Abstract
The important ecosystem services of the high altitude tropical afro-alpine Erica arborea L. forests are under increasing environmental and human pressure. The Erica treeline ecotone in the Ethiopian highlands forms a temperature-responsive vegetation boundary that is potentially affected by climate change. The cambium of 10 Erica arborea trees in Lib Amba Mountain and Ferrah Amba Mountain in the North Ethiopian highlands was marked in 2012, and corresponding tree disks were sampled after 498 days. Microphotographs of these cambial marks confirmed the formation of annual growth rings (0.76 ± 0.24 mm) with higher vessel density in earlywood and radially flattened fibers in the last layers of the latewood. In-continuum measurements of vessel size and density on microphotographs indicated the formation of inter-annual density fluctuations (IADFs) related to early rainfall in March-May. The same stem disks and 40 increment cores were used for detailed tree-ring analyses—a tree-ring chronology with 18 trees spanning from 1966 to 2014 could be derived. A significant (p < 0.1) positive correlation with minimum temperature in the growing season (August) and a negative correlation with minimum temperature in the spring season (March) were indicated as the most important climate factors regulating tree growth of Erica trees in the afro-alpine forest. The existence of annual tree rings and the proven potential for chronology building encourages further tree-ring analyses of Erica arborea in the afro-alpine tropical highlands in order to link it with climate variability and climate change.
1. Introduction
Tree ring series contain valuable information on tree and forest history, as influenced by biotic and environmental factors [1]. This linkage between tree rings and environmental factors (e.g., temperature, precipitation) provides a good opportunity to estimate environmental dynamics retrospectively [2], which may help to understand forest dynamics in relationship to their controls.
Dendrochronological studies are gradually increasing beyond the borders of temperate and boreal regions [3]. Annual growth rings have been identified on a number of species in the tropics and have partly ended a lively debate about the existence of annual tree rings in the tropical regions [4,5,6]. Tree rings in the temperate and boreal zone result typically from intra-annual variation of temperature and solar irradiance. Because temperature variations in the tropics are relatively small, tree rings in these regions are potentially a result of intra-annual rainfall periodicity [4]. However, non-periodic cycles in water availability can cause non-annual tree rings [3]. This still causes major challenges for dendrochronological studies in the dry tropics [3]. Within the European context, similar challenges are encountered in the Mediterranean region, where summer drought and winter rain patterns are less predictable [7].
Due to the occurrence of indistinct growth rings and ring anomalies, dendrochronological studies in the tropics and especially in sub-Saharan Africa remain scarce [4]. However, in recent decades, reliable climate-sensitive tree-ring chronologies have been developed for western Africa [8,9] and Ethiopia [10,11], and drought-sensitive chronologies were indicated in Zambia [12], Namibia [13], and Zimbabwe [14].
In the semi-arid savanna lowlands of Ethiopia, a ring-width chronology of 68 years was developed on the basis of Acacia tree-ring measurements [11]. This chronology has strong positive correlations with rainfall patterns, which indicate the annual formation of tree rings [15]. Still, tree-ring formation is proven complex, as parts of the stem disks and wood increment cores had to be discarded due to rings that are only present on parts of the circumference [11]. Tree-ring chronologies of Juniperus procera L. in the Ethiopian highlands were established with varying results [16]. Distinct annual ring borders are often disturbed due to high inter- and intra-annual rainfall variability. Four typical tree-ring types have been identified by Wils et al. [16]: indistinct rings, multiple rings, annual rings, and multiple missing rings dependent on the rainfall regime and tree sensitivity to water availability.
At present, little is known about tree-ring formation in the afro-alpine Erica arborea L. forests of Ethiopia, although these mountain forests provide important ecosystem services [17,18,19]. The University of East Anglia performed a dendrochronological study of Erica arborea tree samples in 1996, with the conclusion that Erica trees in the North Ethiopian highlands only have a limited potential for dendrochronological research [20]. However, Erica arborea wood samples show a moderately good visibility of growth boundaries [21]. Therefore, Kaeppeli [21] conducted a detailed dendrochronolgical analysis in 1998 of Erica arborea trees in the North Ethiopian highlands and concluded that distinctness of the Erica arborea tree-ring boundaries varied within the trees and between individual trees. There were trees with high probability of annual ring formation, but there were also indistinct boundaries or extremely narrow tree rings [21]. The hypothesis of Kaeppeli [21] is that rainfall outside the rain season can be held responsible for inter-annual density fluctuations (IADFs) (as dormancy is drought-induced). Age determination is therefore difficult, but approximate ages can be derived, which are sufficient to study treeline shift [21]. Treeline shift was studied by Paulsen et al. [22] in 2000 through measurement of tree height and tree age with increasing elevation. They found that the potential of Erica arborea for dendroclimatology is limited due to eccentric growth, partly indistinct ring boundaries, and high human impact. Similar difficulties were found in Erica shrub studies in the Mediterranean regions [23]. Erica arborea shrubs in the Mediterranean have proven to form IADFs in response to site-specific water stress [24]. The complex tree-growth pattern of Erica trees at the treeline ecotone in the North Ethiopian highlands forms the subject of this paper. The aim was to gain improved understanding of tree growth of Erica arborea trees in the North Ethiopian highlands, i.e., (i) understanding tree-ring formation and the link with climate variables, and (ii) understanding the relation between tree growth, the presence and origin of IADFs, and climate.
