Changes of Tree Species Composition and Distribution Patterns in Mts. Jiri and Baegun, Republic of Korea over 15 Years
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Data Collection
2.2. Data Analysis
- Model 1: f(E(species diversity)) ~ (latitude) + s(longitude) + s(elevation) + s(slope) + s(TWI) + s(curvature) + factor(aspect) + factor(soil type);
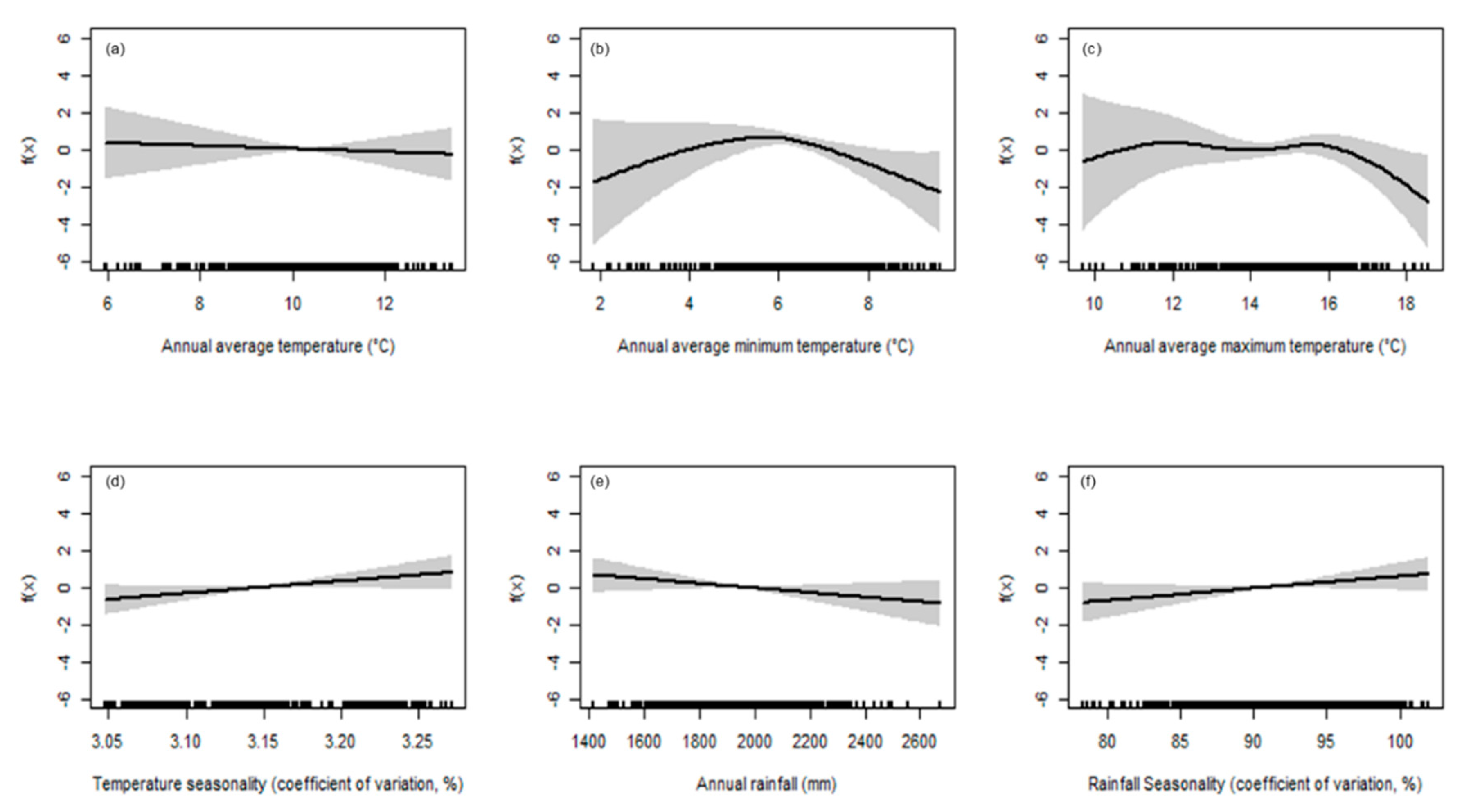
- Model 2: f(E(species diversity)) ~ s(monthly mean of daily minimum temperature) + s(monthly mean of daily maximum temperature) + s(monthly mean of daily average temperature) s(monthly mean of total precipitation) + s(monthly mean of temperature seasonality) + s(monthly mean of precipitation seasonality)
3. Results
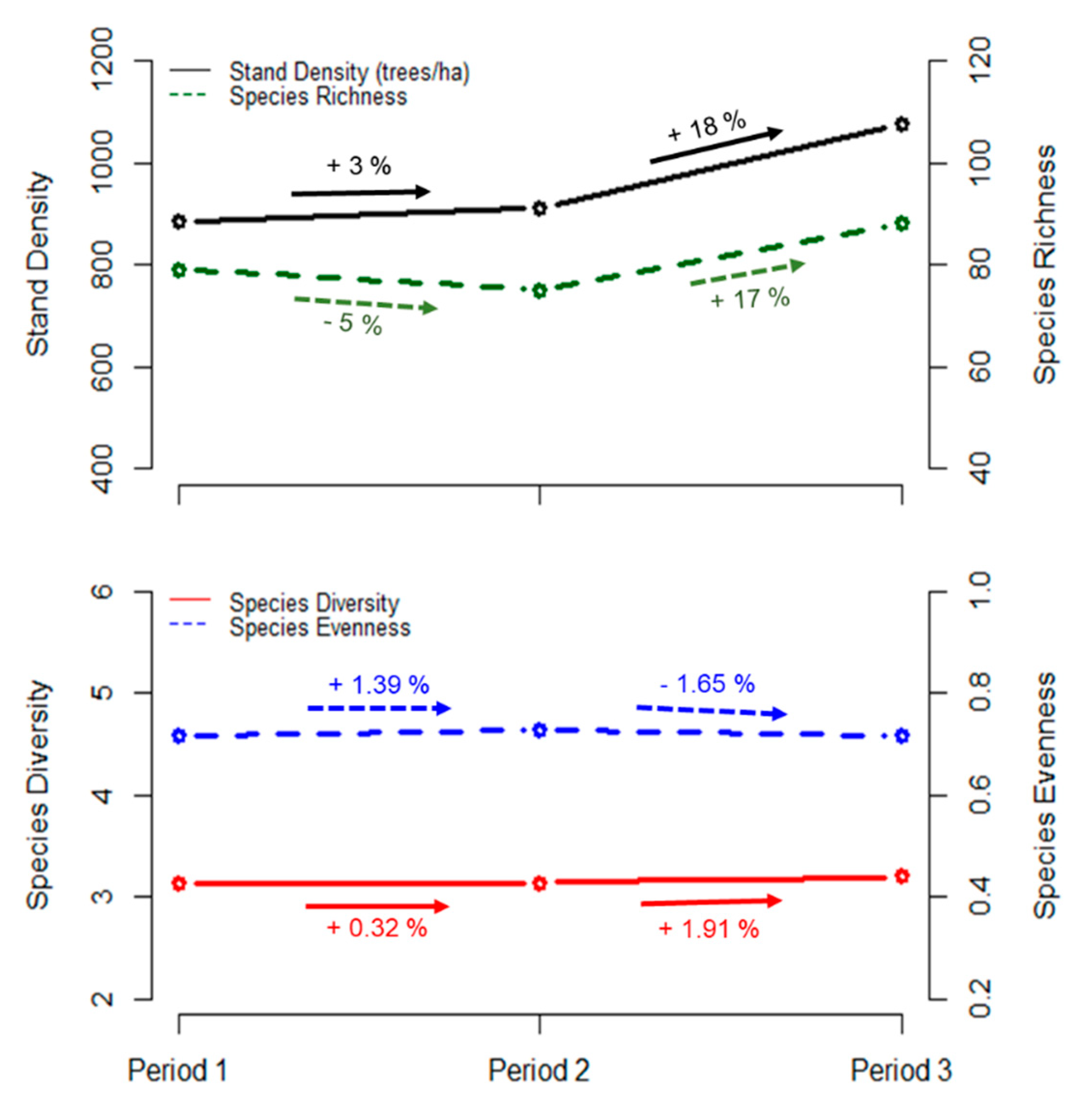
3.1. Overall Changes in Stand Density, Richness, Diversity, and Evenness
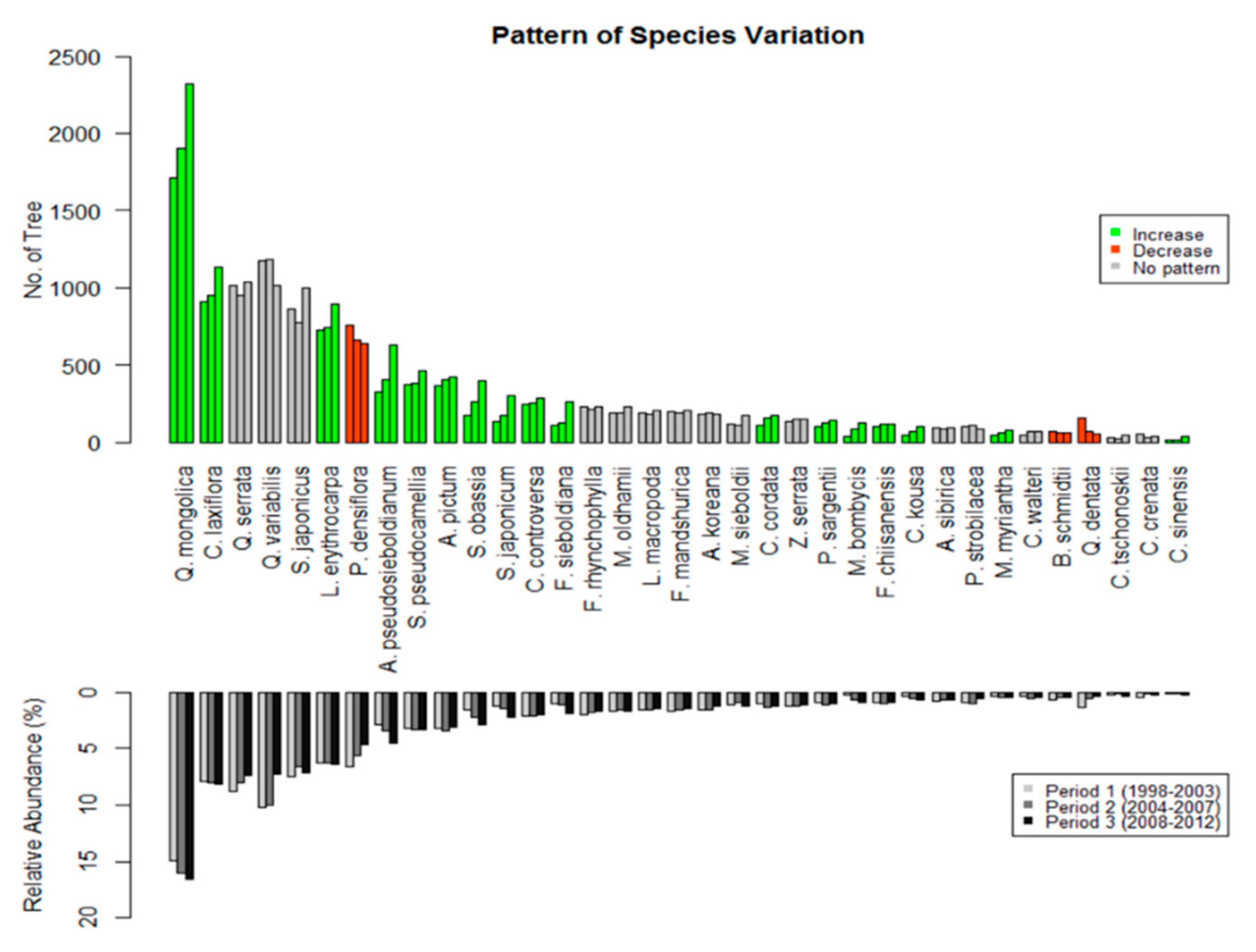
3.2. Shift in Species-Level Composition
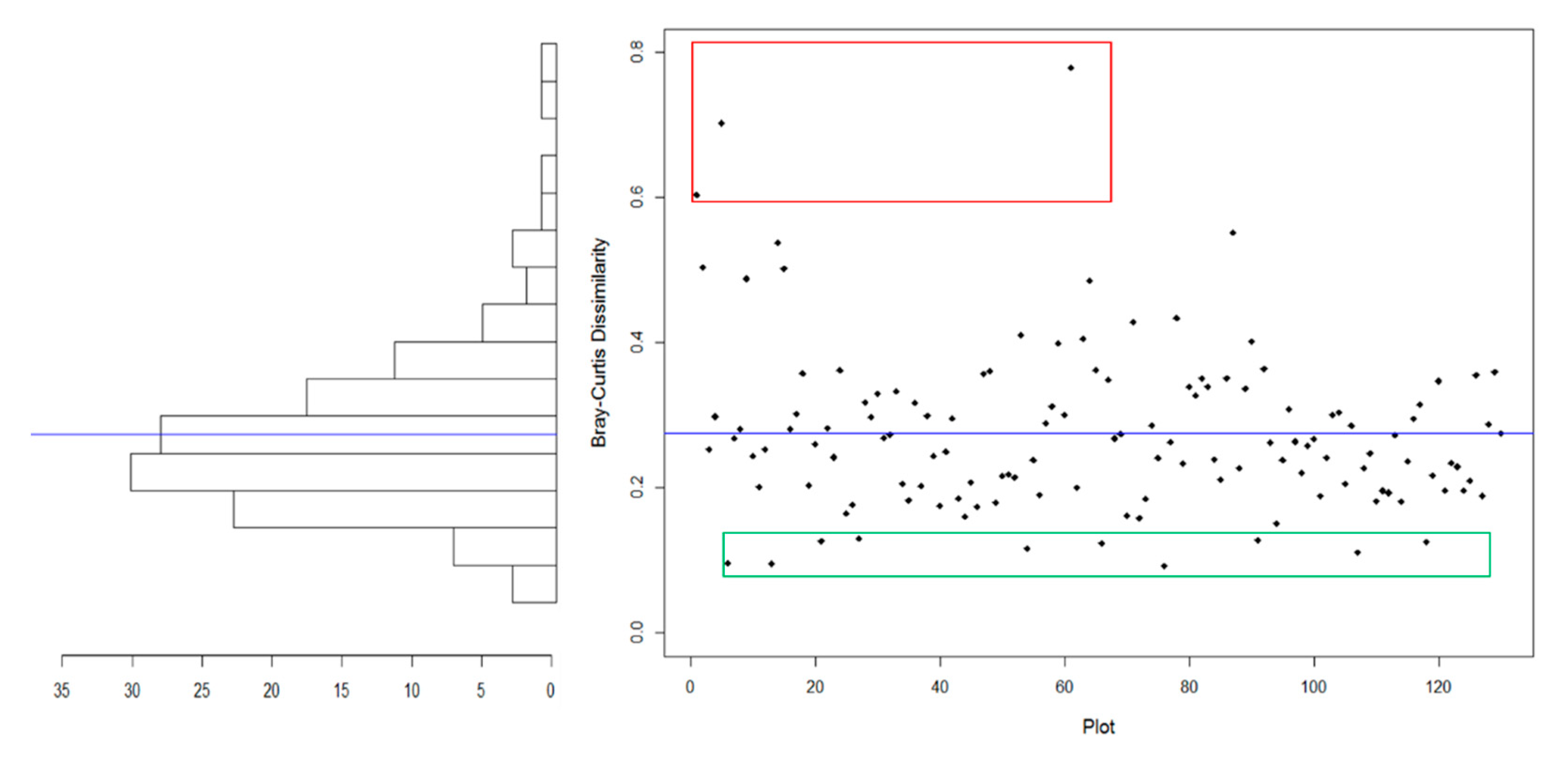
3.3. Shift in Community-Level Composition
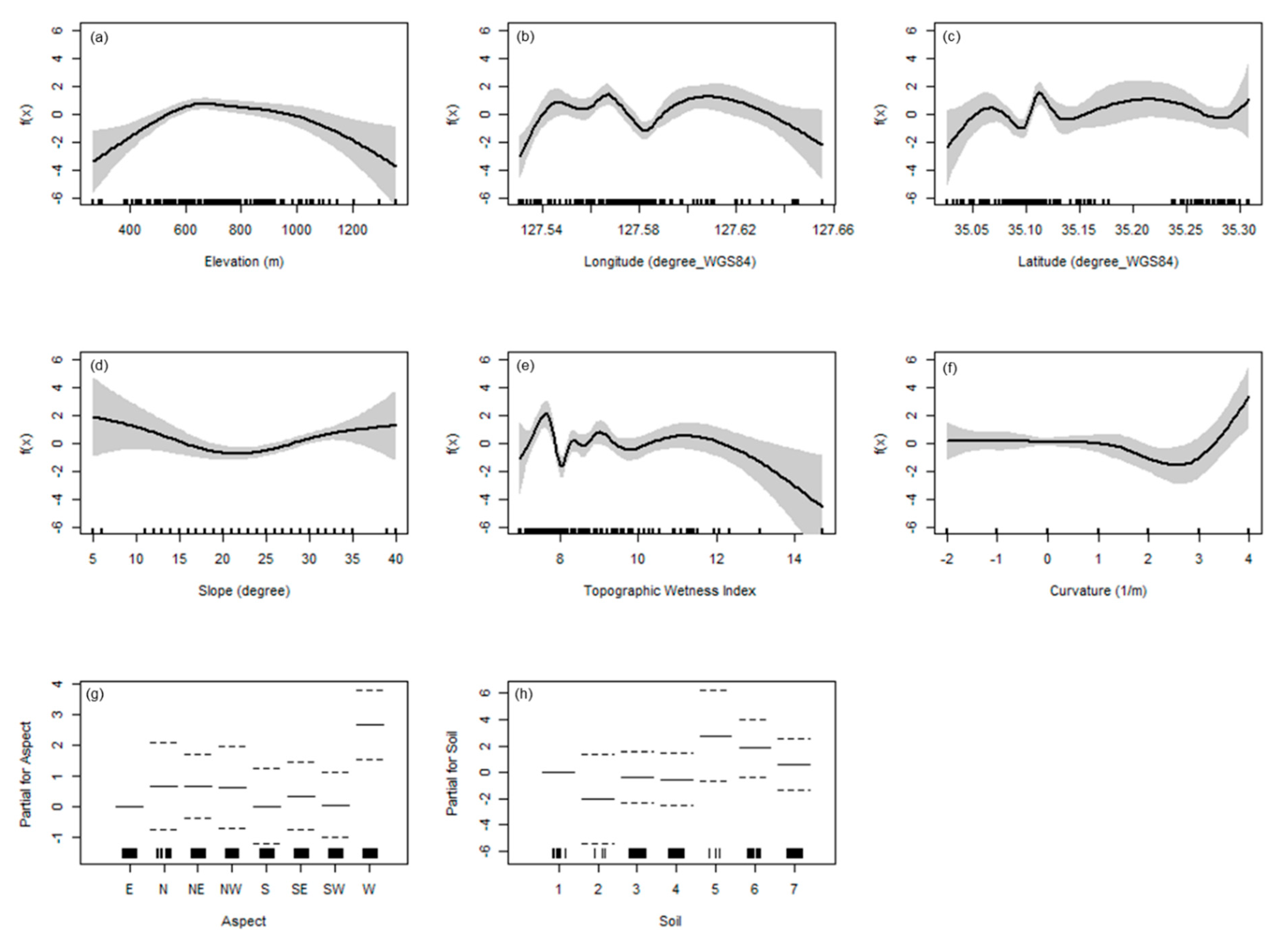
3.4. Distribution of Species Diversity Gradients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37. [Google Scholar] [CrossRef] [PubMed]
