Abstract
The development of plant and soil microbial communities is one of the basic preconditions for the restoration of functional ecosystems. However, nutrients are concurrently used by plants and microbes, and the dynamics of this interaction during ecosystem development have seldom been studied. The aim of our study, thus, was to describe the dynamics of nutrient availability in soil and, at the same time, the nutrient accumulation in plant and microbial biomass along an unassisted primary succession heading toward broadleaf forest. The growth of the understory plant Arrhenatherum elatius on soils originating from three (16, 22, and 45 years’ old) successional stages of a post-mining area and the development of the microbial community in the presence or absence of this plant were studied in a pot experiment. Both, the plant biomass and carbon (C) in microbial biomass in intermediate and late middle successional stages were higher than those in the early stage. In soil, extractable organic C, extractable organic nitrogen (N), and inorganic N increased with proceeding succession, but Olsen phosphorus (P) peaked in the intermediate successional stage. The amounts of N and P in plant and microbial biomass increased during succession. In the late middle successional stage, the amount of P in microbial biomass exceeded that of plant bound P approximately twice, and this increase was higher in pots with plants than without. The results imply that the competition between plants and microbes for available P may increase microbial P uptake and, thus, hinder plant growth in later successional stages.
1. Introduction
Afforestation is a common way of restoring post-mining land [1]. In some cases, primary succession is a sensible approach to restore a functional forest ecosystem [2,3], in which primary production is predominantly limited by nutrient availability [4]. Consequently, restoring nutrient dynamics and nutrient pools is an important step in the restoration of a functional forest ecosystem [5].
The most common limiting nutrients in terrestrial ecosystems are nitrogen (N) and phosphorus (P) [6]. Nitrogen supply is low especially in the early stages of primary succession because of its absence in the majority of primary substrates [7]. On the contrary, Olsen phosphorus (P) becomes more limited later as succession proceeds because of its gradual depletion from soil minerals. The availability of these nutrients in soil is largely driven by microorganisms, which take a key role in transforming nutrients into a form that is accessible to plants [8]. At the same time, however, microbes can compete with plants for the uptake of nutrients [9]. The impact of these somewhat counteracting effects of microorganisms on overall plant productivity is still not fully understood. Microbes, though, are suggested to regulate plant productivity, especially when the soil system is poor in nutrients [8].
For microorganisms, carbon (C) is recognized as the element most often limiting their growth [10,11,12], though nutrient (N and P) limitations are also known [13,14,15].
In our study, we explore the nutrient distribution between plants and microbes during primary succession towards broadleaf forest. We benefit from a well-established and intensively studied chronosequence of post-mining sites where it is possible to observe the development of broadleaf forest from the basic post-mining overburden in combination with the continual development of soil [2]. In our pot experiment study, we measured the amount of soil C available for microbes in the form of extractable organic carbon (EOC) that is considered as the most bioavailable fraction of soil organic C [16]. Nitrogen was determined in the form of extractable organic N (EON), which is considered to contain large amounts of N bioavailable to plants and microorganisms [9], and inorganic N in the form of ammonium (NH4+) and nitrate (NO3−), which are further important N sources for both plants and microorganisms [17]. Phosphorus was determined as Olsen P—the plant available fraction of soil P (i.e., orthophosphate ions; [18]). These parameters, together with the evaluation of nutrient (N and P) contents in plant and microbial biomass, provide new insights into the dynamics of nutrient uptake by plant and microbial biomass during proceeding primary succession. As a model plant, the common grass Arrhenetherium elatius (L.) P.Beauv. ex J.Presl & C.Presl, 1819, growing in the understory of the sites, was used.
We hypothesized that (1) plants and microbes will compete for nutrients (N and P), resulting in differences between plant and microbial biomass. This competition should be reflected in a possible decrease in nutrient availability. We further hypothesized that (2) this decrease will be observed mainly in P concentrations, as P very often represents a limiting nutrient.
