Adaptation of Forest Trees to Rapidly Changing Climate
Abstract
:1. Introduction
2. Forest Threats Related to Global Warming
2.1. Direct Impact of Global Warming on Forests-Abiotic Factors
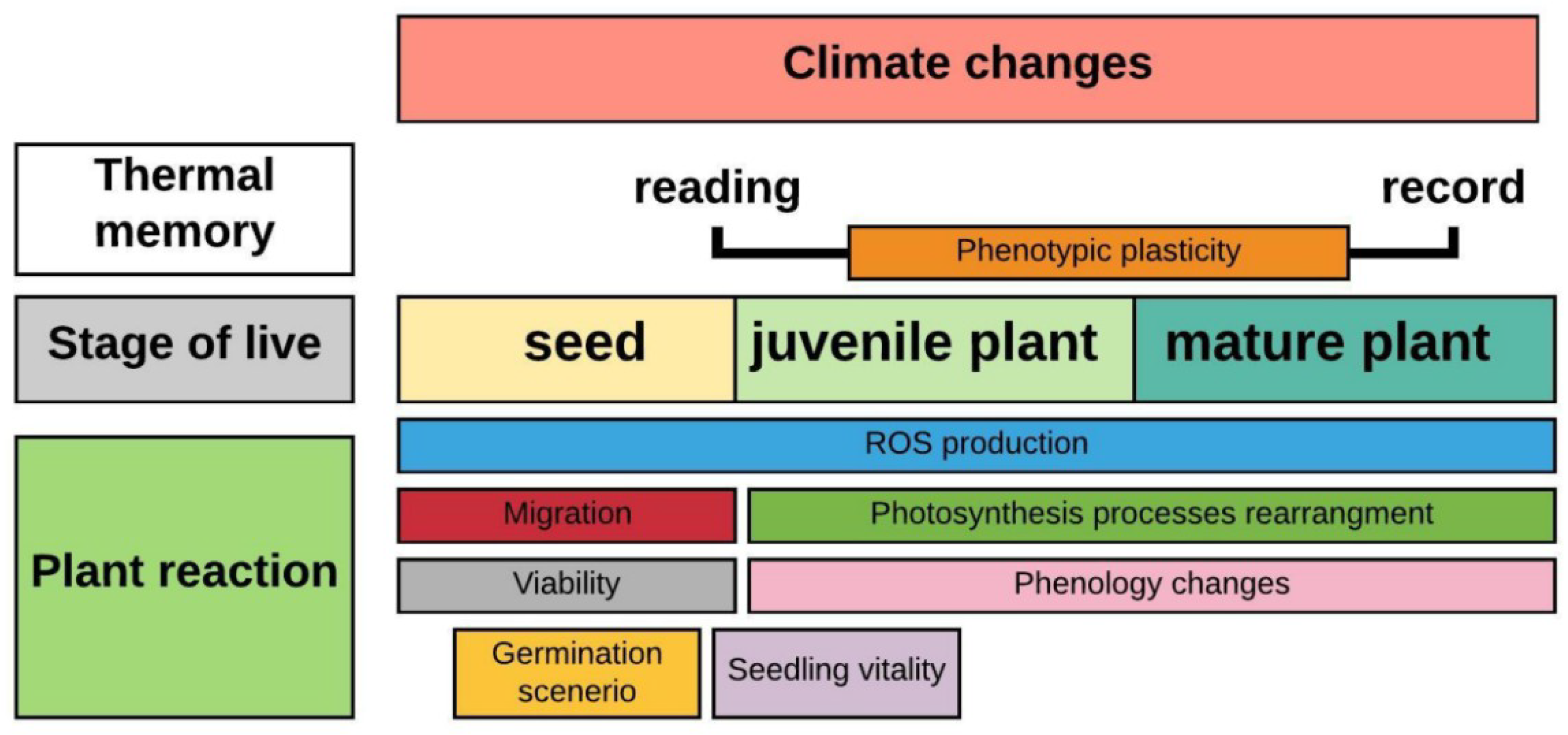
2.1.1. Responses at the Seed Level
2.1.2. Biochemical and Physiological Plant Responses
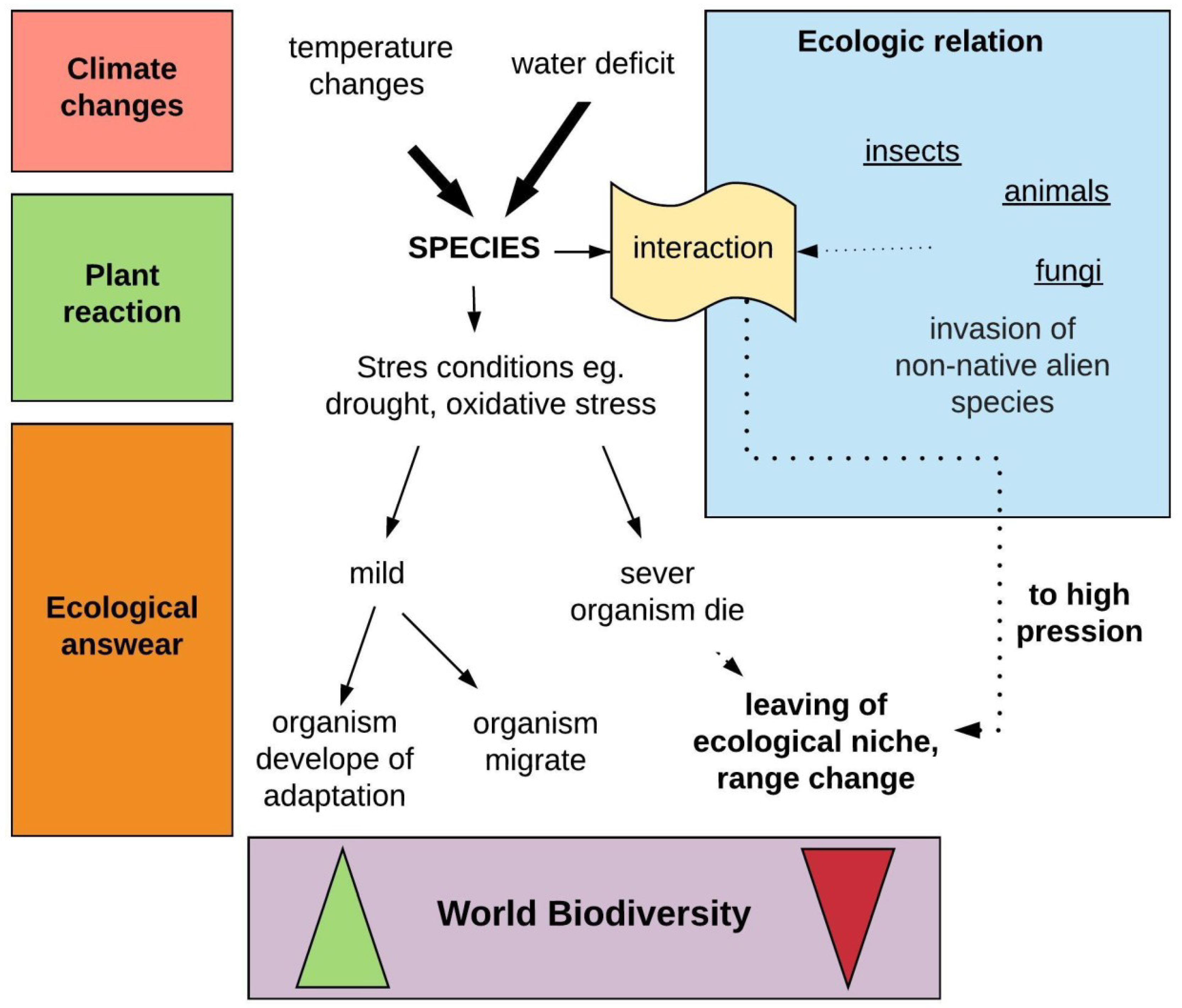
2.2. Indirect Impact of Global Warming on Forests-Biotic Threats
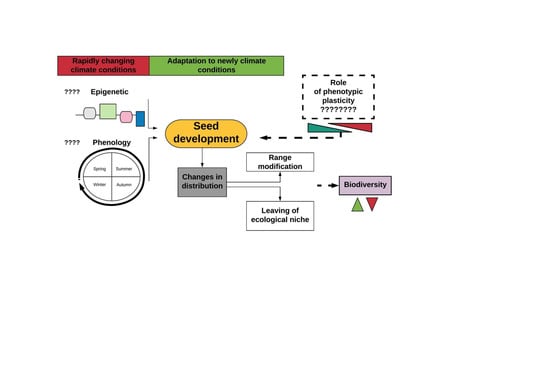
3. Novel Environmental Conditions as Changes Generator
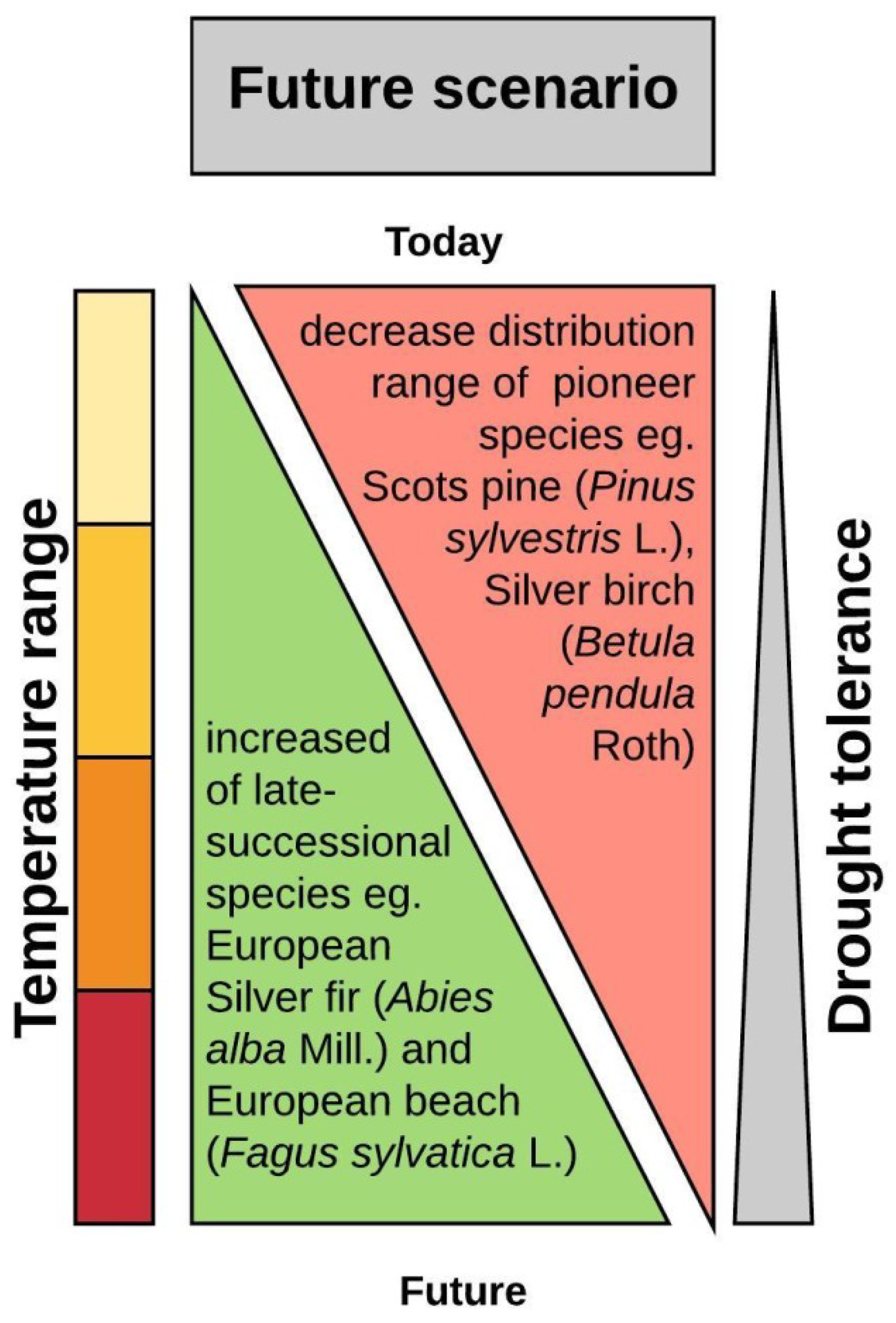
Climate Is an Important Selective Factor in Populations
4. The Impact of Phylogeny on Plant Phenology
5. Phenotypic Plasticity Allows Species to Survive under Novel Conditions
6. Future Perspective for Research and Practice Solution in Terms of Adaptation of Trees to a Rapidly Changing Climate
6.1. Preventing Harmful Climatic Events by Forecasting the Responses of Tree Species
6.2. When Adaptation Is not Fast Enough—Assisted Migration
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ebele, N.E.; Emodi, N.V. Climate Change and Its Impact in Nigerian Economy. J. Sci. Res. Rep. 2016, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Costinot, A.; Donaldson, D.; Smith, C. Evolving Comparative Advantage and the Impact of Climate Change in Agricultural Markets: Evidence from 1.7 Million Fields around the World. J. Political Econ. 2016, 124, 205–248. [Google Scholar] [CrossRef] [Green Version]
- Stolpe, M.B.; Medhaug, I.; Knutti, R. Contribution of Atlantic and Pacific Multidecadal Variability to Twentieth-Century Temperature Changes. J. Clim. 2017, 30, 6279–6295. [Google Scholar] [CrossRef]
- Anderson-Frey, A.K. Statistical Examination of Tornado Report and Warning Near-Storm Environments; The Pennsylvania State University: University Park, PA, USA, 2017; ISBN 9780355329568. [Google Scholar]
- Vogel, M.M.; Zscheischler, J.; Wartenburger, R.; Dee, D.; Seneviratne, S.I. Concurrent 2018 Hot Extremes Across Northern Hemisphere Due to Human-Induced Climate Change. Earth’s Future 2019, 7, 692–703. [Google Scholar] [CrossRef]
- Wang, T.; O’Neill, G.A.; Aitken, S.N. Integrating environmental and genetic effects to predict responses of tree populations to climate. Ecol. Appl. 2010, 20, 153–163. [Google Scholar] [CrossRef]
- Jakobson, L.; Vihma, T.P.; Jakobson, E. How does the shrinking sea ice influence changing wind speed over the Arctic Ocean? Presented at the AGU Fall Meeting, San Francisco, CA, USA, 12–16 December 2016. [Google Scholar]
- Chambers, A. The Arctic’s Shrinking Sea Ice Gives Way to Increasingly Navigable Shipping Corridors: GIS Based Analysis by Satellite Imagery and Radar Observation; Western Washington University: Washington, DC, USA, 2017. [Google Scholar]
- Wdowinski, S.; Bray, R.; Kirtman, B.P.; Wu, Z. Increasing flooding hazard in coastal communities due to rising sea level: Case study of Miami Beach, Florida. Ocean. Coast. Manag. 2016, 126, 1–8. [Google Scholar] [CrossRef]
- Young, D.J.; Stevens, J.T.; Earles, J.M.; Moore, J.; Ellis, A.; Jirka, A.L.; Latimer, A.M. Long-term climate and competition explain forest mortality patterns under extreme drought. Ecol. Lett. 2017, 20, 78–86. [Google Scholar] [CrossRef]
- Dyderski, M.K.; Jagodziński, A.M. Drivers of invasive tree and shrub natural regeneration in temperate forests. Boil. Invasions 2018, 20, 2363–2379. [Google Scholar] [CrossRef] [Green Version]
- Bigler, C.; Bugmann, H. Climate-induced shifts in leaf unfolding and frost risk of European trees and shrubs. Sci. Rep. 2018, 8, 9865. [Google Scholar] [CrossRef] [Green Version]
- Solarik, K.A.; Messier, C.; Ouimet, R.; Bergeron, Y.; Gravel, D. Local adaptation of trees at the range margins impacts range shifts in the face of climate change. Glob. Ecol. Biogeogr. 2018, 27, 1507–1519. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Flint, A.; Huang, C.-Y.; Flint, L.; Berry, J.A.; Davis, F.W.; Sperry, J.S.; Field, C.B. Tree mortality predicted from drought-induced vascular damage. Nat. Geosci. 2015, 8, 367–371. [Google Scholar] [CrossRef] [Green Version]
- Berg, N.; Hall, A. Increased Interannual Precipitation Extremes over California under Climate Change. J. Clim. 2015, 28, 6324–6334. [Google Scholar] [CrossRef]
- Williams, A.P.; Seager, R.; Abatzoglou, J.T.; Cook, B.I.; Smerdon, J.E.; Cook, E.R.; Williams, P. Contribution of anthropogenic warming to California drought during 2012–2014. Geophys. Res. Lett. 2015, 42, 6819–6828. [Google Scholar] [CrossRef] [Green Version]
- Eyre, F.H. Forest Cover Types of the United States and Canada; SAF: Washington, DC, USA, 1980; ISBN 978-0686306979. [Google Scholar]
- De Andrés, E.G. Interactions between Climate and Nutrient Cycles on Forest Response to Global Change: The Role of Mixed Forests. Forests 2019, 10, 609. [Google Scholar] [CrossRef] [Green Version]
- McDowell, N.G.; Allen, C.D. Darcy’s law predicts widespread forest mortality under climate warming. Nat. Clim. Chang. 2015, 5, 669–672. [Google Scholar] [CrossRef]
- Williams, A.; Allen, C.D.; Macalady, A.K.; Griffin, D.; Woodhouse, C.A.; Meko, D.M.; Swetnam, T.W.; Rauscher, S.A.; Seager, R.; Grissino-Mayer, H.D.; et al. Temperature as a potent driver of regional forest drought stress and tree mortality. Nat. Clim. Change. 2013, 3, 292–297. [Google Scholar] [CrossRef]
- Fernández-Martínez, M.; Vicca, S.; Janssens, I.A.; Espelta, J.M.; Peñuelas, J. The North Atlantic Oscillation synchronises fruit production in western European forests. Ecography 2017, 40, 864–874. [Google Scholar] [CrossRef] [Green Version]
- Koenig, W.D.; Knops, J.M.H.; Pesendorfer, M.B.; Zaya, D.N.; Ashley, M.V. Drivers of synchrony of acorn production in the valley oak (Quercus lobata) at two spatial scales. Ecology 2017, 98, 3056–3062. [Google Scholar] [CrossRef]
