Abstract
Pinus koraiensis (Sieb.et Zucc) is an economically and ecologically important tree species, naturally distributed in northeastern China. Conservation efforts and genetic improvement for this species began in the 1960s and 1980s, with the establishment of several primary seed orchards based on range-wide provenance evaluations. The original breeding objective was to improve growth and wood yield, but during the recent decade, it was redefined to include other traits, such as an enhancement of wood properties, seed oil content, cone yield, and the development of elite provenance with families, clones, and varieties with good tolerance to biotic and abiotic stresses. However, improvement processes are slow due to a long breeding cycle, and the number of improved varieties is still low. In this review, we summarize the recent progress in the selective improvement of P. koraiensis varieties, such as elite provenance, family, and clones, using various breeding procedures. We collate information on advances in the improvement of P. koraiensis, based on conventional breeding and molecular marker-assisted breeding methods; identify gaps in our understanding of the tree improvement processes; and propose future research directions, which will provide new insight for subsequent genetic breeding research on P. koraiensis.
1. Introduction
Pinus koraiensis (Sieb.et Zucc), an evergreen coniferous tree species belonging to the Pinaceae family, is an important afforestation tree species with high value for timber production in northeastern China [1,2]. Under natural conditions, it has a life span of over 1000 years and can attain a height of more than 40 m. Large-diameter timber of P. koraiensis is often processed for the manufacture of furniture, ships, and other materials. Its wood and seeds can also be used as industrial raw materials in food, cosmetics, medicine, and polymer material production, owing to its abundant polyphenols and resins [3,4]. Pine polyphenols also have various biomedical applications, including free radical scavenging, inhibition of lipid peroxidation, anti-inflammatory and analgesic properties, and antibacterial, antiviral, anti-cancer, anti-tumor, and anti-radiation actions. They can also play a role in lowering blood sugar, blood pressure, and blood lipids [5,6]. The nuts of P. koraiensis are edible and rich in fatty acids, proteins, carbohydrates, and other nutrients [7]. In addition, P. koraiensis is a strong pioneer species, which establishes successfully through natural succession, with significant ecological bearing in northeast China owing to its stable population structure and strong environmental adaptability.
The natural distribution range of P. koraiensis is mainly in northeast China, but there are few natural populations of P. koraiensis sporadically distributed in the Far East region of Russia, the Korean Peninsula, and Japan [8,9]. Its distribution from the Xiaoxinganling Mountains to the Changbai Mountains in northeast China includes more than half of P. koraiensis forests in the world (Figure 1). However, natural populations of P. koraiensis have declined sharply due to an increasing demand for timber. As a result, P. koraiensis was listed as a second-class nationally protected plant in the Chinese Red Data Book in 1999 [10] and a low-risk species in the 2013 International Union for Conservation of Nature (IUCN) red list [11]. Strategies for protection and utilization of its populations in China, genetic evaluation of natural populations, and establishment of a state-level improved genotype base were limited until the 1960s. Thereafter, major conservation efforts were implemented, followed by efforts at genetic improvement in the 1980s, with the establishment of several primary seed orchards based on range-wide provenance evaluations.
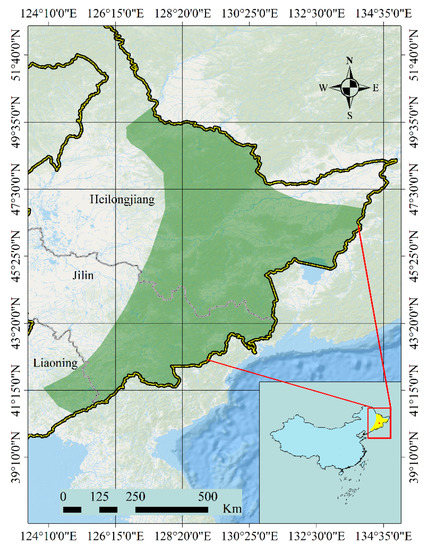
Figure 1.
Distribution of Pinus koraiensis in China.
This review collates information on advances in the improvement of P. koraiensis, based on conventional breeding and molecular marker-assisted breeding methods, and to identify gaps in our understanding of tree improvement processes and propose future research directions. We also considered other related research works for improving the growth and regeneration of P. koraiensis, and highlight the achievements, challenges, and future directions in its breeding, with the aim to expedite the breeding cycle and achieve better genetic gain in targeted traits.
2. Research Progress on Conventional Breeding of Pinus koraiensis
Conventional plant breeding has been carried out for centuries, and remains in common use. Plant breeders select superior plants and cross them to create new and improved varieties, which increases their productivity and quality. The study of genetic and breeding improvement for forest trees had commenced later than for crops, and remains in the traditional breeding stage. Genetic improvement of P. koraiensis began in the 1980s, with the aim of improving tree yield and quality in order to increase economic and social benefits. Primary breeding objectives included the selection of genotypes with good timber quality, including fiber length, fiber width, lignin content, wood density, and stem straightness. With increasing awareness of P. koraiensis’ value aside from timber production, breeding objectives were broadened to include improvements in cone/seed yield, carbon sequestration potential, and resistance to biotic stressors, such as disease. Consequently, several studies have been conducted to quantify variations in those traits among provenances and the selection of families and clones (Table 1). Provenance selection is an important breeding measure for forest improvement, and can provide rich breeding materials for forest genetic improvement research [12]. Because of the differences in geographical and climatic conditions, different provenances will yield natural variation in morphology, physiology, structure, and gene expression in long-term phylogenetic processes and natural selection in plants [13,14].

Table 1.
Studies made on genetic improvement of Pinus koraiensis.
