Uranium Vertical and Lateral Distribution in a German Forested Catchment
Abstract
1. Introduction
2. Materials and Methods
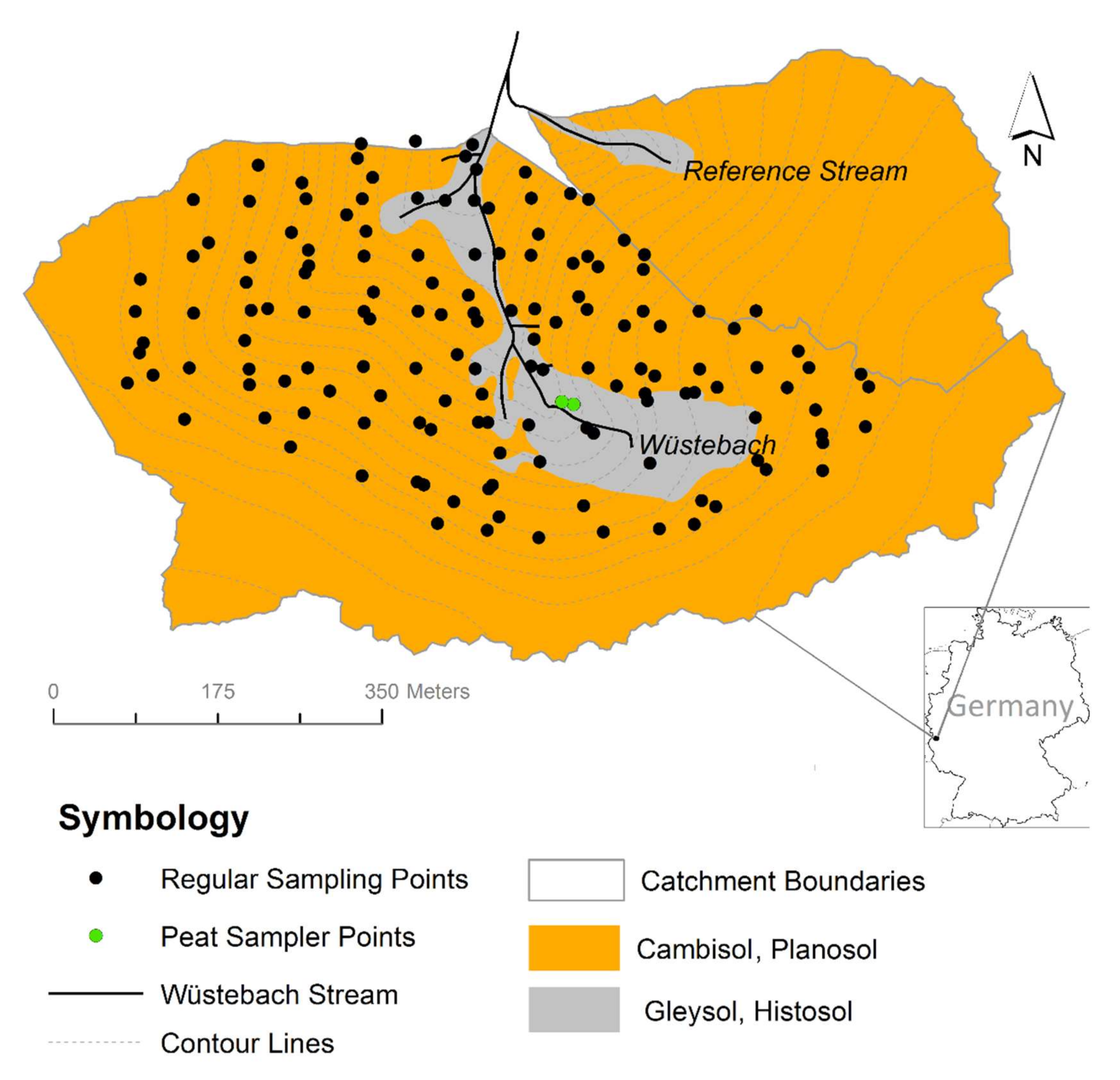
2.1. Study Area
2.2. Datasets
2.3. Data Analysis
3. Results
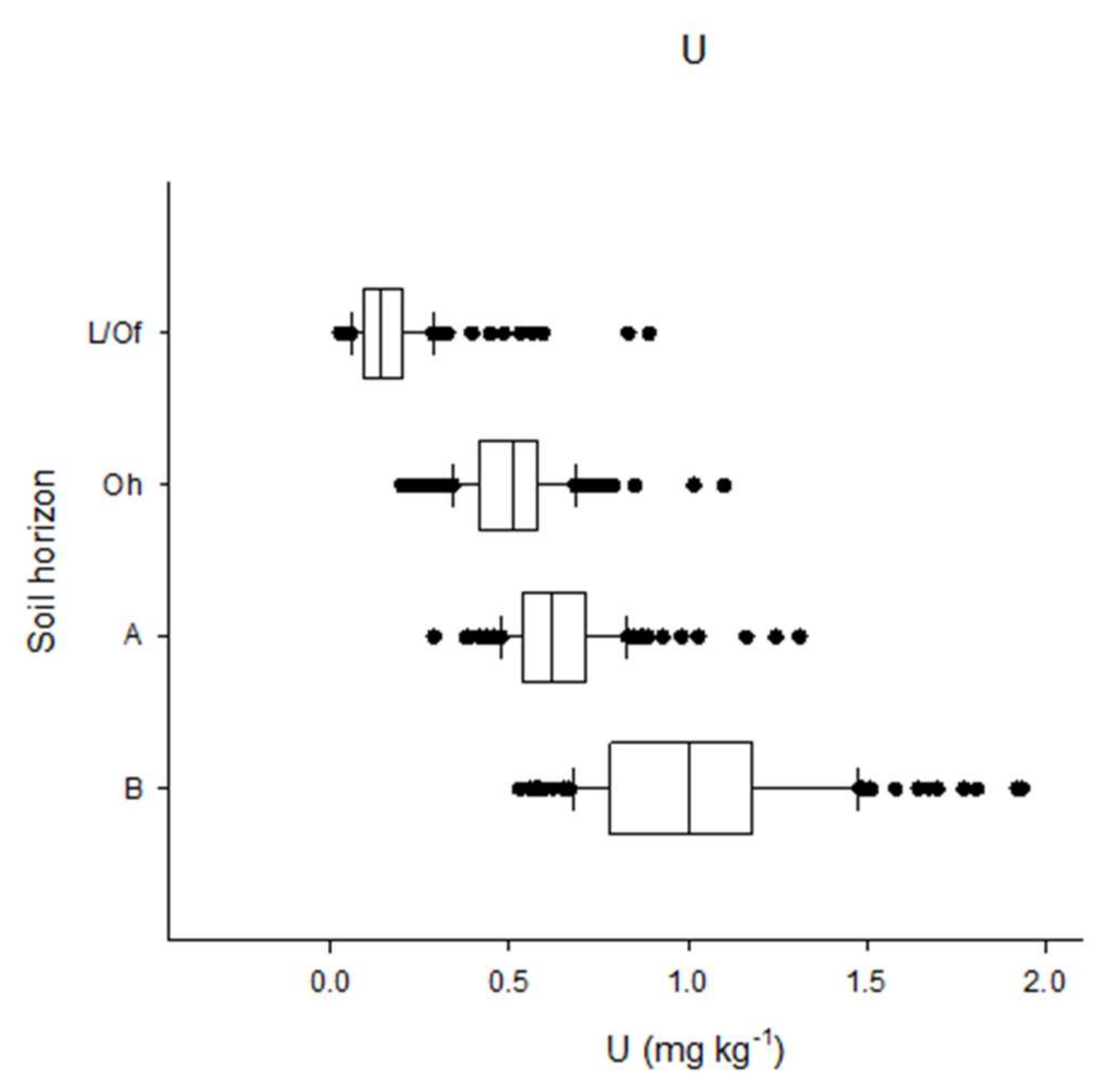
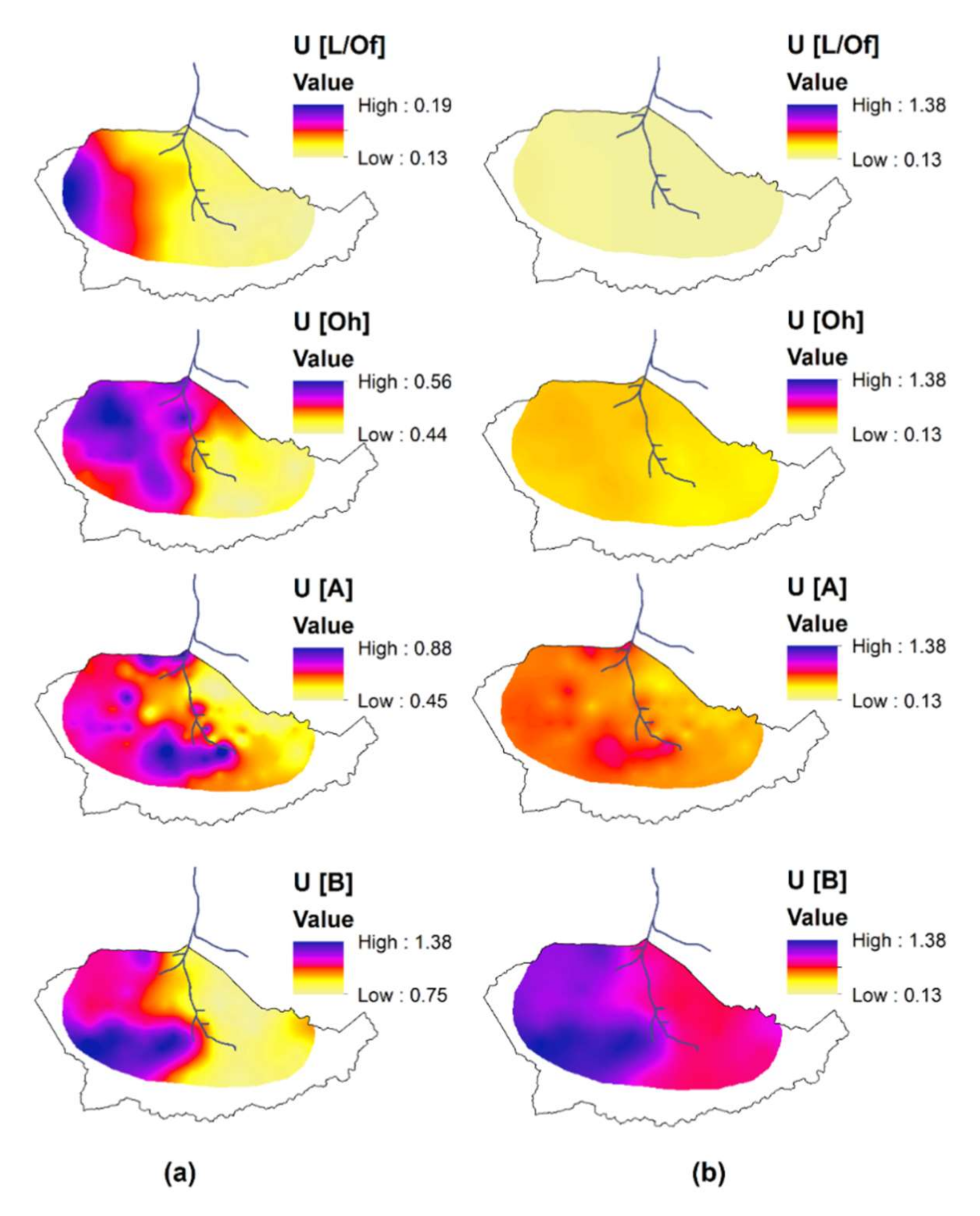
3.1. Uranium Concentration and Spatial Patterns on the Catchment Scale
3.2. Correlations between U and Other Elements
4. Discussion
4.1. Uranium Concentration on the Catchment Scale
4.2. Correlations between U and Other Elements
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nanos, N.; Martín, J.A.R. Multiscale analysis of heavy metal contents in soils: Spatial variability in the Duero river basin (Spain). Geoderma 2012, 189, 554–562. [Google Scholar] [CrossRef]
- Reimann, C.; Fabian, K.; Birke, M.; Filzmoser, P.; Demetriades, A.; Négrel, P.; Oorts, K.; Matschullat, J.; De Caritat, P.; Albanese, S.; et al. GEMAS: Establishing geochemical background and threshold for 53 chemical elements in European agricultural soil. Appl. Geochem. 2018, 88, 302–318. [Google Scholar] [CrossRef]