2. Materials and Methods
2.1. Study Area
To meet our objectives, stem disks and wood increment cores of in total 50 trees were sampled in the North Ethiopian highlands. Erica arborea wood samples were collected from two forests on a northern slope with a slope gradient of approximately 30°, located on Lib Amba Mountain of the Abune Yosef Mountain range (12°04′ N, 39°22′ E, 3993 m a.s.l.) and Ferrah Amba Mountain (12°52′ N, 39°30′ E, 3939 m a.s.l.). Due to its volcanic history, the study area is characterized by inactive volcanic plugs and structural relief, forming steep escarpments [25]. The dominant soil type of the high altitude soils are well developed Andosols [25]. The study sites are situated on the western shoulder of the Rift Valley, on the water divide between the hydrological basin of the East African Rift in the east and the Tekeze and Nile Basin in the west (Figure 1). The two mountains were selected because both are ranging above the present treeline elevation (approximately 3700 m).
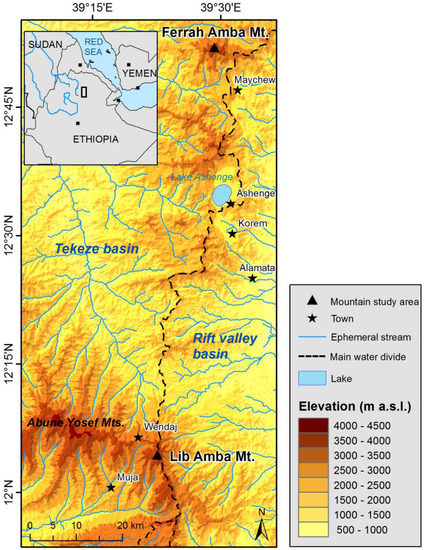
Figure 1.
Location of the study area.
The vegetation belts encountered in the study area are the same belts as mapped in the Simen Mountains by Hurni and Stähli [26], but at different vegetation growth limits, related to local biophysical constraints. These vegetation belts are the Acacia upper limit at around 2730 m; the Hagenia, Juniperus, and Olea montane forest limit at around 3200 m; the Erica arborea treeline limit at around 3700 m; and the upper grass-steppe vegetation limit at around 4225 m. The afro-alpine high altitude vegetation is dominated by Erica arborea, which forms the upper treeline ecotone [25].
Rainfall in the study area shows a bimodal distribution with an unreliable short “azmera” spring rain season (March–May) preceding the main “kiremt” (summer) rain season (June–September) with a peak from July to August (Figure 2). The mean annual precipitation varies with elevation and is approximately 862 mm near the afro-alpine belt in Lib Amba. Mean air temperature is nearly constant throughout the year (8.8 ± 0.7 °C), whereas the daily temperature variation is high (9.5 ± 1 °C). The climate data were obtained in a meteorological station installed near the upper forest in Lib Amba (2012–2014).
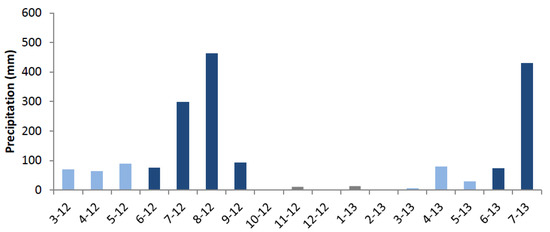
Figure 2.
Rainfall patterns between March 2012 and July 2013 with the azmera (spring) rain season in light blue, the kiremt (summer) rain season in dark blue, and rainfall during the dry season in gray. The period plotted corresponds with the period between cambial marking and sampling of the stem disks.
2.2. Data Collection and Tree-Ring Chronology
At each of the sites, five trees received a cambial mark in early March 2012. Such cambial marks are mechanical injuries of the cambium that cause a wound response in the tree, which acts as a time marker [27]. In each site, the marked stem disks were sampled at the end of July 2013. These stem disks were air-dried and transverse sections were sanded (up to 1200 grit) until the tree rings were completely visible. In addition, more samples were collected in the two study areas in November 2014 using an increment borer along a transect crossing the mountain forest upslope with a 20 m elevation interval. At each interval level, five trees were sampled with two cores each. The trees were cored as close to the ground level as possible, while still allowing room for handling the increment borer, in order to cover maximum tree ages [28] (Figure 3). For each sampled tree, tree diameter and height were measured. Because tree height was relatively low (less than 5 m), heights were directly measured using a pole. All Erica arborea samples are stored in the Xylarium of the Royal Museum for Central Africa (Tervuren Wood Collection, accession numbers stem disks: Tw64881-64890), Tervuren, Belgium. In this paper, the analysis was based on the cambial marked stem disks and the increment core data of one complete transect for each site.

Figure 3.
Wood sample collection using an increment borer in Lib Amba Mountain: (A,B) tree coring and core extraction; (C) Erica arborea tree at Lib Amba Mountain.
