Aspects Determining the Dominance of Fomitopsis pinicola in the Colonization of Deadwood and the Role of the Pathogenicity Factor Oxalate
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Pure Cultures
2.2. Substrate Arrangements
2.3. Fungal Interactions in Glass Tubes 200 mm
2.4. Oxidoreductase Enzymes in Substrate Samples
2.5. Oxalate Quantification
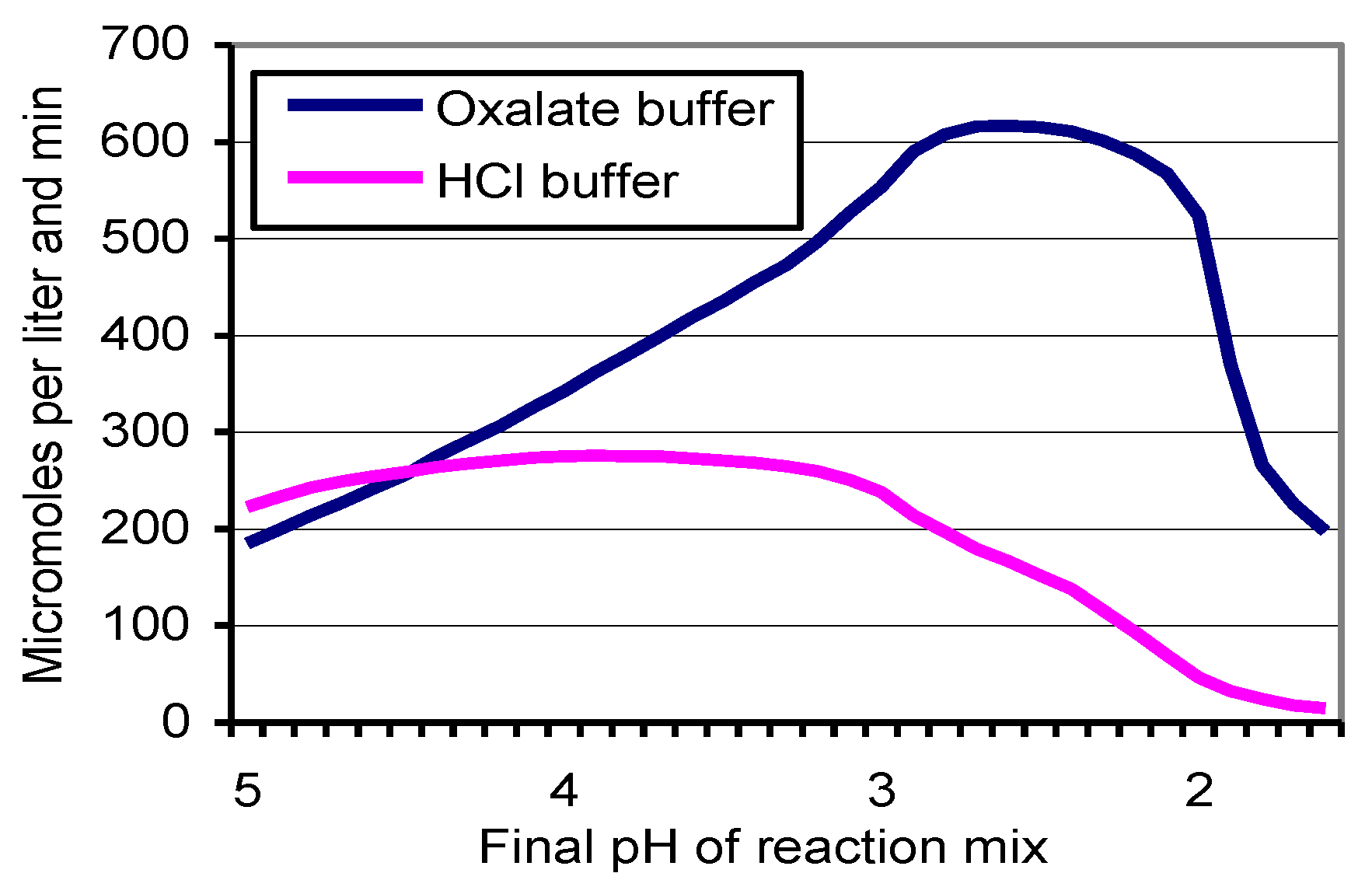
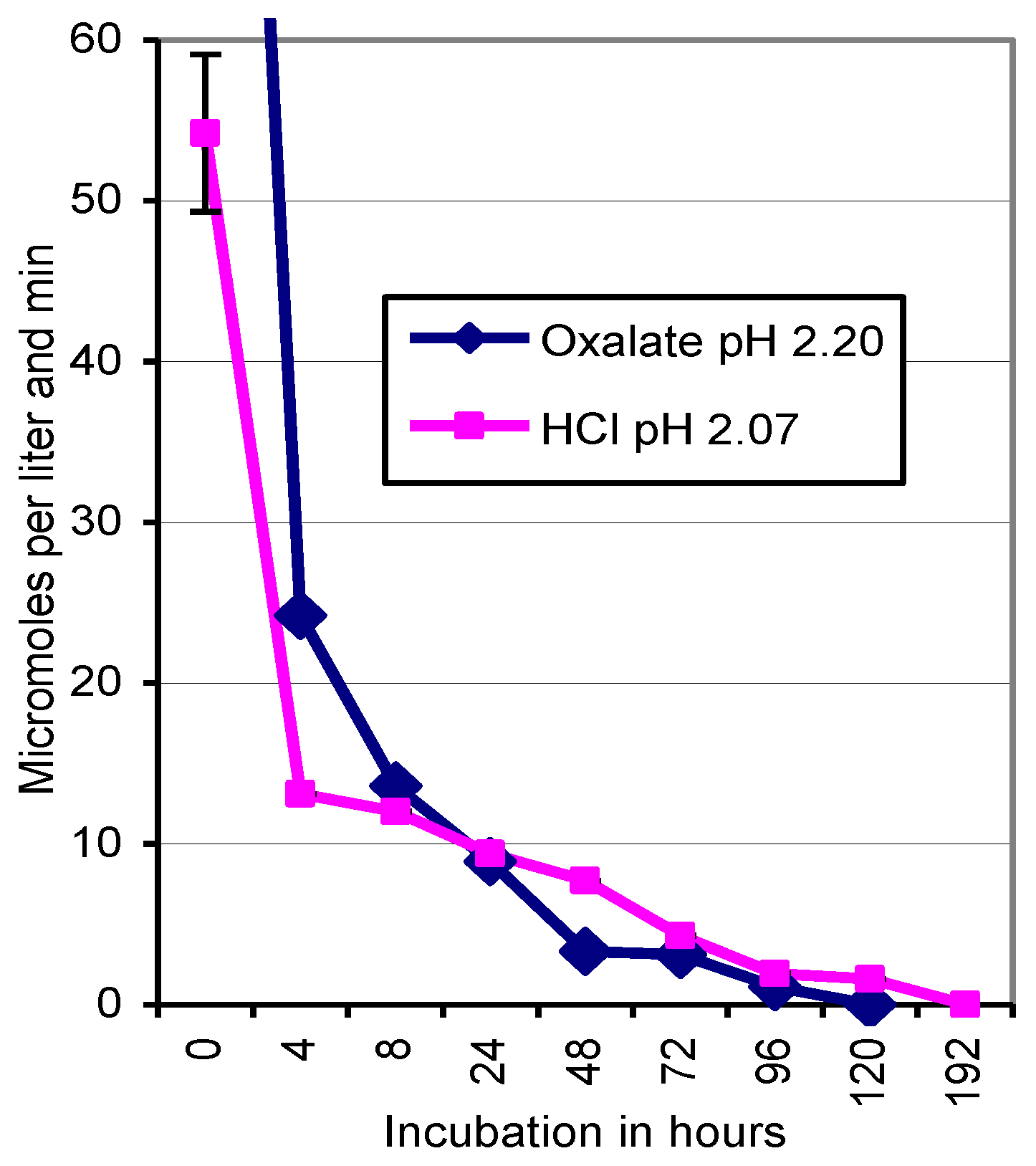
2.6. Inactivation of Laccase by Oxalate and HCl
2.7. Oxalate Tolerance of K. mutabilis Mycelia
2.8. Collection of Volatile Terpenes from 0.3 L Packing Jars
2.9. Statistical Treatments
3. Results
3.1. Dual Cultures in Glass Tubes
3.1.1. Oxalate Production
3.1.2. Production of Oxidoreductases by K. mutabilis Km 10
3.2. Altering Oxalate Release with Calcium
3.3. Stability of Km 10 Laccase at Low pH Conditions
3.4. Colonization of Oxalate-Amended Beech Wood Dust by K. Mutabilis
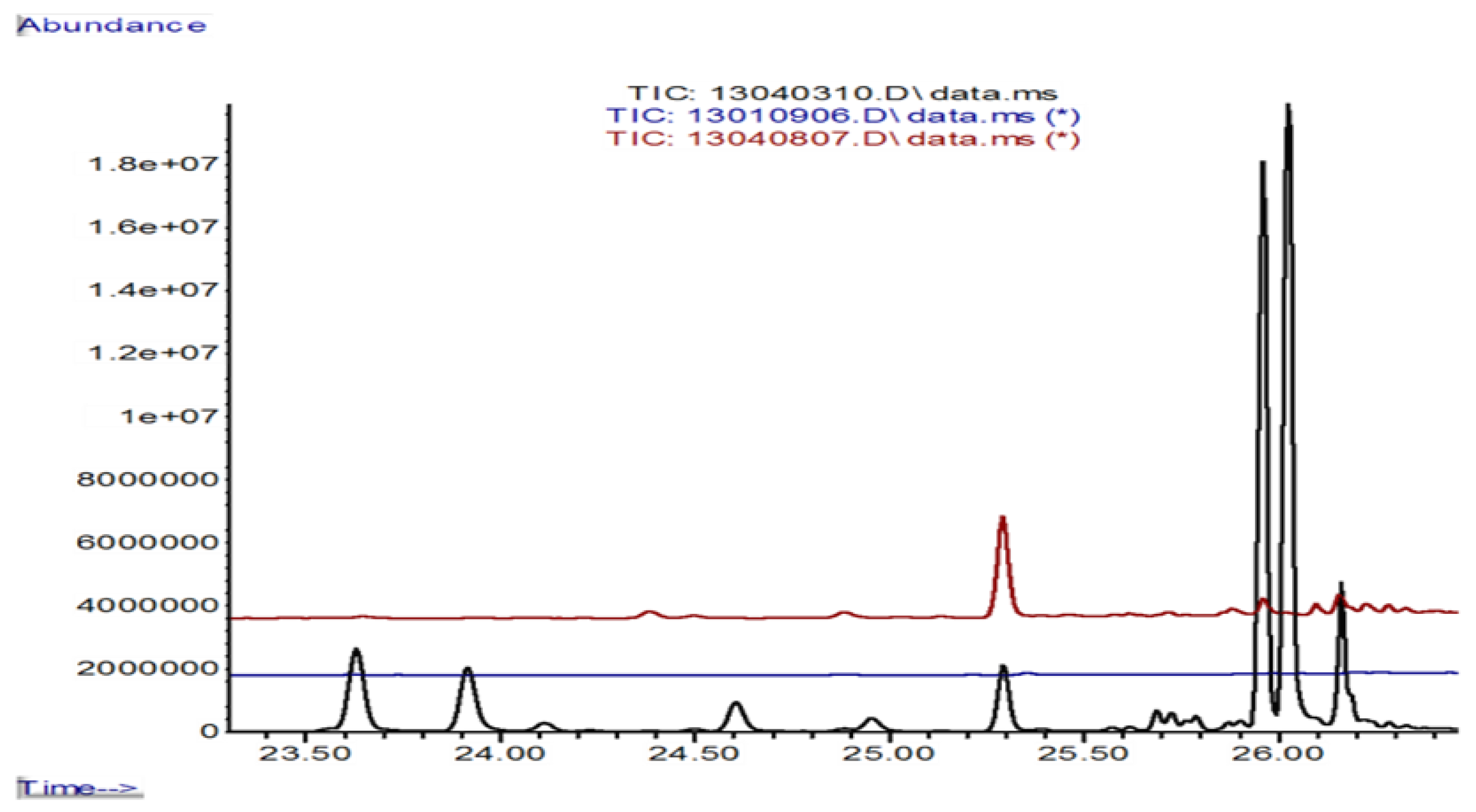
3.5. Formation of Volatile Sesquiterpenes in 0.3 L Jars
4. Discussion
4.1. Dynamics of Interaction
4.2. Volatile Sesquiterpene Components in the Interaction
4.3. The Role of Oxalate
4.4. Further Volatile Biocidal Terpenes of F. pinicola
5. Conclusions
Conflicts of Interest
References
- Gramss, G. Potential contributions of oxidoreductases from alfalfa plants to soil enzymology and biotechnology: A review. J. Nat. Sci. Sust. Technol. 2012, 6, 169–223. [Google Scholar]
- Hofrichter, M.; Ullrich, R.; Pecyna, M.J.; Liers, C.; Lundell, T. New and classic families of secreted fungal heme peroxidases. Appl. Microbiol. Biotechnol. 2010, 87, 871–897. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.W.S. Structure and action mechanism of ligninolytic enzymes. Appl. Biochem. Biotechnol. 2008, 157, 174–209. [Google Scholar] [CrossRef] [PubMed]
- Baldrian, P. Fungal laccases – occurrence and properties. FEMS Microbiol. Rev. 2006, 30, 215–242. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.M.; Taylor, A.F.S.; Burke, R.M.; Cairney, J.W.G. Identification of genes for lignin peroxidases and manganese peroxidases in ectomycorrhizal fungi. New Phytol. 2001, 152, 151–158. [Google Scholar] [CrossRef]
- Steffen, K.; Hofrichter, M.; Hatakka, A. Mineralisation of 14C-labelled synthetic lignin and ligninolytic enzyme activities of litter-decomposing basidiomycetous fungi. Appl. Microbiol. Biotechnol. 2000, 54, 819–825. [Google Scholar] [CrossRef]
