRef]
- Saxe, H.; Cannell, M.G.R.; Johnsen, B.; Ryan, M.G.; Vourlitis, G. Tree and forest functioning in response to global warming. New Phytol. 2001, 139, 395–436. [Google Scholar] [CrossRef]
- Fritts, H. Tree Rings and Climate; Academic Press: New York, NY, USA, 1976; pp. 25–27. [Google Scholar]
- Duchesne, L.; Houle, D.; D’Orangeville, L. Influence of climate on seasonal patterns of stem increment of balsam fir in a boreal forest of Québec, Canada. Agric. For. Meteorol. 2012, 162–163, 108–114. [Google Scholar] [CrossRef]
- van der Maaten, E.; Bouriaud, O.; van der Maaten-Theunissen, M.; Mayer, H.; Spieker, H. Meteorological forcing of day-to-day stem radius variations of beech is highly synchronic on opposing aspects of a valley. Agric. For. Meteorol. 2013, 181, 85–93. [Google Scholar] [CrossRef]
- Gričar, J.; Rathgeber, C.B.K.; Fonti, P. Monitoring seasonal dynamics of wood formation. Dendrochronologia 2011, 29, 123–125. [Google Scholar] [CrossRef]
- Zweifel, R.; Haeni, M.; Buchmann, N.; Eugster, W. Are trees able to grow in periods of stem shrinkage? New Phytol. 2016, 211, 839–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zweifel, R.; Zimmermann, L.; Zeugin, F.; Newbery, D.M. Intra-annual radial growth and water relations of trees: Implications towards a growth mechanism. J. Exp. Bot. 2006, 57, 1445–1459. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.; Hölttä, T.; Berninger, F.; Mäkkinen, H.; Nöjd, P.; Mencuccini, M.; Nikinmaa, E. Separating water-potential induced swelling and shrinking from measured radial stem variations reveals a cambial growth and osmotic concentration signal. Plant Cell Environ. 2016, 39, 233–244. [Google Scholar] [CrossRef]
- Steppe, K.; Sterck, F.; Deslauriers, A. Diel growth dynamics in tree stems: Linking anatomy and ecophysiology. Trends Plant Sci. 2015, 20, 335–343. [Google Scholar] [CrossRef]
- Zweifel, R.; Zimmermann, L.; Newbery, D. Modelling tree water deficit from microclimate: An approach to quantifying drought stress. Tree Physiol. 2005, 25, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, L.; Zweifel, R.; Kahmen, A. Daily stem diameter variations can predict the canopy water status of mature temperate trees. Tree Physiol. 2018, 38, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Oberhuber, W.; Gruber, A.; Kofler, W.; Swidrak, I. Radial stem growth in response to microclimate and soil moisture in a drought-prone mixed coniferous forest at an inner Alpine site. Eur. J. For. Res. 2014, 133, 467–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drew, D.M.; Downes, G.M. The use of precision dendrometers in research on daily stem size and wood property variation: A review. Dendrochronologia 2009, 27, 159–172. [Google Scholar] [CrossRef]
- Cuny, H.E.; Rathgeber, C.B.K.; Frank, D.; Fonti, P.; Mäkinen, H.; Prislan, P.; Rossi, S.; del Castillo, E.M. Woody biomass production lags stem-girth increase by over one month in coniferous forests. Nat. Plants 2015, 1, 15160. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Yang, B.; Deslauriers, A.; Bräuning, A. Intra-annual stem radial increment response of Qilian juniper to temperature and precipitation along an altitudinal gradient in northwestern China. Trees 2015, 29, 25–34. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, B.Q.; Dong, M.Y.; Huang, Y.M.; Wang, M.C.; Wang, B. Response of daily stem radial growth of Platycladus orientalis to environmental factors in a semi-arid area of North China. Trees 2015, 29, 87–96. [Google Scholar] [CrossRef]