To derive a chronology, tree rings were marked on the 10 stem disks and counted under a stereomicroscope along three radii. Ring widths were measured from scanned images (2400dpi) with ImageJ 1.47v (open-source software; National Institutes of Health, Bethesda, USA). In addition, information on tree-ring structures were derived from the increment cores. The cores were cut with a core-microtome of the Swiss Federal Institute for Forest, Snow and Landscape Research (WSL) [29] and the tree rings were counted under a stereomicroscope [30]. Moistening the increment cores with water helped to reveal the tree-ring structures. The tree-ring widths were measured using a Lintab measuring table. After the measurements, the tree-ring series were pairwise crossdated at the level of the individual tree to derive an average tree-ring width series per tree (TSAP Win software [31]). Subsequently, these individual tree-ring series were crossdated to find a tree growth chronology for all trees with a similar pattern [32]. The quality of the crossdated pairs were evaluated using three statistics: the Gleichläufigkeit (Glk), the t-value of Baille–Pilcher (tBP), and the cross-correlation (%) [31]. While crossdating, threshold limits of Glk > 60 and tBP > 2.0 were imposed [5]. Next to the mathematical variables for crossdating, visual control was another important condition for accepting or excluding certain trees from a chronology. A threshold of a minimum of four samples per year was set to guarantee a representative tree-ring chronology [33]. The chronologies were tested for age trends using autocorrelation (AC) statistics [31] and standardized using the 5 year moving average procedure of Baillie and Pilcher [34]. The expressed population signal (EPS) indicates how well the sample chronology matches the theoretical population chronology; the threshold for a representative population signal is 0.85 [35].
2.3. Wood Anatomical Analysis
To study the wood anatomy of the tree rings, cubic samples of approximately 1 cm3, containing the cambial marks, were cut from the stem disks of the two study areas. These Erica arborea cubes were softened in water at 80 °C for 2 weeks until microtome sections of 18 µm thick could be sliced. The sections were stained with Safranin and Astra Blue, dehydrated with an ethanol series, and mounted on permanent slides [36]. From these slides, microphotographs were made using Cell B software (Olympus). The microphotographs visualize in detail the number of tree rings and the amount of wood formed since the cambial marking [37]. These microphotographs were used to identify IADFs according to the procedure of De Micco et al. [2]. In this procedure, the vessel size was measured in-continuum along the tree ring on 300-400 µm wide transects and plotted in a dispersion graph. This graph shows the variation in vessel size against the position of the vessel, expressed as percentage of ring width [2].
2.4. Climate–Growth Relationship
The climate growth analysis was subdivided in two parts: (1) the relationship between tree-ring formation, the presence of IADFs, and rainfall variability on the one hand, and (2) the relationship between the tree-ring chronology and climate variability on the other hand.
2.4.1. Relationship between the Occurrence of IADFs and Rainfall
Besides annual, kiremt (summer), and azmera (spring) rainfall, the dry spell indices from Seleshi and Camberlin [38] were adapted to capture rainfall fluctuations. Two binary variables were constructed: rainfall dip and dry spell based on Maychew and Lalibela National Meteorological Agency (NMA) meteostations.
- The variable “rainfall dip” is 1 if there is azmera (spring) rainfall with more than 100 mm followed by a dry spell of more than 10 days between the azmera (spring) and kiremt (summer) rain season, and 0 in all other cases.
- The variable “dry spell” is 1 if there is a dry spell of more than 20 days and 0 in all other cases.
The strength of the relations between IADF formation and rainfall was tested with an ‘N-1’ χ² test and a binary logistic regression in SPSS Statistics (IBM). The ‘N-1’ χ² test was used because the amount of observations was smaller than 5 in three cells of the contingency table [39]. In addition, the φ coefficient was also calculated.
2.4.2. Dendroclimatology
To derive the climate effect on tree growth, the site chronology was correlated with monthly, seasonal, and annual climate data (i.e., precipitation, minimum and maximum temperature). Because the NMA station data for Maychew (1992–2013) is spatially and temporarily limited, climate data were generated with the climate explorer from “het Koninklijk Nederlands Meteorologisch Instituut” (KNMI), covering more than a century of climate data (1901–2013) [40]. The correlation between the local NMA stations and the climate explorer data was, however, very low for rainfall and minimal temperature (Pearson r < 0.15). Therefore, the climate explorer data (although the long record) were not used to match with tree growth. The Maychew NMA station was used because it has the longest climate record (1992–2013) and is the most reliable source of precipitation data, despite the distance of 11 km and 80 km between Maychew station and Maychew and Lalibela sampling sites, respectively, and an elevational difference of approximately 1300 m.
Besides temperature and precipitation effects, the combined aridity index (Equation (1)) defined by de Martonne [41] was used.
where IDM is the de Martonne aridity index, P is the annual mean precipitation in millimeters, and T is the annual mean air temperature in degrees Celcius.
Relationships between climate variability and ring widths of the average tree-ring chronology were calculated with Pearson correlations [33].
3. Results
3.1. Tree-ring Formation
On the microphotographs, there were two growth zones visible after the cambial mark (Figure 4). These growth zones provided evidence for the formation of annual growth rings because they matched with two rainfall peaks between marking and sampling of the trees. The first ring corresponded with the kiremt (summer) rain season in 2012 and the second ring with part of the kiremt (summer) rain season in 2013. However, the growth zones appeared disturbed in response to the cambial pinning. The tree ring of 2012 directly after the pinning was relatively small, and vessel size and density were relatively limited after marking.