- Brugge, D.; De Lemos, J.L.; Oldmixon, B. Exposure pathways and health effects associated with chemical and radiological toxicity of natural uranium: A review. Rev. Environ. Health 2005, 20, 177–194. [Google Scholar] [CrossRef]
- Taylor, D.M.; Taylor, S.K. Environmental uranium and human health. Rev. Environ. Health 1997, 12, 147–158. [Google Scholar] [CrossRef]
- Kebata-Pendias, A.; Mukherjee, A.B. Trace Elements from Soil to Human; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Utermann, J.; Fuchs, M. Uranium in German soils. In Loads and Fate of Fertilizer-Derived Uranium; Backhuys Publishers: Leiden, The Netherlands, 2008; ISBN/EAN: 978-90-5782-193-6. [Google Scholar]
- Environment Canada. Uranium. In Guidelines for Surface Water Quality: Inorganic Chemical Substances; Water Quality Branch, Inland Waters Directorate: Ottawa, ON, Canada, 1983. [Google Scholar]
- Baeza, A.; Del Rio, M.; Jimenez, A.; Miro, C.; Paniagua, J. Influence of geology and soil particle size on the surface-area/volume activity ratio for natural radionuclides. J. Radioanal. Nucl. Chem. 1995, 189, 289–299. [Google Scholar] [CrossRef]
