Abstract
Putrescine (Put) can enhance secondary metabolite production, but its intrinsic regulatory mechanism remains unclear. In this study, Put treatment promoted betulin production and gene expression of lupeol synthase (LUS), one of betulin synthetic enzymes. The maximum betulin content and gene expression level of LUS was 4.25 mg·g−1 DW and 8.25 at 12 h after 1 mmol·L−1 Put treatment, approximately two- and four-times that in the control, respectively. Put treatment increased the content of nitric oxide (NO) and its biosynthetic enzyme activity of nitrate reductase (NR) and NO synthase (NOS). Pretreatment of the birch suspension cells with NO-specific scavenger 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline- 1-oxyl-3-oxide (cPTIO), NR inhibitor sodium azide (NaN3), and NOS inhibitor NG-nitro-L-Arg methyl ester (L-NAME) decreased Put-triggered NO generation and blocked Put-induced betulin production. Put treatment improved the content of NH4+ and its assimilation enzyme activity of glutamate synthase and glutamate dehydrogenase. NH4+ supplementation also promoted NO and betulin production. Thus, the above data indicated that Put-induced NO was essential for betulin production. NO derived from NR, NOS, and NH4+ mediated betulin production in birch suspension cell cultures under Put treatment.
1. Introduction
Polyamines (PAs), including putrescine (Put), spermidine (Spd), and spermine (Spm), are low-molecular-weight aliphatic polycations that are quite common in living organisms [1]. Put is the major diamine in plants and a direct substrate for the synthesis of Spd and Spm [2]. PAs have been suggested to play important roles in morphogenesis, growth, embryogenesis, organ development, leaf senescence, and abiotic and biotic stress responses [3,4]. Besides the above roles, PAs also regulate various fundamental cellular processes as signaling molecules [5]. The major links in PA signaling may be hydrogen peroxide (H2O2) and nitric oxide (NO) [6,7].
NO is considered to cause the groundbreaking bioactive signaling described in plants [8]. Like PAs, NO also participates in various cell processes such as growth and development, respiratory metabolism, senescence, and maturation, as well as plant response to abiotic and biotic stressors [8,9]. Nitric oxide synthase (NOS) and nitrate reductase (NR) are known as two major sources of NO production in plants [10]. Moreover, NO is also produced via nonenzymatic pathways, such as NO induced by the presence of hydrogen sulfide, abscisic acid, PAs, and ammonium cation (NH4+) [11,12].
Recent studies have indicated that PA-induced NO biosynthesis might be a potential linking signal in many of the developmental processes and stress responses in plants. Such as, Put and Spd stimulated arginine-dependent NO formation during dormancy release and germination of apple embryos [13]. NR is known as one of the major sources of NO production, and NO and NH4+ are all products of NR [14]. However, the role of NR, NO, and NH4+ induced by PAs in plants is poorly understood.
Plant cell culture offers a good alternative to whole-plant collection for the production of bioactive secondary metabolites under controlled and reproducible protocols. Many strategies have been used to increase the secondary metabolite production in plant cell cultures, e.g., elicitation, precursor feeding, and nutritional supplement [15,16]. One such bioactive secondary metabolite is botulin, which is extracted from the bark of white birch trees (Betula platyphylla); one of the pentacyclic triterpene compounds, lupeol synthase (LUS), is mainly responsible for betulin biosynthesis through the MVA (mevalonate) pathway. Betulin is a valuable metabolite with antiviral, antibacterial, and antitumor properties [17]. In our previous study, betulin was detected in birch cell suspension cultures and plantlets; PAs or NO induced betulin accumulation [18,19]. However, the regulatory mechanism of PAs or NO in betulin biosynthesis of birch suspension cells remains elusive. The aim of the present study is to determine the response of NO and NH4+ to Put in betulin production and investigate NH4+ assimilation and the sources of NO in betulin production induced by Put.