The natural variation of different geographical provenances is the result of interactions between plant heredity and the environment, which is of great significance to cross breeding and genetic evolution. Characterization of the natural variation in P. koraiensis has attracted extensive attention and a number of conventional breeding studies have been carried out on seed orchard establishment, provenance regionalization, geographical provenance testing, and selection of excellent provenances [24,25,26]. In a previous study, the natural distribution range of P. koraiensis was divided into three sub-regions based on the mountain region and latitude, including the northern sub-region (Xiaoxing’anling forest region), middle sub-region (Wandashan, Zhangguangcailing forest region), and southern sub-region (Changbai Mountain forest region), with the provenance in the Changbai Mountains found to have the largest variation and an excellent genetic resource base [27]. On the basis of this division, a large number of provenance experiments have been carried out since the 1980s for the entire distribution of P. koraiensis, including Heilongjiang, Jilin, and Liaoning provinces, in China. Evaluation of the selected provenances was based on multiple breeding traits, with comprehensive selection in the same environment. For example, in the study of 24 P. koraiensis provenances distributed in the north-east Lushuihe Forestry Bureau area of Jilin Province, significant differences in the growth among provenances were observed, and the Lushuihe and Dahailin provenances were found to be the best [28]. For wood properties, certain radial variation patterns in wood parameters were observed among 26 provenances of 17-year-old tree individuals from the Maoershan Mountain, and the Caohekou provenance was judged to be optimal, followed by the Wangqing provenance [16,29]. This provided a theoretical basis for provenance selection of P. koraiensis. For carbon sequestration, Lushuihe, Linjiang, and Dahailin provenances were found to be the best of 18 in a 27-year-old Lushuihe forestry bureau area in Jilin province [15]. Overall, these provenance experiments have led to the section of many excellent provenances plus helping to improve stand productivity and provide original materials for further selective breeding, cross breeding, ploidy breeding, and targeted improvement of genetic engineering. On this basis, a large number of primary seed orchards and 13 national-level key improved genotype bases for P. koraiensis were established to produce seeds with good genetic quality, which greatly increased the genetic gain in growth and provided improved genotypes (Table 2).

Table 2.
Distribution of national-level Pinus koraiensis improved variety bases in China.
Selective breeding, a conventional breeding method, has the function of controlling the direction of variation, promoting the accumulation of variation, and increasing and creating important economic traits, which can eventually improve important information for tree genetic improvement [30]. Family and clonal selection is an effective means of tree genetic improvement, which is crucial for improving the yield, wood characteristics, and stress resistance of forest trees. Family selection is based on the phenotypic value of a family while clonal selection is a genetic improvement method based on asexual reproduction techniques, which can yield greater genetic gain. There are abundant variation patterns among families, clones, and individuals, and within individuals in P. koraiensis natural forests [11,31]. Recently, the use of clones for directed cultivation of plantations has yielded successful outcomes for species, such as Pinus radiata [32], Dalbergia sissoo [33], Juglans regia [34], and Populus deltoides [35]. Since the 1980s, more than 20 P. koraiensis clonal seed orchards have been established based on provenance and tree selection, and the first clonal seed orchard was flowered and fruited. Many excellent families and clones of P. koraiensis have been screened for various traits, including growth, wood properties, seed yield, and others [36]. Regarding growth and fructification properties, Wang et al. [20] investigated tree height, diameter at breast height (DBH), and seed yield in 68 half-sib families in the P. koraiensis seed orchard in Wangqing, and selected four families with optimized traits. Jiang et al. [22] measured the height, DBH, and seed traits of 170 Korean 38-year-old pine clones in Linjiang Forest Farm in the Linjiang Forestry Bureau area of Jilin Province, and selected 17 excellent 38-year-old clones. Liang et al. [21] measured and analyzed the growth and wood traits of 50 P. koraiensis clones in the Kaishanyu Forest Farm of Longjing City, Jilin Province, and selected five excellent clones.
In recent years, multi-character selection and directional selection have become significant strategies for the selection and breeding of superior tree species. The selection and breeding of new varieties with fast growth, high yield, high timber quality, and strong resistance is an effective means of improving productivity and expanding the forest resources of P. koraiensis. However, progresses in the selection and breeding of improved varieties were slow due to limited genomic information and a longer breeding cycle. A few recent studies have been conducted to carry out cross breeding of P. koraiensis [37,38], but they have not reported on new excellent hybrids cultivated by cross-breeding technology. Owing to the difficulty in asexual propagation of P. koraiensis, such as cuttings, tissue culture, and somatic embryogenesis, the establishment of seed orchards is considered the main approach for genetic improvement and high genetic quality seed production of P. koraiensis. Many conventional breeding processes for P. koraiensis, including superior provenance and family and clonal selection, are based on seed orchards. As the main means of forest tree seed production, seed orchards can improve the genetic gain of forest trees’ growth and improve the genetic quality of seeds, which is of great significance to forest tree germplasm innovation [39,40].
3. Research Progress on Molecular Breeding of Pinus koraiensis
Advanced molecular and genomic tools, such as molecular markers, expressed sequence tags (ESTs), microarrays, and genetic transformations, enable exploration of the genetic basis of biotic and abiotic stress tolerance, leading to the development of improved cultivars. Recent developments in DNA marker technology have helped to develop the concepts of quantitative trait loci (QTL) mapping, marker-aided selection (MAS), and genetic transformation to produce plants of superior quality. In addition, molecular markers can be used to assess genetic diversity, fingerprinting genotypes, separating hybrids from self-crossed progeny, and other uses. Thus, genetic information is important for studying the evolutionary history and genetic improvement of forest trees. However, due to the lack of whole-genome information in P. korainesis, there have been few studies on its molecular genetics.