- Steffen, K.; Hofrichter, M.; Hatakka, A. Purification and characterization of manganese peroxidases from the litter-decomposing basidiomycetes Agrocybe praecox and Stropharia coronilla. Enzym. Microb. Technol. 2002, 30, 550–555. [Google Scholar] [CrossRef]
- Brink, D.; Ravi, K.; Lidén, G.; Gorwa-Grauslund, M.F. Mapping the diversity of microbial lignin catabolism: Experiences from the eLignin database. Appl. Microbiol. Biotechnol. 2019, 103, 3979–4002. [Google Scholar] [CrossRef]
- Gramss, G.; Ziegenhagen, D.; Sorge, S. Degradation of soil humic extract by wood- and soil-associated fungi, bacteria, and commercial enzymes. Microb. Ecol. 1999, 37, 140–151. [Google Scholar] [CrossRef]
- Janusz, G.; Pawlik, A.; Sulej, J.; Świderska-Burek, U.; Jarosz-Wilkolazka, A.; Paszczyński, A. Lignin degradation: Microorganisms, enzymes involved, genomes analysis and evolution. FEMS Microbiol. Rev. 2017, 41, 941–962. [Google Scholar] [CrossRef]
- Müller, J.; Engel, H.; Blaschke, M. Assemblages of wood-inhabiting fungi related to silvicultural management intensity in beech forests in southern Germany. Eur. J. For. Res. 2007, 126, 513–527. [Google Scholar] [CrossRef]
- Kubartová, A.; Ottosson, E.; Stenlid, J. Linking fungal communities to wood density loss after 12 years of log decay. FEMS Microbiol. Ecol. 2015, 91. [Google Scholar] [CrossRef] [PubMed]
- Leonhardt, S.; Hoppe, B.; Stengel, E.; Noll, L.; Moll, J.; Bässler, C.; Dahl, A.; Buscot, F.; Hofrichter, M.; Kellner, H. Molecular fungal community and its decomposition activity in sapwood and heartwood of 13 temperate European tree species. PLoS ONE 2019, 14, e0212120. [Google Scholar] [CrossRef] [PubMed]
- Ottoson, E. Succession of Wood-Inhabiting Fungal Communities. Diversity and Species Interactions During the Decomposition of Norway Spruce. Ph.D. Thesis, Swedish University of Agricultural Sciences, Uppsala, Swedish, 2013. [Google Scholar]
- Wu, B.; Gaskell, J.; Held, B.W.; Toapanta, C.; Vuong, T.; Ahrendt, S.; Lipzen, A.; Zhang, J.; Schilling, J.S.; Master, E.; et al. Substrate-specific differential gene expression and RNA editing in the brown rot fungus Fomitopsis pinicola. Appl. Environ. Microbiol. 2018, 84, e00991-18. [Google Scholar] [CrossRef]
- Allard, G.; FAO; Overview of forest pests Kyrgyz Republic; Forest Resources Development Service; Forest Management Division; Forestry Department. Forest Health & Biosecurity Working Papers; Working Paper FBS/29E; FAO: Rome, Italy, 2007. Available online: http://www.fao.org/forestry/12277-0ac81b7e1d2a6d6861bbab1599f7244b9.pdf (accessed on 19 December 2019).