2. Material and Methods
2.1. Soil Sampling and Experiment Setup
The soil used for the pot experiment was collected from a forest chronosequence in post-mining sites established by gradual heaping of overburden where spontaneous succession has taken place. The sites were located in the Sokolov mining district (50°14′04″ N, 12°41′04″ E, 450–520 m above sea level), the overburden was deposited mainly in the form of alkaline mudstones, which subsequently disintegrated in to smaller particles and amorphous clay. During soil formation, pH has gradually decreased, C and N have accumulated, and P has become more available [2]. The largest change in C and N accumulation happened between early and intermediate stages (Table 1). Early (16-years old), intermediate (22-years old), and late middle (45-years old) successional stages were used for soil sampling. Selected soil characteristics are presented in Table 1. The early successional stage was dominated by herbs and grasses (Tusilago farfara L. and Calamagrostis epigejos (L.) Roth) with scattered Salix caprea L. shrubs. The intermediate successional stage was overgrown by a dense cover of Salix caprea with occasional occurrence of Betula pendula Roth and Populus tremula L. and little or no understory. The late middle successional stage was covered by light broadleaf forest dominated by Betula pendula and Populus tremula. Dense grass and herb understory were observed at this stage. The grass A. elatius used for our experiment is a common plant in the described chronosequence and especially abundant in intermediate and late middle successional stages [2].

Table 1.
Selected parameters of soils in various successional stages used in the experiment and ergosterol content in pots with and without plant at the end of the experiment. Statistically homogeneous groups are marked by the same letter (one-way ANOVA, Tukey post-hoc test, p < 0.05). If no letter presented, no significant difference was found by ANOVA.
Soil was collected from 15 cm depth at each successional stage. The soil was sieved through a 5 mm mesh and put into plastic pots with a volume of 1317 cm3. In total, six treatments in six replications were established: three types of soil (from the early, intermediate, and late middle successional stages) in (a) pots with A. elatius and (b) pots without A. elatius. For (a) treatments, 20 seeds of A. elatius were seeded in every pot. The plant density was adjusted to nine individuals per pot after germination. For (b) treatments, the pots were kept without any seedling during the whole experiment. The pots were kept outside covered by foil and were either supplied with rainfall water or watered if rainfall did not occur during the previous week. The experiment was conducted from July 2007 to October 2007.
2.2. Soil and Plant Analyses
At the end of the experiment, plants, including roots, were harvested and weighted after drying at 60 °C for 48 h. The soil was stored in a refrigerator until processed. EOC and EON, Olsen P, and inorganic N content were measured in the soil samples. The following microbial parameters were determined: soil respiration, ergosterol content, and microbial biomass C, N, and P. The plant biomass was analyzed for N and P.
Soil and plant C and N contents were measured using a NC 2100 soil analyzer (Thermo-Quest Italia.). Samples for EOC and EON were extracted with 50 mL 0.5 M K2SO4 using a 1:50 ratio and shaken for 1 h. The extracts were passed through a 0.45 µm filter. The C and N contents in the filtrates were determined by high-temperature combustion using a TOC/TN analyzer (Formacs, Skalar). The same extracts were further used for the determination of NH4+ and NO3− -N contents; their sum was considered to represent the inorganic nitrogen content. Leachates were centrifuged, and the supernatants were passed through glass fiber filters. The concentrations of NH4+ and NO3- in the filtrates were determined spectrophotometrically (Genesys 6, Thermo Spectronic, USA) using a colorimetric method [22].
Soil respiration was measured at the end of the experiment using an IRGA, S151 CO2 Analyzer (Qubit Systems, Ontario, Canada) in an open flow system. Soil respiration was expressed as CO2 µL g−1 dry soil hour−1. Ergosterol was extracted and quantified as described previously [23] using a Waters Alliance HPLC system (Waters, USA) with methanol as the mobile phase and UV detection at 282 nm. Microbial biomass C and N were measured using the fumigation-extraction method [24,25]. Correction factors of 0.45 and 0.4 were used for microbial biomass C [24] and N [25], respectively. Microbial biomass P was measured using the technique developed by Brookes et al. [26]. A correction factor of 0.4 was used for microbial biomass P [26].
The content of P in plants was measured by treatment with 70% perchloric acid [27]. Orthophosphate ions were quantified by the ascorbic acid and ammonium molybdate method according to Murphy and Riley [28] modified by Watanabe and Olsen [29].
All results are expressed on an oven-dry (105 °C, 24 h) weight basis except for plant parameters that are expressed on an oven-dry basis at 60 °C.
