Male and Female Plants of Salix viminalis Perform Similarly to Flooding in Morphology, Anatomy, and Physiology
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experiment Design
2.2. Growth Characteristics (Height, Diameter, and Biomass)
2.3. Leaf Gas Exchange Parameters
2.4. Chlorophyll Pigment
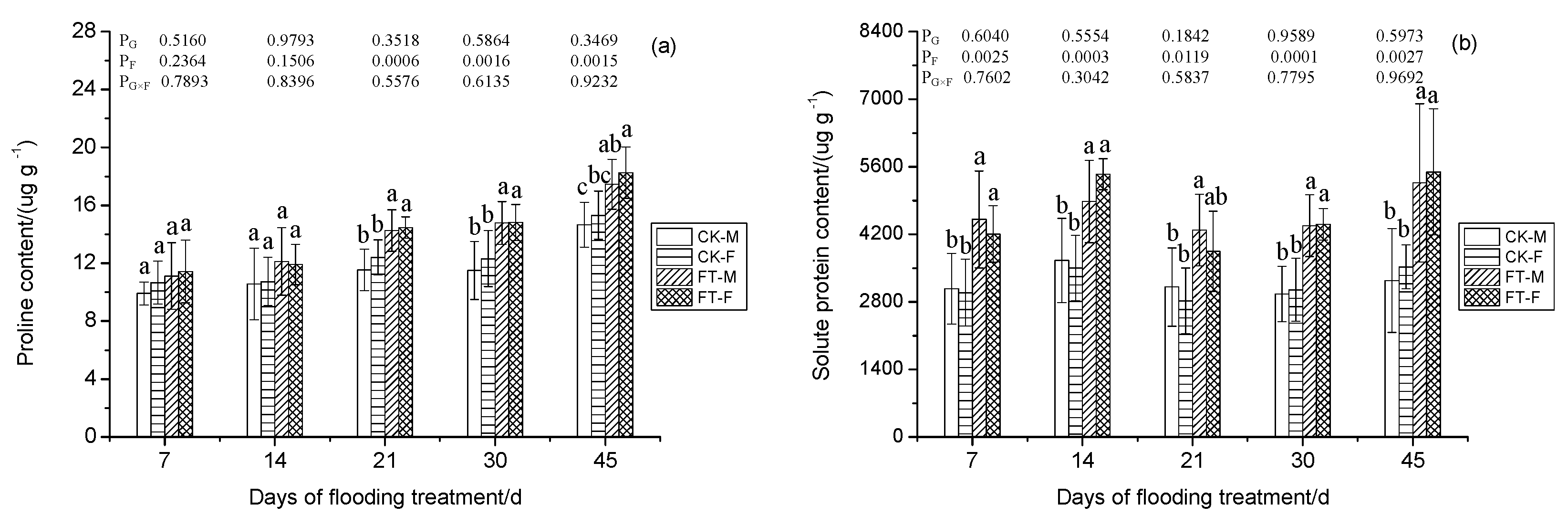
2.5. Proline Content
2.6. Production Ratio of Superoxide Free Radical, Malonaldehyde Content, Solute Proteins Content, and Autioxidant Enzyme Activities Determination
2.7. Anatomical Structure Observation
2.8. Statistical Analysis
3. Results
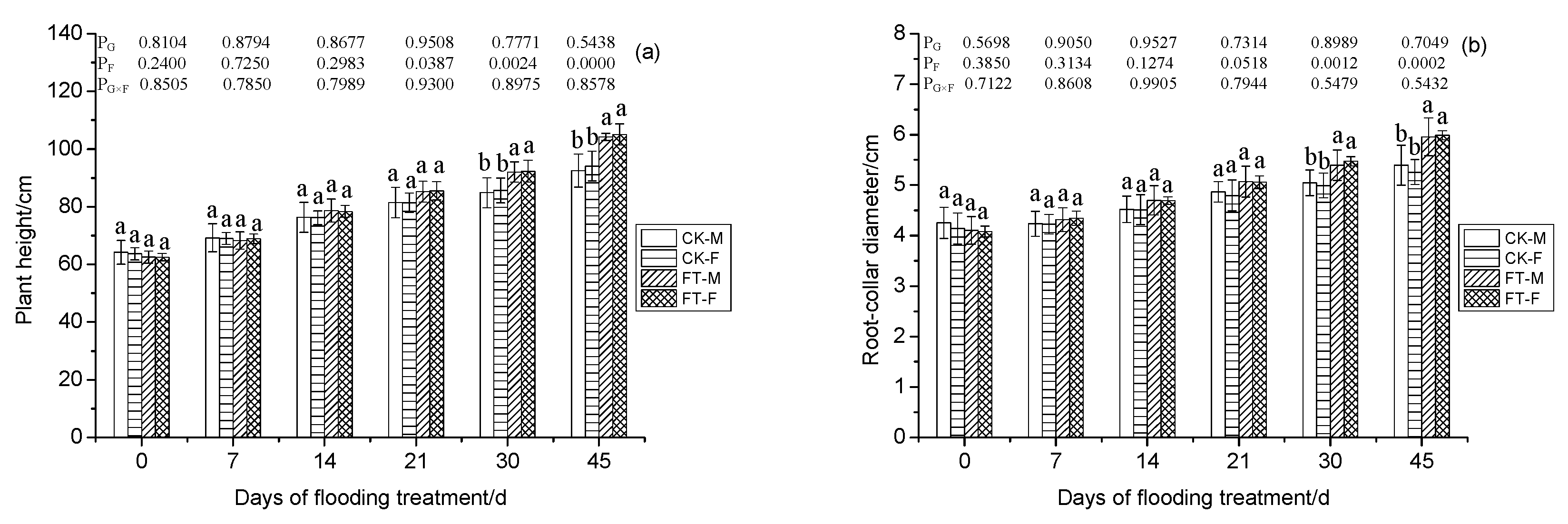
3.1. Gender-Specific Responses of Morphology to Flooding
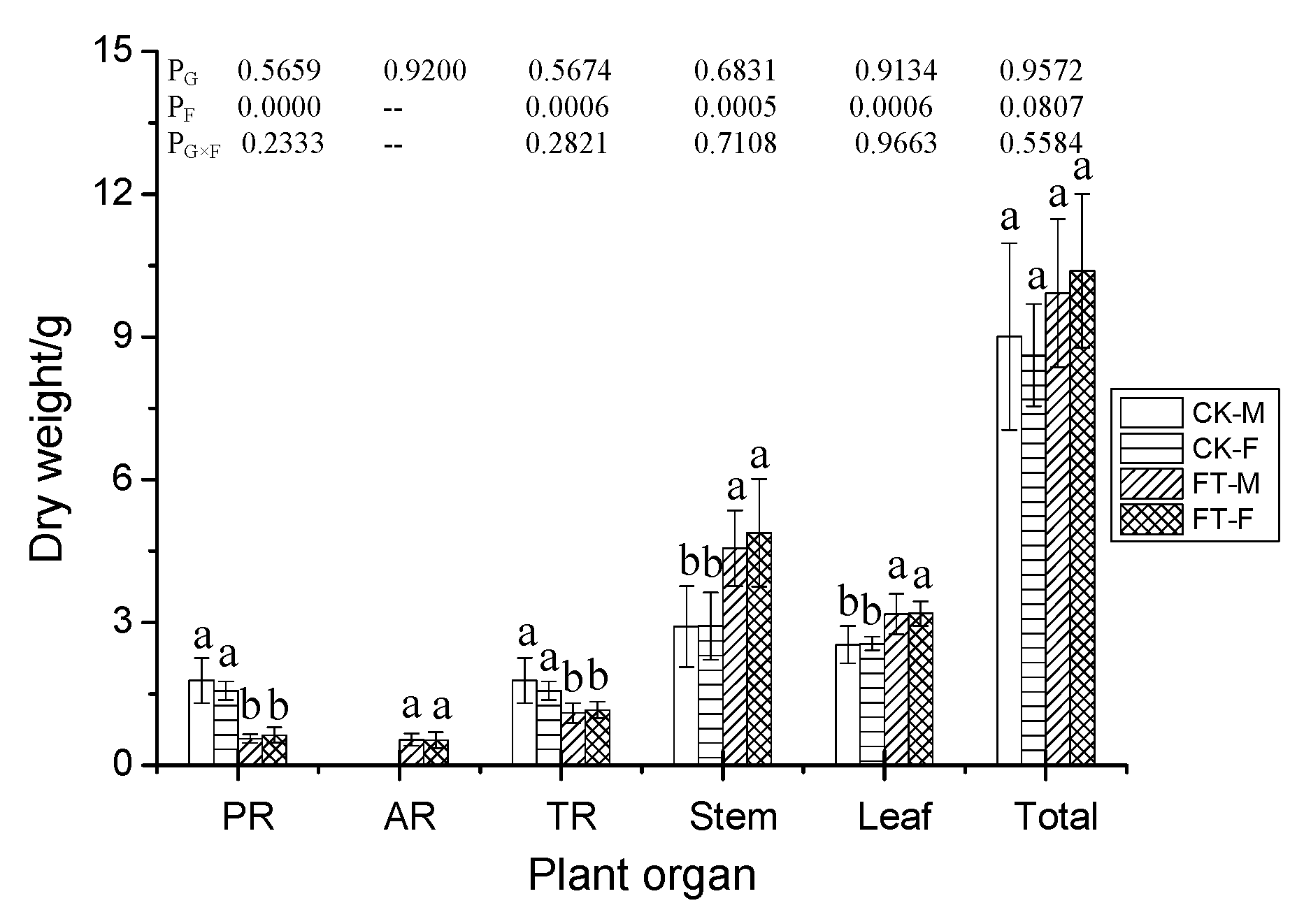
3.2. Gender-Specific Responses of Plant Growth to Flooding
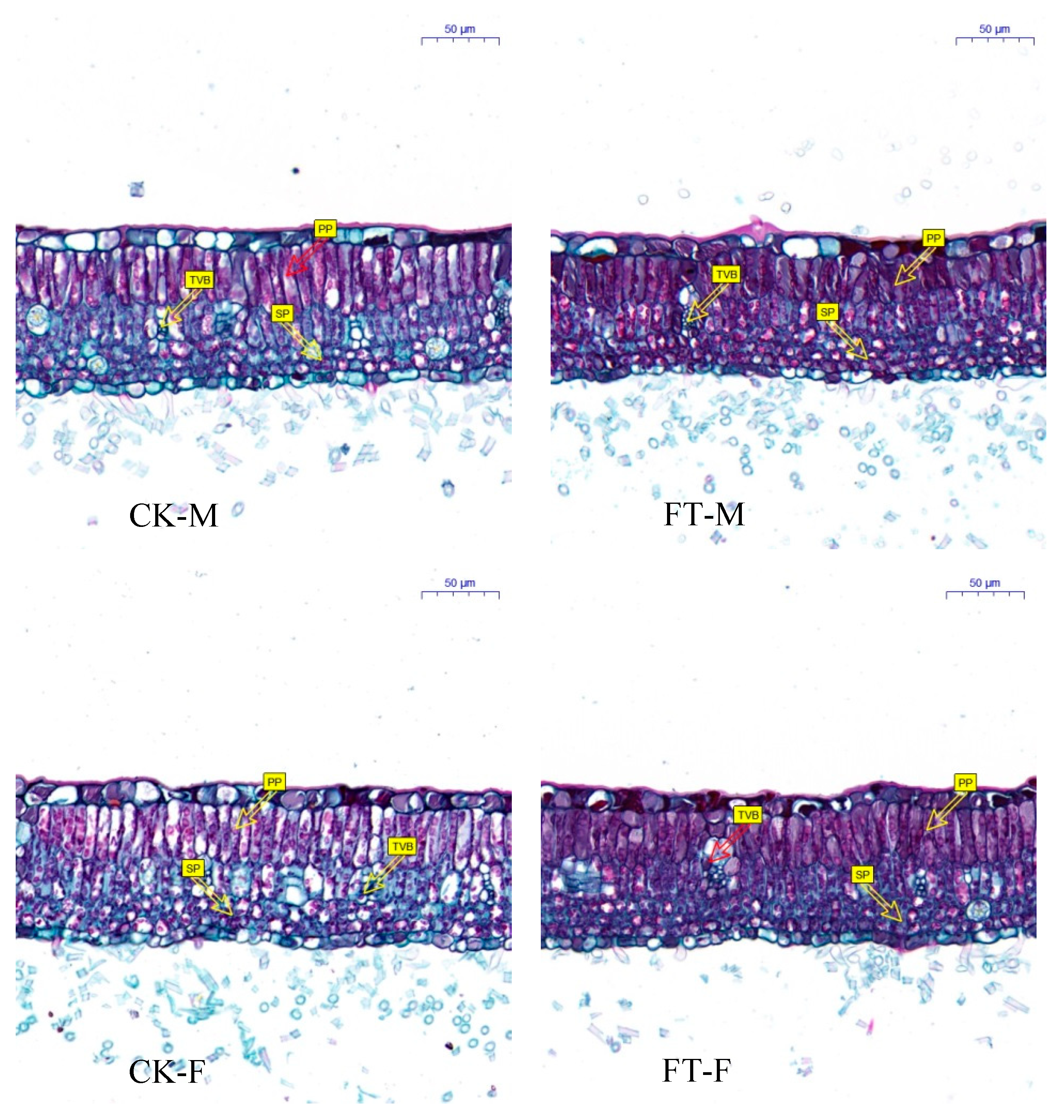
3.3. Gender-Specific Responses of Leaf Anatomical Structure to Flooding
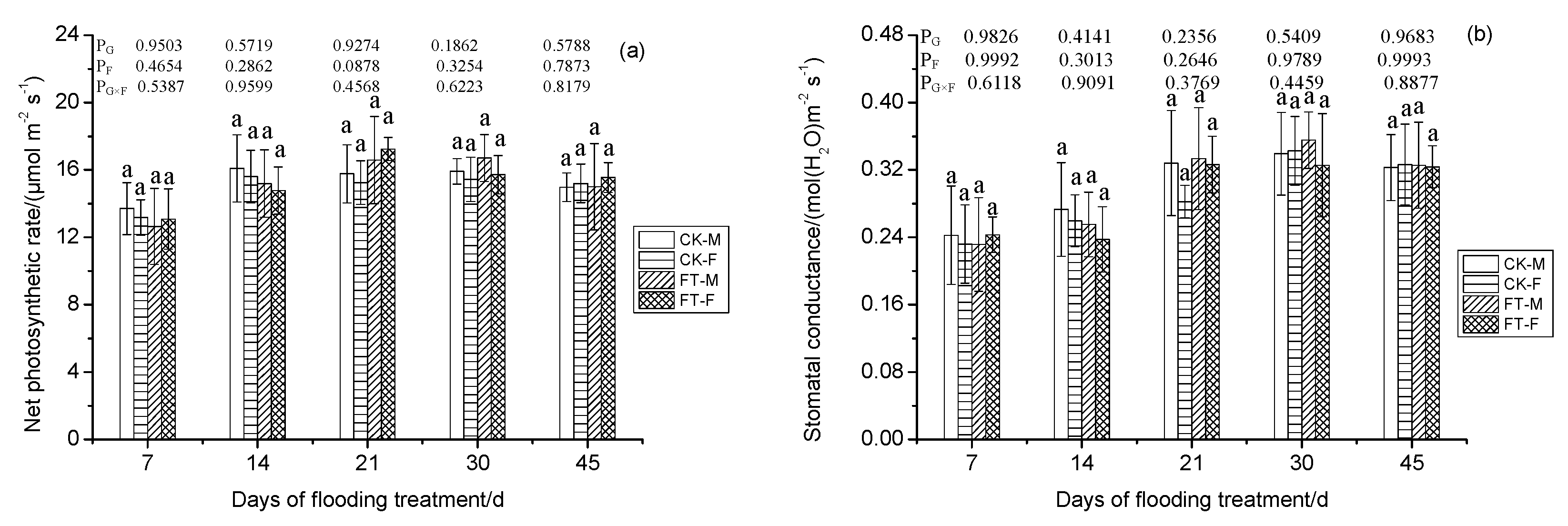
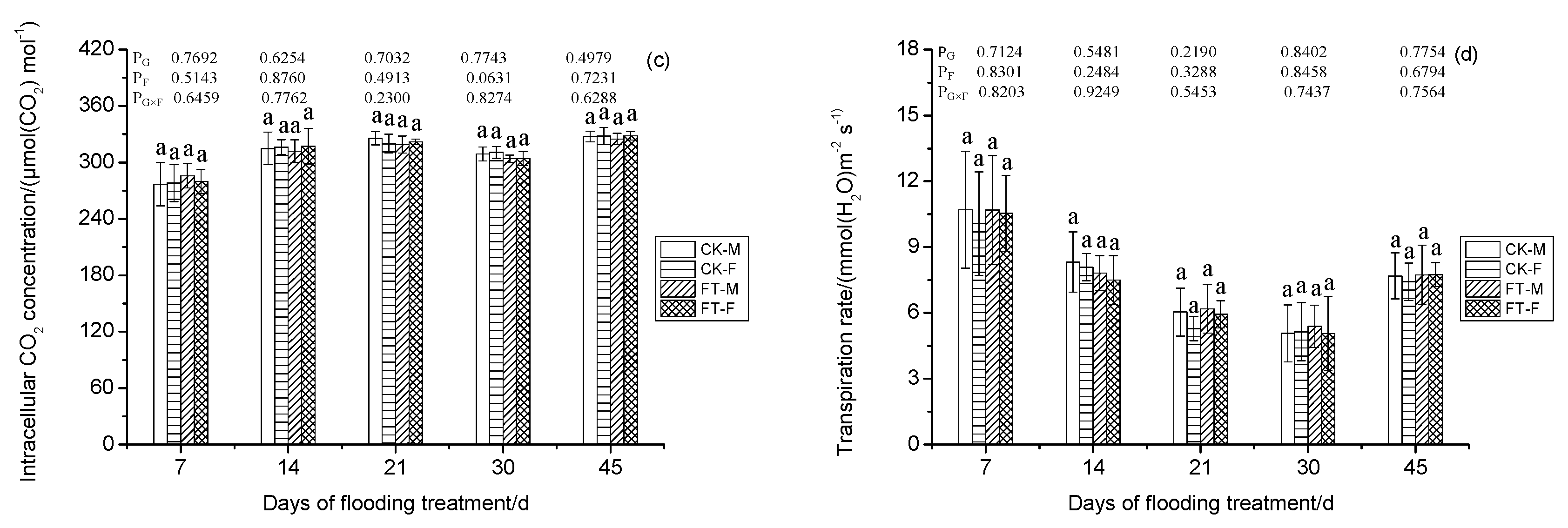
3.4. Gender-Specific Responses of Gas Exchange Parameters to Flooding
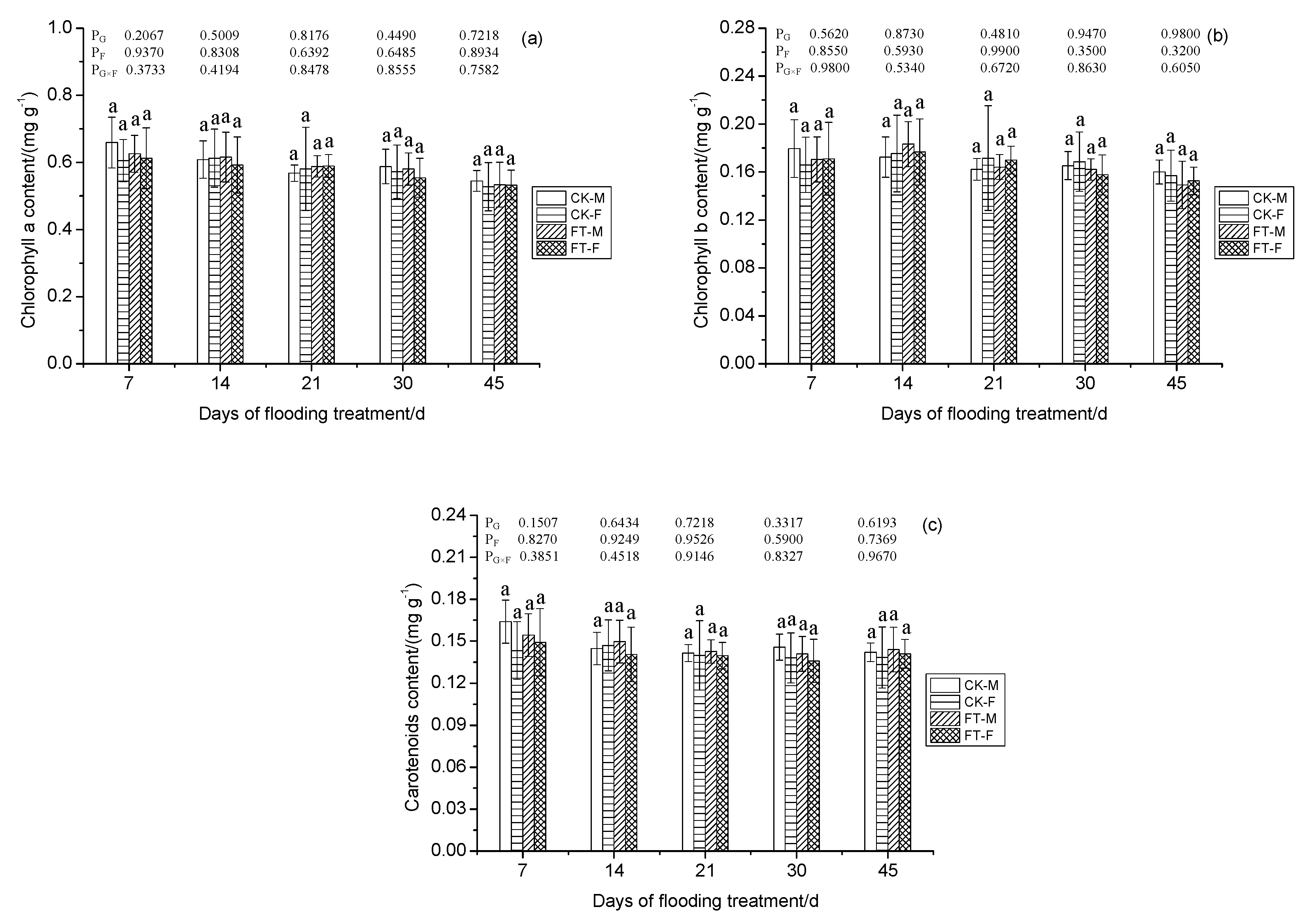
3.5. Gender-Specific Responses of Chlorophyll Pigments to Flooding
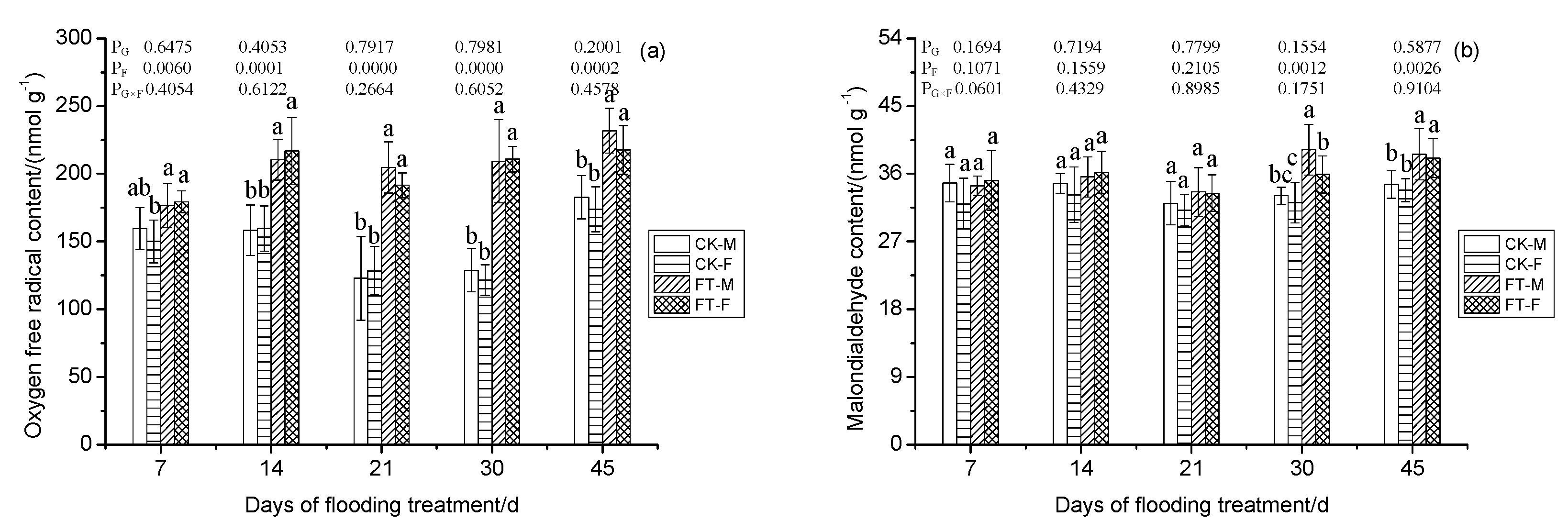
3.6. Gender-Specific Responses of Superoxide Free Radical and Lipid Peroxidation to Flooding
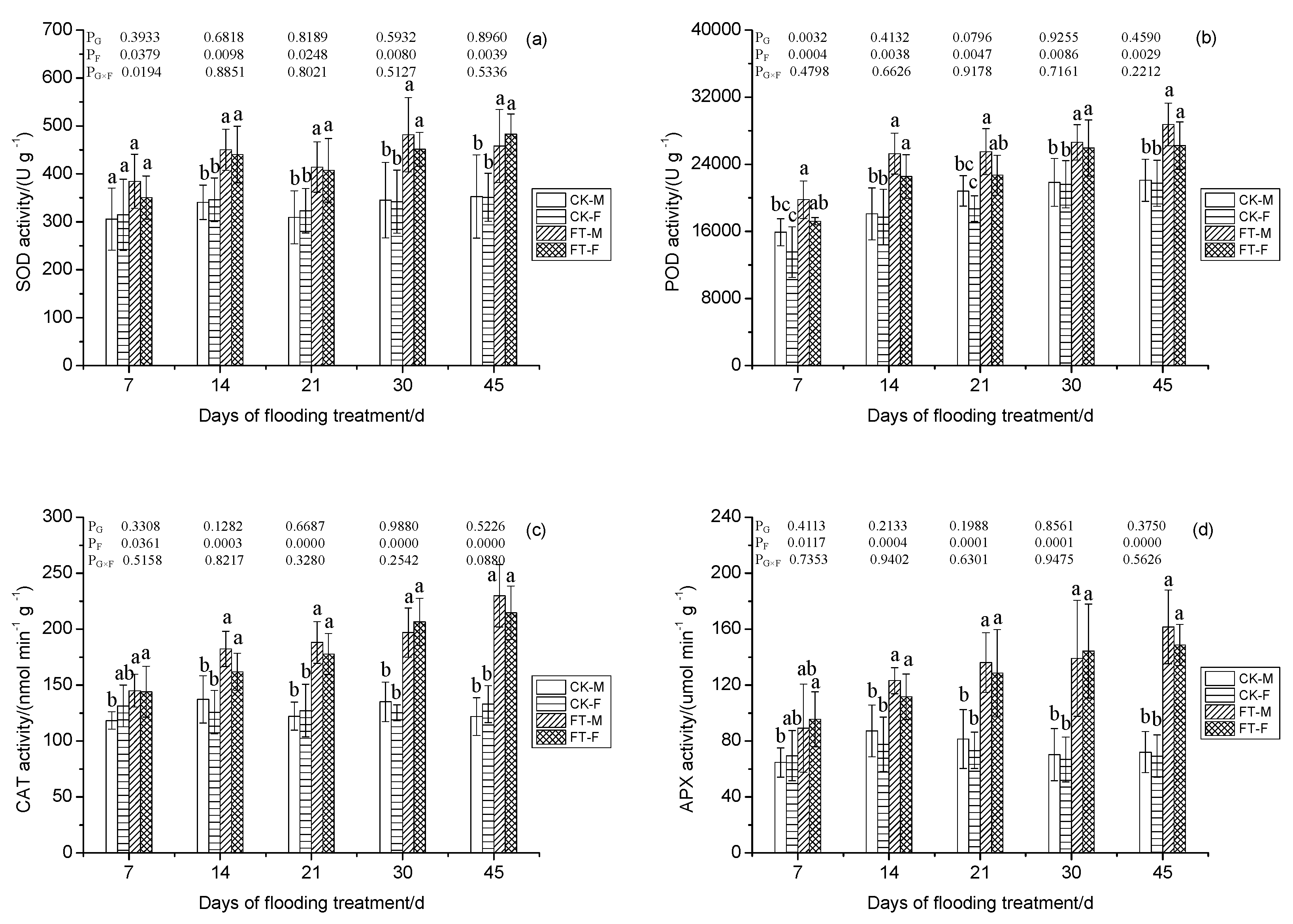
3.7. Gender-Specific Responses of Enzyme Activities to Flooding
3.8. Gender-Specific Responses of Osmotic Regulation to Flooding
4. Discussion
4.1. Flooding Treatment Effects
4.1.1. Morphology, Growth, and Anatomical Structure
4.1.2. Photosynthesis
4.1.3. Oxidative Stress
4.1.4. Antioxidant System
4.1.5. Osmotic Substances
4.2. Gender-Specific Responses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, H.; Zhao, Y.; Zhang, C.; Tao, X.; Xu, X. Growth, leaf gas exchange, and chlorophyll fluorescence responses of two cultivars of Salix integra Thunb, to waterlogging stress. J. Agric. Sci. Technol. Iran 2014, 16, 137–149. [Google Scholar]
- Bailey-Serres, J.; Colmer, T.D. Plant tolerance of flooding stress-recent advances. Plant Cell Environ. 2014, 37, 2211–2215. [Google Scholar] [CrossRef] [PubMed]
- Striker, G.G. Flooding stress on plants: Anatomical, morphological and physiological responses. Botany 2012, 1, 3–28. [Google Scholar]
- Arnell, N.; Liu, C. Climate change 2001: Hydrology and water resources. In Report Intergovernmental Panel on Climate Change; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2001; Available online: http://www.ipcc.ch/ (accessed on 18 January 2019).