- Adams, J.A.; Osmond, J.K.; Rogers, J.J. The geochemistry of thorium and uranium. Phys. Chem. Earth 1959, 3, 298–348. [Google Scholar] [CrossRef]
- ATSDR. Toxicological Profile for Uranium; U.S. Department of Health and Human Services: Atlanta, GA, USA, 1999.
- Sun, Y.; Maekawa, M.; Wu, B.; Amelung, W.; Christensen, B.T.; Pätzold, S.; Bauke, S.L.; Schweitzer, K.; Baumecker, M.; Bol, R. Non-critical uranium accumulation in soils of German and Danish long-term fertilizer experiments. Geoderma 2020, 370, 114336. [Google Scholar] [CrossRef]
- Bigalke, M.; Ulrich, A.; Rehmus, A.; Keller, A. Accumulation of cadmium and uranium in arable soils in Switzerland. Environ. Pollut. 2017, 221, 85–93. [Google Scholar] [CrossRef]
- Cumberland, S.A.; Douglas, G.; Grice, K.; Moreau, J.W. Uranium mobility in organic matter-rich sediments: A review of geological and geochemical processes. Earth Sci. Rev. 2016, 159, 160–185. [Google Scholar] [CrossRef]
- Langmuir, D. Uranium solution-mineral equilibria at low temperatures with applications to sedimentary ore deposits. Geochim. Cosmochim. Acta 1978, 42, 547–569. [Google Scholar] [CrossRef]
- Fayek, M.; Horita, J.; Ripley, E.M. The oxygen isotopic composition of uranium minerals: A review. Ore Geol. Rev. 2011, 41, 1–21. [Google Scholar] [CrossRef]
- Romberger, S.B. Transport and deposition of uranium in hydrothermal systems at temperatures up to 300 °C: Geological implications. In Uranium Geochemistry, Mineralogy, Geology, Exploration and Resources; Springer: Dordrecht, The Netherlands, 1984; pp. 12–17. [Google Scholar]
- Babula, P.; Adam, V.; Opatrilova, R.; Zehnalek, J.; Havel, L.; Kizek, R. Uncommon heavy metals, metalloids and their plant toxicity: A review. Environ. Chem. Lett. 2008, 6, 189–213. [Google Scholar] [CrossRef]
- Canadian Council of Ministers of the Environment (CCME). Canadian Soil Quality Guidelines for Uranium: Environmental and Human Health; Canadian Council of Ministers of the Environment: Hull, QC, Canada, 2007.
