Aboveground Biomass Allometric Models for Evergreen Broad-Leaved Forest Damaged by a Serious Ice Storm in Southern China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Forest Inventory and Sample Tree Selection
2.3. Biomass Measurements
2.4. Allometric Model Development and Evaluation
3. Results
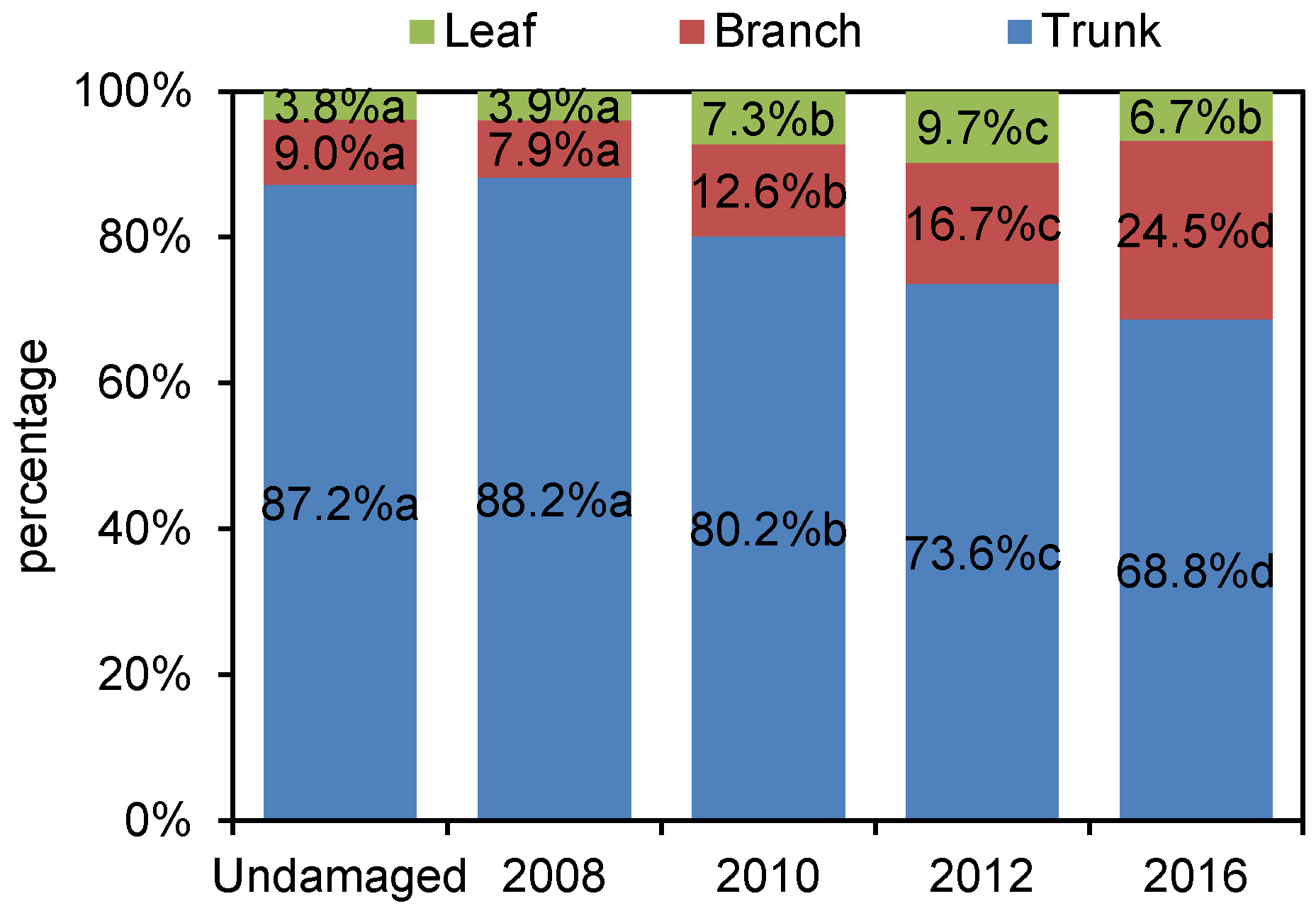
3.1. Dynamic of Biomass Distribution after Disturbance
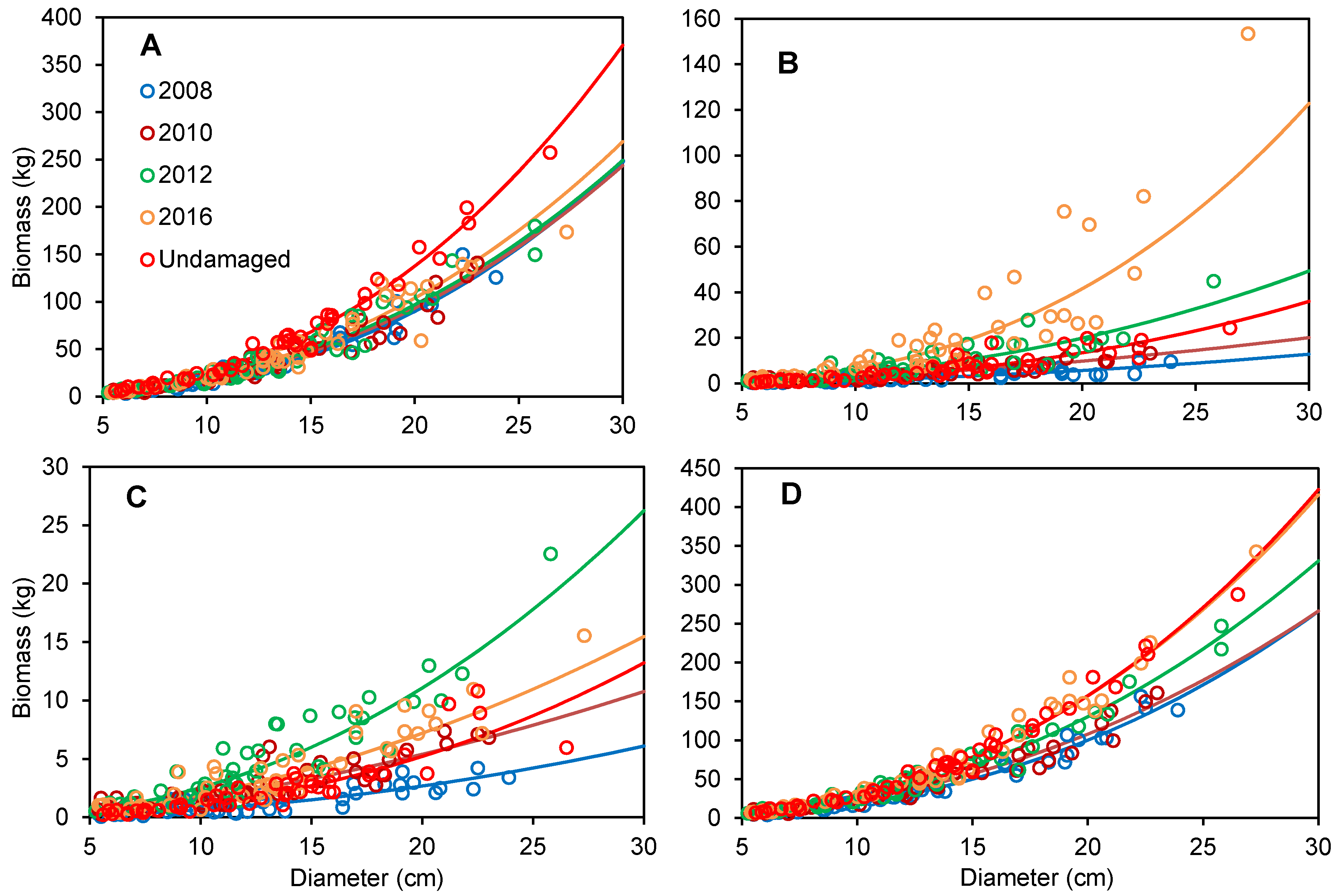
3.2. Biomass Allometric Models at Different Recovery Stages after Disturbance