2.3. Statistical Analyses
The data are presented as means ± standard deviations (SD). Differences between plant and successional stage treatments were analyzed using two-way ANOVA (Tukey post-hoc test). Differences between successional stage treatments, in plant biomass were analyzed using one-way ANOVA (Tukey post-hoc test) and also the parameters which show significant interaction between plant presence and stage by two-way ANOVA was in addition analyzed by one-way ANOVA. In cases in which the data were not normally distributed (this was the case for pH, soil density, EC aboveground biomass, and Olsen P), they were log (n + 1) transformed before ANOVA was applied. ANOVA was performed using Statistica 10.0. (StatSoft, Tulsa, OK, USA)
3. Results
The aboveground as well as belowground plant biomass in intermediate and late middle successional stages were significantly higher than in the early successional stage, but there was no significant difference between those two stages (Figure 1). The amount of above- vs. below-ground biomass did not significantly differ in any of the three successional stages, though in intermediate and late middle successional stages, the amount of belowground biomass tended to be higher than that of aboveground biomass (Figure 1).
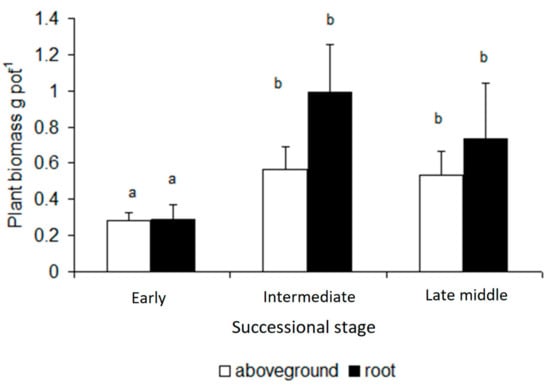
Figure 1.
Aboveground and root biomass of Arrhenatherum elatius grown at post-mining soils of different successional stages. Statistically homogeneous groups are marked by the same letter (one-way ANOVA, Tukey post-hoc test, p < 0.05). Bars represent standard deviations.
Soil respiration was affected by both plant presence and successional stage (two-way ANOVA; Figure 2a). Soil respiration increased with successional stage and was significantly higher in the plant treatment as compared to the no-plant treatment. Microbial biomass C, N, and P, and ergosterol were affected by soil successional stage only (Figure 2b–d; Table 1). While microbial biomass C increased already in the intermediate successional stage (Figure 2b), microbial biomass N did not increase until the late middle successional stage (Figure 2c). Microbial biomass P increased from early to late middle successional stages (Figure 2d).
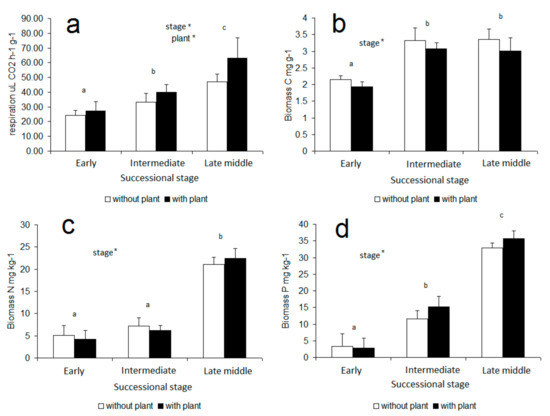
Figure 2.
Soil respiration (a), microbial biomass of carbon (C) (b), microbial biomass of nitrogen (N) (c), and microbial biomass of Olsen phosphorus (P) (d) in post-mining soils originated from different successional stages with and without growing plants (Arrhenatherum elatius). Statistically homogeneous groups are marked by the same letter (one-way ANOVA, Tukey post-hoc test, p < 0.05). Bars represent standard deviations.
Ergosterol contents, as indicator of fungal biomass [30], showed a similar course and significantly increased with successional stage (Table 1). The mass of N in microbial biomass increased from the intermediate to the late middle successional stage in both plant and no-plant treatments and did not differ between these treatments in any of the successional stages (Table 2). Contrary to that, the plants showed an increase in the N mass already from the early to the intermediate successional stage, and the plant N mass was higher than that of the microbial biomass for early and intermediate stages, but not for the late middle successional stage (Table 2).

Table 2.