- Lee, K.W.; Chen, P.W.; Lu, C.A.; Chen, S.; Ho, T.H.D.; Yu, S.M. Coordinated responses to oxygen and sugar deficiency allow rice seedlings to tolerate flooding. Sci. Signal. 2009, 2, ra61. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Serres, J.; Voesenek, L. Flooding stress: Acclimations and genetic diversity. Annu. Rev. Plant Biol. 2008, 59, 313–339. [Google Scholar] [CrossRef]
- Setter, T.; Waters, I.; Sharma, S.; Singh, K.; Kulshreshtha, N.; Yaduvanshi, N.; Ram, P.; Singh, B.; Rane, J.; McDonald, G. Review of wheat improvement for waterlogging tolerance in Australia and India: The importance of anaerobiosis and element toxicities associated with different soils. Ann. Bot.-London 2009, 103, 221–235. [Google Scholar] [CrossRef]
- Chen, X.; Visser, E.J.; de Kroon, H.; Pierik, R.; Voesenek, L.A.; Huber, H. Fitness consequences of natural variation in flooding-induced shoot elongation in Rumex palustris. New Phytol. 2011, 190, 409–420. [Google Scholar] [CrossRef]
- Imaz, J.A.; Giménez, D.O.; Grimoldi, A.A.; Striker, G.G. The effects of submergence on anatomical, morphological and biomass allocation responses of tropical grasses Chloris gayana and Panicum coloratum at seedling stage. Crop Pasture Sci. 2013, 63, 1145–1155. [Google Scholar] [CrossRef]
- Shukor, N.-N.; Abdul-Hamid, H.; Abdu, A.; Ismail, M.-K. Waterlogging effects on growth and physiological characteristics of Azadirachta excelsa seedlings. Am. J. Plant Physiol. 2014, 9, 78–94. [Google Scholar] [CrossRef]
- De Oliveira, V.C.; Joly, C.A. Flooding tolerance of Calophyllum brasiliense Camb.(Clusiaceae): Morphological, physiological and growth responses. Trees 2010, 24, 185–193. [Google Scholar] [CrossRef]
- Parad, G.A.; Zarafshar, M.; Striker, G.G.; Sattarian, A. Some physiological and morphological responses of Pyrus boissieriana to flooding. Trees 2013, 27, 1387–1393. [Google Scholar] [CrossRef]
- Azizi, S.; Tabari, M.; Striker, G.G. Growth, physiology, and leaf ion concentration responses to long-term flooding with fresh or saline water of Populus euphratica. S. Afr. J. Bot. 2017, 108, 229–236. [Google Scholar] [CrossRef]
- Kreuzwieser, J.; Rennenberg, H. Molecular and physiological responses of trees to waterlogging stress. Plant Cell Environ. 2014, 37, 2245–2259. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Zhao, C.; Li, J.; Li, J.; Peng, G. Morphological, physiological, and biochemical responses of Populus euphratica to soil flooding. Photosynthetica 2015, 53, 110–117. [Google Scholar] [CrossRef]
- Yordanova, R.Y.; Popova, L.P. Flooding-induced changes in photosynthesis and oxidative status in maize plants. Acta Physiol. Plant 2007, 29, 535–541. [Google Scholar] [CrossRef]