3.1. Genetic Structure and Genetic Diversity
Pinus. koraiensis is an allogamous hermaphroditice tree species with high heterozygosity and a complex genetic background. In the absence of genomic information, analysis of its natural population structure and genetic diversity is required to rationally formulate a genetic conservation strategy. DNA molecular markers are now widely used in many aspects of research, including population structure, genetic diversity, and genetic relationship and fingerprint mapping. Molecular markers have been used to explore the population structure and genetic diversity of natural populations and clonal seed orchards of P. koraiensis, including single primer amplification reaction (SPAR) [41,42], random amplified polymorphic DNA (RAPD) [43,44], inter-simple sequence repeat (ISSR) [45,46,47], chloroplast microsatellites (cpSSRs) [48], and expressed sequence tag-simple sequence repeat (EST-SSR) [49,50]. Compared with other molecular markers, simple sequence repeat (SSR) markers are characterized by simple operation, good reproducibility, rich polymorphism and co-dominance, and are effective for genetic diversity analysis. Using nine polymorphic nuclear SSRs, Tong et al. [51] evaluated the genetic diversity and population structure of seven populations of P. koraiensis located throughout its native distribution and found high genetic diversity in all populations, with an average expected heterozygosity of 0.610. The northern-most populations (Dailin and Fenglin) showed slightly higher diversity than the other five populations. Yu et al. [52] researched the genetic diversity of P. koraiensis in northeast China using SSR molecular markers developed by magnetic bead enrichment, and found that its genetic variation mainly existed within the population. Researchers also successfully developed 20 microsatellite markers for P. koraiensis using AFLP of sequences containing repeats [53], and used these to study the genetic diversity and population structure throughout the natural distribution area of China. Studies have shown that the genetic diversity of natural P. koraiensis populations in northeast China is higher, but gene flow among the populations is low, and there is a degree of genetic differentiation within populations consistent with the weak differences observed among populations of other wind-pollinated gymnosperms [54]. In addition, some studies have found that due to geographic restrictions and gene flow during the Penultimate Glacial Period, the genetic diversity of P. koraiensis in Zhangguangcailing Mountains and Lesser Khingan Mountains is relatively high, forming a shelter for natural P. koraiensis populations [46]. However, using maternally inherited mtDNA, Aizawa et al. [55] demonstrated that the continental populations exhibited no diversity in mtDNA despite the species’ current extensive range and large populations.
3.2. Transcriptomics
Transcriptome sequencing is an important method of analyzing genes related to important phenotypic traits, such as seed germination, flowering and seed setting, and stress resistance, and provides significant references for research on plant functional genes, molecular marker development, and genetic improvement of germplasm resources. Due to its advantages of high throughput, high sensitivity, and low cost, transcriptome sequencing technology has been applied to many plants, including Oryza sativa [56], Camellia sinensis [57], Pinus halepensis [58], and Acer miaotaiense [59]. However, there have only been a few transcriptomic studies on the genetic improvement of P. koraiensis. Using transcriptome sequencing of various tissues, Zhang et al. [60] analyzed the expression of genes related to secondary metabolites in the growth and development of P. koraiensis, and developed a large number of EST-SSR markers. Liu et al. [61] explored the related genes of polyphenol synthesis by transcriptome sequencing technology during the development of adventitious buds, which provided a theoretical basis for further development and utilization of polyphenols.
3.3. Construction of Genetic Map
A genetic linkage map is constructed using genetic markers of polymorphisms and the exchange rate of two loci. It can be used in genetic analysis and gene mapping on many important traits and is a powerful tool for plant breeding and gene transduction. With the continuous development of sequencing technology, the construction of genetic linkage maps has been widely used in model plants. However, few of these have been developed for many non-model organisms, especially in the genome research of the Pinaceae. Chen et al. [62] constructed the first genetic linkage map of P. koraiensis using molecular markers, such as SRAP, SSR, ISSR, and AFLP, and conducted dynamic QTL mapping for the basal diameter and tree height using a large number of loci.
4. Other Related Research Works on Pinus koraiensis
Several physiological studies focusing particularly on photosynthesis have been carried out on P. koraiensis. Photosynthesis is an important plant physiological trait, and largely determines growth and development processes and environmental adaptability [63,64]. Key photosynthesis characteristics include the stomatal conductance, net photosynthetic rate, transpiration rate, and intercellular CO2 concentration [65,66]. The strength of photosynthesis is closely related to genetic characteristics, and is also affected by external environmental factors, such as temperature, light, and humidity [67,68,69,70]. In P. koraiensis, photosynthetic characteristics were investigated in relation to nitrogen utilization [71], different light conditions [72], various stress treatments [73], CO2 concentration changes [74], and needle age [71]. These studies provide theoretical basis for comprehensive evaluation of P. koraiensis clones and the selection of genotypes and economic traits. It should be noted that photosynthesis is closely related to growth and development, which in turn indirectly affects the survival rate of seedlings and fruiting of large trees [75]. Interestingly, P. koraiensis has a variable demand for light, wherein its light requirements increase with aging [76,77]. Sun et al [78] reported that P. koraiensis seedlings acclimate to forests with relatively weak light intensity by adjusting the morphological characteristics of their needles. Changes in leaf morphology lead directly to corresponding changes in leaf area, which in turn affects the photosynthesis rate of leaves. Zhu et al. [79] found that photosynthesis in P. koraiensis needles of different ages plays a cumulative role during the growing season in such a way that annual needles most strongly affected photosynthesis in the early stages of growth while current year needles were the greater determinant in later growth stages. Furthermore, there is intra- and interspecific variability in photosynthesis parameters as well as among different clones of P. koraiensis [80,81].
5. Research Gaps and Future Research Directions
Considerable conventional breeding work has been carried out over the last three decades, including evaluation of provenance and genetic variations, selection of elite families and clones, establishment of seed orchards, and improvement of propagation techniques. However, the pace of developing improved varieties is still slow. To accelerate the genetic improvement process of P. koraiensis in China, future breeding programs should aim to shorten the breeding cycle, collect and evaluate germplasm resources, carry out comparative experiments, improve high reproductive efficiency, and combine molecular marker-assisted selection and multi-trait improvement.