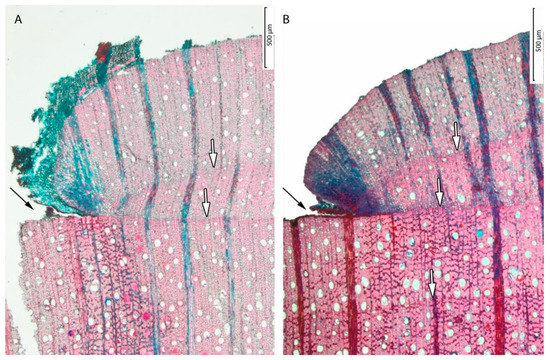
Figure 4.
Microphotograph of the wound tissue formed in response to the cambial pinning. The cambial mark is indicated with black arrows in (A) sample Tw64884 and (B) sample Tw64881. The trees were marked on 15 March 2012 and sampled after 498 days on 25 July 2013. The tree-ring boundaries are indicated with white arrows.
The annual Erica arborea tree rings were mainly marked by radially flattened fibers in the last layers of the latewood and by changes in vessel density. The vessel density was especially higher in the earlywood and decreased towards the latewood (Figure 5). If a layer of flattened fibers was unclear or missing between two rings, this corresponded with an intra-annual density fluctuation (IADF) (Figure 5).
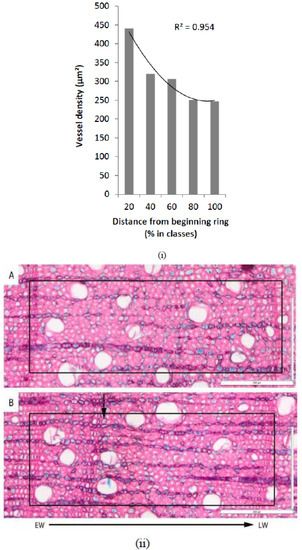
Figure 5.
(i) Variability of the vessel density within a tree ring from the start (0%) to the end of a growth ring (100%), as derived from measurements of 50 tree rings. (ii) Microphotographs from sample Tw64881 showing a typical tree ring (A) without intra-annual density fluctuation (IADF) in 2011 and (B) with IADF in 2009. Figure composition was based on De Micco et al. [2]. The location of the IADF in B is indicated with a black arrow.
A dispersion graph was used to characterize vessel distribution in IADFs based on 50 tree rings of 3 samples (Figure 6). For this graph, IADFs with a clear growth interruption (24% of total tree rings) were combined for all measured years. Partial IADFs (40% of total tree rings) were excluded from the dispersion graph, but were very common and disturbed the overall picture. On the dispersion graph, the normal rings without IADFs (36% of total tree rings) were also plotted as a reference. From this graph, it became clear that IADFs typically occur at the beginning of the annual ring (early IADFs). The graph also shows that the vessel lumen area in normal tree rings decreases from earlywood to latewood.
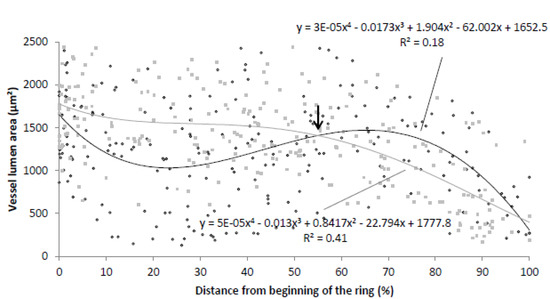
Figure 6.
Dispersion graph of tree-ring formation in Erica arborea showing the vessel lumen area in relation to the distance from the beginning of the tree ring. The dispersion graph indicates the polynomial interpolation line for tree rings with IADFs in black, and the polynomial interpolation line for tree rings without IADFs in gray. The arrow points to the end of the IADF.
3.2. IADFs and Rainfall Variability
IADFs form a major problem for dating tree rings because they are morphologically similar to latewood. However, when detected, IADFs can enhance the quality of tree-ring chronologies [42]. In order to understand the formation of early IADFs in the tree rings, the irregular first spring rain season (azmera) was evaluated as a potential explaining factor. Rainfall patterns and rainfall-derived indices between 1997 and 2013 are presented in Table 1, together with tree-ring derived data. An ‘N-1’ Χ-square test indicated that there was a significant relation between the occurrence of IADFs and dry spells (Χ² = 4.45, p < 0.05). This relation was confirmed by the φ coefficient—there was a significant weak positive association (ϕ: 0.55, p < 0.05) between IADFs and dry spells. This means that the possibility of an IADF increases if there is a rainfall interruption of more than 20 days between the azmera (spring) and kiremt (summer) rain season. A binary logistic regression indicated that the combined effect of the amount of azmera (spring) rainfall, dry spell, and rainfall dip was significant (χ² = 9.99, p < 0.05) and related with IADF formation (Nagelkerke R² = 0.65, classification accuracy 87.5%). Similar regressions indicated that there was no significant relationship between IADF formation and annual rainfall (R² = 0.01; p > 0.05), kiremt (summer) rainfall (R2 = 0.09; p > 0.05), or azmera (spring) rainfall (R2 = 0.02; p > 0.05). IADF formation thus appeared as the result of the combined effect of azmera (spring) rainfall followed by a dry spell.

Table 1.
Rainfall indices and IADF formation.
3.3. Dendrochronology
Despite the tropical environment and the limited success in previous studies, tree-ring series from stem disks and tree cores were crossdated into a 48-year-old tree-ring chronology (Table 2). From the 50 measured samples, 60% were included in the average combined tree-ring chronology (Figure 7). The tree-ring series of the excluded samples (40%) did not match the average tree-ring pattern (Glk > 60, tBP > 2).