- Plantwise Knowledge Bank. Species Page Brown Crumbly Rot, Fomitopsis pinicola. Available online: https://www.plantwise.org/knowledgebank/datasheet/24310 (accessed on 19 December 2019).
- Vogel, S.; Alvarez, B.; Bässler, C.; Müller, J.; Thorn, S. The red-belted bracket (Fomitopsis pinicola) colonizes spruce trees early after bark beetle attack and persists. Fungal Ecol. 2017, 27, 182–188. [Google Scholar] [CrossRef]
- Ruokolainen, A.; Shorohova, E.; Penttilä, R.; Kotkova, V.; Kushnevskaya, H. A continuum of dead wood with various habitat elements maintains the diversity of wood-inhabiting fungi in an old-growth boreal forest. Eur. J. For. Res. 2018, 137, 707–718. [Google Scholar] [CrossRef]
- Gramss, G. Invasion of wood by basidiomycetous fungi. IV. Microbiological approach to the role of kratovirulence in the expression of pathovirulence. J. Basic Microbiol. 1987, 27, 241–251. [Google Scholar] [CrossRef]
- Arfi, Y.; Levasseur, A.; Record, E. Differential Gene Expression in Pycnoporus coccineus during interspecific mycelial interactions with different competitors. Appl. Environ. Microbiol. 2013, 79, 6626–6636. [Google Scholar] [CrossRef]
- Holmer, L.; Renvall, P.; Stenlid, J. Selective replacement between species of wood-rotting basidiomycetes, a laboratory study. Mycol. Res. 1997, 101, 714–720. [Google Scholar] [CrossRef]
- O’Leary, J.; Hiscox, J.; Eastwood, D.C.; Savoury, M.; Langley, A.; McDowell, S.W.; Rogers, H.J.; Boddy, L.; Müller, C.T. The whiff of decay: Linking volatile production and extracellular enzymes to outcomes of fungal interactions at different temperatures. Fungal Ecol. 2019, 39, 336–348. [Google Scholar] [CrossRef]
- Ujor, V.C.; Monti, M.; Peiris, D.; Clements, M.O.; Hedger, J.N. The mycelial response of the white-rot fungus, Schizophyllum commune to the biocontrol agent, Trichoderma viride. Fungal Boil. 2012, 116, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Ujor, V.C.; Adukwu, E.C.; Okonkwo, C.C. Fungal wars: The underlying molecular repertoires of combating mycelia. Fungal Boil. 2018, 122, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Giles, R.L.; Zackeru, J.C.; Galloway, E.R.; Elliott, G.D.; Parrow, M.W. Single versus simultaneous species treatment of wood with Ceriporiopsis subvermispora and Postia placenta for ethanol applications, with observations on interspecific growth inhibition. Int. Biodeterior. Biodegradation 2015, 99, 66–72. [Google Scholar] [CrossRef]
- Minerdi, D.; Bossi, S.; Gullino, M.L.; Garibaldi, A. Volatile organic compounds: A potential direct long-distance mechanism for antagonistic action of Fusarium oxysporum strain MSA 35. Environ. Microbiol. 2009, 11, 844–854. [Google Scholar] [CrossRef]
- Baldrian, P. Interactions of heavy metals with white-rot fungi. Enzym. Microb. Technol. 2003, 32, 78–91. [Google Scholar] [CrossRef]
- Rühl, M. Laccases and Other Ligninolytic Enzymes of the Basidiomycetes Coprinopsis cinerea and Pleurotus ostreatus. Ph.D. Thesis, Georg-August-University, Göttingen, Germany, December 2009. [Google Scholar]
- Arora, D.K.; Upadhyay, R.K. Effect of fungal staling growth substances on colony interaction. Plant Soil 1978, 49, 685–690. [Google Scholar] [CrossRef]
- Park, D.; Robinson, P.M. Isolation and bioassay of a fungal morphogen. Nature 1964, 203, 988–989. [Google Scholar] [CrossRef]
- Gramss, G. Role of soil mycelium in nutrition of wooddestroying basidiomycetous fungi on inoculated wood blocks in soil. J. Basic Microbiol. 1979, 19, 143–145. [Google Scholar]
- Rudman, P. Some observations on the biological activity of the metabolic products of the wood rotting fungus Lentinus lepideus. Holzforschung 1963, 17, 149–151. [Google Scholar] [CrossRef]
- Korn, V.; Inamdar, A.A. Are some fungal volatile organic compounds (VOCs) mycotoxins? Toxins 2015, 7, 3785–3804. [Google Scholar]
- Gramss, G. The Influence of the concomitant microflora on establishment and dieback of decay fungi in standing timber. J. Phytopathol. 1987, 120, 205–215. [Google Scholar] [CrossRef]
- Gao, Y.; Jin, Y.; Li, H.-D.; Chen, H.-J. Volatile organic compounds and their roles in bacteriostasis in five conifer species. J. Integr. Plant Boil. 2005, 47, 499–507. [Google Scholar] [CrossRef]
- Bohm, K.; Martín-Sánchez, L.; Garbeva, P. Microbial volatiles: Small molecules with an important role in intra- and inter-kingdom interactions. Front. Microbiol. 2017, 8. [Google Scholar]
- Yuan, J.; Zhao, M.; Li, R.; Huang, Q.; Raza, W.; Rensing, C.; Shen, Q. Microbial volatile compounds alter the soil microbial community. Environ. Sci. Pollut. Res. 2017, 24, 22485–22493. [Google Scholar] [CrossRef]
- Gramss, G. Approach to the nature of volatile compounds that dominate the ecological niche of basidiomycetous ground fungi in the edaphosphere of grassland. Zentralblatt für Mikrobiologie 1985, 140, 597–606. [Google Scholar] [CrossRef]
- Quintana-Rodríguez, E.; Rivera-Macias, L.; Adame-Álvarez, R.M.; Torres, J.M.; Heil, M. Shared weapons in fungus-fungus and fungus-plant interactions? Volatile organic compounds of plant or fungal origin exert direct antifungal activity in vitro. Fungal Ecol. 2018, 33, 115–121. [Google Scholar] [CrossRef]
- Tortella, G.; Rubilar, O.; Stenström, J.; Cea, M.; Briceno, G.; Quiroz, A.; Diez, M.C.; Parra, L. Using volatile organic compounds to enhance atrazine biodegradation in a biobed system. Biodegradation 2013, 24, 711–720. [Google Scholar] [CrossRef]
- McDougall, B.M. Movement of 14C-photosynthate into the roots of wheat seedlings and exudation of 14C from intact roots. New Phytol. 1970, 69, 37–46. [Google Scholar] [CrossRef]
- Booth, E.; Strobel, G.; Knighton, B.; Sears, J.; Geary, B.; Avci, R. A rapid column technique for trapping and collecting of volatile fungal hydrocarbons and hydrocarbon derivatives. Biotechnol. Lett. 2011, 33, 1963–1972. [Google Scholar] [CrossRef]
- Fischer, G.; Schwalbe, R.; Möller, M.; Ostrowski, R.; Dott, W. Species-specific production of microbial volatile organic compounds (MVOC) by airborne fungi from a compost facility. Chemosphere 1999, 39, 795–810. [Google Scholar] [CrossRef]