- Salminen, R.; Batista, M.J.; Bidovec, M.; Demetriades, A.; De Vivo, B.; De Vos, W.; Duris, M.; Gilucis, A.; Gregorauskiene, V.; Halamic, J.; et al. Geochemical Atlas of Europe, Part 1: Background Information, Methodology and Maps; Geological Survey of Finland: Espoo, Finland, 2005; 526p, ISBN1 951-690-921-3. ISBN2 951-690-960-4. [Google Scholar]
- Peukert, S.; Bol, R.; Roberts, W.; MacLeod, C.J.; Murray, P.J.; Dixon, E.R.; Brazier, R.E. Understanding spatial variability of soil properties: A key step in establishing field- to farm-scale agro-ecosystem experiments. Rapid Commun. Mass Spectrom. 2012, 26, 2413–2421. [Google Scholar] [CrossRef]
- Martín, J.A.; Nanos, N.; Grau, J.M.; Sanchez, L.G.; López-Arias, M. Multiscale analysis of heavy metal contents in Spanish agricultural topsoils. Chemosphere 2008, 70, 1085–1096. [Google Scholar] [CrossRef]
- Taboada, T.; Cortizas, A.M.; García, C.; Garcia-Rodeja, E. Uranium and thorium in weathering and pedogenetic profiles developed on granitic rocks from NW Spain. Sci. Total Environ. 2006, 356, 192–206. [Google Scholar] [CrossRef]
- Yoshida, S.; Muramatsu, Y.; Tagami, K.; Uchida, S. Concentrations of lanthanide elements, Th, and U in 77 Japanese surface soils. Environ. Int. 1998, 24, 275–286. [Google Scholar] [CrossRef]
- Bogena, H.R.; Herbst, M.; Huisman, J.A.; Rosenbaum, U.; Weuthen, A.; Vereecken, H. Potential of wireless sensor networks for measuring soil water content variability. Vadose Zone J. 2010, 9, 1002–1013. [Google Scholar] [CrossRef]
- Zacharias, S.; Bogena, H.; Samaniego, L.; Mauder, M.; Fuß, R.; Pütz, T.; Frenzel, M.; Schwank, M.; Baessler, C.; Butterbach-Bahl, K.; et al. A network of terrestrial environmental observatories in Germany. Vadose Zone J. 2011, 10, 955–973. [Google Scholar] [CrossRef]
- IUSS Working Group. World Reference Base for Soil Resources; World Soil Resources Report 103; FAO: Rome, Italy, 2006. [Google Scholar]
- Rosenbaum, U.; Bogena, H.R.; Herbst, M.; Huisman, J.A.; Peterson, T.J.; Weuthen, A.; Western, A.W.; Vereecken, H. Seasonal and event dynamics of spatial soil moisture patterns at the small catchment scale. Water Resour. Res. 2012, 48, 10. [Google Scholar] [CrossRef]
- Gottselig, N.; Wiekenkamp, I.; Weihermüller, L.; Brüggemann, N.; Berns, A.E.; Bogena, H.R.; Borchard, N.; Klumpp, E.; Lücke, A.; Missong, A.; et al. A three-dimensional view on soil biogeochemistry: A dataset for a forested headwater catchment. J. Environ. Qual. 2017, 46, 210–218. [Google Scholar] [CrossRef]
- Wu, B.; Wiekenkamp, I.; Sun, Y.; Fisher, A.; Clough, R.; Gottselig, N.; Bogena, H.; Pütz, T.; Brüggemann, N.; Vereecken, H.; et al. A dataset for three-dimensional distribution of 39 elements including plant nutrients and other metals and metalloids in the soils of a forested headwater catchment. J. Environ. Qual. 2017, 46, 1510–1518. [Google Scholar] [CrossRef]