Germplasm resources are important materials for forest breeding, particularly for studying the origin and evolution of plants and cultivating new varieties of plants. Because P. koraiensis has historically been mainly used for timber production in northeastern China, the main breeding objective was the genetic improvement of wood properties, including lignin content, cellulose content, and cellulose length. Large-scale exploitative harvesting of natural forests has resulted in a significant loss of resources and a lack of natural germplasm resources of P. koraiensis. Collection and evaluation of natural germplasm resources, along with analysis of the genetic structure and genetic diversity of natural populations among geographic distributions, are urgently needed. In addition, a large-scale comparative experiment of different provenances, families, and clones of P. koraiensis should be conducted to select and cultivate a number of improved varieties to improve the actual genetic gain in important economic traits. Given global climate change predictions and associated risks from pests and diseases, it is of paramount importance to evaluate new germplasm collections for potential resistance to pest and disease outbreaks.
Seed orchards are important for improving the selection of varieties as a fundamental material and also for their momentousness value in the genetic improvement of forest trees. At present, research on P. koraiensis seed orchards mainly focuses on growth, wood quality, and cone/seed traits as well as seed orchards’ upgrading, which lays the foundation for genetic and further orchard development. However, there are still some problems in the genetic improvement of seed orchards of P. koraiensi. Increased collection and evaluation of additional germplasm resources from natural P. koraiensis populations is still required. Additionally, the seed yield in the P. koraiensis seed orchards is low due to the lack of superior provenance and a long growth cycle, and since plantation quality after afforestation is poor, it is unable to meet the growing demand for seed production. Provenance experiments have not been completed, and most P. koraiensis seed orchards are still at the primary level. Advanced generation seed orchards have not been established for scientific development of long-term breeding strategies. Unfortunately, the work of collecting cones is dangerous and expensive in the annual production process due to the height of P. koraiensis; Finally, there is a lack of national-level improved varieties and low use ratio for existing improved varieties. Therefore, it is necessary to cultivate improved varieties and further upgrade the current seed orchards. In the future, the main breeding work for genetic improvement of seed orchards should include the establishment of germplasm resource banks and evaluation of germplasm resources. Studies on height growth control, including the selection of natural dwarf individuals, could overcome the difficulty in collecting cones. For cone traits, orchards could be established by collecting trees from natural forests or seed orchards to produce a large number of high-quality scions. Further, work should also be carried out on the selection and breeding of improved clones based on the cone and seed traits as well as resistance to pests and disease. Improved and advanced generation seed orchards based on natural forests or primary orchards, good provenance, families, and clones can improve the yield and quality of seed orchards. In addition, practical experiments should be developed to increase the flowering rate and seed/fruit set percentage by different treatments, such as hormones, girdling, and fertilizer application, and their combinations. Finally, an evaluation of the photosynthetic efficiency of different provenances, families, and clones should be further studied in order to obtain genetic improvement of P. koraiensis resources. Transcriptome research on P. koraiensis is slow compared with model plants, such as Arabidopsis thaliana, rice, and Populus trichocarpa, and there is a need for more comprehensive, systematic, and in-depth studies in the future.
These breeding objectives may be difficult to achieve in a reasonable time with conventional breeding due to the fragmentation of natural P. koraiensis populations, long reproductive cycle, high genetic heterozygosity, and unclear genetic mechanism of important traits. Molecular breeding could help circumvent this problem, and molecular breeding could play a significant role. In the past decade, molecular biology studies have been initiated in P. koraiensis, especially DNA molecular markers, which have contributed to characterizing genetic diversity and the construction of genetic maps. However, there are still gaps in our understanding of the molecular breeding of P. koraiensis. Functional genes related to some important traits have not yet been discovered, and the work for whole genome sequencing of P. koraiensis is incomplete. Multi-omics analyses, such as transcriptomics and metabolomics, are also lacking, and much genetic information of gene expression regulation networks has not been completely characterized. QTL mapping and genome-wide association analysis of important traits, such as cone, seed, needle, and wood properties, have not been carried out based on various molecule markers, and the relevant mechanisms governing these traits is still unclear. Finally, a genetic transformation system has not yet been established, and relevant research has not yet been carried out. Future research should focus on these issues to lay the foundation for marker-assisted breeding work on P. koraiensis.
Genetic improvement strategies for P. koraiensis should combine both conventional breeding and molecular marker-assisted breeding (Figure 2). Since previous genetic improvement works were based on seed orchard materials and focused on growth and wood quality traits, new germplasm collections from natural populations should be developed. New germplasm should be evaluated for cone/seed characteristics as the main economically important traits. Parallel to traditional breeding using provenance, family, clones, and cross breeding, genomic selection and marker-assisted selection should be applied to speed up the breeding cycle. Thus, the selected improved varieties will greatly improve the genetic gain of important economic traits, solving the demand for cones/seeds and wood and helping to achieve sustainable development in the P. koraiensis industry.
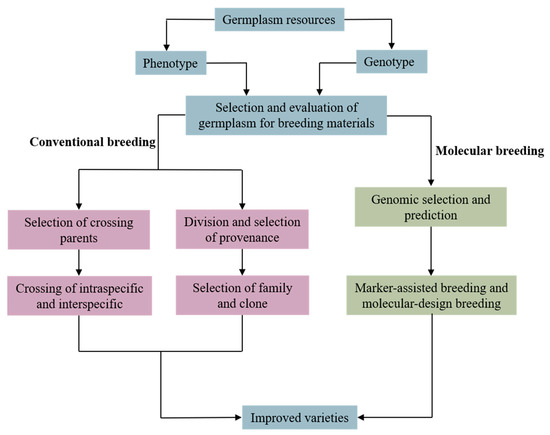
Figure 2.