- Root, T.L.; Price, J.T.; Hall, K.R.; Schneider, S.H.; Rosenzweig, C.; Pounds, J.A. Fingerprints of global warming on wild animals and plants. Nature 2003, 421, 57. [Google Scholar] [CrossRef] [PubMed]
- Walther, G.-R. Community and ecosystem responses to recent climate change. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2019–2024. [Google Scholar] [CrossRef] [PubMed]
- Ahn, U.S.; Kim, D.S.; Yun, Y.S.; Ko, S.H.; Kim, K.S.; Cho, I.S. The inference about the cause of death of Korean Fir in Mt. Halla through the analysis of spatial dying pattern-Proposing the possibility of excess soil moisture by climate changes. Korean J. Agric. For. Meteorol. 2019, 21, 1–28. [Google Scholar]
- Hernández, L.; Cañellas, I.; Alberdi, I.; Torres, I.; Montes, F. Assessing changes in species distribution from sequential large-scale forest inventories. Ann. For. Sci. 2014, 71, 161–171. [Google Scholar] [CrossRef]
- Callahan, J.T. Long-term ecological research. BioScience 1984, 34, 363–367. [Google Scholar] [CrossRef]
- Likens, G.E. Long-Term Studies in Ecology; Springer: Berlin/Heidelberg, Germany, 1989; Volume 500. [Google Scholar]