- Goovaerts, P. Geostatistical tools for characterizing the spatial variability of microbiological and physico-chemical soil properties. Biol. Fertil. Soils 1998, 27, 315–334. [Google Scholar] [CrossRef]
- Hiemstra, P.H.; Pebesma, E.J.; Twenhöfel, C.J.W.; Heuvelink, G.B.M. Real-time automatic interpolation of ambient gamma dose rates from the Dutch Radioactivity Monitoring Network. Comput. Geosci. 2009, 35, 1711–1721. [Google Scholar] [CrossRef]
- Aubert, D.; Probst, A.; Stille, P. Distribution and origin of major and trace elements (particularly REE, U and Th) into labile and residual phases in an acid soil profile (Vosges Mountains, France). Appl. Geochem. 2004, 19, 899–916. [Google Scholar] [CrossRef]
- Yoshida, S.; Muramatsu, Y. Determination of major and trace elements in mushroom, plant and soil samples collected from Japanese forests. Int. J. Environ. Anal. Chem. 1997, 67, 49–58. [Google Scholar] [CrossRef]
- Ahmed, H.; Young, S.D.; Shaw, G. Factors affecting uranium and thorium fractionation and profile distribution in contrasting arable and woodland soils. J. Geochem. Explor. 2014, 145, 98–105. [Google Scholar] [CrossRef]
- Huhle, B.; Kummer, S.; Merkel, B. Mobility of uranium from phosphate fertilizers in sandy soils. In Loads and Fate of Fertilizer-Derived Uranium; Backhuys Publishers: Leiden, The Netherlands, 2008; pp. 47–57. [Google Scholar]
- Sun, Y.; Amelung, W.; Gudmundsson, T.; Wu, B.; Bol, R. Critical accumulation of fertilizer-derived uranium in Icelandic grassland Andosol. Environ. Sci. Eur. 2020, 32, 1–7. [Google Scholar] [CrossRef]
- Gale, J.F.; Laubach, S.E.; Olson, J.E.; Eichhubl, P.; Fall, A. Natural Fractures in shale: A review and new observations. AAPG Bull. 2014, 98, 2165–2216. [Google Scholar] [CrossRef]
- Jobbágy, E.G.; Jackson, R.B. The distribution of soil nutrients with depth: Global patterns and the imprint of plants. Biogeochemistry 2001, 53, 51–77. [Google Scholar] [CrossRef]
- Davis, H.T.; Aelion, C.M.; McDermott, S.; Lawson, A.B. Identifying natural and anthropogenic sources of metals in urban and rural soils using GIS-based data, PCA, and spatial interpolation. Environ. Pollut. 2009, 157, 2378–2385. [Google Scholar] [CrossRef]
- Adriano, D. Trace Elements in Terrestrial Environments: Biogeochemistry, Bioaccessibility and the Risk of Metals, 2nd ed.; Springer: New York, NY, USA, 2001. [Google Scholar]
- Koljonen, T.; Darnley, A.G. The Geochemical Atlas of Finland—Part 2: Till. Econ. Geol. Bull. Soc. Econ. Geol. 1994, 89, 211. [Google Scholar]