- Vacchiano, G.; Ascoli, D.; Berzaghi, F.; Lucas-Borja, M.E.; Caignard, T.; Collalti, A.; Mairota, P.; Palaghianu, C.; Reyer, C.P.; Sanders, T.G.; et al. Reproducing reproduction: How to simulate mast seeding in forest models. Ecol. Model. 2018, 376, 40–53. [Google Scholar] [CrossRef]
- Bogdziewicz, M.; Szymkowiak, J.; Fernández-Martínez, M.; Peñuelas, J.; Espelta, J.M. The effects of local climate on the correlation between weather and seed production differ in two species with contrasting masting habit. Agric. For. Meteorol. 2019, 268, 109–115. [Google Scholar] [CrossRef]
- Fernández-Pascual, E.; Mattana, E.; Pritchard, H.W. Seeds of future past: Climate change and the thermal memory of plant reproductive traits. Biol. Rev. Camb. Philos. Soc. 2019, 94, 439–456. [Google Scholar] [CrossRef] [PubMed]
- Desnoues, E.; de Carvalho, J.F.; Zohner, C.M.; Crowther, T.W. The relative roles of local climate adaptation and phylogeny in determining leaf-out timing of temperate tree species. For. Ecosytems 2017, 4, 2–7. [Google Scholar] [CrossRef] [Green Version]
- Jastrzębowski, S.; Ukalska, J. Dynamics of epicotyl emergence of Quercus robur from different climatic regions is strongly driven by post-germination temperature and humidity conditions. Dendrobiology 2019, 81, 73–85. [Google Scholar] [CrossRef] [Green Version]
- Cooper, H.F.; Grady, K.C.; Cowan, J.A.; Best, R.J.; Allan, G.J.; Whitham, T.G. Genotypic variation in phenological plasticity: Reciprocal common gardens reveal adaptive responses to warmer springs but not to fall frost. Glob. Chang. Biol. 2019, 25, 187–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, X.-W.; Zhang, J.-J.; Xu, D.-H.; Pang, J.; Gao, T.-P.; Zhang, C.-H.; Li, F.-M.; Turner, N.C. Seed germination of Caragana species from different regions is strongly driven by environmental cues and not phylogenetic signals. Sci. Rep. 2017, 7, 11248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daws, M.I.; Cleland, H.; Chmielarz, P.; Gorian, F.; Leprince, O.; Mullins, C.E.; Thanos, C.A.; Vandvik, V.; Pritchard, H.W. Variable desiccation tolerance in Acer pseudoplatanus seeds in relation to developmental conditions: A case of phenotypic recalcitrance? Funct. Plant. Boil. 2006, 33, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Moles, A.T.; Ackerly, D.D.; Webb, C.O.; Tweddle, J.C.; Dickie, J.B.; Pitman, A.J.; Westoby, M. Factors that shape seed mass evolution. Proc. Natl. Acad. Sci. USA 2005, 102, 10540–10544. [Google Scholar] [CrossRef] [Green Version]
- Arène, F.; Affre, L.; Doxa, A.; Saatkamp, A. Temperature but not moisture response of germination shows phylogenetic constraints while both interact with seed mass and lifespan. Seed Sci. Res. 2017, 27, 110–120. [Google Scholar] [CrossRef]
- Karley, A.J.; Leigh, R.A.; Sanders, D. Where do all the ions go? The cellular basis of differential ion accumulation in leaf cells. Trends Plant. Sci. 2000, 5, 465–470. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [Green Version]
- Anjum, N.A.; Sofo, A.; Scopa, A.; Roychoudhury, A.; Gill, S.S.; Iqbal, M.; Lukatkin, A.S.; Pereira, E.; Duarte, A.C.; Ahmad, I. Lipids and proteins--major targets of oxidative modifications in abiotic stressed plants. Environ. Sci. Pollut. Res Int. 2015, 22, 4099–4121. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Shahzad, B.; Kumar, V.; Kohli, S.K.; Sidhu, G.P.S.; Bali, A.S.; Handa, N.; Kapoor, D.; Bhardwaj, R.; Zheng, B. Phytohormones Regulate Accumulation of Osmolytes Under Abiotic Stress. Biomolecules 2019, 9, 285. [Google Scholar] [CrossRef] [Green Version]
- Marriboina, S.; Attipalli, R.R. Hydrophobic cell-wall barriers and vacuolar sequestration of Na+ ions are among the key mechanisms conferring high salinity tolerance in a biofuel tree species, Pongamia pinnata L. pierre. Environ. Exp. Bot. 2020, 171, 103949. [Google Scholar] [CrossRef]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Wang, R.-G.; Mao, G.; Koczan, J.M. Identification of Drought Tolerance Determinants by Genetic Analysis of Root Response to Drought Stress and Abscisic Acid1. Plant Physiol. 2006, 142, 1065–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Jia, W.; Yang, J.; Ismail, A.M. Role of ABA in integrating plant responses to drought and salt stresses. Field Crop. Res. 2006, 97, 111–119. [Google Scholar] [CrossRef]
- Sah, S.K.; Reddy, K.R.; Li, J. Abscisic Acid and Abiotic Stress Tolerance in Crop Plants. Front. Plant Sci. 2016, 7, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nirala, D.; Pant, N.; Rawat, M. Response of tree roots to drought condition: A review. Int. J. Chem. 2019, 7, 824–826. [Google Scholar] [CrossRef]
- Joslin, J.D.; Wolfe, M.H.; Hanson, P.J. Effects of altered water regimes on forest root systems. New Phytol. 2000, 147, 117–129. [Google Scholar] [CrossRef]
- Iqbal, N.; Khan, N.A.; Ferrante, A.; Trivellini, A.; Francini, A.; Khan, M.I.R. Ethylene Role in Plant Growth, Development and Senescence: Interaction with Other Phytohormones. Front. Plant Sci. 2017, 8, 6484. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.A.; Khan, M.I.R.; Ferrante, A.; Poor, P. Editorial: Ethylene: A Key Regulatory Molecule in Plants. Front. Plant Sci. 2017, 8, 1782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rust, S.; Roloff, A. Reduced photosynthesis in old oak (Quercus robur): The impact of crown and hydraulic architecture. Tree Physiol. 2002, 22, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Niinemets, Ü. Controls on the emission of plant volatiles through stomata: A sensitivity analysis. J. Geophys. Res. Space Phys. 2003, 108. [Google Scholar] [CrossRef] [Green Version]
- Tombesi, S.; Nardini, A.; Frioni, T.; Soccolini, M.; Zadra, C.; Farinelli, D.; Poni, S.; Palliotti, A. Stomatal closure is induced by hydraulic signals and maintained by ABA in drought-stressed grapevine. Sci. Rep. 2015, 5, 12449. [Google Scholar] [CrossRef]