Genetic improvement strategies for Pinus koraiensis.
6. Conclusions
Natural P. koraiensis populations provide wood, cones/seeds, and forest by-products, and play an important ecological role in China. Considerable traditional breeding work has been carried out and several excellent families and clones were selected to improve the genetic gain in growth, wood quality, and cone/seed yields. Most of this selection work has been conducted using seed orchard materials, which were initially established based on growth traits. However, breeding progress regarding these important traits is still slow due to a long breeding cycle, and the number of improved varieties is low. To shorten the breeding cycle and improve breeding efficiency, the use of genomic selection and marker-assisted selection (MAS) will have huge potential for improving important economic traits, such as wood properties, cone/seed yield, and biomass. However, further studies are required to design molecular breeding in P. koraiensis, including the construction of a high-density genetic map and genome-wide association study (GWAS) based on polymorphic molecular markers, as well as whole genome sequencing. These issues need to be addressed in future research, particularly using new germplasm collections from natural populations, since previous seed orchards were established based on growth traits only.
Author Contributions
Conceptualization, X.-Y.Z. and X.L.; methodology, X.-T.L. and Y.L.; validation, X.L., X.-Y.Z. and M.T.; resources, X.L. and J.-T.W.; writing—original draft preparation, X.L.; writing—review and editing, X.-Y.Z.; supervision, X.-Y.Z.; project administration, X.-Y.Z.; funding acquisition, X.-Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Heilongjiang Province Applied Technology Research and Development Program Project, grant number GA19B201-4.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Barnes, B.V.; Xü, Z.; Zhao, S. Forest ecosystems in an old-growth pine-mixed hardwood forest of the Changbai Mountain preserve in northeastern China. Can. J. For. Res. 1992, 22, 144–160. [Google Scholar] [CrossRef]
- Park, J.M.; Kwon, S.H.; Lee, H.J.; Na, S.J.; El-Kassaby, Y.A.; Kang, K.S. Integrating fecundity variation and genetic relatedness in estimating the gene diversity of seed crops: Pinus koraiensis seed orchard as an example. Can. J. For. Res. 2016, 47, 366–370. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Wang, H. Physicochemical properties, bioaccessibility and antioxidant activity of the polyphenols from pine cones of Pinus koraiensis. Int. J. Biol. Macromol. 2018, 126, 385–391. [Google Scholar] [CrossRef]
- Shpatov, A.V.; Popov, S.A.; Salnikova, O.I.; Kukina, T.P.; Shmidt, E.N.; Um, B.H. Composition and bioactivity of lipophilic metabolites from needles and twigs of Korean and Siberian Pinus. Chem. Biodivers. 2017, 14, e1600203. [Google Scholar] [CrossRef]
- Lai, F.; Xie, H.; Wang, J. Functions of biology and disease control with pycnogonid from pine bark extract. Chin. Tradit. Herb. Drugs 2005, 36, 127–131. [Google Scholar]
- Liu, R.; Wang, Z.; Cui, J.; Deng, X.; Lu, J. Elicitations on the synthesis polyphenols of Pinus koraiensis. J. Beijing Univ. 2013, 35, 26–31. [Google Scholar]
- Fan, X.; Ruan, C.; Zhang, W.; Ding, J. Dynamic changes of oil content in Pinus koraiensis seed during development and fatty acid composition of its oil. China Oils Fats 2019, 44, 118–120. [Google Scholar]
- Ma, J.; Zhuang, L.; Chen, D.; Li, J. Geographical distribution of Korean pine. J. Northeast For. Univ. 1992, 20, 40–48. [Google Scholar]
- Wang, F.; Liang, D.; Pei, X.; Zhang, Q.; Zhang, P.; Zhang, J.; Lu, Z.; Yang, Y.; Liu, G.; Zhao, X. Study on the physiological indices of Pinus sibirica and Pinus koraiensis seedlings under cold stress. J. For. Res. 2018, 30, 1255–1265. [Google Scholar] [CrossRef]
- Zhang, Y.; Yi, X.; Wang, Y.; Ji, L.; Wu, P. Impact of tree harvesting on the population structure and dynamics of Pinus koraiensis (Pinaceae). Acta Ecol. Sin. 2015, 35, 38–45. [Google Scholar]
- International Union for Conservation of Nature and Natural Resources. Available online: https://www.iucnredlist.org/species/42373/2975987 (accessed on 13 December 2010).