- Pereira, F.J.; Magalhães, P.C.; Souza, T.C.D.; Castro, E.M.D.; Alves, J.D. Antioxidant system activity and aerenchyma formation in’Saracura’maize roots. Pesqui. Agropecu. Bras. 2010, 45, 451–456. [Google Scholar] [CrossRef]
- Shabala, S. Physiological and cellular aspects of phytotoxicity tolerance in plants: The role of membrane transporters and implications for crop breeding for waterlogging tolerance. New Phytol. 2011, 190, 289–298. [Google Scholar] [CrossRef]
- Pérez-Jiménez, M.; Hernández-Munuera, M.; Piñero, M.C.; López-Ortega, G.; del Amor, F.M. Are commercial sweet cherry rootstocks adapted to climate change? Short-term waterlogging and CO2 effects on sweet cherry cv.‘Burlat’. Plant Cell Environ. 2018, 41, 908–918. [Google Scholar] [CrossRef]
- An, Y.Y.; Liang, Z.S.; Zhao, R.K.; Zhang, J.; Wang, X.J. Organ-dependent responses of Periploca sepium to repeated dehydration and rehydration. S. Afr. J. Bot. 2011, 77, 446–454. [Google Scholar] [CrossRef][Green Version]
- An, Y.; Qi, L.; Wang, L. ALA Pretreatment improves waterlogging tolerance of fig plants. PLoS ONE 2016, 11, e0147202. [Google Scholar] [CrossRef]
- Lekshmy, S.; Jha, S.K.; Sairam, R.K. Physiological and molecular mechanisms of flooding tolerance in plants. In Elucidation of Abiotic Stress Signaling in Plants; Pandey, G.K., Ed.; Springer: New York, NY, USA, 2015; pp. 227–242. [Google Scholar]
- Saha, R.; Ahmed, F.; Mokarroma, N.; Rohman, M.; Golder, P. Physiological and biochemical changes in waterlog tolerant sesame genotypes. SAARC J. Agri. 2016, 14, 31–45. [Google Scholar] [CrossRef]
- Vwioko, E.; Adinkwu, O.; El-Esawi, M.A. Comparative physiological, biochemical, and genetic responses to prolonged waterlogging stress in okra and maize given exogenous ethylene priming. Front. Physiol. 2017, 8, 632. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, S.; Li, Y.; Chen, S.; Guo, Z. Physiological response of Phyllostachys rivalis rhizome roots to long-term water stress. For. Res. 2014, 27, 621–625. [Google Scholar]
- Kutschera, U.; Niklas, K.J. Julius von Sachs’ forgotten 1897-article: Sexuality and gender in plants vs. humans. Plant Signal. Behav. 2018, 13, e1489671. [Google Scholar] [CrossRef] [PubMed]
- Renner, S.S. The relative and absolute frequencies of angiosperm sexual systems: Dioecy, monoecy, gynodioecy, and an updated online database. Am. J. Bot. 2014, 101, 1588–1596. [Google Scholar] [CrossRef]
- Jiang, H.; Peng, S.; Zhang, S.; Li, X.; Korpelainen, H.; Li, C. Transcriptional profiling analysis in Populus yunnanensis provides insights into molecular mechanisms of sexual differences in salinity tolerance. J. Exp. Bot. 2012, 63, 3709–3726. [Google Scholar] [CrossRef]