Mass of N and P in mg pot−1 in plants and microbial biomass. Contents in microbial biomass were measured in pots with and without plants. Homogeneous groups are marked by the same letter (one-way ANOVA, Tukey post-hoc test, p < 0.05). Letters behind the numbers mark significant differences in the same treatment between successional stages, letter before numbers mark significant differences between different treatments (with plant without plant) within the same successional stage. If no letter is present, the values do not significantly differ.
Mass of the P content in microbial biomass increased from the early to late middle successional stage, while the P mass did not differ in the early successional stage between plant and no-plant treatments. However, in later successional stages it was significantly higher in the plant treatments. The P content in the plant itself also increased, but only from the early to the intermediate successional stage and while it was higher than that of the microbial biomass in the intermediate stage, it was lower than that of the microbial biomass in the late middle successional stage.
The contents of EOC, EON, Olsen P, and inorganic N in soil were affected by both plant presence and successional stage (two-way ANOVA; Figure 3). EOC, EON, and Olsen P were additionally affected by an interaction of plant presence/absence and successional stage (two-way ANOVA; Figure 3). The contents of EOC, EON, and inorganic N were highest in the late middle successional stage (Figure 3a,b,d). Only Olsen P peaked in the intermediate successional stage (Figure 3c). The results showed that the presence of A. elatius reduced the content of Olsen P in the intermediate and late middle successional stages and also tended to reduce the content of inorganic N, though this effect was not significant (Figure 3d).
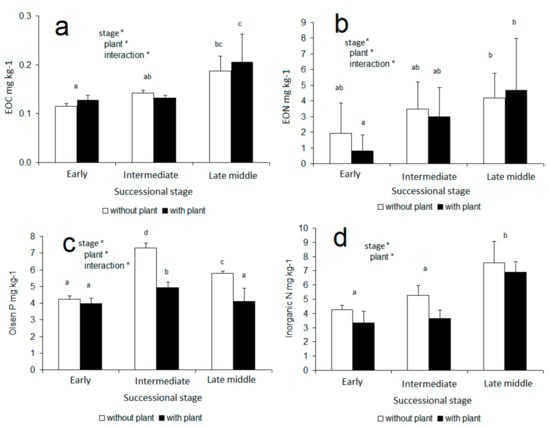
Figure 3.
Extractable organic C (a), extractable organic N (b), Olsen P (c), and total inorganic N (d) in post-mining soils of different successional stages with and without growing plants (Arrhenatherum elatius). Statistically homogeneous groups are marked by the same letter (one-way ANOVA, Tukey post-hoc test, p < 0.05). Bars represent standard deviations.
4. Discussion
Our results show that nutrient availability in soil and, at the same time, nutrient immobilization in plant and microbial biomass increased with proceeding primary succession (Figure 2c,d and Figure 3b,c, and Table 2). The observed increase in nutrient availability during primary succession has also been described by previous studies [4]. The increased availability of N likely corresponds with an accumulation of N in soil during succession due to N fixing bacteria and mycorrhizal fungi [8] and a gradual increase of N stocks in the ecosystem [31]. The major drivers of an increasing availability of P are the gradual weathering of minerals and the activity of plants, which can move P from deeper soil horizons to surface soil horizons [31]. A concurrent immobilization of nutrients in the steadily increasing plant and microbial biomass during succession, though, might lead to a competition between plants and microbes for nutrients. While we did not conduct direct measurements of the plant-microbe competition for nutrients, several indicators suggest that such a competition existed, particularly in the late middle successional stage. The decrease in available P in soil (Olsen P; Figure 3c) was associated with an increase in P (and N) in microbial biomass (Figure 2c,d). At the same time, the presence of plants reduced (significantly or marginally significantly) the amount of available P and total inorganic N in soil (Figure 3c,d). Likewise, in the late middle successional stage, the amount of P and N immobilized in microbial biomass was larger than that immobilized in plant biomass (Table 2), and was higher for microbial biomass in the presence as compared to the absence of plants. This, together with the fact that microorganisms often have a higher P uptake efficiency than plant roots and that some microbes can store large amount of P in polyphosphates [32], indicates that plants compete with microbes for nutrients, especially for P and increasingly so with advancing succession. This may be a specific situation related to primary succession, where the microbial community gradually develops [33], but may also be applicable to secondary successions, such as the succession on abandoned fields, where the microbial biomass is suppressed during agricultural use and re-develops during succession.