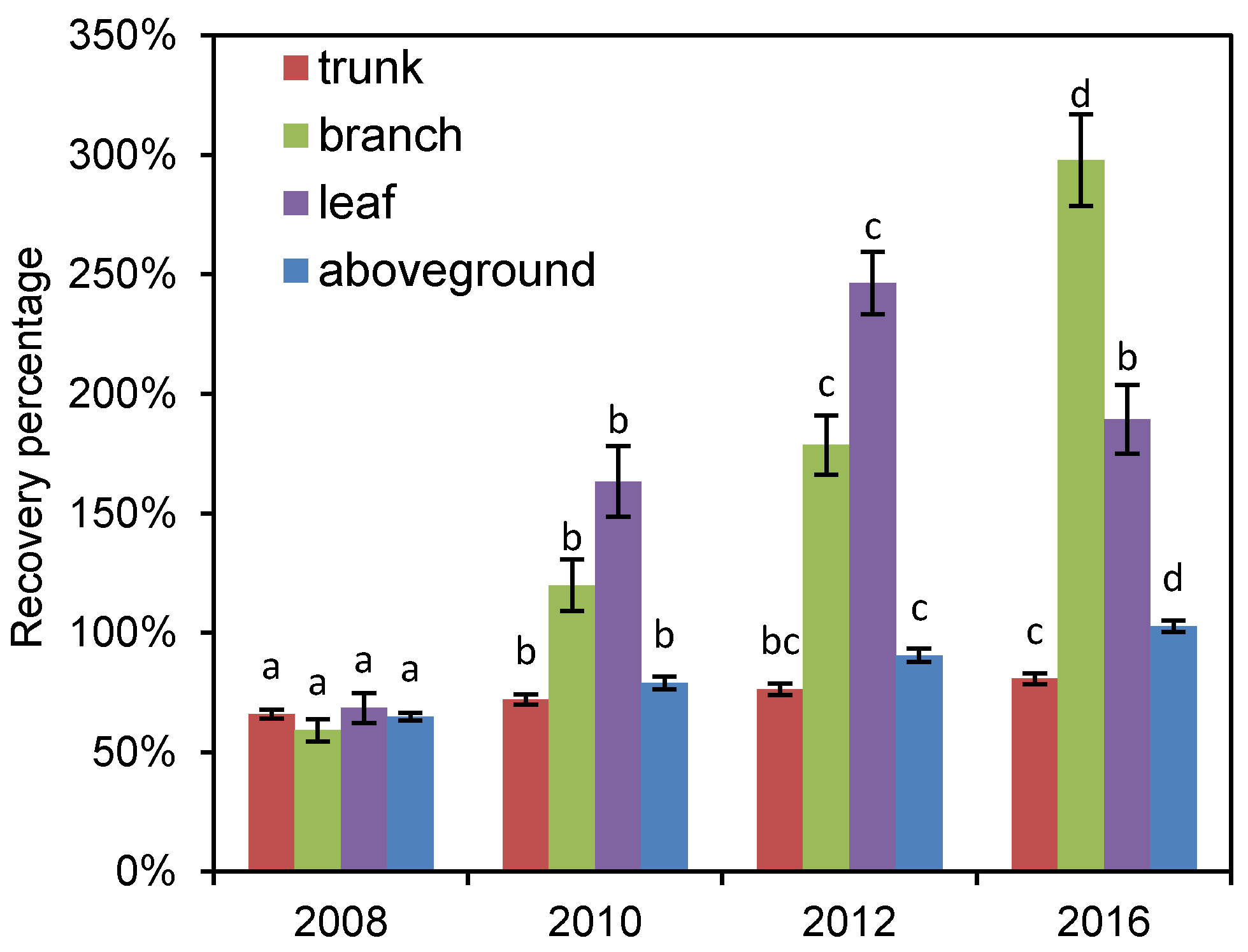
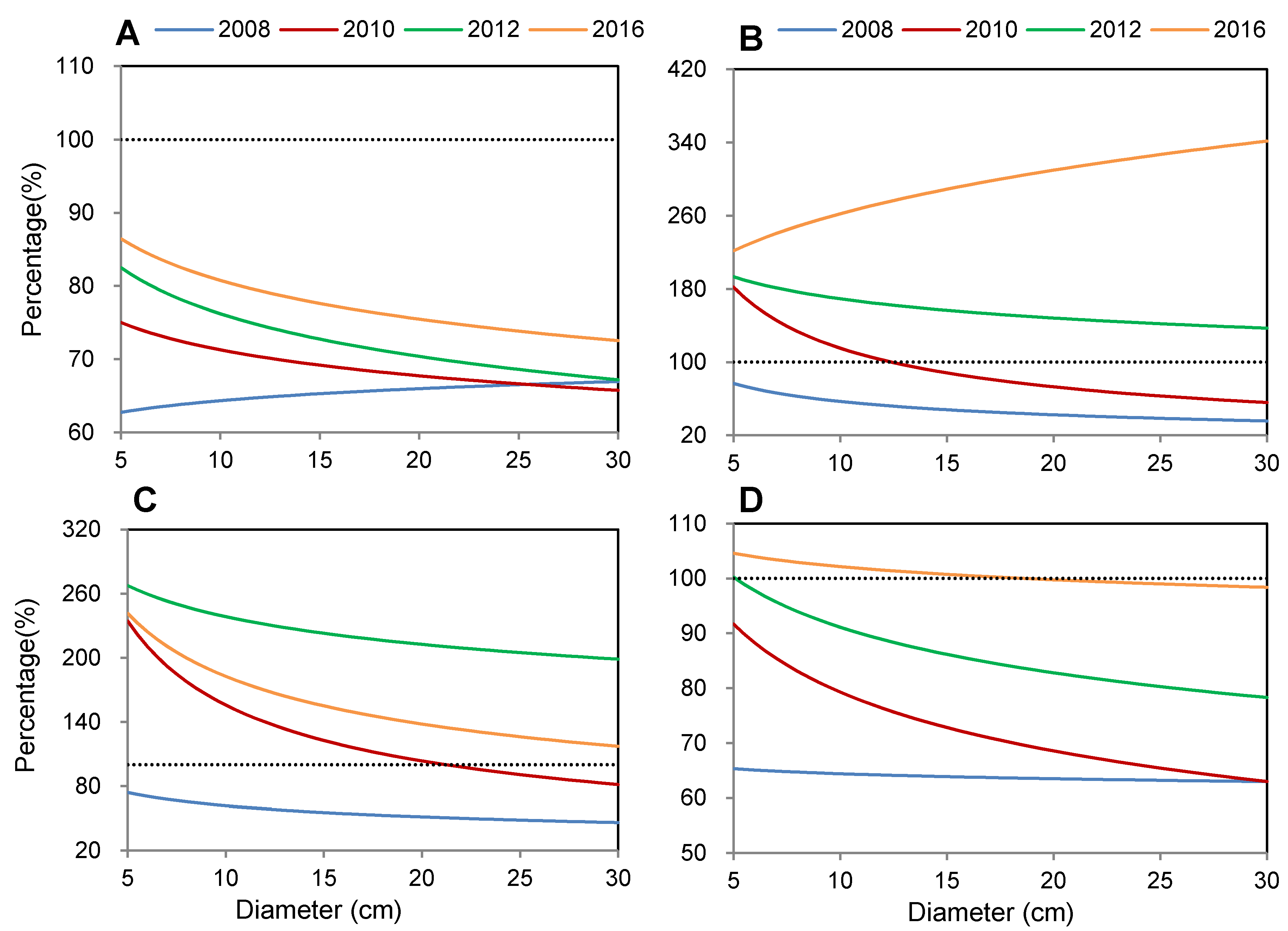
3.3. Recovery Process of Different Tree Components after Disturbance
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WMO (World Meteorological Organization). The Global Climate 2001–2010: A Decade of Climate Extremes; WMO (World Meteorological Organization): Geneva, Switzerland, 2013; Available online: https://library.wmo.int/index.php?lvl=notice_display&id=15112 (accessed on 20 January 2020).