- Zhang, R.; Yuan, Y.; Gou, X.; Zhang, T.; Zou, C.; Ji, C.; Fan, Z.; Qin, L.; Shang, H.; Li, X. Intra-annual radial growth of Schrenk spruce (Picea schrenkiana Fisch. et Mey) and its response to climate on the northern slopes of the Tianshan Mountains. Dendrochronologia 2016, 40, 36–42. [Google Scholar] [CrossRef]
- Krepkowski, J.; Bräuning, A.; Gebrekirstos, A.; Strobl, S. Seasonal growth dynamics and climatic control of different tree life forms in Munessa Forest (Ethiopia). Trees 2011, 25, 59–70. [Google Scholar] [CrossRef]
- Volland-Voigt, F.; Bräuning, A.; Ganzhi, O.; Peters, T.; Maza, H. Radial stem variations of Tabebuia chrysantha (Bignoniaceae) in different tropical forest ecosystems of southern Ecuador. Trees 2011, 25, 39–48. [Google Scholar] [CrossRef]
- Raffelsbauer, V.; Spannl, S.; Peña, K.; Pucha, D.A.; Steppe, K.; Bräuning, A. Tree circumference changes and species-specific growth recovery after extreme dry events in a montane rainforest in southern Ecuador. Front. Plant Sci. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mendivelso, H.A.; Julio Camarero, J.; Gutiérrez, E.; Castañ o-Naranjo, A. Climatic influences on leaf phenology, xylogenesis and radial stem changes at hourly to monthly scales in two tropical dry forests. Agric. For. Meteorol. 2016, 216, 20–36. [Google Scholar] [CrossRef]
- Cocozza, C.; Palombo, C.; Tognetti, R.; Porta, N.L.; Anichini, M.; Giovannelli, A.; Emiliani, G. Monitoring intra-annual dynamics of wood formation with microcores and dendrometers in Picea abies at two different altitudes. Tree Physiol. 2016, 36, 832–846. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Xu, X.; Sun, A.Y.; Wang, K.; Liu, W.; Zhang, X. Is southwestern China experiencing more frequent precipitation extremes? Environ. Res. Lett. 2014, 9, 064002. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Ma, Y.; Li, Y.; Liu, Y. On climatic effects of airstreams over the Ailao Mountains. Geogr. Res. 1992, 31, 461–467. [Google Scholar]
- Wang, H.F.; Lencinas, M.V.; Friedman, C.R.; Zhu, Z.X.; Qiu, J.X. Understory plant diversity assessment of Szemao pine (Pinus kesiya var. langbianensis) plantations in Yunnan, China. Collect. Bot. 2012, 31, 51–65. [Google Scholar] [CrossRef]
- Rossi, S.; Deslauriers, A.; Morin, H. Application of the Gompertz equation for the study of xylem cell development. Dendrochronologia 2003, 21, 33–39. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria.
- Zuur, A.F.; Leno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer: New Youk, NY, USA, 2009. [Google Scholar]
- Pumijumnong, N.; Wanyaphet, T. Seasonal cambial activity and tree-ring formation of Pinus merkusii and Pinus kesiya in Northern Thailand in dependence on climate. For. Ecol. Manag. 2006, 226, 279–289. [Google Scholar] [CrossRef]
- Wagner, F.H.; Hérault, B.; Bonal, D.; Stahl, C.; Anderson, L.O.; Baker, T.R.; Sebastian Becker, G.; Beeckman, H.; Boanerges Souza, D.; Cesar Botosso, P.; et al. Climate seasonality limits leaf carbon assimilation and wood productivity in tropical forests. Biogeosciences 2016, 13, 2537–2562. [Google Scholar] [CrossRef] [Green Version]
- Rossi, S.; Deslauriers, A.; Anfodillo, T.; Morin, H.; Saracino, A.; Motta, R.; Borghetti, M. Conifers in cold environments synchronize maximum growth rates of tree-ring formation with day length. New Phytol. 2006, 170, 301–310. [Google Scholar] [CrossRef]
- Pumijumnong, N.; Eckstein, D. Reconstruction of pre-monsoon weather conditions in northwestern Thailand from the tree-ring widths of Pinus merkusii and Pinus kesiya. Trees 2011, 25, 125–132. [Google Scholar] [CrossRef]
- Chaudhary, V.; Bhattacharyya, A. Suitability of Pinus kesiya in Shillong, Meghalaya for tree-ring analysis. Curr. Sci. 2002, 83, 1010–1015. [Google Scholar]