- Matysik, S.; Herbarth, O.; Mueller, A. Determination of microbial volatile organic compounds (MVOCs) by passive ampling onto charcoal sorbents. Chemosphere 2009, 76, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, K.; Larsen, K.; Simkus, M. Volatile metabolites from mold growth on building materials and synthetic media. Chemosphere 2000, 41, 437–446. [Google Scholar] [CrossRef]
- Benchaar, C.; Calsamiglia, S.; Chaves, A.V.; Fraser, G.; Colombatto, D.; McAllister, T.A.; Beauchemin, K. A review of plant-derived essential oils in ruminant nutrition and production. Anim. Feed. Sci. Technol. 2008, 145, 209–228. [Google Scholar] [CrossRef]
- Fisher, R.F. Allelopathy. In Plant Disease, How Pathogens Induce Disease; Horsfall, J.G., Cowling, E.B., Eds.; Academic Press: New York, USA, 1979; pp. 313–330. [Google Scholar]
- Hamilton-Kemp, T.R.; Andersen, R.A. Volatiles from winter wheat: Identification of additional compounds and effects of tissue source. Phytochemistry 1985, 25, 241–243. [Google Scholar] [CrossRef]
- Gramss, G.; Bergmann, H. Role of plants in the vegetative and reproductive growth of saprobic basidiomycetous ground fungi. Microb. Ecol. 2008, 56, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Huffer, S.; Clark, M.E.; Ning, J.C.; Blanch, H.W.; Clark, U.S. Role of Alcohols in growth, lipid composition, and membrane fluidity of yeasts, bacteria, and archaea. Appl. Environ. Microbiol. 2011, 77, 6400–6408. [Google Scholar] [CrossRef] [PubMed]
- Sikkema, J.; A De Bont, J.; Poolman, B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 1995, 59, 201–222. [Google Scholar] [CrossRef]
- Krokene, P.; Nagy, N.E.; Solheim, H. Methyl jasmonate and oxalic acid treatment of Norway spruce: Anatomically based defense responses and increased resistance against fungal infection. Tree Physiol. 2008, 28, 29–35. [Google Scholar] [CrossRef]
- Walz, A.; Zingen-Sell, I.; Theisen, S.; Kortekamp, A. Reactive oxygen intermediates and oxalic acid in the pathogenesis of the necrotrophic fungus Sclerotinia sclerotiorum. Eur. J. Plant Pathol. 2007, 120, 317–330. [Google Scholar] [CrossRef]
- Gramss, G.; Kirsche, B.; Voigt, K.-D.; Günther, T.; Fritsche, W. Conversion rates of five polycyclic aromatic hydrocarbons in liquid cultures of fifty-eight fungal species and the concomitant production of oxidative enzymes. Mycol. Res. 1999, 103, 1009–1018. [Google Scholar] [CrossRef]
- Leonowicz, A.; Malinowska, M. Purification of the constitutive and inducible forms of laccase of the fungus Pholiota mutabilis by affinity chromatography. Acta Biochim. Pol. 1982, 29. [Google Scholar]
- Chen, Q.; Krügener, S.; Hirth, T.; Rupp, S.; Zibek, S. Co-cultured production of lignin-modifying enzymes with white-rot fungi. Appl. Biochem. Biotechnol. 2011, 165, 700–718. [Google Scholar]
- Chi, Y.; Hatakka, A.; Maijala, P. Can co-culturing of two white-rot fungi increase lignin degradation and the production of lignin-degrading enzymes? Int. Biodeterior. Biodegrad. 2007, 59, 32–39. [Google Scholar] [CrossRef]
- Velázquez-Cedeño, M.; Farnet, A.M.; Mata, G.; Savoie, J.-M. Role of Bacillus spp. in antagonism between Pleurotus ostreatus and Trichoderma harzianum in heat-treated wheat-straw substrates. Bioresour. Technol. 2008, 99, 6966–6973. [Google Scholar] [CrossRef]
- Kersten, P.J.; Cullen, D. Copper radical oxidases and related extracellular oxidoreductases of wood-decay Agaricomycetes. Fungal Genet. Boil. 2014, 72, 124–130. [Google Scholar] [CrossRef]
- Riley, R.; Salamov, A.A.; Brown, D.W.; Nagy, L.G.; Floudas, D.; Held, B.W.; Levasseur, A.; Lombard, V.; Morin, E.; Otillar, R.; et al. Extensive sampling of basidiomycete genomes demonstrates inadequacy of the white-rot/brown-rot paradigm for wood decay fungi. Proc. Natl. Acad. Sci. USA 2014, 111, 9923–9928. [Google Scholar] [CrossRef]
- Arantes, V.; Milagres, A.; Filley, T.R.; Goodell, B. Lignocellulosic polysaccharides and lignin degradation by wood decay fungi: The relevance of nonenzymatic Fenton-based reactions. J. Ind. Microbiol. Biotechnol. 2010, 38, 541–555. [Google Scholar] [CrossRef]
- Shah, F.; Mali, T.; Lundell, T. Polyporales brown rot species Fomitopsis pinicola: Enzyme activity profiles, oxalic acid production, and Fe3+-reducing metabolite secretion. Appl. Environ. Microbiol. 2018, 84, e02662-17. [Google Scholar] [CrossRef]
- Huang, K.-C.; Couttenye, R.A.; Hoag, G.E. Kinetcs of heat-assisted persulfate oxidation of methyl tert-butyl ether (MTBE). Chemosphere 2002, 49, 413–420. [Google Scholar] [CrossRef]
- Hastrup, A.C.S. Aspects of Cellulose Degradation by Brown Rot Fungi. Involvement of Enzymatic and Non-Enzymatic Decay Processes Including Oxalic Acid Regulation and Metal Sequestering. Ph.D. Thesis, University of Copenhagen, Copenhagen, Denmark, June 2011. [Google Scholar]
- Dutton, M.V.; Evans, C.S. Oxalate production by fungi: Its role in pathogenicity and ecology in the soil environment. Can. J. Microbiol. 1996, 42, 881–895. [Google Scholar] [CrossRef]
- Kunz, C.; Vandelle, E.G.G.; Rolland, S.; Poinssot, B.; Bruel, C.; Cimerman, A.; Zotti, C.; Moreau, E.; Vedel, R.; Pugin, A.; et al. Characterization of a new, nonpathogenic mutant of Botrytis cinerea with impaired plant colonization capacity. New Phytol. 2006, 170, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Munir, E.; Yoon, J.-J.; Tokimatsu, T.; Hattori, T.; Shimada, M. New role for glyoxylate cycle enzymes in wood-rotting basidiomycetes in relation to biosynthesis of oxalic acid. J. Wood Sci. 2001, 47, 368–373. [Google Scholar] [CrossRef]
- Jarosz-Wilkołazka, A.; Gadd, G.M. Oxalate production by wood-rotting fungi growing in toxic metal-amended medium. Chemosphere 2003, 52, 541–547. [Google Scholar] [CrossRef]
- Schilling, J.S. Oxalate Production and Cation Translocation During Wood Biodegradation by Fungi. Electronic Theses and Dissertations. 2006. Available online: http://digitalcommons.library.umaine.edu/etd/336 (accessed on 19 December 2019).