The overall increase of nutrients in the microbial biomass cannot only be explained by the plain increase in microbial biomass itself because the largest increase in the amount of microbially bound nutrients occurred between the intermediate and late middle successional stages, when microbial biomass C mostly stagnated (Figure 2b–d). This suggests that a change in microbial community composition may contribute to the observed nutrient increase in the microbial biomass. This is supported by the ergosterol contents (Table 1), which increased relatively more than microbial biomass C, suggesting a shift from a bacterial to a more fungal dominated microbial community in later successional stages. Soil fungi are known to accumulate P in the form of polyphosphate [34]. A relative increase of fungi may also be the reason for the tendency of microbial biomass N and P contents being higher in later successional stages in the presence of plants because plants are often associated with mycorrhizal fungi [35,36]. This can also explain the observed higher microbial respiration in the presence of plants (Figure 2a).
Eventually it should be stated that every mesocosm experiment, also the actual one, should be extrapolated to the field with caution. In particular, the described interactions may be more complicated in the field, e.g., by interactions with Ectomycorrhizal fungi that can bind more C provided by trees or by competition for nutrients and other resources with plant roots of other understory plants and associated arbuscular mycorrhiza fungi [37,38]. Earlier studies conducted on the same sites revealed that such interactions were strong already in mid successional stages but may decrease in late mid succession [39]. Yet, our results suggest that interactions with the non-mycorrhiza part of the microbial community are important. Due to the simplicity and artificial nature of our experiment, we clearly show that the increase of the soil microbial biomass during succession had two aspects: (1) microbial biomass can contribute to the mineralization of organic matter and nutrient release and (2) soil microbial biomass becomes a progressively stronger competitor for nutrients during primary succession.
5. Conclusions
Although overall nutrient availability increased with proceeding succession, we could observe a decrease in P availability in the late middle successional stage in the pots with plants. The microbial community immobilized double the amount of P in comparison to plants in this stage and microbial immobilization was significantly higher than that in the pots without plants. This indicates a possible competition for available P and its limitation for plants in the late middle successional stage. Thus, we postulate that the microbial community is more efficient in covering its P demand as compared to plants.
Author Contributions
Conceptualization, S.K. and J.F.; methodology, S.K. and J.F.; investigation, S.K.; writing—original draft preparation, Š.A, S.K.; writing—review and editing, Š.A.; funding acquisition, J.F. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by the Czech Science Foundation [18-24138S].
Acknowledgments
We would like to thank Jitka Hubačová and Jiří Kalčík for help with laboratory analyses and Gerrit Angst for reading and commenting on the manuscript.
Conflicts of Interest
The authors declare no conflict of interests.
References
- Macdonald, S.E.; Landhäusser, S.M.; Skousen, J.; Franklin, J.; Frouz, J.; Hall, S.; Jacobs, D.F.; Quideau, S. Forest restoration following surface mining disturbance: Challenges and solutions. New For. 2015, 46, 703–732. [Google Scholar] [CrossRef]
- Frouz, J.; Prach, K.; Pižl, V.; Háněl, L.; Starý, J.; Tajovský, K.; Materna, J.; Balík, V.; Kalčík, J.; Řehounková, K. Interactions between soil development, vegetation and soil fauna during spontaneous succession in post mining sites. Eur. J. Soil Biol. 2008, 44, 109–121. [Google Scholar] [CrossRef]