- Agurla, S.; Gahir, S.; Munemasa, S.; Murata, Y.; Raghavendra, A.S. Mechanism of Stomatal Closure in Plants Exposed to Drought and Cold Stress. Kidney Dev. Dis. 2018, 215–232. [Google Scholar] [CrossRef]
- Leuschner, C.; Wedde, P.; Lübbe, T. The relation between pressure–volume curve traits and stomatal regulation of water potential in five temperate broadleaf tree species. Ann. For. Sci. 2019, 76, 60. [Google Scholar] [CrossRef]
- Le Provost, G.; Domergue, F.; Lalanne, C.; Campos, P.R.; Grosbois, A.; Bert, D.; Meredieu, C.; Danjon, F.; Plomion, C.; Gion, J.M. Soil water stress affects both cuticular wax content and cuticle-related gene expression in young saplings of maritime pine (Pinus pinaster Ait). BMC Plant Biol. 2013, 13, 95. [Google Scholar] [CrossRef] [Green Version]
- Griñán, I.; Rodríguez, P.; Nouri, H.; Wang, R.; Huang, G.; Morales, D.; Corell, M.; Pérez-López, D.; Centeno, A.; Martin-Palomo, M.; et al. Leaf mechanisms involved in the response of Cydonia oblonga trees to water stress and recovery. Agric. Water Manag. 2019, 221, 66–72. [Google Scholar] [CrossRef]
- Abdulrahman, A.A.; Oladele, F.A. Leaf size and transpiration rates in Agave americana and Aloe vera. Phytol. Balcan. 2017, 23, 95–100. [Google Scholar] [CrossRef]
- Lazaridou, M.; Koutroubas, S.D. Drought effect on water use efficiency of berseem clover at various growth stages. In New Directions for a Diverse Planet: Proceedings of the 4th International Crop Science Congress Brisbane; Australian Society of Agronomy Inc.: Melbourne, Australia, 2004; Volume 26, ISBN 1920842225. [Google Scholar]
- Sanchez, A.C.; Haq, N.; Assogbadjo, A.E. Variation in baobab (Adansonia digitata L.) leaf morphology and its relation to drought tolerance. Genet. Resour Crop Evol. 2010, 57, 17–25. [Google Scholar] [CrossRef]
- Flexas, J.; Medrano, H. Drought-inhibition of Photosynthesis in C3 Plants: Stomatal and Non-stomatal Limitations Revisited. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Van Lun, M.; Hub, J.S.; Van Der Spoel, D.; Andersson, I. CO2 and O2 Distribution in Rubisco Suggests the Small Subunit Functions as a CO2 Reservoir. J. Am. Chem. Soc. 2014, 136, 3165–3171. [Google Scholar] [CrossRef]
- Rennenberg, H.; Loreto, F.; Polle, A.; Brilli, F.; Fares, S.; Beniwal, R.S.; Gessler, A. Physiological Responses of Forest Trees to Heat and Drought. Plant Boil. 2006, 8, 556–571. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Tikkanen, M.; Aro, E.-M. Thylakoid protein phosphorylation in dynamic regulation of photosystem II in higher plants. Bioenergetics 2012, 1817, 232–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haldimann, P.; Feller, U. Inhibition of photosynthesis by high temperature in oak (Quercus pubescens L.) leaves grown under natural conditions closely correlates with a reversible heat-dependent reduction of the activation state of ribulose-1,5-bisphosphate carboxylase/oxygenase. Plant Cell Environ. 2004, 27, 1169–1183. [Google Scholar] [CrossRef]
- Johnson, M.P. Photosynthesis. Essays Biochem. 2016, 31, 255–273. [Google Scholar] [CrossRef]
- Foyer, C.H. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ. Exp. Bot. 2018, 154, 134–142. [Google Scholar] [CrossRef]
- Hideg, Éva A comparative study of fluorescent singlet oxygen probes in plant leaves. Open Life Sci. 2008, 3, 273–284. [CrossRef]
- Dietz, K.-J.; Turkan, I.; Krieger-Liszkay, A. Redox- and Reactive Oxygen Species-Dependent Signaling into and out of the Photosynthesizing Chloroplast. Plant Physiol. 2016, 171, 1541–1550. [Google Scholar] [CrossRef]
- Sweetlove, L.J.; Lytovchenko, A.; Morgan, M.; Nunes-Nesi, A.; Taylor, N.L.; Baxter, C.J.; Eickmeier, I.; Fernie, A.R. Mitochondrial uncoupling protein is required for efficient photosynthesis. Proc. Natl. Acad. Sci. USA 2006, 103, 19587–19592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noctor, G.; De Paepe, R.; Foyer, C.H. Mitochondrial redox biology and homeostasis in plants. Trends Plant Sci. 2007, 12, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Schwarzländer, M.; Finkemeier, I. Mitochondrial energy and redox signaling in plants. Antioxid. Redox Signal. 2013, 18, 2122–2144. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Williams, E.; Cadenas, E. Mitochondrial respiratory chain-dependent generation of superoxide anion and its release into the intermembrane space. Biochem. J. 2001, 353, 411. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Pospíšil, P. Production of Reactive Oxygen Species by Photosystem II as a Response to Light and Temperature Stress. Front. Plant Sci. 2016, 7, 1950. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; Van Breusegem, F. ROS signaling: The new wave? Trends Plant Sci. 2011, 16, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Sun, K.; Li, M.; Li, H.; Li, X.; Li, Y.; Wang, Y. The expression, function and regulation of mitochondrial alternative oxidase under biotic stresses. Mol. Plant Pathol. 2010, 11, 429–440. [Google Scholar] [CrossRef]
- Vanlerberghe, G.C. Alternative Oxidase: A Mitochondrial Respiratory Pathway to Maintain Metabolic and Signaling Homeostasis during Abiotic and Biotic Stress in Plants. Int. J. Mol. Sci. 2013, 14, 6805–6847. [Google Scholar] [CrossRef]
- Saha, B.; Borovskii, G.; Panda, S.K. Alternative oxidase and plant stress tolerance. Plant Signal. Behav. 2016, 11, e1256530. [Google Scholar] [CrossRef]
- Brosché, M.; Overmyer, K.; Wrzaczek, M.; Kangasjärvi, J.; Kangasjärvi, S. Stress signaling III: Reactive oxygen species (ROS). In Abiotic Stress Adaptation in Plants; Pareek, A., Sopory, S., Bohnert, H., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 91–102. ISBN 978-90-481-3112-9. [Google Scholar]