- Hawkins, T.S.; Schiff, N.M.; Leininger, T.D.; Gardiner, E.S.; Devall, M.S.; Hamel, P.B.; Wilson, A.D.; Connor, K.F. Growth and intraspecific competitive abilities of the dioecious Lindera melissifolia (Lauraceae) in varied flooding regimes. J. Torrey Bot. Soc. 2009, 136, 91–101. [Google Scholar] [CrossRef]
- Kutschera, U. Sex-gender-conflicts in aquatic hermaphrodites: Are genes immortal? J. Marine Sci. Res. Dev. 2017, 7, 1–4. [Google Scholar] [CrossRef]
- Dawson, T.E.; Ehleringer, J.R. Gender-specific physiology, carbon isotope discrimination, and habitat distribution in boxelder, Acer Negundo. Ecology 1993, 74, 798–815. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, X.; Zhang, Y.; Korpelainen, H.; Li, C. Nitrogen deposition limits photosynthetic response to elevated CO2 differentially in a dioecious species. Oecologia 2011, 165, 41–54. [Google Scholar] [CrossRef]
- Chen, F.; Chen, L.; Zhao, H.; Korpelainen, H.; Li, C. Sex-specific responses and tolerances of Populus cathayana to salinity. Physiol. Plant. 2010, 140, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chen, L.; Duan, B.; Korpelainen, H.; Li, C. Populus cathayana males exhibit more efficient protective mechanisms than females under drought stress. For. Ecol. Manag. 2012, 275, 68–78. [Google Scholar] [CrossRef]
- Han, Y.; Wang, L.; Zhang, X.; Korpelainen, H.; Li, C. Sexual differences in photosynthetic activity, ultrastructure and phytoremediation potential of Populus cathayana exposed to lead and drought. Tree Physiol. 2013, 33, 1043–1060. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Luo, Y.; Zong, S.; Wang, R.; Luo, H. Comparison in leaf anatomical structure and drought resistance of different sex and varieties of sea buckthorn. J. Beijing For. Univ. 2012, 34, 34–41. [Google Scholar]
- Randriamanana, T.R.; Nissinen, K.; Moilanen, J.; Nybakken, L.; Julkunen-Tiitto, R. Long-term UV-B and temperature enhancements suggest that females of Salix myrsinifolia plants are more tolerant to UV-B than males. Environ. Exp. Bot. 2015, 109, 296–305. [Google Scholar] [CrossRef]
- Cao, Y.; Ma, C.; Chen, G.; Zhang, J.; Xing, B. Physiological and biochemical responses of Salix integra Thunb. under copper stress as affected by soil flooding. Environ. Pollut. 2017, 225, 644–653. [Google Scholar] [CrossRef]
- Nielsen, J.L.; Rood, S.B.; Pearce, D.W.; Letts, M.G.; Jiskoot, H. Streamside trees: Responses of male, female and hybrid cottonwoods to flooding. Tree Physiol. 2010, 30, 1479–1488. [Google Scholar] [CrossRef]
- Rood, S.B.; Nielsen, J.L.; Shenton, L.; Gill, K.M.; Letts, M.G. Effects of flooding on leaf development, transpiration, and photosynthesis in narrowleaf cottonwood, a willow-like poplar. Photosynth. Res. 2010, 104, 31–39. [Google Scholar] [CrossRef]