- Lacaze, J.F.; Fao, R.F.D. Advances in species and provenance selection. Unasylva 1978, 31, 371–375. [Google Scholar]
- Lauteri, M.; Scartazza, A.; Guido, M.C.; Brugnoli, E. Genetic variation in photosynthetic capacity, carbon isotope discrimination and mesophyll conductance in provenances of Castanea sativa adapted to different environments. Funct. Ecol. 2003, 11, 675–683. [Google Scholar] [CrossRef]
- Reinhardt, K.; Castanha, C.; Germino, M.J.; Kueppers, L.M. Ecophysiological variation in two provenances of Pinus flexilis seedlings across an elevation gradient from forest to alpine. Tree Physiol. 2011, 31, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Zhang, L.; Wei, Z.; Zhou, C.; Xia, D. Genetic variation of Pinus koraiensis from provenances and high carbon storage provenance selection. Bull. Bot. Res. 2016, 36, 452–460. [Google Scholar]
- Gao, J.; Zhang, H. Provenance selection test of building materials in Pinus koraiensis. For. Technol. Dev. 2004, 2, 20–22. [Google Scholar]
- Wang, Y.; Yang, D.; Wang, Z.; Li, G.; Gao, W.; Yang, F. Geographical variation law of biennia Pinus koraiensis seedlings and selection of best provenance in Maoershan Mountain. For. Sci. Technol. 1995, 20, 15–16. [Google Scholar]
- Zhang, Q.; Wang, H.; Jiang, G.; Shen, G.; Wang, L.; Li, Y.; Wang, L.; Wang, L.; LI, Y.; Li, R.; et al. Variation analysis and selection of Pinus koraiensis half-sib families. Bull. Bot. Res. 2019, 39, 557–567. [Google Scholar]
- Wang, F.; Wang, Y.; Wang, C.; Zhang, W.; Liu, W.; Lu, Z.; Yang, Y. Variation of the growth, fruiting and resistance to disease and insect of the half-sib families of Pinus koraiensis superior trees. Chin. J. Appl. Ecol. 2019, 30, 1679–1686. [Google Scholar]
- Wang, Z.; Hao, X.; Hu, X.; Yang, D.; Lang, F.; Wang, D.; Mu, H. Family selection of Pinus koraiensis based on growth and fructification. J. Beihua Univ. Nat. Sci. 2019, 20, 26–32. [Google Scholar]
- Liang, D.; Ding, C.; Zhao, G.; Leng, W.; Zhang, M.; Zhao, X.; Qu, G. Variation and selection analysis of Pinus koraiensis clones in northeast China. J. For. Res. 2018, 29, 611–622. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, J.; Zhang, P.; Liang, D.; Zhang, Q.; Wang, B.; Pei, X.; Zhao, X. Variation and selection of growth and fruit traits among 170 Pinus koraiensis clones. For. Res. 2019, 32, 58–64. [Google Scholar]
- Liang, D.; Jin, Y.; Zhao, G.; Dong, Y.; Len, W.; Chen, C.; Wang, H.; Zhao, X. Variance analyses of growth and wood characteristics of 50 Pinus koraiensis clones. J. Beijing Univ. 2016, 38, 51–59. [Google Scholar]
- Yang, J.; Wang, Y.; Wang, Y.; Zhou, C.; Wang, H. Selection of excellent provenance of Pinus koraiensis. Jilin For. Technol. 1998, 14–16. [Google Scholar]
- Wang, Y.; Dong, Y.; Wu, P.; Han, Y.; Wu, D.; Wu, Z. Effect analysis of establishing seedling seed orchard of Pinus koraiensis. Jilin For. Technol. 2007, 36, 1–5. [Google Scholar]
- Wang, X.; Hou, D.; Xia, D.; Yang, C.; Wei, Z. Correlation characteristics variation of Pinus koraiensis provenance and its excellent provenance selection. Prot. For. Sci. Technol. 2016, 11–16. [Google Scholar]
- Xia, D.; Yang, S.; Yang, C.; Lv, Q.; Liu, G.; Zhang, P. Study of Pinus koraiensis provenance test (1):Provenance regionalization. J. Northeast Univ. 1991, 122–128. [Google Scholar]
- Zhou, S.; Lin, S.; Zhang, D.; Zhao, S.; Lv, Z.; Zhao, H.; Zhu, H.; Chen, Y.; Wu, Y.; Zhang, G.; et al. Study on the genetic improvement of Pinus koraiensis Sieb.et Zucc. in river of Lushui and its germplasm preservation and utilization. Chin. Countrys. Well Off Technol. 2010, 59–61. [Google Scholar]
- Wang, H.; Xia, D.; Wang, W.; Yang, S. Genetic variations of wood properties and growth characters of Korean pines from different provenances. J. For. Res. 2002, 13, 277–280. [Google Scholar]
- Hill, W.G. Selective breeding. Brenners Encycl. Genet. 2013, 1, 371–373. [Google Scholar]
- Liu, D. Research progress on the selection and breeding of Pinus koraiensis in China. Prot. For. Sci. Technol. 2017, 96–99. [Google Scholar]
- Hawkins, B.J.; Xue, J.; Bown, H.E.; Clinton, P.W. Relating nutritional and physiological characteristics to growth of Pinus radiata clones planted on a range of sites in New Zealand. Tree Physiol. 2010, 30, 1174–1191. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.J.; Deng, J.; Ting, D.U.; Shi, L.; Zhou, C.L. Superior plant and clone selection and evaluation of introduced dalbergia sissoo provenances. For. Res. 2017, 30, 916–920. [Google Scholar]
- Sharma, R.M.; Kour, K.; Singh, B.; Yadav, S.; Kotwal, N.; Rana, J.C.; Anand, R. Selection and characterization of elite walnut (Juglans regia L.) clone from seedling origin trees in north western himalayan region of India. Aust. J. Crop. Sci. 2014, 8, 257–262. [Google Scholar]
- Liu, Y.; Yan, K.; He, W.; Pan, H. Genetic variation of fiber traits in Populus Deltoides clones. J. Nanjing For. Univ. 2019, 1–11. [Google Scholar]