- Magnuson, J.J. Long-term ecological research and the invisible present. BioScience 1990, 40, 495–501. [Google Scholar] [CrossRef]
- Condit, R. Research in large, long-term tropical forest plots. Trends Ecol. Evol. 1995, 10, 18–22. [Google Scholar] [CrossRef]
- Condit, R. Tropical Forest Census Plots: Methods and Results from Barro Colorado Island, Panama and a Comparison with Other Plots; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Hobbie, J.E.; Carpenter, S.R.; Grimm, N.B.; Gosz, J.R.; Seastedt, T.R. The US long term ecological research program. BioScience 2003, 53, 21–32. [Google Scholar] [CrossRef]
- Knapp, A.K.; Smith, M.D.; Hobbie, S.E.; Collins, S.L.; Fahey, T.J.; Hansen, G.J.; Landis, D.A.; La Pierre, K.J.; Melillo, J.M.; Seastedt, T.R. Past, present, and future roles of long-term experiments in the LTER network. BioScience 2012, 62, 377–389. [Google Scholar] [CrossRef]
- Laurance, W.F.; Oliveira, A.A.; Laurance, S.G.; Condit, R.; Nascimento, H.E.; Sanchez-Thorin, A.C.; Lovejoy, T.E.; Andrade, A.; D’Angelo, S.; Ribeiro, J.E. Pervasive alteration of tree communities in undisturbed Amazonian forests. Nature 2004, 428, 171. [Google Scholar] [CrossRef] [PubMed]