- Jackson, M.B.; Attwood, P.A. Roots of willow (Salix viminalis L.) show marked tolerance to oxygen shortage in flooded soils and in solution culture. Plant Soil 1996, 187, 37–45. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic membranes. Method. Enzymol. 1987, 148, 350–383. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Berlin, S.; Fogelqvist, J.; Lascoux, M.; Lagercrantz, U.; Rönnberg-Wästljung, A.C. Polymorphism and divergence in two willow species, Salix viminalis L. and Salix schwerinii E. Wolf. G3 Genes Genom. Genet. 2011, 1, 387–400. [Google Scholar]
- Kozlowski, T.; Pallardy, S. Acclimation and adaptive responses of woody plants to environmental stresses. Bot. Rev. 2002, 68, 270–334. [Google Scholar] [CrossRef]
- Sasidharan, R.; Voesenek, L.A. Ethylene-mediated acclimations to flooding stress. Plant Physiol. 2015, 169, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhou, Z.; Tong, R.; Hu, X.; Du, K. Anatomy and ultrastructure adaptations to soil flooding of two full-sib poplar clones differing in flood-tolerance. Flora 2017, 233, 90–98. [Google Scholar] [CrossRef]
- Yang, F.; Wang, Y.; Wang, J.; Deng, W.; Liao, L.; Li, M. Different eco-physiological responses between male and female Populus deltoides clones to waterlogging stress. For. Ecol. Manag. 2011, 262, 1963–1971. [Google Scholar] [CrossRef]
- Du, K.; Xu, L.; Wu, H.; Tu, B.; Zheng, B. Ecophysiological and morphological adaption to soil flooding of two poplar clones differing in flood-tolerance. Flora 2012, 207, 96–106. [Google Scholar] [CrossRef]
- Adams, A.E.; Kazenel, M.R.; Rudgers, J.A. Does a foliar endophyte improve plant fitness under flooding? Plant Ecol. 2017, 218, 711–723. [Google Scholar] [CrossRef]
- Lei, S.; Zeng, B.; Xu, S.; Zhang, X. Response of basal metabolic rate to complete submergence of riparian species Salix variegata in the Three Gorges reservoir region. Sci. Rep. UK 2017, 7, 13885. [Google Scholar] [CrossRef]
- Perata, P.; Armstrong, W.; Voesenek, L.A. Plants and flooding stress. New Phytol. 2011, 190, 269–273. [Google Scholar] [CrossRef]
- Phukan, U.J.; Mishra, S.; Shukla, R.K. Waterlogging and submergence stress: Affects and acclimation. Crit. Rev. Biotechnol. 2016, 36, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.Q.; Zeng, B.; Meng, J.L.; Wu, M.; Zhang, X.P. Responses in shoot elongation, carbohydrate utilization and growth recovery of an invasive species to submergence at different water temperatures. Sci. Rep. UK 2018, 8, 306. [Google Scholar] [CrossRef] [PubMed]