- Yi, C.; Pendall, E.; Ciais, P. Focus on extreme events and the carbon cycle. Environ. Res. Lett. 2015, 10, 070201:1–07201:10. [Google Scholar] [CrossRef]
- Stott, P. How climate change affects extreme weather events. Science 2016, 352, 1517–1518. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Gu, L.; Ding, Y.; Shao, L.; Wu, Z.; Yang, X.; Li, C.; Li, Z.; Wang, X.; Cao, Y.; et al. The great 2008 Chinese ice storm: Its socioeconomic-ecological impact and sustainability lessons learned. B. Am. Meteorol. Soc. 2011, 92, 47–60. [Google Scholar] [CrossRef]
- Changnon, S.A. Characteristics of ice storms in the United States. J. Appl. Meteorol. Clim. 2003, 42, 630–639. [Google Scholar] [CrossRef]
- Stone, R. Natural disasters: Ecologists report huge storm losses in China’s forests. Science 2008, 319, 1318–1319. [Google Scholar] [CrossRef]
- Shao, Q.; Huang, L.; Liu, J.; Kuang, W.; Li, J. Analysis of forest damage caused by the snow and ice chaos along a transect across southern China in spring 2008. J. Geogr. Sci. 2011, 21, 219–234. [Google Scholar] [CrossRef]
- Zhou, B.; Li, Z.; Wang, X.; Cao, Y.; An, Y.; Deng, Z.; Letu, G.; Wang, G.; Gu, L. Impact of the 2008 ice storm on moso bamboo plantations in southeast China. J. Geophys. Res. 2011, 116, G00H06. [Google Scholar] [CrossRef]
- Wang, X.; Yang, F.; Gao, X.; Wang, W.; Zha, X. Evaluation of forest damaged area and severity caused by ice-snow frozen disasters over southern china with remote sensing. Chinese. Geogr. Sci. 2019, 29, 405–416. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, X.; Cao, Y.; Ge, X.; Gu, L.; Meng, J. Damage assessment to subtropical forests following the 2008 Chinese ice storm. Iforest 2017, 10, 406–415. [Google Scholar] [CrossRef]
- Sun, Y.; Gu, L.; Dickinson, R.E.; Zhou, B. Forest greenness after the massive 2008 Chinese ice storm: Integrated effects of natural processes and human intervention. Environ. Res. Lett. 2012, 7, 035702:1–035702:8. [Google Scholar] [CrossRef]
- Bragg, D.C. Initial mortality rates and extent of damage to loblolly and longleaf pine plantations affected by an ice storm in South Carolina. For. Sci. 2016, 62, 574–585. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, Z.; Qiu, Z.; Li, Z.; Zhou, G. Effects of stump characteristics and soil fertility on stump resprouting of schima superba. Cerne 2018, 24, 249–258. [Google Scholar] [CrossRef]
- Wang, X.; Huang, S.; Li, J.; Zhou, G.; Shi, L. Sprouting response of an evergreen broad-leaved forest to a 2008 winter storm in Nanling Mountains, southern China. Ecosphere 2016, 7, e01395:1–e01395:10. [Google Scholar] [CrossRef]
- Huang, R.; Jia, X.; Ou, Y.; Xu, M.; Xie, P.; Su, Z. Monitoring canopy recovery in a subtropical forest following a huge ice storm using hemispherical photography. Environ. Monit. Assess. 2019, 191, 355. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Xiong, G.; Wang, Z.; Zhang, M.; Zhao, C.; Shen, G.; Xu, W.; Xie, Z. Altered dynamics of broad-leaved tree species in a Chinese subtropical montane mixed forest: The role of an anomalous extreme 2008 ice storm episode. Ecol. Evol. 2015, 5, 1484–1493. [Google Scholar] [CrossRef]
- Sholes, O.D.V. Effects of ice storm damage on radial growth of Quercus spp. J. Torrey Bot. Soc. 2013, 3, 364–368. [Google Scholar] [CrossRef]
- Covey, K.R.; Barrett, A.L.; Ashton, M.S. Ice storms as a successional pathway for Fagus grandifolia advancement in Quercus rubra dominated forests of southern New England. Can. J. Forest Res. 2015, 45, 1628–1635. [Google Scholar] [CrossRef]