- Źróbek-Sokolnik, A. Temperature stress and responses of plants. In Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Ahmad, P., Prasad, M.N.V., Eds.; Springer: New York, NY, USA, 2012; pp. 113–134. ISBN 978-1-4614-0814-7. [Google Scholar]
- Ruelland, E.; Zachowski, A. How plants sense temperature. Environ. Exp. Bot. 2010, 69, 225–232. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Kitao, M.; Lei, T.T.; Koike, T.; Tobita, H.; Maruyama, Y.; Matsumoto, Y.; Ang, L.-H. Temperature response and photoinhibition investigated by chlorophyll fluorescence measurements for four distinct species of dipterocarp trees. Physiol. Plant 2000, 109, 284–290. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Suzuki, N.; Miller, G.; Salazar, C.; Mondal, H.A.; Shulaev, E.; Cortes, D.F.; Shuman, J.L.; Luo, X.; Shah, J.; Schlauch, K.; et al. Temporal-spatial interaction between reactive oxygen species and abscisic acid regulates rapid systemic acclimation in plants. Plant Cell 2013, 25, 3553–3569. [Google Scholar] [CrossRef] [Green Version]
- Pukacka, S.; Malec, M.; Ratajczak, E. ROS production and antioxidative system activity in embryonic axes of Quercus robur seeds under different desiccation rate conditions. Acta Physiol. Plant. 2011, 33, 2219–2227. [Google Scholar] [CrossRef]
- Pukacka, S.; Ratajczak, E. Production and scavenging of reactive oxygen species in Fagus sylvatica seeds during storage at varied temperature and humidity. J. Plant Physiol. 2005, 162, 873–885. [Google Scholar] [CrossRef]
- Choudhury, S.; Panda, P.; Sahoo, L.; Panda, S.K. Reactive oxygen species signaling in plants under abiotic stress. Plant Signal. Behav. 2013, 8, e23681. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, N.; Koussevitzky, S.; Mittler, R.; Miller, G. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 2012, 35, 259–270. [Google Scholar] [CrossRef]
- Kalemba, E.M.; Suszka, J.; Ratajczak, E. The role of oxidative stress in determining the level of viability of black poplar (Populus nigra) seeds stored at different temperatures. Funct. Plant. Boil. 2015, 42, 630–642. [Google Scholar] [CrossRef] [Green Version]
- Ratajczak, E.; Małecka, A.; Bagniewska-Zadworna, A.; Kalemba, E.M. The production, localization and spreading of reactive oxygen species contributes to the low vitality of long-term stored common beech (Fagus sylvatica L.) seeds. J. Plant Physiol. 2015, 174, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, E.; Staszak, A.M.; Wojciechowska, N.; Bagniewska-Zadworna, A.; Dietz, K.J. Regulation of thiol metabolism as a factor that influences the development and storage capacity of beech seeds. J. Plant Physiol. 2019, 239, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Mittler, R. Reactive oxygen species and temperature stresses: A delicate balance between signaling and destruction. Physiol. Plant 2006, 126, 45–51. [Google Scholar] [CrossRef]
- Repetto, M.; Semprine, J.; Boveris, A. Lipid Peroxidation: Chemical Mechanism, Biological Implications and Analytical Determination. Lipid Peroxidation 2012, 1, 3–30. [Google Scholar]
- Leprince, O.; Buitink, J.; Hoekstra, F.A. Axes and cotyledons of recalcitrant seeds of Castanea sativa Mill. exhibit contrasting responses of respiration to drying in relation to desiccation sensitivity. J. Exp. Bot. 1999, 50, 1515–1524. [Google Scholar] [CrossRef]
- Chmielowska-Bäk, J.; Izbiaå-Ska, K.; Deckert, J.; Izbiańska, K. Products of lipid, protein and RNA oxidation as signals and regulators of gene expression in plants. Front. Plant Sci. 2015, 6, 405. [Google Scholar] [CrossRef]
- Bailly, C.; El-Maarouf-Bouteau, H.; Corbineau, F. From intracellular signaling networks to cell death: The dual role of reactive oxygen species in seed physiology. Comptes Rendus Boil. 2008, 331, 806–814. [Google Scholar] [CrossRef]
- Berwal, M.K.; Ram, C.H. Superoxide Dismutase: A Stable Biochemical Marker for Abiotic Stress Tolerance in Higher Plants. In Superoxide Dismutase; Intech Open: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Caverzan, A.; Casassola, A.; Brammer, S.P. Reactive Oxygen Species and Antioxidant Enzymes Involved in Plant Tolerance to Stress. In Abiotic and Biotic Stress in Plants; Intech Open: London, UK, 2016; pp. 463–480. ISBN 978-953-51-2250-0. [Google Scholar]
- Ratajczak, E.; Pukacka, S. Decrease in beech (Fagus sylvatica) seed viability caused by temperature and humidity conditions as related to membrane damage and lipid composition. Acta Physiol. Plant 2005, 27, 3–12. [Google Scholar] [CrossRef]
- Nievola, C.C.; Carvalho, C.P.; Carvalho, V.; Rodrigues, E. Rapid responses of plants to temperature changes. Temperature 2017, 4, 371–405. [Google Scholar] [CrossRef]
- Jactel, H.; Petit, J.; Desprez-Loustau, M.L.; Delzon, S.; Piou, D.; Battisti, A.; Koricheva, J. Drought effects on damage by forest insects and pathogens: A meta-analysis. Glob. Change Biol. 2012, 18, 267–276. [Google Scholar] [CrossRef]
- Bentz, B.J.; Jönsson, A.M. Modeling Bark Beetle Responses to Climate Change. In Bark Beetles; Elsevier BV: Amsterdam, The Nederlands, 2015; pp. 533–553. ISBN 978-0-12-417156-5. [Google Scholar]
- Bentz, B.J. Mountain pine beetle population sampling: Inferences from Lindgren pheromone traps and tree emergence cages. Can. J. For. Res. 2006, 36, 351–360. [Google Scholar] [CrossRef]
- Keeling, C.I.; Yuen, M.M.S.; Liao, N.Y.; Docking, T.R.; Chan, S.K.; Taylor, G.A.; Palmquist, D.L.; Jackman, S.D.; Nguyen, A.; Li, M.; et al. Draft genome of the mountain pine beetle, Dendroctonus ponderosae Hopkins, a major forest pest. Genome Boil. 2013, 14, R27. [Google Scholar] [CrossRef] [Green Version]
- Marini, L.; Ayres, M.P.; Battisti, A.; Faccoli, M. Climate affects severity and altitudinal distribution of outbreaks in an eruptive bark beetle. Clim. Chang. 2012, 115, 327–341. [Google Scholar] [CrossRef]