Table 2.
Characteristics of the Erica chronologies for the stem disks, tree cores, and combined chronology.
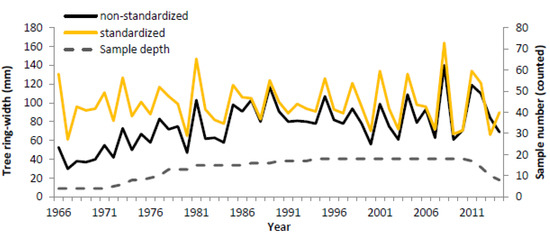
Figure 7.
Tree-ring width chronology of Erica arborea in the North Ethiopian highlands (1966–2014). On the graph, the non-standardized tree-ring chronology is given in black and the standardized chronology in orange. The sample number is given with a dashed line, and a minimum of four samples per year is used as a threshold.
The combined chronology revealed no strong age trend (all autocorrelation (AC) values were below 0.5; Table 2), and the change in mean sensitivity (MS) before and after standardization was only 1%. The expressed population service (EPS) of the crossdated trees was lower than the required threshold of 0.85. However, despite low cross-dating values (low correlation values, no significant Pearson correlation), visual inspection [33] suggested correct crossdating between the trees, given that the time series was very short compared to standard dendrochronological studies.
3.4. Dendroclimatology
The NMA data of Maychew was used to derive the climate effect on tree growth. Moreover, during the crossdating process, tree growth was proven to be more sensitive to climate patterns under limited conditions higher up the mountain and more northerly in Ferrah Amba Mountain, near to the Maychew station (higher Glk and tBP values).
The tree-ring chronology was significantly (p < 0.1) correlated with minimum temperature in March and August (Figure 8). In March, during the azmera (spring) rain season, this correlation was negative and in August, in the kiremt (summer) rain season, this correlation was positive. For the maximum temperature and for rainfall, there were no significant correlations with tree growth. There were also no significant correlations (p > 0.1) between tree growth and the de Martonne aridity index. A multiple linear regression with minimum temperature in March and August was significant and was able to explain 24% of the variation in tree growth (R²: 0.24; p < 0.05).
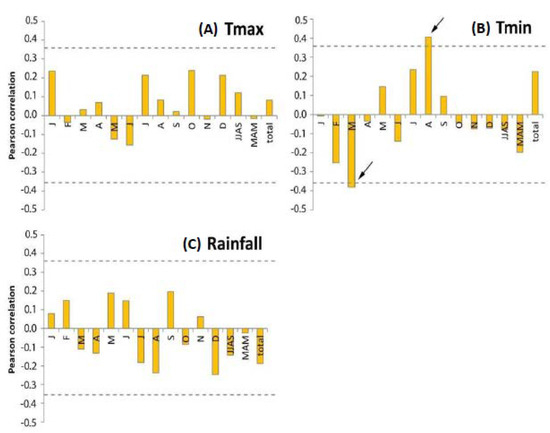
Figure 8.
Correlation coefficients (n = 22) between monthly and seasonal climate variability and tree-ring width of Erica arborea (1992–2013). (A) Maximum temperature; (B) minimum temperature; (C) rainfall. The dotted line represents the p < 0.1 level. Significant correlations are indicated with a black arrow.
4. Discussion
Radially flattened fibers in the last layers of the latewood mainly characterize the tree-ring boundaries of Erica arborea in the tropics. In the Mediterranean, Erica tree rings are typically formed by a transition of vessel density and size from earlywood to latewood; vessel density and size are higher in the earlywood and decrease towards the latewood [24]. A similar transition is observed for Erica arborea in the tropics, but is less pronounced. The possibility for IADF formation in Erica in Ethiopia is higher when there is a dry spell between the azmera (spring) and kiremt (summer) rain season, especially when this is combined with high rainfall amounts during the azmera (spring) rain season. Similarly, the formation of IADFs in the Mediterranean is also related to drought stress [24,42]. IADF detection is important to understand wood density-climate interactions in Spain [42], which show high inter-annual variability in growth [43]. IADFs in the Mediterranean form as the combined result of high temperature in the summer, rainfall, and soil water variability. The formation of IADFs is much higher (with more than 70% of the total rings) in the Mediterranean region [24] in comparison to IADF formation in the tropics (24%).
The tree-ring chronology presented here is not the first for Erica arborea in the tropics. Kaeppeli [21] made a chronology of Erica arborea in 1998 spanning a period of 20 years (1976–1996) at Nebir Mekemecha (3800 m a.s.l.) in the Simen Mountains. This tree-ring chronology of Kaeppeli [21] was used to compare with the overlapping chronology established in this study; however, the correlation was very weak (Pearson r = 0.04). This can be attributed to the variable climatological conditions in the Simen Mountains. Kaeppeli [21] did not consider dendroclimatology with Erica arborea due to wedging rings, human impact, and the occurrence of IADFs, whereas this study indicated that Erica arborea could potentially be used for dendroclimatology. The relation between tree growth and minimum temperature was only significant at the p < 0.1 significance level. This limited significance was mainly due to the restricted available climate data of only 22 years (1992–2013), whereas the chronology spanned a period of 48 years (1966–2014). The low EPS might also explain the low correlation with climate because the sample size was small and possibly not representative for the whole population of Erica arborea. Nevertheless, at the p < 0.1 significance level, there were two significant relations between tree growth and minimum temperature. There was a positive correlation between tree growth and minimum temperature in the growing season (August). A similar positive relation between temperature and Cedrela montana tree growth in the growing season was observed in the humid mountain rainforests of southern Ecuador by Bräuning et al. [44] in 2009. This indicated that the minimum temperature in the growing season controls tree growth and that warmer temperatures can enhance growth. In March, there was a negative relation between minimum temperature and tree growth. This was potentially related with the formation of IADFs. However, more research is required to verify this relationship.