- Xu, J.X.; Xue, L.; Su, Z.Y. Impacts of forest gaps on soil properties after a severe ice storm in a Cunninghamia lanceolata stand. Pedosphere 2016, 26, 408–416. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, G.; Liu, S.; Du, H.; Mo, L.; Shi, Y.; Jiang, H.; Zhou, Y.; Liu, E. Implications of ice storm damages on the water and carbon cycle of bamboo forests in southeastern China. Agr. Forest Meteorol. 2013, 177, 35–45. [Google Scholar] [CrossRef]
- Powell, S.L.; Cohen, W.B.; Kennedy, R.E.; Healey, S.P.; Huang, C. Observation of trends in biomass loss as a result of disturbance in the conterminous U.S.: 1986–2004. Ecosystems 2014, 17, 142–157. [Google Scholar] [CrossRef]
- Hill, T.C.; Ryan, C.M.; Williams, M. A framework for estimating forest disturbance intensity from successive remotely sensed biomass maps: Moving beyond average biomass loss estimates. Carbon. Bal. Manag. 2015, 10, 27:1–27:9. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Sha, Z.; Yu, M. Remote sensing imagery in vegetation mapping: A review. J. Plant. Ecol. 2008, 1, 9–23. [Google Scholar] [CrossRef]
- Henry, M.; Picard, N.; Trotta, C.; Manlay, R.J.; Valentini, R.; Bernoux, M.; Saintandré, L. Estimating tree biomass of sub-Saharan African forests: A review of available allometric equations. Silva. Fenn. 2011, 45, 477–569. [Google Scholar] [CrossRef]
- Zianis, D.; Mencuccini, M. On simplifying allometric analyses of forest biomass. Forest Ecol. Manag. 2004, 187, 311–332. [Google Scholar] [CrossRef]
- Hounzandji, A.P.I.; Jonard, M.; Nys, C.; Saint-André, L.; Ponette, Q. Improving the robustness of biomass functions: From empirical to functional approaches. Ann. Forest Sci. 2015, 72, 795–810. [Google Scholar] [CrossRef][Green Version]
- Ribeiro, G.; Suwa, R.; Marra, D.; Lima, A.; Kajimoto, T.; Ishizuka, M.; Higuchi, N. Allometry for juvenile trees in an Amazonian forest after wind disturbance. Jap. Agric. Res. Quar. 2014, 48, 213–219. [Google Scholar] [CrossRef][Green Version]
- Mahmood, H.; Siddique, M.R.H.; Abdullah, S.M.R.; Akhter, M.; Islam, S. Manual for Building Tree Volume and Biomass Allometric Equation for Bangladesh; Bangladesh Forest Department, Ministry of Environment and Forests: Dhaka, Bangladesh; Food and Agricultural Organization of the United Nations: Dhaka, Bangladesh, 2016.
- Moussa, M.; Mahamane, L. Allometric models for estimating aboveground biomass and carbon in Faidherbia albida and Prosopis africana under agroforestry parklands in drylands of Niger. J. Forestry Res. 2018, 29, 1703–1717. [Google Scholar] [CrossRef]
- Zhao, H.; Zhou, G.; Li, Z.; Qiu, Z.; Wu, Z. Site-specific allometric models for prediction of above- and belowground biomass of subtropical forests in Guangzhou, southern China. Forests 2019, 10, 862. [Google Scholar] [CrossRef]
- Xiang, W.; Zhou, J.; Ouyang, S.; Zhang, S.; Lei, P.; Li, J.; Deng, X.; Fang, X.; Forrester, D.I. Species-specific and general allometric equations for estimating tree biomass components of subtropical forests in southern China. Eur. J. Forest Res. 2016, 135, 1–17. [Google Scholar] [CrossRef]