- Chave, J.; Condit, R.; Muller-Landau, H.C.; Thomas, S.C.; Ashton, P.S.; Bunyavejchewin, S.; Dattaraja, H.S.; Davies, S.J.; Esufali, S.; Ewango, C.E. Assessing evidence for a pervasive alteration in tropical tree communities. PLoS Biol. 2008, 6, e45. [Google Scholar] [CrossRef] [PubMed]
- Feeley, K.J.; Davies, S.J.; Perez, R.; Hubbell, S.P.; Foster, R.B. Directional changes in the species composition of a tropical forest. Ecology 2011, 92, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.M.T.; Staudhammer, C.L.; Brandeis, T.J.; Escobedo, F.J.; Zipperer, W. Temporal dynamics of a subtropical urban forest in San Juan, Puerto Rico, 2001–2010. Landsc. Urban Plan. 2013, 120, 96–106. [Google Scholar] [CrossRef]
- Shen, Z.; Fei, S.; Feng, J.; Liu, Y.; Liu, Z.; Tang, Z.; Wang, X.; Wu, X.; Zheng, C.; Zhu, B. Geographical patterns of community-based tree species richness in Chinese mountain forests: The effects of contemporary climate and regional history. Ecography 2012, 35, 1134–1146. [Google Scholar] [CrossRef]