The environmental lapse rate of temperature is −0.72 °C per 100 m for the study area, whereas for rainfall there is no significant trend with elevation [45]. The rainfall patterns in Maychew are not suitable to understand the relation with tree growth at the afro-alpine forest. Rainfall data from near to the forest is needed to understand the impact of rainfall patterns on tree growth. The effect of rainfall on tree growth is indicated as being positive in subtropical treelines in Northwestern Argentina [46], but negative in the humid mountain rainforest in southern Ecuador [44]. The difference between these studies is caused by different rainfall conditions. In Northwestern Argentina, rainfall strongly decreases with elevation up to <200 mm above 4000 m, whereas average annual rainfall in southern Ecuador exceeds 2000 mm [44,46]. Rainfall thus impedes tree growth under wet conditions and enhances tree growth under dry conditions. This trend is also visible in the correlation between tree growth and rainfall variability (Figure 8). In the azmera (spring) and kiremt (summer) rain season, tree growth was found to be negatively correlated with rainfall (however, this relationship was not significant). This indicates the need for detailed climate data at higher elevations. The strength of the correlation would potentially rise if more climate data would be available at high elevations and if more samples were included. An improved correlation between tree growth and climate could potentially allow climate reconstructions, as given by Schöngart et al. [9] for West Africa in 2006.
In the European Alps, tree height decreases with approximately 1 meter over 100 vertical meters [47]. Smaller size and slower growth at higher elevation are generally adaptations to colder and windier conditions [48]. Trees at the treeline elevation are therefore lower in height in comparison to trees 100 vertical meters downslope, but not necessarily younger in age. The sampled tree ages gave no evidence for a recent upwards shift of the treeline.
5. Conclusions and Outlook
The presented 48 years of tree-ring chronology of Erica arborea (1966–2014) proved the potential of tropical Erica trees for dendrochronology. Stem disks and increment cores were collected in the tropical highlands of North Ethiopia in Lib Amba and Ferrah Amba Mountain. The formation of annual growth rings was illustrated by microphotographs of cambial marked stem disks. Erica arborea tree rings were characterized by radially flattened fibers in the last layers of the latewood and by decreasing vessel density and size from earlywood to latewood. IADFs were observed in relation with early rainfall followed by a dry spell. Tree growth was found to be significantly and positively correlated with minimum temperature in the growing season, but negatively correlated with tree growth in the azmera (spring) rain season (potentially explaining higher risks for IADF formation). Rainfall might have a negative impact during the rain season, but more research is needed to better understand the rainfall effect. The strength of the correlations would potentially rise if more climate data were available at high elevations and if more samples were included. The Erica arborea chronology presented in this study provides a successful example for further dendrochronological studies. IADF formation shows the best opportunities for climate reconstructions. Wood density measurements and repeated cambial pinning on more trees combined with site-specific climate monitoring are suggested to further disentangle the link between climate and tree growth.
Author Contributions
Conceptualization, M.J.; Investigation, M.J., H.B. and M.V.; Methodology, M.J. and M.d.R.; Software, M.d.R.; Supervision, J.N. and H.B.; Validation, M.d.R.; Writing—original draft, M.J.; Writing—review editing, M.d.R., T.G., J.N. and H.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the Belgian Special Research Fund (Bijzonder Onderzoeksfonds, Ghent University). Field assistance was provided by Mekelle University and the VLIR Institutional University Cooperation program. We also acknowledge the Herbaxylaredd project supported by the Belgian Science Policy of the Belgian Federal Government (BR/143/A3/HERBAXYLAREDD). Special thanks also goes to the local communities for their assistance and hospitality during the fieldwork.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rossi, S.; Deslauriers, A. Intra-annual time scales in tree rings. Dendrochronologia 2007, 25, 75–77. [Google Scholar] [CrossRef]
- De Micco, V.; Battipaglia, G.; Cherubini, P.; Aronne, G. Comparing methods to analyse anatomical features of tree rings with and without intra-annual density fluctuations (IADFs). Dendrochronologia 2014, 32, 1–6. [Google Scholar] [CrossRef]