- Raffa, K.F.; Aukema, B.H.; Bentz, B.J.; Carroll, A.L.; Hicke, J.A.; Kolb, T.E.; Björkman, C.; Niemelä, P. Responses of tree-killing bark beetles to a changing climate. In Climate Change and Insect Pests; CABI: Warszawa, Poland, 2015; pp. 173–201. ISBN 9781780643786. [Google Scholar]
- Starzyk, J.R. Species characteristics (Charakterystyka gatunku). In The bark beetle and its role in forest ecosystems (Kornik drukarz i jego rola w ekosystemach leśnych); Grodzki, W., Ed.; CILP: Warszawa, Poland, 2013; pp. 99–103. ISBN 978-83-63895-08-2. [Google Scholar]
- Brzeziecki, B.; Hilszczański, J.; Kowalski, T.; Łakomy, P.; Małek, S.; Miścicki, S.; Modrzyński, J.; Sowa, J.; Starzyk, J.R. The problem of mortality of spruce stands in the “Puszcza Białowieska” Leśny Kompleks Promocyjny (Problem masowego zamierania drzewostanów świerkowych w Leśnym Kompleksie Promocyjnym “Puszcza Białowieska”). Sylwan 2018, 162. [Google Scholar]
- Starzyk, J.R. Abiotic and anthropogenic factors (Czynniki abiotyczne i antropogeniczne). In The Bark Beetle and its Role in Forest Ecosystems (Kornik drukarz i jego rola w ekosystemach leśnych); Grodzki, W., Ed.; CILP: Warszawa, Poland, 2013; pp. 99–103. ISBN 978-83-63895-08-2. [Google Scholar]
- Virtanen, T.; Neuvonen, S.; Nikula, A.; Varama, M.; Niemelä, P. Climate change and the risks of Neodiprion sertifer outbreaks on Scots pine. Silva. Fenn. 1996, 30, 169–177. [Google Scholar] [CrossRef]
- Ramsfield, T.; Bentz, B.; Faccoli, M.; Jactel, H.; Brockerhoff, E.G. Forest health in a changing world: Effects of globalization and climate change on forest insect and pathogen impacts. Forests 2016, 89, 245–252. [Google Scholar] [CrossRef]
- Jaworski, J.; Hilszczański, T. Impact of temperature and humidity changes on development cycles and the importance of insects in forest ecosystems in connection with probable climate changes. (Wpływ zmian temperatury i wilgotności na cykle rozwojowe i znaczenie owadów w ekosystemach leśnych w związku z prawdopodobnymi zmianami klimatycznymi). For. Res. Pap. 2013, 74, 345–355. [Google Scholar] [CrossRef]
- Hódar, J.A.; Zamora, R. Herbivory and climatic warming: A Mediterranean outbreaking caterpillar attacks a relict, boreal pine species. Biodivers. Conserv. 2004, 13, 493–500. [Google Scholar] [CrossRef]
- Dickie, I.A.; Bufford, J.L.; Cobb, R.C.; Desprez-Loustau, M.-L.; Grelet, G.; Hulme, P.E.; Klironomos, J.; Makiola, A.; Nuñez, M.A.; Pringle, A.; et al. The emerging science of linked plant-fungal invasions. New Phytol. 2017, 215, 1314–1332. [Google Scholar] [CrossRef] [Green Version]
- Britton, K.O.; Liebhold, A.M. One world, many pathogens! New Phytol. 2013, 197, 9–10. [Google Scholar] [CrossRef]
- Lonsdale, D. Review of oak mildew, with particular reference to mature and veteran trees in Britain. Arboric. J. 2015, 37, 61–84. [Google Scholar] [CrossRef]
- Guo, C.; Xu, J.; Yang, L.; Guo, X.; Liao, J.; Zheng, X.; Zhang, Z.; Chen, X.; Yang, K.; Wang, M. Life cycle evaluation of greenhouse gas emissions of a highway tunnel: A case study in China. J. Clean. Prod. 2019, 211, 972–980. [Google Scholar] [CrossRef]
- Marçais, B.; Desprez-Loustau, M.L. European oak powdery mildew: Impact on trees, effects of environmental factors, and potential effects of climate change. Ann. For. Sci. 2014, 71, 633–642. [Google Scholar] [CrossRef] [Green Version]
- Jarvis, W.R.; Gubler, W.D.; Grove, G.G. Epidemiology of powdery mildews in agricultural pathosystems. In The Powdery Mildews, A Comprehensive Treatise; Bélanger, R.R., Bushnell, W.R., Dik, A.J., Carver, T.L.W., Eds.; APS Press: St. Paul, MI, USA, 2002; pp. 169–199. [Google Scholar]
- Asher, M.J.C.; Williams, G.E. Forecasting the national incidence of sugar-beet powdery mildew from weather data in Britain. Plant Pathol. 1991, 40, 100–107. [Google Scholar] [CrossRef]
- Mmbaga, M.T. Ascocarp formation and survival and primary inoculum production in Erysiphe (sect. Microsphaera) pulchra in dogwood powdery mildew. Ann. Appl. Boil. 2002, 141, 153–161. [Google Scholar] [CrossRef]
- Penczykowski, R.M.; Walker, E.; Soubeyrand, S.; Laine, A.L. Linking winter conditions to regional disease dynamics in a wild plant–pathogen metapopulation. New Phytol. 2015, 205, 1142–1152. [Google Scholar] [CrossRef]
- Camenen, E.; Porté, A.J.; Garzón, M.B. American trees shift their niches when invading Western Europe: Evaluating invasion risks in a changing climate. Ecol. Evol. 2016, 6, 7263–7275. [Google Scholar] [CrossRef] [Green Version]
- Johnsen, O.; Daehlen, O.G.; Østreng, G.; Skrøppa, T.; Dæhlen, O.G. Daylength and temperature during seed production interactively affect adaptive performance of Picea abies progenies. New Phytol. 2005, 168, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Caignard, T.; Kremer, A.; Firmat, C.; Nicolas, M.; Venner, S.; Delzon, S. Increasing spring temperatures favor oak seed production in temperate areas. Sci. Rep. 2017, 7, 8555. [Google Scholar] [CrossRef]
- Rossi, S. Local adaptations and climate change: Converging sensitivity of bud break in black spruce provenances. Int. J. Biometeorol. 2015, 59, 827–835. [Google Scholar] [CrossRef]
- Ford, K.R.; Harrington, C.A.; Bansal, S.; Gould, P.J.; Clair, J.B.S. Will changes in phenology track climate change? A study of growth initiation timing in coast Douglas-fir. Glob. Chang. Boil. 2016, 22, 3712–3723. [Google Scholar] [CrossRef] [PubMed]
- Lankau, R.A.; Zhu, K.; Ordonez, A. Mycorrhizal strategies of tree species correlate with trailing range edge responses to current and past climate change. Ecology 2015, 96, 1451–1458. [Google Scholar] [CrossRef] [Green Version]
- Lau, J.A.; Lennon, J.T.; Heath, K.D. Trees harness the power of microbes to survive climate change. Proc. Natl. Acad. Sci. USA 2017, 114, 11009–11011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavin, L.; Jump, A.S. Highest drought sensitivity and lowest resistance to growth suppression are found in the range core of the tree Fagus sylvatica L. not the equatorial range edge. Glob. Chang. Boil. 2016, 23, 362–379. [Google Scholar] [CrossRef] [Green Version]