- Sproull, G.J.; Quigley, M.F.; Sher, A.; González, E. Long-term changes in composition, diversity and distribution patterns in four herbaceous plant communities along an elevational gradient. J. Veg. Sci. 2015, 26, 552–563. [Google Scholar] [CrossRef]
- Umeki, K.; Kikuzawa, K. Long-term growth dynamics of natural forests in Hokkaido, northern Japan. J. Veg. Sci. 1999, 10, 815–824. [Google Scholar] [CrossRef]
- Yamada, T.; Aiba, S.-I.; Kubota, Y.; Okubo, K.; Miyata, I.; Suzuki, E.; Maenaka, H.; Nagano, M. Dynamics of species diversity in a Japanese warm-temperate secondary forest. Ecosphere 2011, 2, 1–19. [Google Scholar] [CrossRef]
- Dai, L.; Qi, L.; Wang, Q.; Su, D.; Yu, D.; Wang, Y.; Ye, Y.; Jiang, S.; Zhao, W. Changes in forest structure and composition on Changbai Mountain in Northeast China. Ann. For. Sci. 2011, 68, 889. [Google Scholar] [CrossRef]
- Suzuki, S.N.; Ishihara, M.I.; Hidaka, A. Regional-scale directional changes in abundance of tree species along a temperature gradient in Japan. Glob. Chang. Biol. 2015, 21, 3436–3444. [Google Scholar] [CrossRef]