- Paul, K.I.; Roxburgh, S.H.; England, J.R.; Ritson, P.; Hobbs, T.; Brooksbank, K.; Raison, R.J.; Larmour, J.S.; Murphy, S.; Norris, J. Development and testing of allometric equations for estimating above-ground biomass of mixed-species environmental plantings. Forest Ecol. Manag. 2013, 310, 483–494. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, J.; Franklin, S.B.; Liang, J.; Ding, P.; Luo, Y.; Lu, Z.; Bao, D.; Jiang, M. Improving allometry models to estimate the above- and belowground biomass of subtropical forest, China. Ecosphere 2015, 6, 1–15. [Google Scholar] [CrossRef]
- Lin, K.; Lyu, M.; Jiang, M.; Chen, Y.; Li, Y.; Chen, G.; Xie, J.; Yang, Y. Improved allometric equations for estimating biomass of the three Castanopsis carlesii H. forest types in subtropical China. New Forest 2017, 48, 1–21. [Google Scholar] [CrossRef]
- Zeng, B. Aboveground biomass partitioning and leaf development of Chinese subtropical trees following pruning. Forest Ecol. Manag. 2003, 173, 135–144. [Google Scholar] [CrossRef]
- Souza, J.P.; Albino, A.L.S.; Prado, C.H.B.A. Evidence of the effects of fire on branching and leaf development in cerrado trees. Acta Botanica Brasilica 2017, 31, 677–685. [Google Scholar] [CrossRef]
- Schutz, A.E.N.; Bond, W.J.; Cramer, M.D. Defoliation depletes the carbohydrate reserves of resprouting Acacia saplings in an African savanna. Plant. Ecol. 2011, 212, 2047–2055. [Google Scholar] [CrossRef]
- Zhu, W.; Xiang, J.; Wang, S.; Li, M. Resprouting ability and mobile carbohydrate reserves in an oak shrubland decline with increasing elevation on the eastern edge of the Qinghai-Tibet Plateau. Forest Ecol. Manag. 2012, 278, 118–126. [Google Scholar] [CrossRef]
- Kays, J.S.; Canham, C.D. Effects of time and frequency of cutting on hardwood root reserves and sprout growth. Forest Sci. 1991, 37, 524–539. [Google Scholar]
- Lutz, J.A.; Halpern, C.B. Tree mortality during early forest development: A long-term study of rates, causes, and consequences. Ecol. Monogr. 2006, 76, 257–275. [Google Scholar] [CrossRef]
- Rydberg, D. Initial sprouting, growth and mortality of European aspen and birch after selective coppicing in central Sweden. Forest Ecol. Manag. 2000, 130, 27–35. [Google Scholar] [CrossRef]
- Li, C.; Huang, S.; Barclay, H.; Filipescu, C.N. Estimation of compensatory growth of coastal Douglas-fir following pre-commercial thinning across a site quality gradient. Forest Ecol. Manag. 2018, 429, 308–316. [Google Scholar] [CrossRef]
- Guillet, C.; Bergstrom, R. Compensatory growth of fast-growing willow (Salix) coppice in response to simulated large herbivore browsing. Oikos 2006, 113, 33–42. [Google Scholar] [CrossRef]
- Lieurance, D.M. Biomass allocation of the invasive tree Acacia auriculiformis and refoliation following hurricane-force winds. J. Torrey Bot. Soc. 2007, 134, 389–397. [Google Scholar] [CrossRef]
- Angela, G.B.; Neil, C.; Contreras, T.A.; Fahrig, L. Crown loss and subsequent branch sprouting of forest trees in response to a major ice storm. J. Torrey Bot. Soc. 2004, 131, 169–176. [Google Scholar] [CrossRef]
- Myers, J.A.; Kitajima, K. Carbohydrate storage enhances seedling shade and stress tolerance in a neotropical forest. J. Ecol. 2007, 95, 383–395. [Google Scholar] [CrossRef]
- Nzunda, E.F.; Griffiths, M.E.; Lawes, M.J. Resource allocation and storage relative to resprouting ability in wind disturbed coastal forest trees. Evol. Ecol. 2014, 28, 735–749. [Google Scholar] [CrossRef]
- Mascaro, J.; Perfecto, I.; Barros, O.; Boucher, D.H.; Cerda, I.G.D.L.; Ruiz, J.; Vandermeer, J. Aboveground biomass accumulation in a tropical wet forest in Nicaragua following a catastrophic hurricane disturbance. Biotropica 2005, 37, 600–608. [Google Scholar] [CrossRef]