- Wang, B.; Zhao, X.; Wang, H.; Wang, G.; Shen, G.; Wang, L.; Li, Y.; Lin, J.; Wang, Z. Variance analysis of growth characteristics of 30 Pinus koraiensis half-sib families. J. Northeast Univ. 2019, 47, 10–13. [Google Scholar]
- Mo, C.; Zhang, H.; Zhang, L.; Hou, D.; Zhang, H. Variations in nutrition compositions and morphology characteristics in different hybrid combination of Korean Pine (Pinus koraiensis). Bull. Bot. Res. 2017, 37, 700–708. [Google Scholar]
- Liang, D.; Wang, B.; Song, S.; Wang, J.; Wang, L.; Wang, Q.; Ren, X.; Zhao, X. Analysis of genetic effects on a complete diallel cross test of Pinus koraiensis. Euphytica 2019, 215, 1–12. [Google Scholar] [CrossRef]
- Sweet, G.B. Seed orchards in development. Tree Physiol. 1995, 15, 527–530. [Google Scholar] [CrossRef]
- Svensson, G.P.; Wang, H.-L.; Jirle, E.V.; Rosenberg, O.; Liblikas, I.; Chong, J.M.; Löfstedt, C.; Anderbrant, O. Challenges of pheromone-based mating disruption of Cydia strobilella and Dioryctria abietella in spruce seed orchards. J. Pest Sci. 2018, 91, 639–650. [Google Scholar] [CrossRef]
- Feng, F.; Chen, M.; Zhang, D.; Sui, X.; Han, S. Application of SRAP in the genetic diversity of Pinus koraiensis of different provenances. Afr. J. Biotechnol. 2010, 8, 1000–1008. [Google Scholar]
- Zhang, D. Stuty on Genetic Structure in Different Populations of Pinus Koraiensis by SRAP. Master’s Thesis, Northeast Forestry University, Harbin, China, 2008. [Google Scholar]
- Xia, M.; Zhou, X.; Zhao, S. RAPD analysis on genetic diversity of natural populations of Pinus koraiensis. Acta Ecol. Sin. 2000, 21, 730–737. [Google Scholar]
- Kim, B.Z.S.; Hwang, J.W.; Lee, S.W.; Yang, C.; Gorovoy, P.G. Genetic variation of Korean Pine (Pinus koraiensis Sieb. et Zucc.) at allozyme and RAPD markers in Korea, China and Russia. Silvae Genet. 2005, 54, 235–246. [Google Scholar] [CrossRef]
- Feng, F.; Sui, X.; Zhang, D. Study on genetic diversity of Korean pine from different provenance. For. Sci. Technol. 2008, 33, 1–4. [Google Scholar]
- Jiang, Y. Genetic Diversity of Piuns Koraiensis Populations from Hei Longjiang Area Revealed by ISSR Analysis. Master’s Thesis, Liaoning Normal University, Dalian, China, 2010. [Google Scholar]
- Jia, J. Analysis of Genetic Diversity of Jilin Natural Pinus Koraiensis Population by ISSR Marker. Master’s Thesis, Liaoning Normal University, Dalian, China, 2011. [Google Scholar]
- Shao, D.; Pei, Y.; Zhang, H. cpSSR analysis of variation of genetic diversity in temporal dimension of natural population of Pinus koraiensis in Liangshui National Nature Reserve. Bull. Bot. Res. 2007, 27, 473–477. [Google Scholar]
- Zhang, Y.; Yi, X.; Ji, L. Screening of Pinus koraiensis microsatellite makers from relative species of Pinus and analysis of population genetic diversity. Chin. J. Ecol. 2013, 32, 2307–2313. [Google Scholar]
- Tong, Y.W.; Lewis, B.J.; Zhou, W.M.; Mao, C.R.; Wang, Y.; Zhou, L.; Yu, D.P.; Dai, L.M.; Qi, L. Genetic diversity and population structure of natural Pinus koraiensis populations. Forests 2020, 11, 39. [Google Scholar] [CrossRef]
- Jia, D.; Zhen, Z.; Zhang, H.; Tang, J. EST–SSR marker development and transcriptome sequencing analysis of different tissues of Korean pine (Pinus koraiensis Sieb. et Zucc.). Biotechnol. Biotechnol. Equip. 2017, 31, 1–11. [Google Scholar] [CrossRef]
- Yu, J.-H.; Chen, C.-M.; Tang, Z.-H.; Yuan, S.-S.; Wang, C.-J.; Zu, Y.-G. Isolation and characterization of 13 novel polymorphic microsatellite markers for Pinus koraiensis (Pinaceae). Am. J. Bot. 2012, 99, e421–e424. [Google Scholar] [CrossRef]
- Li, Y.; Liang, L.; Ge, X.J. Development of microsatellite loci for Pinus koraiensis (Pinaceae). Am. J. Bot. 2010, 97, e39–e41. [Google Scholar] [CrossRef]
- Yun, J.; Juan, Z.; Ying, W.; Wei-Bing, F.; Gui-Fang, Z.; Zhong-Hu, L. Effects of geological and environmental events on the diversity and genetic divergence of four closely related Pines: Pinus koraiensis, P. armandii, P. griffithii, and P. pumila. Front. Plant Sci. 2018, 9, 1264. [Google Scholar]
- Aizawa, M.; Kim, Z.; Yoshimaru, H. Phylogeography of the Korean pine (Pinus koraiensis) in northeast Asia: Inferences from organelle gene sequences. J. Plant Res. 2012, 125, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Zha, M.; Imran, M.; Wang, Y.; Xu, J.; Ding, Y.; Wang, S. Transcriptome analysis revealed the interaction among strigolactones, auxin, and cytokinin in controlling the shoot branching of rice. Plant Cell Rep. 2019, 38, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, X.; Li, Q.; Chen, X.; Li, X. Comparative transcriptome analysis to elucidate the enhanced thermotolerance of tea plants (Camellia sinensis) treated with exogenous calcium. Planta 2019, 249, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Fox, H.; Doron-Faigenboim, A.; Kelly, G.; Bourstein, R.; David-Schwartz, R. Transcriptome analysis of Pinus halepensis under drought stress and during recovery. Tree Physiol. 2017, 38, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Meng, L.; Lu, H.; Zhiyong, Z.; Xiaoming, P.; Yingyue, L. De novo transcriptome assembly and population genetic analyses for an endangered Chinese endemic Acer miaotaiense (Aceraceae). Genes 2018, 9, 378. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Z.; Hanguo, Z.; Chi, M.; Lei, Z. Transcriptome sequencing analysis and development of EST-SSR markers for Pinus koraiensis. Sci. Silvae Sin. 2015, 51, 114–120. [Google Scholar]