- Chung, J.-M.; Hwang, S.-M.; Kim, Y.-M.; Shin, J.-K.; Kim, M.-S. Stand structure and dynamics in forests around Nari Basin of Ulleung Island, Korea. Korean J. Agric. For. Meteorol. 2010, 12, 23–35. [Google Scholar] [CrossRef]
- Jang, J.-H.; Han, B.-H.; Lee, K.-J.; Choi, J.-W.; Noh, T.-H. A study on the characteristics and changes of vegetation structure of the plant community in Mt. Kwanak. Korean J. Environ. Ecol. 2013, 27, 344–356. [Google Scholar]
- Pielou, E.C. Species-diversity and pattern-diversity in the study of ecological succession. J. Theor. Biol. 1966, 10, 370–383. [Google Scholar] [CrossRef]
- Magurran, A.E. Ecological Diversity and Its Measurement; Princeton University Press: Princeton, NJ, USA, 1988. [Google Scholar]
- Lee, C.-B.; Chun, J.-H. Relative importance of climatic and habitat factors on plant richness along elevation gradients on the Mt. Baekhwa, South Korea. Korean J. Agric. For. Meteorol. 2018, 20, 233–242. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R. Generalized Additive Models. In Chapman Hall & CRC. Monographs on Statistics & Applied Probability; Chapman and Hall/CRC: New York, FL, USA, 1990; Volume 1. [Google Scholar]