| Species | Sample Number | Min DBH (cm) | Max DBH (cm) | 5–10 cm | 10–15 cm | 15–20 cm | >20 cm |
|---|---|---|---|---|---|---|---|
| Castanopsis fissa | 11 | 5.5 | 27.3 | 2 | 3 | 3 | 3 |
| Aleurites montana | 8 | 10.3 | 22.7 | - | 3 | 3 | 2 |
| Castanopsis carlesii | 6 | 6.7 | 19.8 | 1 | 3 * | 2 | - |
| Lithocarpus harlandii | 5 | 7.1 | 19.2 | 2 | 2 | 1 | - |
| Diospyros morrisiana | 4 | 5.3 | 13.3 | 2 | 2 | - | - |
| Castanopsis tibetana | 2 | 5.5 | 13.5 | 1 | 1 | - | - |
| Machilus chinensis | 2 | 5.9 | 12.7 | 1 | 1 | - | - |
| Machilus kwangtungensis | 2 | 5.4 | 7.7 | 2 * | - | - | - |
| Manglietia moto | 2 | 6.3 | 9.1 | 2 | - | - | - |
| Sapium discolor | 2 | 10.3 | 14.8 | - | 2 * | - | - |
| Castanopsis eyrei | 1 | 5.5 | 7.3 | 1 | - | - | - |
| Cinnamomum porrectum | 1 | 6.1 | 8.6 | 1 | - | - | - |
| Machilus thunbergii | 1 | 12.6 | 15 | - | 1 | - | - |
| Components | Year | Coefficient Symbol | R2 | CV (%) | Bias (%) | |
|---|---|---|---|---|---|---|
| a | b | |||||
| Trunk | 2008 | −2.926 ± 0.184 | 2.482 ± 0.073 | 0.949 | 20.69% | −1.82% |
| 2010 | −2.571 ± 0.181 | 2.372 ± 0.073 | 0.944 | 20.85% | −2.20% | |
| 2012 | −2.409 ± 0.196 | 2.331 ± 0.078 | 0.942 | 22.67% | −2.12% | |
| 2016 | −2.390 ± 0.159 | 2.347 ± 0.064 | 0.913 | 26.14% | −1.80% | |
| Undamaged | −2.401 ± 0.126 | 2.445 ± 0.050 | 0.981 | 12.44% | −0.91% | |
| Branch | 2008 | −4.275 ± 0.436 | 2.007 ± 0.173 | 0.703 | 42.19% | −10.37% |
| 2010 | −3.033 ± 0.349 | 1.774 ± 0.141 | 0.777 | 37.34% | −7.97% | |
| 2012 | −3.727 ± 0.384 | 2.242 ± 0.153 | 0.820 | 42.87% | −8.05% | |
| 2016 | −4.286 ± 0.349 | 2.675 ± 0.139 | 0.767 | 64.58% | −8.43% | |
| Undamaged | −4.694 ± 0.306 | 2.434 ± 0.122 | 0.795 | 42.46% | −5.27% | |
| Leaf | 2008 | −5.099 ± 0.529 | 2.031 ± 0.210 | 0.706 | 41.80% | −16.66% |
| 2010 | −3.423 ± 0.356 | 1.705 ± 0.144 | 0.777 | 34.98% | −8.08% | |
| 2012 | −3.973 ± 0.333 | 2.129 ± 0.133 | 0.892 | 30.04% | −6.52% | |
| 2016 | −3.693 ± 0.356 | 1.891 ± 0.142 | 0.844 | 33.03% | −9.11% | |
| Undamaged | −5.224 ± 0.297 | 2.295 ± 0.118 | 0.761 | 46.80% | −5.21% | |
| Aboveground | 2008 | −2.638 ± 0.156 | 2.417 ± 0.062 | 0.963 | 17.13% | −1.32% |
| 2010 | −1.995 ± 0.179 | 2.228 ± 0.072 | 0.951 | 18.70% | −2.13% | |
| 2012 | −2.021 ± 0.173 | 2.300 ± 0.069 | 0.970 | 15.97% | −1.60% | |
| 2016 | −2.145 ± 0.130 | 2.404 ± 0.052 | 0.971 | 16.24% | −1.16% | |
| Undamaged | −2.246 ± 0.111 | 2.438 ± 0.044 | 0.984 | 11.44% | −0.71% | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Li, Z.; Zhou, G.; Qiu, Z.; Wu, Z. Aboveground Biomass Allometric Models for Evergreen Broad-Leaved Forest Damaged by a Serious Ice Storm in Southern China. Forests 2020, 11, 320. https://doi.org/10.3390/f11030320
Zhao H, Li Z, Zhou G, Qiu Z, Wu Z. Aboveground Biomass Allometric Models for Evergreen Broad-Leaved Forest Damaged by a Serious Ice Storm in Southern China. Forests. 2020; 11(3):320. https://doi.org/10.3390/f11030320
Chicago/Turabian StyleZhao, Houben, Zhaojia Li, Guangyi Zhou, Zhijun Qiu, and Zhongmin Wu. 2020. "Aboveground Biomass Allometric Models for Evergreen Broad-Leaved Forest Damaged by a Serious Ice Storm in Southern China" Forests 11, no. 3: 320. https://doi.org/10.3390/f11030320
APA StyleZhao, H., Li, Z., Zhou, G., Qiu, Z., & Wu, Z. (2020). Aboveground Biomass Allometric Models for Evergreen Broad-Leaved Forest Damaged by a Serious Ice Storm in Southern China. Forests, 11(3), 320. https://doi.org/10.3390/f11030320