- Savi, T.; Bertuzzi, S.; Branca, S.; Tretiach, M.; Nardini, A. Drought-induced xylem cavitation and hydraulic detorientation: Risk factors for urban trees under climate change? New Phytol. 2015, 205, 1106–1116. [Google Scholar] [CrossRef] [Green Version]
- Pretzsch, H.; Biber, P.; Uhl, E.; Dahlhausen, J.; Schütze, G.; Perkins, D.; Rötzer, T.; Caldentey, J.; Koike, T.; Van Con, T.; et al. Climate change accelerates growth of urban trees in metropolises worldwide. Sci. Rep. 2017, 7, 15403. [Google Scholar] [CrossRef]
- Medlyn, B.E.; Dreyer, E.; Ellsworth, D.; Forstreuter, M.; Harley, P.C.; Kirschbaum, M.U.F.; Le Roux, X.; Montpied, P.; Strassemeyer, J.; Walcroft, A.; et al. Temperature response of parameters of a biochemically based model of photosynthesis. II. A review of experimental data. Plant Cell Environ. 2002, 25, 253–264. [Google Scholar] [CrossRef] [Green Version]
- De Kort, H.; Vander Mijnsbrugge, K.; Vandepitte, K.; Mergeay, J.; Ovaskainen, O.; Honnay, O. Evolution, plasticity and evolving plasticity of phenology in the tree species Alnus glutinosa. J. Evol. Biol. 2016, 29, 253–264. [Google Scholar] [CrossRef] [Green Version]
- Jump, A.S.; Peñuelas, J. Running to stand still: Adaptation and the response of plants to rapid climate change. Ecol. Lett. 2005, 8, 1010–1020. [Google Scholar] [CrossRef]
- Williams, B.L.; Brawn, J.D.; Paige, K.N. Landscape scale genetic effects of habitat fragmentation on a high gene flow species: Speyeria idalia (Nymphalidae). Mol. Ecol. 2003, 12, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Gutschick, V.P.; BassiriRad, H. Extreme events as shaping physiology, ecology, and evolution of plants: Toward a unified definition and evaluation of their consequences. New Phytol. 2003, 160, 21–42. [Google Scholar] [CrossRef]
- DeWitt, T.J.; Sih, A.; Wilson, D.S. Costs and limits of phenotypic plasticity. Trends Ecol. Evol. 1998, 13, 77–81. [Google Scholar] [CrossRef]
- Peñuelas, J.; Lloret, F.; Montoya, R. Severe drought effects on Mediterranean woody flora in Spain. For. Sci. 2001, 47, 214–218. [Google Scholar] [CrossRef]
- Peñuelas, J.; Boada, M. A global change-induced biome shift in the Montseny mountains (NE Spain). Glob. Chang. Boil. 2003, 9, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Walther, G.R. Plants in a warmer world. In Perspectives in Plant Ecology, Evolution and Systematics; Edwards, P., Ed.; Stockton Press: Jena, Germany, 2003; Volume 6, pp. 169–185. ISBN 14338319. [Google Scholar]
- Pliūra, A.; Jankauskienė, J.; Bajerkevičienė, G.; Lygis, V.; Suchockas, V.; Labokas, J.; Verbylaitė, R. Response of juveniles of seven forest tree species and their populations to different combinations of simulated climate change-related stressors: Spring-frost, heat, drought, increased UV radiation and ozone concentration under elevated CO2 level. J. Plant Res. 2019, 132, 789–811. [Google Scholar] [CrossRef]
- De Jong, G. Evolution of phenotypic plasticity: Patterns of plasticity and the emergence of ecotypes. New Phytol. 2005, 166, 101–118. [Google Scholar] [CrossRef]
- Dobrowolska, D. Vitality of European Beech (Fagus sylvatica L.) at the Limit of Its Natural Range in Poland. Pol. J. Ecol. 2015, 63, 260–272. [Google Scholar] [CrossRef]
- Arana, M.V.; Gonzalez-Polo, M.; Martinez-Meier, A.; Gallo, L.A.; Benech-Arnold, R.L.; Sánchez, R.A.; Batlla, D. Seed dormancy responses to temperature relate to Nothofagus species distribution and determine temporal patterns of germination across altitudes in Patagonia. New Phytol. 2016, 209, 507–520. [Google Scholar] [CrossRef]
- Walck, J.L.; Hidayati, S.N.; Dixon, K.W.; Thompson, K.; Poschlod, P. Climate change and plant regeneration from seed. Glob. Chang. Boil. 2011, 17, 2145–2161. [Google Scholar] [CrossRef]
- O’Sullivan, O.S.; Heskel, M.A.; Reich, P.B.; Tjoelker, M.G.; Weerasinghe, L.K.; Penillard, A.; Zhu, L.; Egerton, J.J.G.; Bloomfield, K.J.; Creek, D.; et al. Thermal limits of leaf metabolism across biomes. Glob. Chang. Boil. 2016, 23, 209–223. [Google Scholar] [CrossRef] [Green Version]
- Baskin, J.M.; Baskin, C.C. Seed germination ecophysiology of jeffersonia diphylla, a perennial herb of mesic deciduous forests. Am. J. Bot. 1989, 76, 1073–1080. [Google Scholar] [CrossRef]
- Jansen, P.A.; Bongers, F.; Hemerik, L. Seed mass and mast seeding enhance dispersal by a neotropical scatter-hoarding rodent. Ecol. Monogr. 2004, 74, 569–589. [Google Scholar] [CrossRef]
- Gómez, J.M. Bigger is not always better: Conflicting selective pressures on seed size in quercus ILEX. Evolution 2004, 58, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.C.; Willis, C.G.; Primack, R.B.; Miller-Rushing, A.J. The importance of phylogeny to the study of phenological response to global climate change. Philos. Trans. R. Soc. B Boil. Sci. 2010, 365, 3201–3213. [Google Scholar] [CrossRef] [Green Version]
- Carta, A.; Hanson, S.; Müller, J.V. Plant regeneration from seeds responds to phylogenetic relatedness and local adaptation in Mediterranean Romulea (Iridaceae) species. Ecol. Evol. 2016, 6, 4166–4178. [Google Scholar] [CrossRef]
- Willis, C.G.; Ruhfel, B.; Primack, R.B.; Miller-Rushing, A.J.; Davis, C.C. Phylogenetic patterns of species loss in Thoreau’s woods are driven by climate change. Proc. Natl. Acad. Sci. USA 2008, 105, 17029–17033. [Google Scholar] [CrossRef] [Green Version]
- Davies, T.J.; Wolkovich, E.M.; Kraft, N.J.B.; Salamin, N.; Allen, J.M.; Ault, T.R.; Betancourt, J.L.; Bolmgren, K.; Cleland, E.E.; Cook, B.I.; et al. Phylogenetic conservatism in plant phenology. J. Ecol. 2013, 101, 1520–1530. [Google Scholar] [CrossRef]