- Frouz, J.; Dvorščík, P.; Vávrová, A.; Doušová, O.; Kadochová, Š.; Matějíček, L. Development of canopy cover and woody vegetation biomass on reclaimed and unreclaimed post-mining sites. Ecol. Eng. 2015, 84, 233–239. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Matson, P.A.; Van Cleve, K. Nitrogen availability and nitrification during succession: Primary, secondary, and old-field seres. Plant Soil 1989, 115, 229–239. [Google Scholar] [CrossRef]
- Prescott, C.E.; Frouz, J.; Grayston, S.J.; Quideau, S.A.; Straker, J. Rehabilitating forest soils after disturbance. Dev. Soil Sci. 2019, 36, 309–343. [Google Scholar]
- Vitousek, P.M.; Porder, S.; Houlton, B.Z.; Oliver, A.; Vitousek, P.M.; Porder, S.; Houlton, B.Z.; Chadwick, O.A. Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen—Phosphorus interactions. Ecol. Appl. 2010, 20, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Vitousek, P.M.; Farrington, H. Nutrient limitation and soil development: Experimental test of a biogeochemical theory. Biogeochemistry 1997, 37, 63–75. [Google Scholar] [CrossRef]
- Van Der Heijden, M.G.A.; Bardgett, R.D.; Van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef]
- Schimel, J.P.; Bennett, J. Nitrogen mineralization: Challenges of a changing paradigm. Ecology 2004, 85, 591–602. [Google Scholar] [CrossRef]
- Demoling, F.; Figueroa, D.; Bååth, E. Comparison of factors limiting bacterial growth in different soils. Soil Biol. Biochem. 2007, 39, 2485–2495. [Google Scholar] [CrossRef]
- Ekblad, A.; Nordgren, A. Is growth of soil microorganisms in boreal forests limited by carbon or nitrogen availability? Plant Soil 2002, 242, 115–122. [Google Scholar] [CrossRef]
- Spohn, M.; Kuzyakov, Y. Phosphorus mineralization can be driven by microbial need for carbon. Soil Biol. Biochem. 2013, 61, 69–75. [Google Scholar] [CrossRef]
- Čapek, P.; Kotas, P.; Manzoni, S.; Šantrůčková, H. Drivers of phosphorus limitation across soil microbial communities. Funct. Ecol. 2016, 30, 1705–1713. [Google Scholar] [CrossRef]
- Darcy, J.L.; Schmidt, S.K.; Knelman, J.E.; Cleveland, C.C.; Castle, S.C.; Nemergut, D.R. Phosphorus, not nitrogen, limits plants and microbial primary producers following glacial retreat. Sci. Adv. 2018, 4, eaaq0942. [Google Scholar] [CrossRef] [PubMed]
- Hart, S.C.; Stark, J.M. Nitrogen limitation of the microbial biomass in an old-growth forest soil. Écoscience 1997, 4, 91–98. [Google Scholar] [CrossRef]
- Marschner, B.; Kalbitz, K. Controls of bioavailability and biodegradability of dissolved organic matter in soils. Geoderma 2003, 113, 211–235. [Google Scholar] [CrossRef]
- Burger, M.; Jackson, L.E. Microbial immobilization of ammonium and nitrate in relation to ammonification and nitrification rates in organic and conventional cropping systems. Soil Biol. Biochem. 2003, 35, 29–36. [Google Scholar] [CrossRef]
- Richardson, A.E.; Barea, J.M.; McNeill, A.M.; Prigent-Combaret, C. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 2009, 321, 305–339. [Google Scholar] [CrossRef]
- Bartuška, M.; Pawlett, M.; Frouz, J. Particulate organic carbon at reclaimed and unreclaimed post-mining soils and its microbial community composition. CATENA 2015, 131, 92–98. [Google Scholar] [CrossRef]
- Bartuška, M.; Frouz, J. Carbon accumulation and changes in soil chemistry in reclaimed open-cast coal mining heaps near Sokolov using repeated measurement of chronosequence sites. Eur. J. Soil Sci. 2015, 66, 104–111. [Google Scholar] [CrossRef]
- Harantová, L.; Mudrák, O.; Kohout, P.; Elhottová, D.; Frouz, J.; Baldrian, P. Development of microbial community during primary succession in areas degraded by mining activities. Land Degrad. Dev. 2017, 28, 2574–2584. [Google Scholar] [CrossRef]
- Zbíral, J.; Honsa, I.; Malý, S. Soil Analyses, Part III; Czech Central Institute for Supervising and Testing in Agriculture: Brno, Czech, 1997. [Google Scholar]