- Ran, L. The Mechanism of Pine Polyphenols Synthesis in Pinus Koraiensis Cell Regulated by Elicitor. Ph.D. Thesis, Noreheast Forestry University, Harbin, China, 2015. [Google Scholar]
- Chen, M.; Feng, F.; Sui, X.; Han, S. Comparison of application of three molecular marker methods in map construction of Pinus koraiensis. Nonwood For. Res. 2011, 29, 13–16. [Google Scholar]
- Schaedle, M. Tree photosynthesis. Annu. Rev. Plant Physiol. 2003, 26, 101–115. [Google Scholar] [CrossRef]
- Tanai, C.; Shengxi, S.; Peter, J.N. Enhancing photosynthesis in plants: The light reactions. Essays Biochem. 2018, 62, 85–94. [Google Scholar]
- Sui, X.; Shan, N.; Hu, L.; Zhang, C.; Yu, C.; Ren, H.; Turgeon, R.; Zhang, Z. The complex character of photosynthesis in cucumber fruit. J. Exp. Bot. 2017, 68, 1625–1637. [Google Scholar] [CrossRef]
- Sello, S.; Meneghesso, A.; Alboresi, A.; Baldan, B.; Morosinotto, T. Plant biodiversity and regulation of photosynthesis in the natural environment. Planta 2019, 249, 1217–1228. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Wang, W.Y.; Meng, J.X.; Liu, H.; Xing, Z.; Mao, J.-F.; Wang, X.-R.; Li, Y. Comparison of the photosynthetic characteristics of hybrid and parental species of Pinus tabuliformis ×P. yunnanensis and P. densata. J. Beijing Univ. 2016, 38, 37–43. [Google Scholar]
- Takagi, K.; Hirata, R.; Ide, R.; Ueyama, M.; Ichii, K.; Saigusa, N.; Hirano, T.; Asanuma, J.; Li, S.-G.; Machimura, T. Spatial and seasonal variations of CO2 flux and photosynthetic and respiratory parameters of larch forests in East Asia. Soil Sci. Plant Nutr. 2014, 61, 61–75. [Google Scholar] [CrossRef]
- Ambrosini, V.G.; Rosa, D.J.; Melo, G.W.B.D.; Zalamena, J.; Brunetto, G. High copper content in vineyard soils promotes modifications in photosynthetic parameters and morphological changes in the root system of ‘Red Niagara’ plantlets. Plant Physiol. Biochem. 2018, 128, 89–98. [Google Scholar] [CrossRef]
- Shang, B.; Feng, Z.; Li, P.; Yuan, X.; Xu, Y.; Calatayud, V. Ozone exposure-and flux-based response relationships with photosynthesis, leaf morphology and biomass in two poplar clones. Sci. Total Environ. 2017, 603, 185–195. [Google Scholar] [CrossRef]
- Cheng, X.B.; Wu, J.; Han, S.J.; Zhou, Y.M.; Wang, X.X.; Wang, C.G.; Zhao, J.; Hu, Q.H. Photosynthesis, leaf morphology and chemistry of Pinus koraiensis and Quercus mongolicain broadleaved Korean pine mixed forest. Photosynthetica 2012, 50, 56–66. [Google Scholar] [CrossRef]
- Zhou, Y.M.; Han, S.J. Photosynthetic response and stomatal behaviour of Pinus koraiensis during the fourth year of exposure to elevated CO2 concentration. Photosynthetica 2005, 43, 445–449. [Google Scholar] [CrossRef]
- Huo, H.; Wang, C. Effects of canopy position and leaf age on photosynthesis and transpiration of Pinus koraiensis. Chin. J. Appl. Ecol. 2007, 18, 9–14. [Google Scholar]
- Dong, Y.; Liu, Y. Changes in the response of leaf traits in Pinus koraiensis (Korean pine) seedlings of different ages to controlled temperatures and light conditions. Acta Ecol. Sin. 2017, 37, 5662–5672. [Google Scholar]
- Wang, W.J.; Zu, Y.G.; Wang, H.M.; Li, X.Y.; Koike, T. Newly-formed photosynthates and the respiration rate of girdled stems of Korean pine (Pinus koraiensis Sieb. et Zucc.). Photosynthetica 2006, 44, 147–150. [Google Scholar] [CrossRef]
- Sun, Y.; Zhu, J.; Yu, L.; Yan, Q.; Wang, K. Photosynthetic characteristics of Pinus koraiensis seedlings under different light regime. Chin. J. Ecol. 2009, 28, 72–79. [Google Scholar]
- Mou, W.; Yan, T.; Li, J.; Meng, W. Overview of studies on photosynthesis of Korean pine. Agric. Internet Inf. 2009, 99–102. [Google Scholar]
- Sun, Y.; Zhu, J.; Sun, O.J.; Yan, Q. Photosynthetic and growth responses of Pinus koraiensis seedlings to canopy openness: Implications for the restoration of mixed-broadleaved Korean pine forests. Environ. Exp. Bot. 2016, 129, 118–126. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, K.; Sun, Y.; Yan, Q. Response of Pinus koraiensis seedling growth to different light conditions based on the assessment of photosynthesis in current and one-year-old needles. J. Res. 2014, 25, 53–62. [Google Scholar] [CrossRef]
- Liang, D.; Jiang, Y.; Zhao, G.; Dong, Y.; Len, W.; Zhao, X. Comparative study of photosynthetic characteristics of Pinus koraiensis clones. Genomics Appl. Biol. 2018, 37, 3996–4006. [Google Scholar]
- Wang, M. Comparison of photosynthetic and growth characteristics between Korean pine and Pinus sibirica seedlings. J. Liaoning For. Sci. Technol. 2017, 35, 33–35. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).