- Schmull, M.; Thomas, F.M. Morphological and physiological reactions of young deciduous trees (Quercus robur L., Q. petraea [Matt.] Liebl, Fagus sylvatica L.) to waterlogging. Plant Soil 2000, 225, 227–242. [Google Scholar] [CrossRef]
- Yin, D.; Zhang, Z.; Luo, H. Anatomical responses to waterlogging in Chrysanthemum zawadskii. Sci. Hortic. Amst. 2012, 146, 86–91. [Google Scholar] [CrossRef]
- Elcan, J.; Pezeshki, S. Effects of flooding on susceptibility of Taxodium distichum L. seedlings to drought. Photosynthetica 2002, 40, 177–182. [Google Scholar] [CrossRef]
- Bertolde, F.; Almeida, A.A.; Pirovani, C.; Gomes, F.; Ahnert, D.; Baligar, V.; Valle, R. Physiological and biochemical responses of Theobroma cacao L. genotypes to flooding. Photosynthetica 2012, 50, 447–457. [Google Scholar] [CrossRef]
- Li, C.X.; Wei, H.; Geng, Y.H.; Schneider, R. Effects of submergence on photosynthesis and growth of Pterocarya stenoptera (Chinese wingnut) seedlings in the recently-created Three Gorges Reservoir region of China. Wetl. Ecol. Manag. 2010, 18, 485–494. [Google Scholar] [CrossRef]
- Herrera, A. Responses to flooding of plant water relations and leaf gas exchange in tropical tolerant trees of a black-water wetland. Front. Plant Sci. 2013, 4, 106. [Google Scholar] [CrossRef]
- Zhou, C.; Bai, T.; Wang, Y.; Wu, T.; Zhang, X.; Xu, X.; Han, Z. Morpholoical and enzymatic responses to waterlogging in three Prunus species. Sci. Hortic. Amst. 2017, 221, 62–67. [Google Scholar] [CrossRef]
- Pereira, E.S.; Silva, O.N.; Argemiro Filho, P.; Felipe, J.P.; Alves, G.A.; Lobato, A.K. Antioxidant enzymes efficiently control leaf and root cell damage in young Euterpe oleracea plants exposed to waterlogging. Ind. J. Plant Physiol. 2015, 20, 213–219. [Google Scholar] [CrossRef]
- Lin, K.H.; Kuo, W.S.; Chiang, C.M.; Hsiung, T.C.; Chiang, M.C.; Lo, H.F. Study of sponge gourd ascorbate peroxidase and winter squash superoxide dismutase under respective flooding and chilling stresses. Scientia Hortic. Amst. 2013, 162, 333–340. [Google Scholar] [CrossRef]
- Yu, M.; Zhou, Z.; Deng, X.; Li, J.; Mei, F.; Qi, Y. Physiological mechanism of programmed cell death aggravation and acceleration in wheat endosperm cells caused by waterlogging. Acta Physiol. Plant. 2017, 39, 23. [Google Scholar] [CrossRef]
- Liu, M.; Jiang, Y. Genotypic variation in growth and metabolic responses of perennial ryegrass exposed to short-term waterlogging and submergence stress. Plant Physiol. Bioch. 2015, 95, 57–64. [Google Scholar] [CrossRef]