- Šnajdr, J.; Valášková, V.; Merhautová, V.; Cajthaml, T.; Baldrian, P. Activity and spatial distribution of lignocellulose-degrading enzymes during forest soil colonization by saprotrophic basidiomycetes. Enzyme Microb. Technol. 2008, 43, 186–192. [Google Scholar] [CrossRef]
- Joergensen, R.G. The fumigation-extraction method to estimate soil microbial biomass: Calibration of the kEC value. Soil Biol. Biochem. 1996, 28, 25–31. [Google Scholar] [CrossRef]
- Jonasson, S.; Michelsen, A.; Schmidt, I.K.; Nielsen, E.V.; Terry, V.; Michelsen, A.; Nielsen, E.V.; Callaghan, T.V. Microbial Biomass C, N and P in Two Arctic Soils and Responses to Addition of NPK Fertilizer and Sugar: Implications for Plant Nutrient Uptake. Oecologia 1996, 106, 507–515. [Google Scholar] [CrossRef]
- Brookes, P.C.; Powlson, D.S.; Jenkinson, D.S. Phosphorus in the soil microbial biomass. Soil Biol. Biochem. 1984, 16, 169–175. [Google Scholar] [CrossRef]
- Sommers, L.E.; Nelson, D.W. Determination of Total Phosphorus in Soils: A Rapid Perchloric Acid Digestion Procedure1. Soil Sci. Soc. Am. J. 1972, 36, 902–904. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Watanabe, F.S.; Olsen, S.R. Test of an Ascorbic Acid Method for Determining Phosphorus in Water and NaHCO3 Extracts from Soil1. Soil Sci. Soc. Am. J. 1965, 29, 677–678. [Google Scholar] [CrossRef]
- Gessner, M.O. Ergosterol as a measure of fungal biomass. In Methods to Study Litter Decomposition: A Practical Guide; Graça, M.A.S., Bärlocher, F., Eds.; Springer: Dordrech, The Netherlands, 2005; pp. 189–195. ISBN 9781402034664. [Google Scholar]
- Šourková, M.; Frouz, J.; Šantrůčková, H. Accumulation of carbon, nitrogen and phosphorus during soil formation on alder spoil heaps after brown-coal mining, near Sokolov (Czech Republic). Geoderma 2005, 124, 203–214. [Google Scholar] [CrossRef]
- Deubel, A.; Merbach, W. Influence of Microorganisms on Phosphorus Bioavailability in Soils. In Microorganisms in Soils: Roles in Genesis and Functions; Springer: Berlin/Heidelberg, Germany, 2005; pp. 177–191. [Google Scholar]
- Frouz, J.; Nováková, A. Development of soil microbial properties in topsoil layer during spontaneous succession in heaps after brown coal mining in relation to humus microstructure development. Geoderma 2005, 129, 54–64. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Smernik, R.J.; Doolette, A.L.; Marschner, P.; Stonor, R.; Wakelin, S.A.; McNeill, A.M. Forms of phosphorus in bacteria and fungi isolated from two Australian soils. Soil Biol. Biochem. 2008, 40, 1908–1915. [Google Scholar] [CrossRef]
- Ezawa, T.; Cavagnaro, T.R.; Smith, S.E.; Smith, F.A.; Ohtomo, R. Rapid accumulation of polyphosphate in extraradical hyphae of an arbuscular mycorrhizal fungus as revealed by histochemistry and a polyphosphate kinase/luciferase system. New Phytol. 2004, 161, 387–392. [Google Scholar] [CrossRef]
- Bolan, N.S. A critical review on the role of mycorrhizal fungi in the uptake of phosphorus by plants. Plant Soil 1991, 134, 189–207. [Google Scholar] [CrossRef]
- Mudrák, O.; Hermová, M.; Tesnerová, C.; Rydlová, J.; Frouz, J. Above-ground and below-ground competition between the willow Salix caprea and its understorey. J. Veg. Sci. 2016, 27, 156–164. [Google Scholar] [CrossRef]
- Knoblochová, T.; Kohout, P.; Püschel, D.; Doubková, P.; Frouz, J.; Cajthaml, T.; Kukla, J.; Vosátka, M.; Rydlová, J. Asymmetric response of root-associated fungal communities of an arbuscular mycorrhizal grass and an ectomycorrhizal tree to their coexistence in primary succession. Mycorrhiza 2017, 27, 775–789. [Google Scholar] [CrossRef] [PubMed]
- Frouz, J.; Moradi, J.; Püschel, D.; Rydlová, J. Earthworms affect growth and competition between ectomycorrhizal and arbuscular mycorrhizal plants. Ecosphere 2019, 10, e02736. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).