- Johnston, K.; Ver Hoef, J.M.; Krivoruchko, K.; Lucas, N. ArcGIS Geostatistical Analyst; ESRI (Environmental Systems Research Institute): Redlands, CA, USA, 2001. [Google Scholar]
- Soil Science Division Staff. Soil Survey Manual; USDA Handbook 18; Ditzler, C., Scheffe, K., Monger, H.C., Eds.; Government Printing Office: Washington, DC, USA, 2017.
- Package Statistical Functions (Stats) in R. Available online: https://stat.ethz.ch/R-manual/R-devel/library/stats/html/stats-package.html (accessed on 15 June 2016).
- Beals, E.W. Bray-Curtis ordination: An effective strategy for analysis of multivariate ecological data. In Advances in Ecological Research; Elsevier: Amsterdam, The Netherlands, 1984; Volume 14, pp. 1–55. [Google Scholar]
- Faith, D.P.; Minchin, P.R.; Belbin, L. Compositional dissimilarity as a robust measure of ecological distance. Vegetatio 1987, 69, 57–68. [Google Scholar] [CrossRef]
- Qian, S.S. Environmental and Ecological Statistics with R; Chapman and Hall/CRC: New York, FL, USA, 2016. [Google Scholar]
- Package Generalized Additive Models (Gam) in R. Available online: https://cran.r-project.org/web/packages/gam/gam.pdf (accessed on 27 June 2016).