- Wils, T.; Sass-Klaassen, U.; Eshetu, Z.; Bräuning, A.; Gebrekirstos, A.; Couralet, C.; Robertson, I.; Touchan, R.; Koprowski, M.; Conway, D.; et al. Dendrochronology in the dry tropics: The Ethiopian case. Trees 2010, 25, 345–354. [Google Scholar] [CrossRef]
- Worbes, M. One hundred years of tree-ring research in the tropics: A brief history and an outlook to future challenges. Dendrochronologia 2002, 20, 217–231. [Google Scholar] [CrossRef]
- Trouet, V.; Esper, J.; Beeckman, H. Climate/growth relationships of Brachystegia spiciformis from the miombo woodland in south central Africa. Dendrochronologia 2010, 28, 161–171. [Google Scholar] [CrossRef]
- De Ridder, M.; Trouet, V.; Van den Bulcke, J.; Hubau, W.; van Acker, J.; Beeckman, H. A tree-ring based comparison of Terminalia superba climate-growth relationships in West and Central Africa. Trees-Struct. Funct. 2013, 27, 1225–1238. [Google Scholar] [CrossRef]
- Cherubini, P.; Gartner, B.L.; Tognetti, R.; Bräker, O.U.; Schoch, W.; Innes, J.L. Identification, measurement and interpretation of tree rings in woody species from mediterranean climates. Biol. Rev. Camb. Philos. Soc. 2003, 78, 119–148. [Google Scholar] [CrossRef] [PubMed]
- Worbes, M.; Staschel, R.; Roloff, A.; Junk, W. Tree ring analysis reveals age structure, dynamics and wood production of a natural forest stand in Cameroon. For. Ecol. Manag. 2003, 173, 105–123. [Google Scholar] [CrossRef]
- Schöngart, J.; Orthmann, B.; Hennenberg, K.; Porembski, S.; Worbes, M. Climate-growth relationships of tropical tree species in West Africa and their potential for climate reconstruction. Glob. Chang. Biol. 2006, 12, 1139–1150. [Google Scholar] [CrossRef]
- Couralet, C.; Sass-Klaassen, U.; Sterck, F.; Bekele, T.; Zuidema, P. Combining dendrochronology and matrix modelling in demographic studies: An evaluation for Juniperus procera in Ethiopia. For. Ecol. Manag. 2005, 216, 317–330. [Google Scholar] [CrossRef]
- Gebrekirstos, A.; Mitlöhner, R.; Teketay, D.; Worbes, M. Climate—Growth relationships of the dominant tree species from semi-arid savanna woodland in Ethiopia. Trees 2008, 22, 631–641. [Google Scholar] [CrossRef]
- Trouet, V.; Coppin, P.; Beeckman, H. Annual growth ring patterns in Brachystegia spiciformis reveal influence of precipitation on tree growth. Biotropica 2006, 38, 375–382. [Google Scholar] [CrossRef]
- Fichtler, E.; Trouet, V.; Beeckman, H.; Coppin, P.; Worbes, M. Climatic signals in tree rings of Burkea africana and Pterocarpus angolensis from semiarid forests in Namibia. Trees 2004, 18, 442–451. [Google Scholar] [CrossRef]
- Therrell, M.; Stahle, D.; Ries, L.; Shugart, H. Tree-ring reconstructed rainfall variability in Zimbabwe. Clim. Dyn. 2006, 26, 677–685. [Google Scholar] [CrossRef]
- Stahle, D. Useful strategies for the development of tropical tree-ring chronologies. IAWA J. 1999, 20, 249–253. [Google Scholar] [CrossRef]
- Wils, T.; Robertson, I.; Eshetu, Z.; Touchan, R.; Sass-Klaassen, U.; Koprowski, M. Crossdating Juniperus procera from North Gondar, Ethiopia. Trees 2010, 25, 71–82. [Google Scholar] [CrossRef]
- Miehe, G.; Miehe, S. Ericaceous Forests and Heathlands in the Bale Mountains of South Ethiopia: Ecology and Man’s Impact; Stiftung Walderhaltung in Afrika: Hamburg, Germany, 1994. [Google Scholar]
- Aerts, R.; November, E.; Behailu, M.; Deckers, J.; Hermy, M.; Muys, B. Forest rehabilitation: One approach to water conservation in central Tigray. Water Sci. Technol. 2002, 6, 34–37. [Google Scholar]
- Nyssen, J.; Poesen, J.; Moeyersons, J.; Deckers, J.; Haile, M.; Lang, A. Human impact on the environment in the Ethiopian and Eritrean highlands—A state of the art. Earth-Sci. Rev. 2004, 64, 273–320. [Google Scholar] [CrossRef]
- Conway, D.; Brooks, N.; Briffa, K.R.; Desta, S.; Merrin, P.D.; Jones, P.D. Exploring the Potential for Dendroclimatic Analysis in Northern Ethiopia; University of East Anglia, Climate Research Unit: Norwich, UK, 1997. [Google Scholar]
- Kaeppeli, M. Regeneration and Age structure of Relict Ericaceous Forests—A Dendrochronological Study Near the Timberline in the Simen Mountains, Ethiopia; University of Berne: Berne, Switzerland, 1998. [Google Scholar]
- Paulsen, J.; Weber, U.M.; Körner, C. Tree growth near treeline: Abrupt or gradual reduction with altitude? Arct. Antarct. Alp. Res. 2000, 32, 113–119. [Google Scholar] [CrossRef]
- Gea-Izquierdo, G.; Cherubini, P.; Battipaglia, G.; Gärtner, H. Xylem Adjustment in Erica Arborea to Temperature and Moisture Availability in Contrasting Climates. IAWA J. 2013, 34, 109–126. [Google Scholar] [CrossRef]