- Bateman, D.F. An induced mechanism of tissue resistance to polygalacturonase in Rhizoctonia infected hypocotyls of bean. Phytopathology 1964, 54, 438–445. [Google Scholar]
- Bateman, D.F.; Beer, S.V. Simultaneous production and synergistic action of oxalic acid and polygalcturonase during pathogenesis by Sclerotium rolfsii. Phytopathology 1965, 55, 204–211. [Google Scholar]
- Punja, Z.; Huang, J.-S.; Jenkins, S. Relationship of mycelial growth and production of oxalic acid and cell wall degrading enzymes to virulence in Sclerotium Rolfsii. Can. J. Plant Pathol. 1985, 7, 109–117. [Google Scholar] [CrossRef]
- Kurian, P. The synergistic role of oxalic acid and endopolygalacturonase in bean leaves infected by Cristulariella pyramidalis. Phytopathology 1979, 69, 1301. [Google Scholar] [CrossRef]
- Gramss, G. Invasion of wood by basidiomycetous fungi. V. The role of fungal elicitors of plant cell necrobiosis in the expression of pathovirulence and kratovirulence. J. Basic Microbiol. 1989, 29, 563–579. [Google Scholar] [CrossRef]
- De Boer, W.; Folman, L.B.; Gunnewiek, P.J.K.; Svensson, T.; Bastviken, D.; Öberg, G.; Del Río, J.C.; Boddy, L. Mechanism of antibacterial activity of the white-rot fungus Hypholoma fasciculare colonizing wood. Can. J. Microbiol. 2010, 56, 380–388. [Google Scholar] [CrossRef]
- Desriac, N.; Broussolle, V.; Postollec, F.; Mathot, A.-G.; Sohier, D.; Coroller, L.; Leguerinel, I. Bacillus cereus cell response upon exposure to acid environment: Toward the identification of potential biomarkers. Front. Microbiol. 2013, 4. [Google Scholar] [CrossRef]
- Hayaishi, O.; Jakoby, W.B.; Ohmura, E. Enzymatic decarboxylation of oxalic acid. J. Boil. Chem. 1956, 222. [Google Scholar]
- Hofrichter, M.; Ziegenhagen, D.; Vares, T.; Friedrich, M.; Jäger, M.; Fritsche, W.; Hatakka, A. Oxidative decomposition of malonic acid as basis for the action of manganese peroxidase in the absence of hydrogen peroxide. FEBS Lett. 1998, 434, 362–366. [Google Scholar] [CrossRef]
- Sutherland, G.R.; Aust, S.D. The effects of calcium on the thermal stability and activity of manganese peroxidase. Arch. Biochem. Biophys. 1996, 332, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Ren, K.; Jia, R.; Chen, W.; Sun, R. Expression of a fungal manganese peroxidase in Escherichia coli: A comparison between the soluble and refolded enzymes. BMC Biotechnol. 2016, 16, 87. [Google Scholar] [CrossRef] [PubMed]
- Sato, M. Inhibition by oxalates of spinach chloroplast phenolase in unfrozen and frozen states. Phytochemistry 1980, 19, 1613–1617. [Google Scholar] [CrossRef]
- Sterjiades, R.; Dean, J.; Eriksson, K.-E.L. Laccase from sycamore maple (Acer pseudoplatanus) polymerizes monolignols. Plant Physiol. 1992, 99, 1162–1168. [Google Scholar] [CrossRef]
- Wariishi, H.; Valli, K.; Gold, M.H. Manganese(II) oxidation by manganese peroxidase from the basidiomycete Phanerochaete chrysosporium. Kinetic mechanism and role of chelators. J. Boil. Chem. 1992, 267, 23688–23695. [Google Scholar]
- Luthardt, W. Wood Inhabiting Fungi. Cultivation and Wood Mycology. In Holzbewohnende Pilze. Anzucht und Holzmykologie; A. Ziemsen: Wittenberg, Germany, 1969. [Google Scholar]
- Park, N.; Park, S.-S. Purification and characterization of a novel laccase from Fomitopsis pinicola mycelia. Int. J. Boil. Macromol. 2014, 70, 583–589. [Google Scholar] [CrossRef]
- Evans, J.A.; Eyre, C.A.; Rogers, H.; Boddy, L.; Muller, C. Changes in volatile production during interspecific interactions between four wood rotting fungi growing in artificial media. Fungal Ecol. 2008, 1, 57–68. [Google Scholar] [CrossRef]
- Flores, C.; Vidal, C.; Trejo-Hernandez, M.R.; Galindo, E.; Serrano-Carreón, L. Selection of Trichoderma strains capable of increasing laccase production by Pleurotus ostreatus and Agaricus bisporus in dual cultures. J. Appl. Microbiol. 2009, 106, 249–257. [Google Scholar] [CrossRef]
- Hiscox, J.; Baldrian, P.; Rogers, H.; Boddy, L. Changes in oxidative enzyme activity during interspecific mycelial interactions involving the white-rot fungus Trametes versicolor. Fungal Genet. Boil. 2010, 47, 562–571. [Google Scholar] [CrossRef]