- Richardson, B.A.; Chaney, L.; Shaw, N.L.; Still, S.M. Will phenotypic plasticity affecting flowering phenology keep pace with climate change? Glob. Change Biol. 2017, 23, 2499–2508. [Google Scholar] [CrossRef]
- Levin, N.A. Flowering Phenology in Relation to Adaptive Radiation. Syst. Bot. 2006, 31, 239–246. [Google Scholar] [CrossRef]
- De Casas, R.R.; Kovach, K.; Dittmar, E.; Barua, D.; Barco, B.; Donohue, K. Seed after-ripening and dormancy determine adult life history independently of germination timing. New Phytol. 2012, 194, 868–879. [Google Scholar] [CrossRef]
- Elwell, A.L.; Gronwall, D.S.; Miller, N.D.; Spalding, E.P.; Durham Brooks, T.L. Separating parental environment from seed size effects on next generation growth and development in Arabidopsis. Plant Cell Environ. 2011, 34, 291–301. [Google Scholar] [CrossRef]
- Billington, H.L.; Pelham, J. Genetic Variation in the Date of Budburst in Scottish Birch Populations: Implications for Climate Change. Funct. Ecol. 1991, 5, 403. [Google Scholar] [CrossRef]
- Dahlquist, R.M.; Prather, T.S.; Stapleton, J.J. Time and Temperature Requirements for Weed Seed Thermal Death. Weed Sci. 2007, 55, 619–625. [Google Scholar] [CrossRef]
- Kim, D.H.; Han, S.H. Direct Effects on Seed Germination of 17 Tree Species under Elevated Temperature and CO2 Conditions. Open Life Sci. 2018, 13, 137–148. [Google Scholar] [CrossRef]
- Peterson, M.E.; Daniel, R.M.; Danson, M.J.; Eisenthal, R. The dependence of enzyme activity on temperature: Determination and validation of parameters. Biochem. J. 2007, 402, 331–337. [Google Scholar] [CrossRef]
- De Villalobos, A.; Pelaez, D.; Boo, R.; Mayor, M.; Elia, O. Effect of high temperatures on seed germination of Prosopis caldenia Burk. J. Arid. Environ. 2002, 52, 371–378. [Google Scholar] [CrossRef]
- Harrington, C.A.; Gould, P.J.; St.Clair, J.B. Modeling the effects of winter environment on dormancy release of Douglas-fir. For. Ecol. Manag. 2010, 259, 798–808. [Google Scholar] [CrossRef]
- Duputié, A.; Rutschmann, A.; Ronce, O.; Chuine, I. Phenological plasticity will not help all species adapt to climate change. Glob. Chang. Boil. 2015, 21, 3062–3073. [Google Scholar] [CrossRef]
- Vegis, A. Dormancy in Higher Plants. Annu. Rev. Plant Physiol. 1964, 15, 185–224. [Google Scholar] [CrossRef]
- Fu, Y.H.; Piao, S.; Vitasse, Y.; Zhao, H.; De Boeck, H.J.; Liu, Q.; Yang, H.; Weber, U.; Hänninen, H.; Janssens, I.A. Increased heat requirement for leaf flushing in temperate woody species over 1980–2012: Effects of chilling, precipitation and insolation. Glob. Chang. Boil. 2015, 21, 2687–2697. [Google Scholar] [CrossRef]
- Hänninen, H.; Kramer, K. A framework for modelling the annual cycle of trees in boreal and temperate regions. Silva. Fenn. 2007, 41, 167–205. [Google Scholar] [CrossRef] [Green Version]
- West-Eberhard, M.J. Toward a Modern Revival of Darwin’s Theory of Evolutionary Novelty. Philos. Sci. 2008, 75, 899–908. [Google Scholar] [CrossRef]
- Jump, A.S.; Marchant, R.; Peñuelas, J.; Marchant, R. Environmental change and the option value of genetic diversity. Trends Plant Sci. 2009, 14, 51–58. [Google Scholar] [CrossRef]
- Nicotra, A.; Atkin, O.; Bonser, S.; Davidson, A.; Finnegan, E.J.; Mathesius, U.; Poot, P.; Purugganan, M.; Richards, C.; Valladares, F.; et al. Plant phenotypic plasticity in a changing climate. Trends Plant Sci. 2010, 15, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Lande, R. Adaptation to an extraordinary environment by evolution of phenotypic plasticity and genetic assimilation. J. Evol. Boil. 2009, 22, 1435–1446. [Google Scholar] [CrossRef] [PubMed]
- Van Kleunen, M.; Fischer, M. Adaptive evolution of plastic foraging responses in a clonal plant. Ecology 2001, 82, 3309–3319. [Google Scholar] [CrossRef]
- Van Kleunen, M.; Lenssen, J.P.M.; Fischer, M.; De Kroon, H. Selection on phenotypic plasticity of morphological traits in response to flooding and competition in the clonal shore plant Ranunculus reptans. J. Evol. Boil. 2007, 20, 2126–2137. [Google Scholar] [CrossRef] [PubMed]
- Bräutigam, K.; Vining, K.J.; Lafon-Placette, C.; Fossdal, C.G.; Mirouze, M.; Marcos, J.G.; Fluch, S.; Fraga, M.F.; Guevara, M.; Ángeles, L.; et al. Epigenetic regulation of adaptive responses of forest tree species to the environment. Ecol. Evol. 2013, 3, 399–415. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Fischer, M.; Colot, V.; Bossdorf, O. Epigenetic variation creates potential for evolution of plant phenotypic plasticity. New Phytol. 2013, 197, 314–322. [Google Scholar] [CrossRef]
- Sow, M.D.; Segura, V.; Chamaillard, S.; Jorge, V.; Delaunay, A.; Lafon-Placette, C.; Fichot, R.; Faivre-Rampant, P.; Villar, M.; Brignolas, F.; et al. Narrow-sense heritability and PST estimates of DNA methylation in three Populus nigra L. populations under contrasting water availability. Tree Genet. Genomes 2018, 14, 78. [Google Scholar] [CrossRef]
- Gourcilleau, D.; Bogeat-Triboulot, M.-B.; Thiec, D.; Lafon-Placette, C.; Delaunay, A.; Abu El-Soud, W.; Brignolas, F.; Maury, S. DNA methylation and histone acetylation: Genotypic variations in hybrid poplars, impact of water deficit and relationships with productivity. Ann. For. Sci. 2010, 67, 208. [Google Scholar] [CrossRef] [Green Version]
- Le Gac, A.-L.; Lafon-Placette, C.; Chauveau, D.; Segura, V.; Delaunay, A.; Fichot, R.; Marron, N.; Le Jan, I.; Berthelot, A.; Bodineau, G.; et al. Winter-dormant shoot apical meristem in poplar trees shows environmental epigenetic memory. J. Exp. Bot. 2018, 69, 4821–4837. [Google Scholar] [CrossRef] [Green Version]
- Raj, S.; Bräutigam, K.; Hamanishi, E.T.; Wilkins, O.; Thomas, B.R.; Schroeder, W.; Mansfield, S.D.; Plant, A.L.; Campbell, M.M. Clone history shapes Populus drought responses. Proc. Natl. Acad. Sci. USA 2011, 108, 12521–12526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lafon-Placette, C.; Faivre-Rampant, P.; Delaunay, A.; Street, N.; Brignolas, F.; Maury, S. Methylome of DN ase I sensitive chromatin in Populus trichocarpa shoot apical meristematic cells: A simplified approach revealing characteristics of gene-body DNA methylation in open chromatin state. New Phytol. 2013, 197, 416–430. [Google Scholar] [CrossRef]