- Battipaglia, G.; DE Micco, V.; Brand, W.A.; Saurer, M.; Aronne, G.; Linke, P.; Cherubini, P. Drought impact on water use efficiency and intra-annual density fluctuations in Erica arborea on Elba (Italy). Plant. Cell Environ. 2014, 37, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Wesche, K.; Miehe, G.; Kaeppeli, M. The significance of fire for afroalpine ericaceous vegetation. Mt. Res. Dev. 2000, 20, 340–347. [Google Scholar] [CrossRef]
- Hurni, H.; Stähli, P. Simen Mountains, Ethiopia: Climate and Dynamics of Altitudinal Belts from the Last Cold Period to the Present Day; Geographisches Institut der Universität Bern: Bern, Switzerland, 1982; Volume 13. [Google Scholar]
- Mariaux, A. Les cernes dans les bois tropicaux africains nature et périodicité. Bois Forêts des Trop. 1967, 113, 3–14. [Google Scholar]
- Grissino Mayer, H. A manual and tutorial for the proper use of an increment borer. Tree-Ring Res. 2003, 59, 63–79. [Google Scholar]
- Gärtner, H.; Nievergelt, D. The core-microtome: A new tool for surface preparation on cores and time series analysis of varying cell parameters. Dendrochronologia 2010, 28, 85–92. [Google Scholar] [CrossRef]
- Gärtner, H.; Banzer, L.; Schneider, L.; Schweingruber, F.; Bast, A. Preparing micro sections of entire (dry) conifer increment cores for wood anatomical time-series analyses. Dendrochronologia 2015, 34, 19–23. [Google Scholar] [CrossRef]
- Rinn, F. TSAP-WinTM User Reference; RinnTech: Heidelberg, Germany, 2003. [Google Scholar]
- Douglass, A. Crossdating in Dendrochronology. J. For. 1941, 39, 825–831. [Google Scholar]
- De Ridder, M.; Toirambe, B.; van den Bulcke, J.; Bourland, N.; van Acker, J.; Beeckman, H. Dendrochronological potential in a semi-deciduous rainforest: The case of Pericopsis elata in Central Africa. Forests 2014, 5, 3087–3106. [Google Scholar] [CrossRef]
- Baillie, M.; Pilcher, J. A simple program for tree-ring research. Tree-Ring Bull. 1973, 33, 7–14. [Google Scholar]
- Wigley, T.; Briffa, K.; Jones, P. On the average value of correlated time series, with applications in dendroclimatology and hydrometeorology. J. Clim. Appl. Meteorol. 1984, 23, 201–213. [Google Scholar] [CrossRef]
- Gärtner, H.; Cherubini, P.; Fonti, P.; von Arx, G.; Schneider, L.; Nievergelt, D.; Verstege, A.; Bast, A.; Schweingruber, F.; Büntgen, U. A technical perspective in modern tree-ring research-How to overcome dendroecological and wood anatomical challenges. J. Vis. Exp. 2015, 97, 52337. [Google Scholar] [CrossRef] [PubMed]
- Verheyden, A.; Kairo, J.G.; Beeckman, H.; Koedam, N. Growth rings, growth ring formation and age determination in the mangrove Rhizophora mucronata. Ann. Bot. 2004, 94, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Seleshi, Y.; Camberlin, P. Recent changes in dry spell and extreme rainfall events in Ethiopia. Theor. Appl. Climatol. 2005, 83, 181–191. [Google Scholar] [CrossRef]
- Campbell, I. Chi-squared and Fisher-Irwin tests of two-by-two tables with small sample recommendations. Stat. Med. 2007, 26, 3661–3675. [Google Scholar] [CrossRef]
- Trouet, V.; Van Oldenborgh, G. KNMI Climate Explorer: A web-based research tool for high-resolution paleoclimatology. Tree-Ring Res. 2013, 69, 3–13. [Google Scholar] [CrossRef]
- De Martonne, E. L’indice d’aridité. Bulletin de l’Associaction de Géographes Francais 1926, 9, 3–5. [Google Scholar] [CrossRef]
- Olivar, J.; Bogino, S.; Spiecker, H.; Bravo, F. Changes in climate-growth relationships and IADF formation over time of pine species (Pinus halepensis, P. pinaster and P. sylvestris) in Mediterranean environments. For. Syst. 2015, 24. [Google Scholar] [CrossRef]
- Olivar, J.; Rathgeber, C.; Bravo, F. Climate change, tree-ring width and wood density of pines in Mediterranean environments. IAWA 2015, 36, 267–269. [Google Scholar] [CrossRef]
- Bräuning, A.; Volland-Voigt, F.; Burchardt, I.; Ganzhi, O.; Nauss, T.; Peters, T. Climatic control of radial growth of Cedrela montana in a humid mountain rainforest in southern Ecuador. Erdkunde 2009, 63, 337–345. [Google Scholar] [CrossRef]
- Jacob, M. Treeline Dynamics and Forest Cover Change in Afroalpine Ethiopia, As Affected by Climate Change and Anthropo-Zoogenic Impacts. Ph.D. Thesis, Ghent University, Belgium, Germany, 2015. [Google Scholar]
- Morales, M.; Villalba, R.; Grau, R.; Paolini, L. Rainfall-controlled tree growth in high-elevation subtropical treelines. Ecology 2004, 85, 3080–3089. [Google Scholar] [CrossRef]
- Paulsen, J.; Körner, C. A climate-based model to predict potential treeline position around the globe. Alp. Bot. 2014, 124, 1–12. [Google Scholar] [CrossRef]
- Körner, C. Plant adaptation to cold climates. F1000Research 2016, 5, 2769. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).