- Bell, A.A.; Wheeler, M.H. Biosynthesis and functions of fungal melanins. Annu. Rev. Phytopath. 1986, 24, 411–451. [Google Scholar] [CrossRef]
- Baldrian, P. Wood-inhabiting ligninolytic basidiomycetes in soils: Ecology and constraints for applicability in bioremediation. Fungal Ecol. 2008, 1, 4–12. [Google Scholar] [CrossRef]
- Jeon, J.-R.; Baldrián, P.; Murugesan, K.; Chang, Y.-S. Laccase-catalysed oxidations of naturally occurring phenols: From in vivo biosynthetic pathways to green synthetic applications. Microb. Biotechnol. 2012, 5, 318–332. [Google Scholar] [CrossRef]
- Decros, G.; Baldet, P.; Beauvoit, B.; Stevens, R.; Flandin, A.; Colombié, S.; Gibon, Y.; Pétriacq, P. Get the balance right: ROS homeostasis and redox signalling in fruit. Front. Plant Sci. 2019, 10, 1091. [Google Scholar] [CrossRef]
- Silar, P. Peroxide accumulation and cell death in filamentous fungi induced by contact with a contestant. Mycol. Res. 2005, 109, 137–149. [Google Scholar] [CrossRef]
- González-Mas, M.C.; Rambla, J.L.; López-Gresa, M.P.; Blázquez, M.A.; Granell, A. Volatile compounds in citrus essential oils: A comprehensive review. Front. Plant Sci. 2019, 10, 12. [Google Scholar] [CrossRef]
- Martins, C.D.M.; Nascimento, E.A.D.; De Morais, S.A.L.; De Oliveira, A.; Chang, R.; Cunha, L.C.S.; Martins, M.M.; Martins, C.H.G.; Moraes, T.D.S.; Rodrigues, P.V.; et al. Chemical constituents and evaluation of antimicrobial and cytotoxic activities of Kielmeyera coriacea Mart. & Zucc. essential oils. Evid.-Based Complement. Altern. Med. 2015, 2015, 1–9. [Google Scholar]
- Rokbeni, N.; Mrabet, Y.; Dziri, S.; Chaabane, H.; Jemli, M.; Fernandez, X.; Boulila, A. Variation of the chemical composition and antimicrobial activity of the essential oils of natural populations of Tunisian Daucus carota L. (Apiaceae). Chem. Biodivers. 2013, 10, 2278–2290. [Google Scholar] [CrossRef]
- Bougatsos, C.; Meyer, J.J.M.; Magiatis, P.; Vagias, C.; Chinou, I.B. Composition and antimicrobial activity of the essential oils of Helichrysum kraussii Sch. Bip. and H. rugulosum Less. from South Africa. Flavour Fragr. J. 2003, 18, 48–51. [Google Scholar] [CrossRef]
- Carneiro, N.S.; Alves, C.C.F.; Alves, J.M.; Egea, M.B.; Martins, C.H.G.; Silva, T.S.; Bretanha, L.C.; Balleste, M.P.; Caon, T.; Silveira, E.V.; et al. Chemical composition, antioxidant and antibacterial activities of essential oils from leaves and flowers of Eugenia klotzschiana Berg (Myrtaceae). Anais da Academia Brasileira de Ciências 2017, 89, 1907–1915. [Google Scholar] [CrossRef]
- Kazemi, M.; Rostami, H. Chemical composition, antimicrobial and antioxidant activities of the essential oil of Psammogeton canescens. Nat. Prod. Res. 2014, 29, 277–280. [Google Scholar] [CrossRef]
- De Melo, N.I.; Magalhães, L.G.; De Carvalho, C.E.; Wakabayashi, K.A.L.; Aguiar, G.D.P.; Ramos, R.C.; Mantovani, A.L.L.; Turatti, I.C.C.; Rodrigues, V.; Groppo, M.; et al. Schistosomicidal activity of the essential oil of Ageratum conyzoides L. (Asteraceae) against adult Schistosoma mansoni Worms. Molecules 2011, 16, 762–773. [Google Scholar] [CrossRef]
- Bohlmann, J.; Crock, J.; Jetter, R.; Croteau, R. Terpenoid-based defenses in conifers: cDNA cloning, characterization, and functional expression of wound-inducible (E)-α-bisabolene synthase from grand fir (Abies grandis). Proc. Natl. Acad. Sci. USA 1998, 95, 6756–6761. [Google Scholar] [CrossRef]
- Manter, D.K.; Kelsey, R.G.; Karchesy, J.J. Antimicrobial activity of extractable conifer heartwood compounds toward Phytophthora ramorum. J. Chem. Ecol. 2007, 33, 2133–2147. [Google Scholar] [CrossRef]
- Erkel, G.; Lorenzen, K.; Anke, T.; Velten, R.; Gimenez, A.; Steglich, W. Kuehneromycins A and B, two new biological active compounds from a Tasmanian Kuehneromyces sp. (Strophariaceae, Basidiomycetes). Z. Naturforsch. 1995, 50, 1–10. [Google Scholar] [CrossRef]
- Malhautier, L.; Khammar, N.; Bayle, S.; Fanlo, J.-L. Biofiltration of volatile organic compounds. Appl. Microbiol. Biotechnol. 2005, 68, 16–22. [Google Scholar] [CrossRef]