- Arbona, V.; Hossain, Z.; López-Climent, M.F.; Pérez-Clemente, R.M.; Gómez-Cadenas, A. Antioxidant enzymatic activity is linked to waterlogging stress tolerance in citrus. Physiol. Plant. 2008, 132, 452–466. [Google Scholar] [CrossRef]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef]
- Ma, L.T.; Chen, S.L. Physiological responses of Guadua amplexifolia to NaCl stress. Chinese J. Ecol. 2008, 27, 1487–1491. [Google Scholar]
- Duhan, S.; Kumari, A.; Bala, S.; Sharma, N.; Sheokand, S. Effects of waterlogging, salinity and their combination on stress indices and yield attributes in pigeonpea (Cajanus cajan L. Millsp.) genotypes. Ind. J. Plant Physiol. 2018, 23, 65–76. [Google Scholar] [CrossRef]
- Vurayai, R.; Emongor, V.; Moseki, B. Physiological responses of bambara groundnut (Vigna subterranea L. Verdc) to short periods of water stress during different developmental stages. Asian J. Agric. Sci. 2011, 3, 37–43. [Google Scholar]
- Ji, X.M.; Xu, L.; Xie, Y.F.; Guo, C.X.; Chen, W.D. Effects of soil water conditions on growth and physiology of Acorus tatarinowii Schott. Southwest China J. Agric. Sci. 2013, 26, 2285–2288. [Google Scholar]
- Jing, S.W.; Coley, P.D. Dioecy and herbivory: The effect of growth rate on plant defense in Acer negundo. Oikos 1990, 58, 369–377. [Google Scholar] [CrossRef]
- Nybakken, L.; Julkunen-Tiitto, R. Gender differences in Salix myrsinifolia at the pre-reproductive stage are little affected by simulated climatic change. Physiol. Plant. 2012, 147, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, D.; Villar-Salvador, P.; García-Fayos, P.; Verdú, M. Genders in Juniperus thurifera have different functional responses to variations in nutrient availability. New Phytol. 2012, 193, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Randriamanana, T.R.; Nybakken, L.; Lavola, A.; Aphalo, P.J.; Nissinen, K.; Julkunen-Tiitto, R. Sex-related differences in growth and carbon allocation to defence in Populus tremula as explained by current plant defence theories. Tree Physiol. 2014, 34, 471–487. [Google Scholar] [CrossRef] [PubMed]
- Dudley, L.S.; Galen, C. Stage-dependent patterns of drought tolerance and gas exchange vary between sexes in the alpine willow, Salix glauca. Oecologia 2007, 153, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Zeng, B.; Lin, F.; Qiao, P.; Ayi, Q.; Huang, W. How does long-term complete submergence influence sex ratio and resource allocation of a dioecious shrub, Salix variegata Franch.? Ecol. Eng. 2016, 87, 218–223. [Google Scholar] [CrossRef]
- Ueno, N.; Seiwa, K. Gender-specific shoot structure and functions in relation to habitat conditions in a dioecious tree, Salix sachalinensis. J. For. Res. 2003, 8, 9–16. [Google Scholar] [CrossRef]