- Deslauriers, A.; Morin, H.; Urbinati, C.; Carrer, M. Daily weather response of balsam fir (Abies balsamea (L.) Mill.) stem radius increment from dendrometer analysis in the boreal forests of Québec (Canada). Trees 2003, 17, 477–484. [Google Scholar] [CrossRef]
- Deslauriers, A.; Rossi, S.; Anfodillo, T. Dendrometer and intra-annual tree growth: What kind of information can be inferred? Dendrochronologia 2007, 25, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Gruber, A.; Zimmermann, J.; Wieser, G.; Oberhuber, W. Effects of climate variables on intra-annual stem radial increment in Pinus cembra (L.) along the alpine treeline ecotone. Ann. For. Sci. 2009, 6, 503–603. [Google Scholar] [CrossRef] [PubMed]
- Steppe, K.; De Pauw, D.J.; Lemeur, R.; Vanrolleghem, P.A. A mathematical model linking tree sap flow dynamics to daily stem diameter fluctuations and radial stem growth. Tree Physiol. 2006, 26, 257–273. [Google Scholar] [CrossRef] [Green Version]
- Gebrekirstos, A.; Noordwijk, M.; van Neufeldt, H.; Mitlöhner, R. Relationships of stable carbon isotopes, plant water potential and growth an approach to assess water use efficiency and growth strategies of dry land agroforestry species. Trees 2011, 25, 95–102. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef]
- King, G.; Fonti, P.; Nievergelt, D.; Büntgen, U.; Frank, D. Climatic drivers of hourly to yearly tree radius variations along a 6 °C natural warming gradient. Agric. For. Meteorol. 2013, 168, 36–46. [Google Scholar] [CrossRef]
- Biondi, F.; Hartsough, P. Using automated point dendrometers to analyze tropical treeline stem growth at Nevado de Colima, Mexico. Sensors 2010, 10, 5827–5844. [Google Scholar] [CrossRef]
- Rosado, B.H.P.; Joly, C.A.; Burgess, S.S.O.; Oliveira, R.S.; Aidar, M.P.M. Changes in plant functional traits and water use in Atlantic rainforest: Evidence of conservative water use in spatio-temporal scales. Trees 2016, 30, 47–61. [Google Scholar] [CrossRef]
| # | Variable | Estimate | SE | df | t-Value | p-Value |
| GRO rate | Intercept | −4.05 | 0.09 | 1307 | −44.90 | <0.001 |
| PRE | 0.266 | 0.025 | 1307 | 10.64 | <0.001 | |
| RH | 0.401 | 0.0072 | 1307 | 5.54 | <0.001 | |
| TWD | Intercept | −3.65 | 0.155 | 642 | −23.59 | <0.001 |
| WS | 0.097 | 0.046 | 642 | 2.102 | 0.036 | |
| PRE | −0.126 | 0.049 | 642 | −2.586 | 0.009 | |
| VPD | 0.299 | 0.104 | 642 | 2.891 | 0.004 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Z.-X.; Bräuning, A.; Fu, P.-L.; Yang, R.-Q.; Qi, J.-H.; Grießinger, J.; Gebrekirstos, A. Intra-Annual Radial Growth of Pinus kesiya var. langbianensis Is Mainly Controlled by Moisture Availability in the Ailao Mountains, Southwestern China. Forests 2019, 10, 899. https://doi.org/10.3390/f10100899
Fan Z-X, Bräuning A, Fu P-L, Yang R-Q, Qi J-H, Grießinger J, Gebrekirstos A. Intra-Annual Radial Growth of Pinus kesiya var. langbianensis Is Mainly Controlled by Moisture Availability in the Ailao Mountains, Southwestern China. Forests. 2019; 10(10):899. https://doi.org/10.3390/f10100899
Chicago/Turabian StyleFan, Ze-Xin, Achim Bräuning, Pei-Li Fu, Rao-Qiong Yang, Jin-Hua Qi, Jussi Grießinger, and Aster Gebrekirstos. 2019. "Intra-Annual Radial Growth of Pinus kesiya var. langbianensis Is Mainly Controlled by Moisture Availability in the Ailao Mountains, Southwestern China" Forests 10, no. 10: 899. https://doi.org/10.3390/f10100899
APA StyleFan, Z.-X., Bräuning, A., Fu, P.-L., Yang, R.-Q., Qi, J.-H., Grießinger, J., & Gebrekirstos, A. (2019). Intra-Annual Radial Growth of Pinus kesiya var. langbianensis Is Mainly Controlled by Moisture Availability in the Ailao Mountains, Southwestern China. Forests, 10(10), 899. https://doi.org/10.3390/f10100899