- Schenkel, D.; Macia-Vicente, J.G.; Bissell, A.; Splivallo, R. Fungi indirectly affect plant root architecture by modulating soil volatile organic compounds. Front. Microbiol. 2018, 9, 1847. [Google Scholar] [CrossRef]
- Noyes, R.; Hancock, J. Role of oxalic acid in the Sclerotinia wilt of sunflower. Physiol. Plant Pathol. 1981, 18, 123–132. [Google Scholar] [CrossRef]
- Pardo, I.; Rodríguez-Escribano, D.; Aza, P.; De Salas, F.; Martinez, M.J.; Camarero, S. A highly stable laccase obtained by swapping the second cupredoxin domain. Sci. Rep. 2018, 8, 15669. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, Z.; Chen, G.; Wang, Z. An improved method to encapsulate laccase from Trametes versicolor with enhanced stability and catalytic activity. Catalysts 2018, 8, 286. [Google Scholar] [CrossRef]
- Barron, G.L. Microcolonies of bacteria as a nutrient source for lignicolous and other fungi. Can. J. Bot. 1988, 66, 2505–2510. [Google Scholar] [CrossRef]
- Kertesz, M.; Thai, M. Compost bacteria and fungi that influence growth and development of Agaricus bisporus and other commercial mushrooms. Appl. Microbiol. Biotechnol. 2018, 102, 1639–1650. [Google Scholar] [CrossRef]
- Dresch, P.; D’aguanno, M.N.; Rosam, K.; Grienke, U.; Rollinger, J.; Peintner, U. Fungal strain matters: Colony growth and bioactivity of the European medicinal polypores Fomes fomentarius, Fomitopsis pinicola and Piptoporus betulinus. AMB Express 2015, 5, 687. [Google Scholar] [CrossRef]
- Grienke, U.; Zöll, M.; Peintner, U.; Rollinger, J.M. European medicinal polypores – A modern view on traditional uses. J. Ethnopharmacol. 2014, 154, 564–583. [Google Scholar] [CrossRef]
- Rösecke, J.; König, W.A. Steroids from the fungus Fomitopsis pinicola. Phytochemistry 1999, 52, 1621–1627. [Google Scholar]
- Zjawiony, J. Biologically Active Compounds from Aphyllophorales (Polypore) Fungi⊥. J. Nat. Prod. 2004, 67, 300–310. [Google Scholar] [CrossRef]
- Rösecke, J.; Pietsch, M.; König, W.A. Volatile constituents of wood-rotting basidiomycetes. Phytochemistry 2000, 54, 747–750. [Google Scholar]
- Reuter, A. Über 100,000 Hektar müssen Wieder Aufgeforstet Werden. Available online: https://www.tagesschau.de/inland/wald-stuerme-borkenkaefer-101.html (accessed on 10 January 2020).
- Waldschäden: 2019 Bereits mehr als 5 Millionen Kubikmeter Schadholz | in Südthüringen.de. Available online: https://www.insuedthueringen.de/region/thueringen/thuefwthuedeu/Waldschaeden-2019-bereits-mehr-als-5-Millionen-Kubikmeter-Schadholz;art83467,7014843 (accessed on 10 January 2020).
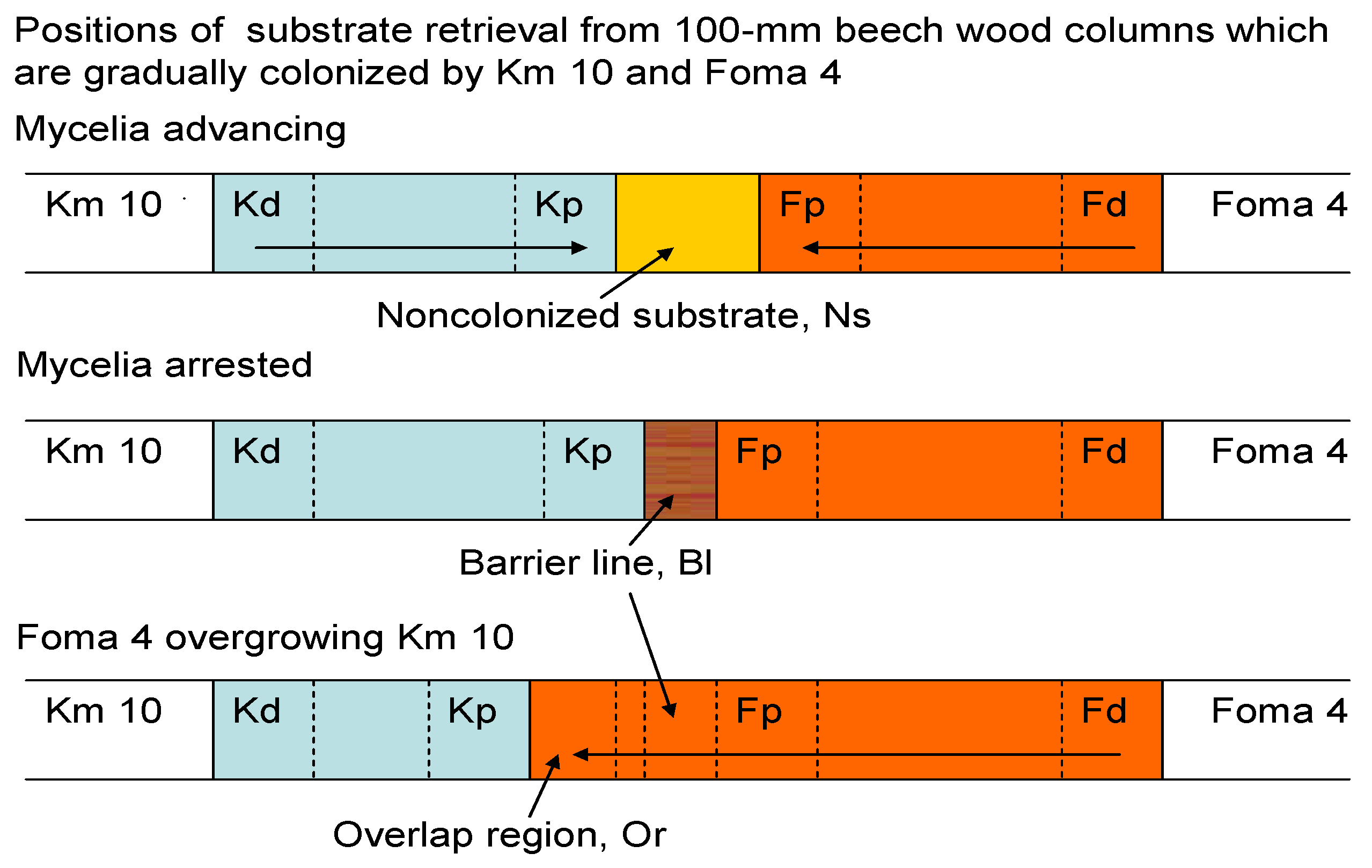
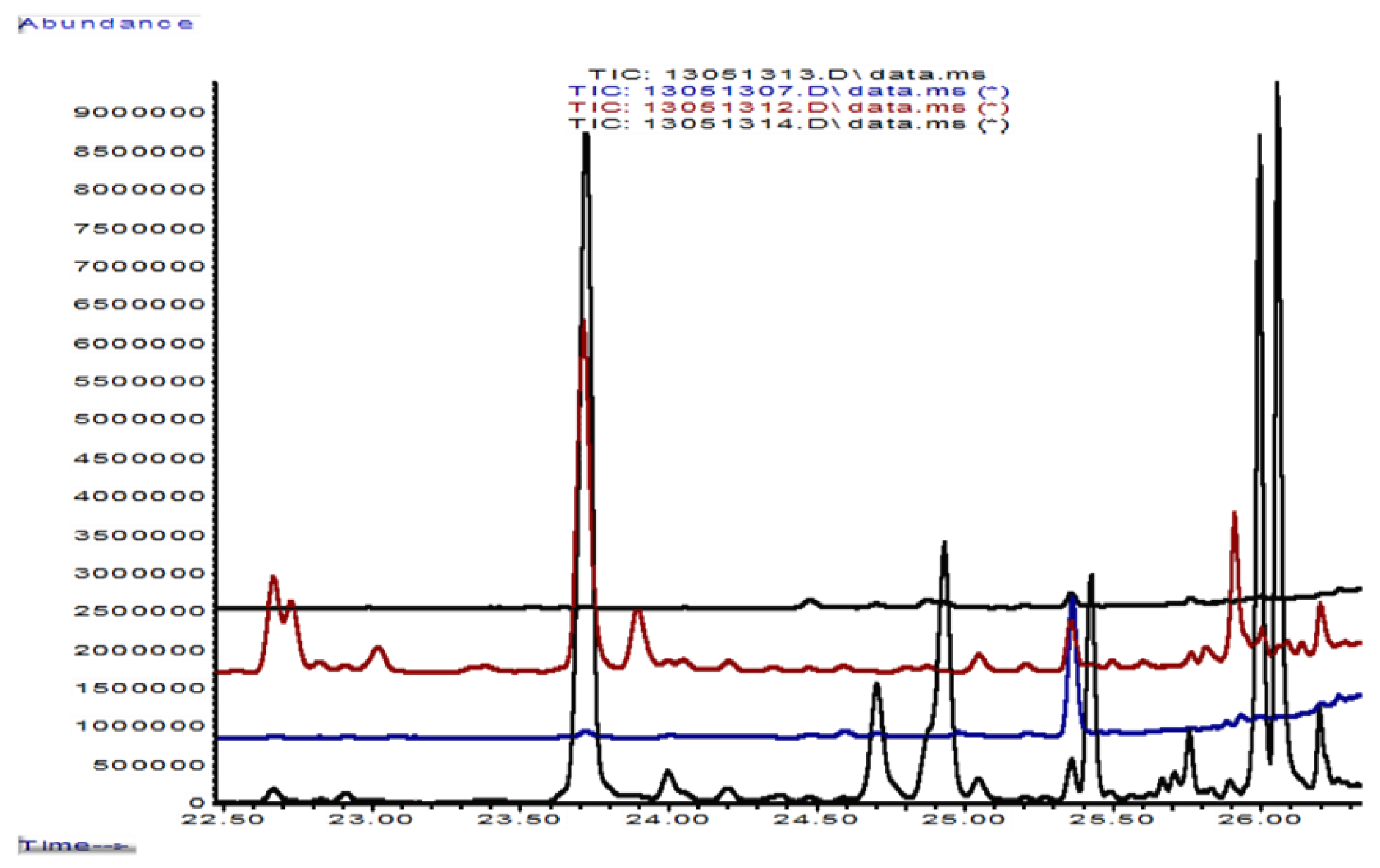
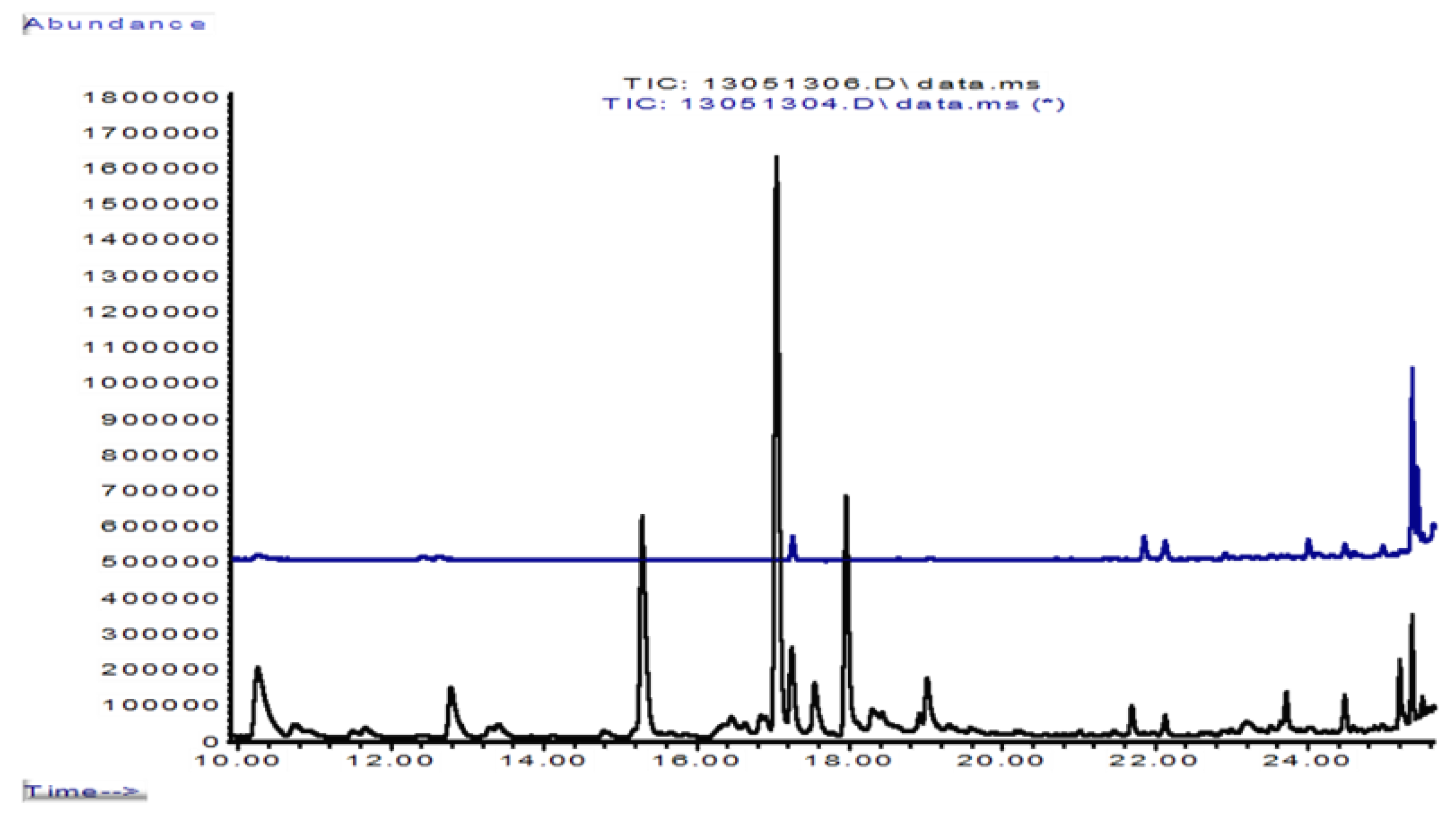
| Variant (No. of Replicates) | Retrieval at | pHaqu 1:2.5 w:v | Oxalate | Laccase (ABTS) | MnP | Distan-ce d-p 1 |
|---|---|---|---|---|---|---|
| Km, monoxenic (2) | Kd | 3.21–3.47 | ND | 22–75 | 0–12 | 71–77 |
| Kp | 4.42–4.44 | 0.034 | 5610–5690 a | 251–365 a | ||
| Ns | 4.36–4.38 | ND | 48–49 | 0 | ||
| Foma, monoxenic (2) | Fd | 3.32–3.40 | 1.76–2.30 | 0 | 0 | 65 |
| Fp | 1.97–2.2 | 15–21.3 a | 0 | 0 | ||
| Ns | 2.45–2.94 | 1.95–6.11 | 0 | 0 | ||
| Km and Foma approaching; Ns, 19-22 mm (3) | Kd | 3.49–3.62 | ND | 5.4–28 | 20–53 | |
| Kp | 3.92–4.15 | ND | 1400–1430 b | 788–901 b | 29–42 | |
| Ns | 4.42–4.61 | 0.043–0.051 | 32–68 | 4.9–7.8 | ||
| Fp | 2.29–2.53 | 9.85–12.8 b | 0 | 0 | 21 | |
| Fd | 1.95–2.04 | 16.2–17.1 | 0 | 0 | ||
| Km and Foma contacting (4) | Kd | 3.28–3.32 | ND | 2.3–4.6 | 27–141 | |
| Kp | 3.54–3.63 | 0.022–0.035 | 995–1944 b | 1146–1983 b,c | 46 | |
| Fp | 2.06–2.23 | 9.27–9.75 b,c | 0.7–1 | 0 | 30 | |
| Fd | 1.74–2.13 | 12.3–19.3 | 0 | 0 | ||
| Km and Foma arrested for 11 d; barrier line 4 mm. (6) | Kd | 3.13–3.29 | 0.013–0.026 | 19–39 | 4.6–16 | |
| Kp | 3.15–.31 | 0–0.027 | 130–175 c | 15–53 d | 44–48 | |
| Bl | 3.21–3.40 | 0.019–0.035 | 724–2820 | 15–140 | ||
| Fp | 1.69–1.75 | 23.8–25.1 d | 0 | 0 | 31–40 | |
| Fd | 1.40–1.56 | 24–29.8 | 0 | 0 | ||