- Tozawa, M.; Ueno, N.; Seiwa, K. Compensatory mechanisms for reproductive costs in the dioecious tree Salix integra. Botany 2009, 87, 315–323. [Google Scholar] [CrossRef]
- Moritz, K.K.; Björkman, C.; Parachnowitsch, A.L.; Stenberg, J.A. Female Salix viminalis are more severely infected by Melampsora spp. but neither sex experiences associational effects. Ecol. Evol. 2016, 6, 1154–1162. [Google Scholar] [CrossRef]
- Zhai, F.F.; Liu, J.X.; Mao, J.M.; Peng, X.Y.; Han, L.; Sun, Z.Y. Physiological differences and variations in male and female plants of Salix viminalis under high temperature stress. J. Beijing For. Univ. 2016, 38, 43–49. [Google Scholar]
- Letts, M.G.; Phelan, C.A.; Johnson, D.R.; Rood, S.B. Seasonal photosynthetic gas exchange and leaf reflectance characteristics of male and female cottonwoods in a riparian woodland. Tree Physiol. 2008, 28, 1037–1048. [Google Scholar] [CrossRef]
- Hughes, F.M.R.; Johnamsson, M.; Xiong, S.; Carlborg, E.; Hawkins, D.; Svedmark, M.; Hayes, A.; Goodall, A.; Richards, K.S.; Nilsson, C. The influence of hydrological regimes on sex ratios and spatial segregation of the sexes in two dioecious riparian shrub species in northern Sweden. Plant Ecol. 2010, 208, 77–92. [Google Scholar] [CrossRef]
- Ueno, N.; Suyama, Y.; Seiwa, K. What makes the sex ratio female-biased in the dioecious tree Salix sachalinensis? J. Ecol. 2007, 95, 951–959. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, F.-f.; Li, H.-d.; Zhang, S.-w.; Li, Z.-j.; Liu, J.-x.; Qian, Y.-q.; Ju, G.-s.; Zhang, Y.-x.; Liu, L.; Han, L.; et al. Male and Female Plants of Salix viminalis Perform Similarly to Flooding in Morphology, Anatomy, and Physiology. Forests 2020, 11, 321. https://doi.org/10.3390/f11030321
Zhai F-f, Li H-d, Zhang S-w, Li Z-j, Liu J-x, Qian Y-q, Ju G-s, Zhang Y-x, Liu L, Han L, et al. Male and Female Plants of Salix viminalis Perform Similarly to Flooding in Morphology, Anatomy, and Physiology. Forests. 2020; 11(3):321. https://doi.org/10.3390/f11030321
Chicago/Turabian StyleZhai, Fei-fei, Hai-dong Li, Shao-wei Zhang, Zhen-jian Li, Jun-xiang Liu, Yong-qiang Qian, Guan-sheng Ju, Yun-xing Zhang, Long Liu, Lei Han, and et al. 2020. "Male and Female Plants of Salix viminalis Perform Similarly to Flooding in Morphology, Anatomy, and Physiology" Forests 11, no. 3: 321. https://doi.org/10.3390/f11030321
APA StyleZhai, F.-f., Li, H.-d., Zhang, S.-w., Li, Z.-j., Liu, J.-x., Qian, Y.-q., Ju, G.-s., Zhang, Y.-x., Liu, L., Han, L., & Sun, Z.-y. (2020). Male and Female Plants of Salix viminalis Perform Similarly to Flooding in Morphology, Anatomy, and Physiology. Forests, 11(3), 321. https://doi.org/10.3390/f11030321