- Bossdorf, O.; Richards, C.L.; Pigliucci, M. Epigenetics for ecologists. Ecol Lett. 2008, 11, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Sáez-Laguna, E.; Guevara, M.-A.; Díaz, L.-M.; Sánchez-Gómez, D.; Collada, C.; Aranda, I.; Cervera, M.-T. Epigenetic Variability in the Genetically Uniform Forest Tree Species Pinus pinea L. PLoS ONE 2014, 9, e103145. [Google Scholar] [CrossRef] [Green Version]
- Skrøppa, T.; Johnsen, Ø. Patterns of adaptive genetic variation in forest tree species; the reproductive enviroment as an evolutionary force in Picea abies. Agrofor. Sci. Policy Pract. 2000, 63, 49–58. [Google Scholar]
- Skrøppa, T.; Tollefsrud, M.M.; Sperisen, C.; Johnsen, Ø. Rapid change in adaptive performance from one generation to the next in Picea abies—Central European trees in a Nordic environment. Tree Genet.Genomes 2010, 6, 93–99. [Google Scholar] [CrossRef]
- Ashcroft, M.B.; Gollan, J.R.; Batley, M. Combining citizen science, bioclimatic envelope models and observed habitat preferences to determine the distribution of an inconspicuous, recently detected introduced bee (Halictus smaragdulus Vachal Hymenoptera: Halictidae) in Australia. Biol. Invasions 2012, 14, 515–527. [Google Scholar] [CrossRef]
- Deb, J.C.; Phinn, S.; Butt, N.; McAlpine, C.A. The impact of climate change on the distribution of two threatened Dipterocarp trees. Ecol. Evol. 2017, 7, 2238–2248. [Google Scholar] [CrossRef]
- Hanewinkel, M.; Cullmann, D.A.; Schelhaas, M.-J.; Nabuurs, G.-J.; Zimmermann, N.E. Climate change may cause severe loss in the economic value of European forest land. Nat. Clim. Chang. 2013, 3, 203–207. [Google Scholar] [CrossRef]
- Leech, S.M.; Almuedo, P.L.; O’Neill, G. Assisted migration: Adapting forest management to a changing climate. J. Ecol. Manag. 2011, 12, 18–34. [Google Scholar]
- Dumroese, R.K.; Williams, M.I.; Stanturf, J.A.; Clair, J.B.S. Considerations for restoring temperate forests of tomorrow: Forest restoration, assisted migration, and bioengineering. New For. 2015, 46, 947–964. [Google Scholar] [CrossRef]
- Erickson, V.; Aubry, C.; Berrang, P.; Blush, T.; Bower, A.; Crane, B.; Johnson, R. Genetic Resource Management and Climate Change: Genetic Options for Adapting National Forests to Climate Change; USDA Forest Service Forest Management: Washington, DC, USA, 2012. [Google Scholar]
- Grady, K.C.; Kolb, T.E.; Ikeda, D.H.; Whitham, T.G. A bridge too far: Cold and pathogen constraints to assisted migration of riparian forests. Restor. Ecol. 2015, 23, 811–820. [Google Scholar] [CrossRef]
- O’Neill, G.A.; Ukrainetz, N.K.; Carlson, M.R.; Cartwright, C.V.; Jaquish, B.C.; King, J.N.; Krakowski, J.; Russell, J.H.; Stoehr, M.; Xie, C.Y.; et al. Assisted Migration to Address Climate Change in British Columbia: Recommendations for Interim Seed Transfer Standards; BC Ministry of Forests and Range, Forest Sci Branch: Victoria, BC, Canada, 2008; Tech Rep. 048; ISBN 978-0-7726-6076-3.
- St.Clair, J.B.; Howe, G.T. Strategies for conserving forest genetic resources in the face of climate change. Turk. J. Bot. 2011, 35, 403–409. [Google Scholar] [CrossRef]
- Keppel, G.; Van Niel, K.P.; Wardell-Johnson, G.W.; Yates, C.J.; Byrne, M.; Mucina, L.; Schut, A.G.T.; Hopper, S.D.; Franklin, S.E. Refugia: Identifying and understanding safe havens for biodiversity under climate change. Glob. Ecol. Biogeogr. 2012, 21, 393–404. [Google Scholar] [CrossRef]
| Stage of Development | Species | Type of Adaptation | References |
|---|---|---|---|
| Seed stage | Quercus petraea (Matt.) Liebl. Quercus robur L. | Increasing temperature improve seed resistance to environmental stress and increase germination efficiency | [124] |
| Germination stage | Caragana ssp. | Germination strategies tightly linked to the ecological niche of the species | [30] |
| Quercus robur L. | Offspring of the population occurring in the continental climate has a wide range of tolerance to abiotic environmental conditions | [28] | |
| Further developmental stages | Picea abies L. | Epigenetic “memory”: impact of simulated length of day and temperature, in which parent plants were kept, on the growth of their progenies | [123] |
| Populus fremonti Wats. | Offspring of the populations occurring in warmer conditions shows four times greater plasticity, unlike the populations that adaptedto cooler conditions | [29] | |
| Picea mariana Mill. | The wide range of abiotic conditions in which the species occurs causes the formation of populations that vary in terms of specific adaptations to local conditions | [125] | |
| Pseudotsuga menziesii Mirb. | Growth initiation on Douglas-fir will track progressive changes in favorable climatic conditions at high elevations and latitudes | [12] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kijowska-Oberc, J.; Staszak, A.M.; Kamiński, J.; Ratajczak, E. Adaptation of Forest Trees to Rapidly Changing Climate. Forests 2020, 11, 123. https://doi.org/10.3390/f11020123
Kijowska-Oberc J, Staszak AM, Kamiński J, Ratajczak E. Adaptation of Forest Trees to Rapidly Changing Climate. Forests. 2020; 11(2):123. https://doi.org/10.3390/f11020123
Chicago/Turabian StyleKijowska-Oberc, Joanna, Aleksandra M. Staszak, Jan Kamiński, and Ewelina Ratajczak. 2020. "Adaptation of Forest Trees to Rapidly Changing Climate" Forests 11, no. 2: 123. https://doi.org/10.3390/f11020123
APA StyleKijowska-Oberc, J., Staszak, A. M., Kamiński, J., & Ratajczak, E. (2020). Adaptation of Forest Trees to Rapidly Changing Climate. Forests, 11(2), 123. https://doi.org/10.3390/f11020123