| Foma overgrowing Km by 17–26 mm (5) | Kd | 3.12–3.67 | 0.029–0.040 | 0–3.7 | 0 | |
| Kp | 2.96–3.16 | 0.017–0.045 | 0–12 d | 0 | 17–29 | |
| Or | 1.50–2.34 | 16.3–29.3 | 0 | 0 | ||
| Fp | 1.78–2.71 | 3.92–16.1 a,d | 0 | 0 | 34–40 | |
| Fd | 2.65–3.63 | 0.016–0.126 | 0 | 0 | ||
| Km overgrown by Foma for 23 d (4) | Or | 2.70–3.34 | 0.015–0.032 | 0 | 0 | 88 |
| Fp | 2.64–2.69 | 0.015–0.019 e | 0 | 0 | ||
| Fd | 2.67–2.85 | 0.017–0.057 | 0 | 0 | 34 |
| Oxalate Amendment in mg g−1 | Number of Samples Colonized | Initial Substrate pH | pH of the Colonized Substrate |
|---|---|---|---|
| 0 | 4 of 4 | 5.08 ± 0.01 | 4.04 ± 0.03 |
| 5 | 4 of 4 | 3.10 ± 0.14 | 4.02 ± 0.02 |
| 10 | 1 of 4 | 2.61 ± 0.06 | 4.06 |
| 50 | 0 of 4 | 1.68 ± 0.10 | − |
| Rt, elution sequence | Isoprenoid | Formula | 6 g of Beech Dust | Km 10 | Foma 4 | Km 10/HCl Solution | Km/Foma, First Contact | Km/Foma, Barrier Line | Foma over-Grew Km |
|---|---|---|---|---|---|---|---|---|---|
| 10.094 | Alpha-pinene | C10H16 | 220,000 1 | ||||||
| 10.574 | Camphene | C10H16 | 40,000 1 | ||||||
| 12.608 | Delta-3-carene | C10H16 | 240,000 1 | ||||||
| 13.106 | Para-cymene | C10H16 | 30,000 | ||||||
| 13.221 | Limonene | C10H16 | 60,000 1 | ||||||
| 14.192 | Gamma-terpinene | C10H16 | 27,000 1 | ||||||
| 16.832 | Camphor | C10H16O | 47,000 1 | ||||||
| 17.385 | Pinocarvone | C10H14O | 57,000 | ||||||
| 17.846 | 4-Terpineol | C10H18O | 36,000 | ||||||
| 18.392 | Myrtenal | C10H14O | 110,000 | ||||||
| 18.778 | Berbenone (Verbenone) | C10H14O | 200,000 | ||||||
| 20.946 | (-)-Bornyl acetate | C12H20O2 | 70,000 1 | ||||||
| 21.66–21.86 | Alpha-gurjunene | C15H24 | 65,000 | 95,000 1 | 33,000 | ||||
| 22.71–22.83 | Alpha-cubebene | C15H24 | 40,000 1 | 310,000 1 | 680,000 1 | ||||
| 23.44 | Alpha-copaene | C15H24 | 170,000 1 | ||||||
| 23.85 | Alpha-cedrene | C15H24 | 470,000 1 | ||||||
| 23.80–24.00 | Beta-cubebene | C15H24 | 250,000 1 | 1,900,000 1 | 2,500,000 1 | ||||
| 24.00–24.20 | (+)-Sativene | C15H24 | 2,400,000 | 300,000 1 | 350,000 1 | ||||
| 24.25 | Iso-longifolene | C15H24 | 140,000 1 | ||||||
| 24.38–24.48 | Junipene | C15H24 | 8,000 | 60,000 1 | 70,000 1 | 40,000 1 | |||
| 24.61–24.89 | Gamma-muurolene | C15H24 | 10,100,000 1 | 1,100,000 1 | 120,000 | 60,000 | |||
| 24.88–25.05 | Germacrene B and D | C15H24 | 175,000 1 | 360,000 | 380,000 1 | 700,000 1 | |||
| 25.64–26.01 | Alpha-amorphene | C15H24 | 55,000 1 | ||||||
| 25.67–25.91 | Gamma-curcumene | C15H24 | 300,000 | 2,000,000 | |||||
| 25.91–25.96 | Alpha-muurolene | C15H24 | 90,000 1 | 20,000 | 235,000 1 | ||||
| 25.97 | Trans-alpha-bisabolene | C15H24 | 4,750,000 1 | 12,500,000 1 | 26,000,000 1 | ||||
| 26.06 | Beta-bisabolene | C15H24 | 35,000 | 4,900,000 1 | 16,000,000 1 | 30,000,000 1 | |||
| 26.15–26.20 | Delta-cadinene | C15H24 | 100,000 1 | 50,000 1 | 950,000 1 | 2,400,000 1 | 5,600,000 1 | ||
| 26.25 | Cis-alpha-bisabolene | C15H24 | 1,000,000 1 | ||||||
| 26.28 | Alpha-cadinene | C15H24 | 110,000 1 | ||||||
| Total abundance of terpenes | 1,562,000 | 28,000 | 730,000 | 24,345,000 | 35,230,000 | 69,153,000 | 60,000 | ||
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gramss, G. Aspects Determining the Dominance of Fomitopsis pinicola in the Colonization of Deadwood and the Role of the Pathogenicity Factor Oxalate. Forests 2020, 11, 290. https://doi.org/10.3390/f11030290
Gramss G. Aspects Determining the Dominance of Fomitopsis pinicola in the Colonization of Deadwood and the Role of the Pathogenicity Factor Oxalate. Forests. 2020; 11(3):290. https://doi.org/10.3390/f11030290
Chicago/Turabian StyleGramss, Gerhard. 2020. "Aspects Determining the Dominance of Fomitopsis pinicola in the Colonization of Deadwood and the Role of the Pathogenicity Factor Oxalate" Forests 11, no. 3: 290. https://doi.org/10.3390/f11030290
APA StyleGramss, G. (2020). Aspects Determining the Dominance of Fomitopsis pinicola in the Colonization of Deadwood and the Role of the Pathogenicity Factor Oxalate. Forests, 11(3), 290. https://doi.org/10.3390/f11030290