- Mercadier, J.; Cuney, M.; Lach, P.; Boiron, M.-C.; Bonhoure, J.; Richard, A.; Leisen, M.; Kister, P. Origin of uranium deposits revealed by their rare earth element signature. Terra Nova 2011, 23, 264–269. [Google Scholar] [CrossRef]
| L/Of | Oh | A | B | |
|---|---|---|---|---|
| TC | −0.76 ** | −0.34 ** | −0.65 ** | −0.15 |
| TN | −0.17 * | −0.28 ** | −0.65 ** | -0.02 |
| K | −0.31 ** | −0.37 ** | −0.23 | 0.26 ** |
| Ca | −0.32 ** | −0.31 ** | −0.60 ** | 0.35 ** |
| S | 0.07 | −0.38 ** | −0.63 ** | −0.18 * |
| P | 0.16 * | 0.20 * | −0.07 | 0.40 ** |
| Mg | 0.63 ** | 0.12 | 0.15 | −0.32 ** |
| Fe | 0.87 ** | 0.51 ** | 0.58 ** | 0.23 ** |
| Cu | 0.68 ** | 0.35 ** | 0.09 | 0.59 ** |
| Mn | −0.47 | 0.17 * | 0.64 ** | 0.20 * |
| Mo | 0.63 ** | 0.10 | −0.13 | 0.42 ** |
| Zn | 0.31 ** | 0.10 | 0.39 | 0.07 |
| P | 0.16 * | 0.20 * | −0.07 | 0.40 ** |
| Mg | 0.63 ** | 0.12 | 0.16 | −0.32 ** |
| L/Of | Oh | A | B | |
|---|---|---|---|---|
| Cr | 0.87 ** | 0.47 ** | 0.55 * | 0.12 |
| Co | 0.84 ** | 0.30 ** | 0.55 * | 0.14 |
| As | 0.86 ** | 0.47 ** | −0.20 | 0.28 ** |
| Pb | 0.74 ** | 0.24 ** | −0.41 | 0.23 ** |
| Ni | 0.74 ** | 0.42 ** | 0.23 | −0.03 |
| Ga | 0.54 ** | 0.36 ** | 0.036 | −0.11 |
| Hg | 0.33 ** | −0.05 | −0.22 | 0.40 ** |
| Cd | 0.46 ** | −0.003 | −0.15 | 0.16 |
| L/Of | Oh | A | B | |
|---|---|---|---|---|
| La | 0.93 ** | 0.70 ** | 0.25 ** | 0.35 ** |
| Ce | 0.93 ** | 0.74 ** | 0.25 ** | 0.52 ** |
| Pr | 0.93 ** | 0.78 ** | 0.19 * | 0.45 ** |
| Nd | 0.92 ** | 0.79 ** | 0.32 ** | 0.47 ** |
| Sm | 0.93 ** | 0.80 ** | 0.39 ** | 0.54 ** |
| Eu | 0.93 ** | 0.77 ** | 0.42 ** | 0.52 ** |
| Gd | 0.94 ** | 0.76 ** | 0.30 ** | 0.61 ** |
| Tb | 0.93 ** | 0.77 ** | 0.48 ** | 0.74 ** |
| Dy | 0.94 ** | 0.77 ** | 0.63 ** | 0.78 ** |
| Ho | 0.92 ** | 0.75 ** | 0.67 ** | 0.78 ** |
| Er | 0.93 ** | 0.74 ** | 0.57 ** | 0.80 ** |
| Tm | 0.93 ** | 0.67 ** | 0.68 ** | 0.82 ** |
| Yb | 0.93 ** | 0.73 ** | 0.66 ** | 0.83 ** |
| Lu | 0.92 ** | 0.69 ** | 0.69 ** | 0.82 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Wu, B.; Wiekenkamp, I.; Kooijman, A.M.; Bol, R. Uranium Vertical and Lateral Distribution in a German Forested Catchment. Forests 2020, 11, 1351. https://doi.org/10.3390/f11121351
Sun Y, Wu B, Wiekenkamp I, Kooijman AM, Bol R. Uranium Vertical and Lateral Distribution in a German Forested Catchment. Forests. 2020; 11(12):1351. https://doi.org/10.3390/f11121351
Chicago/Turabian StyleSun, Yajie, Bei Wu, Inge Wiekenkamp, Annemieke M. Kooijman, and Roland Bol. 2020. "Uranium Vertical and Lateral Distribution in a German Forested Catchment" Forests 11, no. 12: 1351. https://doi.org/10.3390/f11121351
APA StyleSun, Y., Wu, B., Wiekenkamp, I., Kooijman, A. M., & Bol, R. (2020). Uranium Vertical and Lateral Distribution in a German Forested Catchment. Forests, 11(12), 1351. https://doi.org/10.3390/f11121351