- Crow, T.R. A rainforest chronicle: A 30-year record of change in structure and composition at El Verde, Puerto Rico. Biotropica 1980, 12, 42–55. [Google Scholar] [CrossRef]
- Schoonmaker, P.; McKee, A. Species composition and diversity during secondary succession of coniferous forests in the western Cascade Mountains of Oregon. For. Sci. 1988, 34, 960–979. [Google Scholar]
- Wittmann, F.; Junk, W.J. Sapling communities in Amazonian white-water forests. J. Biogeogr. 2003, 30, 1533–1544. [Google Scholar] [CrossRef]
- Lewis, S.L.; Malhi, Y.; Phillips, O.L. Fingerprinting the impacts of global change on tropical forests. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2004, 359, 437–462. [Google Scholar] [CrossRef]
- Lewis, S.L. Tropical forests and the changing earth system. Philos. Trans. R. Soc. B Biol. Sci. 2005, 361, 195–210. [Google Scholar] [CrossRef]
- Grime, J. Control of species density in herbaceous vegetation. J Environ. Manag. 1973, 1, 151–167. [Google Scholar]
- Bruun, H.H.; Moen, J.; Virtanen, R.; Grytnes, J.-A.; Oksanen, L.; Angerbjörn, A. Effects of altitude and topography on species richness of vascular plants, bryophytes and lichens in alpine communities. J. Veg. Sci. 2006, 17, 37–46. [Google Scholar] [CrossRef]
- Kessler, M. Patterns of diversity and range size of selected plant groups along an elevational transect in the Bolivian Andes. Biodivers. Conserv. 2001, 10, 1897–1921. [Google Scholar] [CrossRef]
- Austrheim, G. Plant diversity patterns in semi-natural grasslands along an elevational gradient in southern Norway. Plant Ecol. 2002, 161, 193–205. [Google Scholar] [CrossRef]
- Legendre, P.; Dale, M.R.; Fortin, M.-J.; Casgrain, P.; Gurevitch, J. Effects of spatial structures on the results of field experiments. Ecology 2004, 85, 3202–3214. [Google Scholar] [CrossRef]
- Comita, L.S.; Engelbrecht, B.M. Seasonal and spatial variation in water availability drive habitat associations in a tropical forest. Ecology 2009, 90, 2755–2765. [Google Scholar] [CrossRef]
- Balvanera, P.; Quijas, S.; Pérez-Jiménez, A. Distribution patterns of tropical dry forest trees along a mesoscale water availability gradient. Biotropica 2011, 43, 414–422. [Google Scholar] [CrossRef]
- Ter steege, H.; Pitman, N.C.; Phillips, O.L.; Chave, J.; Sabatier, D.; Duque, A.; Molino, J.-F.; Pr´evost, M.-F.; Spichiger, R.; Castellanos, H. Continental-scale patterns of canopy tree composition and function across Amazonia. Nature 2006, 443, 444–447. [Google Scholar] [CrossRef]
- Engelbrecht, B.M.; Comita, L.S.; Condit, R.; Kursar, T.A.; Tyree, M.T.; Turner, B.L.; Hubbell, S.P. Drought sensitivity shapes species distribution patterns in tropical forests. Nature 2007, 447, 80–82. [Google Scholar] [CrossRef]
- John, R.; Dalling, J.W.; Harms, K.E.; Yavitt, J.B.; Stallard, R.F.; Mirabello, M.; Hubbell, S.P.; Valencia, R.; Navarrete, H.; Vallejo, M. Soil nutrients influence spatial distributions of tropical tree species. Proc. Natl. Acad. Sci. USA 2007, 104, 864–869. [Google Scholar] [CrossRef]
- Gentili, R.; Armiraglio, S.; Sgorbati, S.; Baroni, C. Geomorphological disturbance affects ecological driving forces and plant turnover along an altitudinal stress gradient on alpine slopes. Plant Ecol. 2013, 214, 571–586. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, B.; Park, J.; Lee, H.; Kim, T.K.; Cho, S.; Yoon, J.; Kim, H.S. Changes of Tree Species Composition and Distribution Patterns in Mts. Jiri and Baegun, Republic of Korea over 15 Years. Forests 2020, 11, 186. https://doi.org/10.3390/f11020186
Lee B, Park J, Lee H, Kim TK, Cho S, Yoon J, Kim HS. Changes of Tree Species Composition and Distribution Patterns in Mts. Jiri and Baegun, Republic of Korea over 15 Years. Forests. 2020; 11(2):186. https://doi.org/10.3390/f11020186
Chicago/Turabian StyleLee, Boknam, Juhan Park, Hoontaek Lee, Tae Kyung Kim, Sunhee Cho, Jongguk Yoon, and Hyun Seok Kim. 2020. "Changes of Tree Species Composition and Distribution Patterns in Mts. Jiri and Baegun, Republic of Korea over 15 Years" Forests 11, no. 2: 186. https://doi.org/10.3390/f11020186
APA StyleLee, B., Park, J., Lee, H., Kim, T. K., Cho, S., Yoon, J., & Kim, H. S. (2020). Changes of Tree Species Composition and Distribution Patterns in Mts. Jiri and Baegun, Republic of Korea over 15 Years. Forests, 11(2), 186. https://doi.org/10.3390/f11020186