2. Results
2.1. Put Enhanced Betulin Production
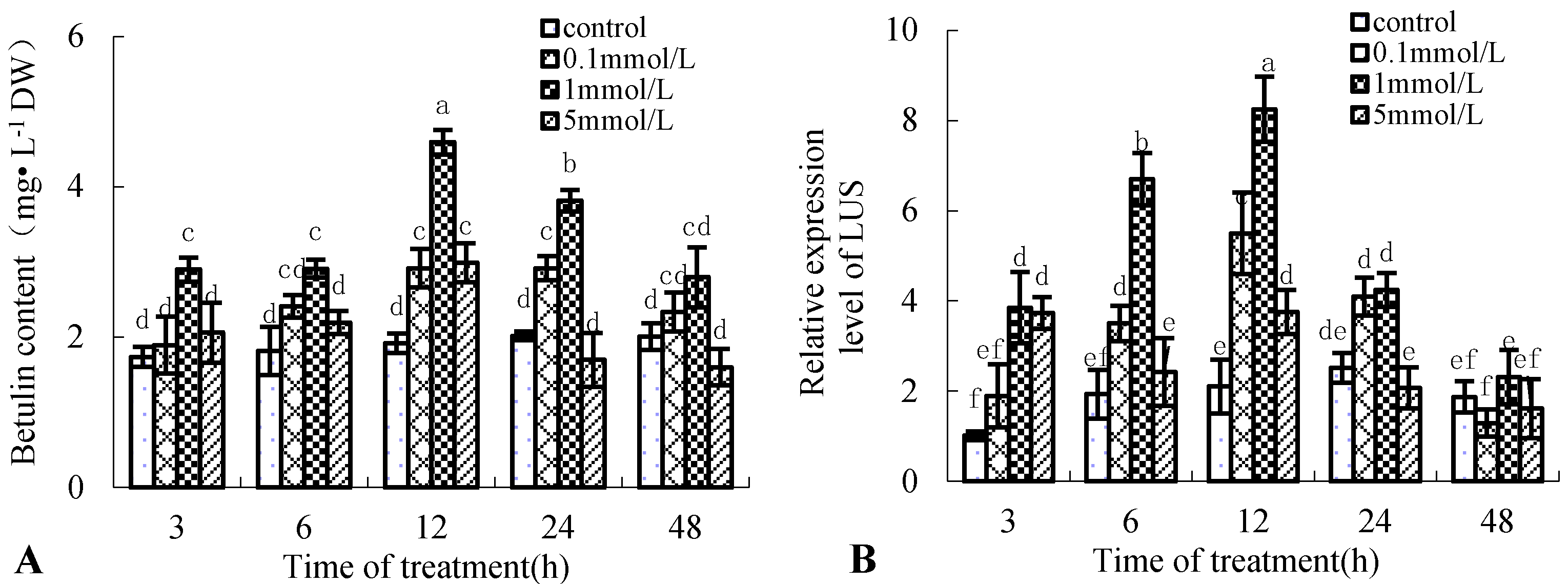
To improve betulin content in birch cell suspension cultures, the induced effect of 0.1, 1, and 5 mmol·L−1 Put on betulin production was used for analysis. Figure 1A shows that betulin production in birch suspension cells induced by Put is time-dependent and dose-dependent. Betulin content peaked at 12 h after 0.1, 1, and 5 mmol·L−1 Put treatments, which were 51.89%, 139.23%, and 55.69% higher than those of the control, respectively. In addition, 5 mmol·L−1 Put treatment decreased betulin content at 24 and 48 h, which were 15.81% and 20.21% lower than those of the control, respectively.
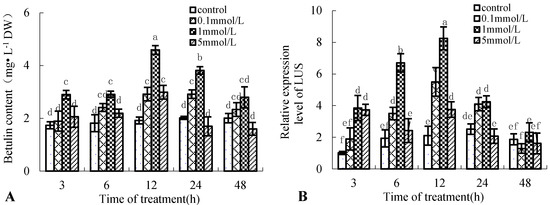
Figure 1.
Effect of putrescine (Put) on betulin production and gene expression of lupeol synthase (LUS) in suspension cells of birch. Eight-day-old suspension cells were treated with 0.1, 1, and 5 mmol·L−1 Put and harvested at the indicated time points after treatment. Suspension cells treated with the same volume of distilled water were used as controls. Different letters show significant differences among means (p < 0.05, Tukey’s test); the following figures are the same. (A), betulin content in suspension cells of birch; (B), gene expression of LUS in suspension cells of birch.
Betulin is a lupane-type triterpene; one of its synthetic key enzymes is lupeol synthase (LUS) [19]. Figure 1B shows that the gene expression of LUS has the same varying tendency with betulin content in the suspension cells of birch treated with Put; the maximum gene expression level of LUS was 8.25 at 12 h after 1 mmol·L−1 Put treatment, approximately four times that of the control.
2.2. Put Induced NO Production
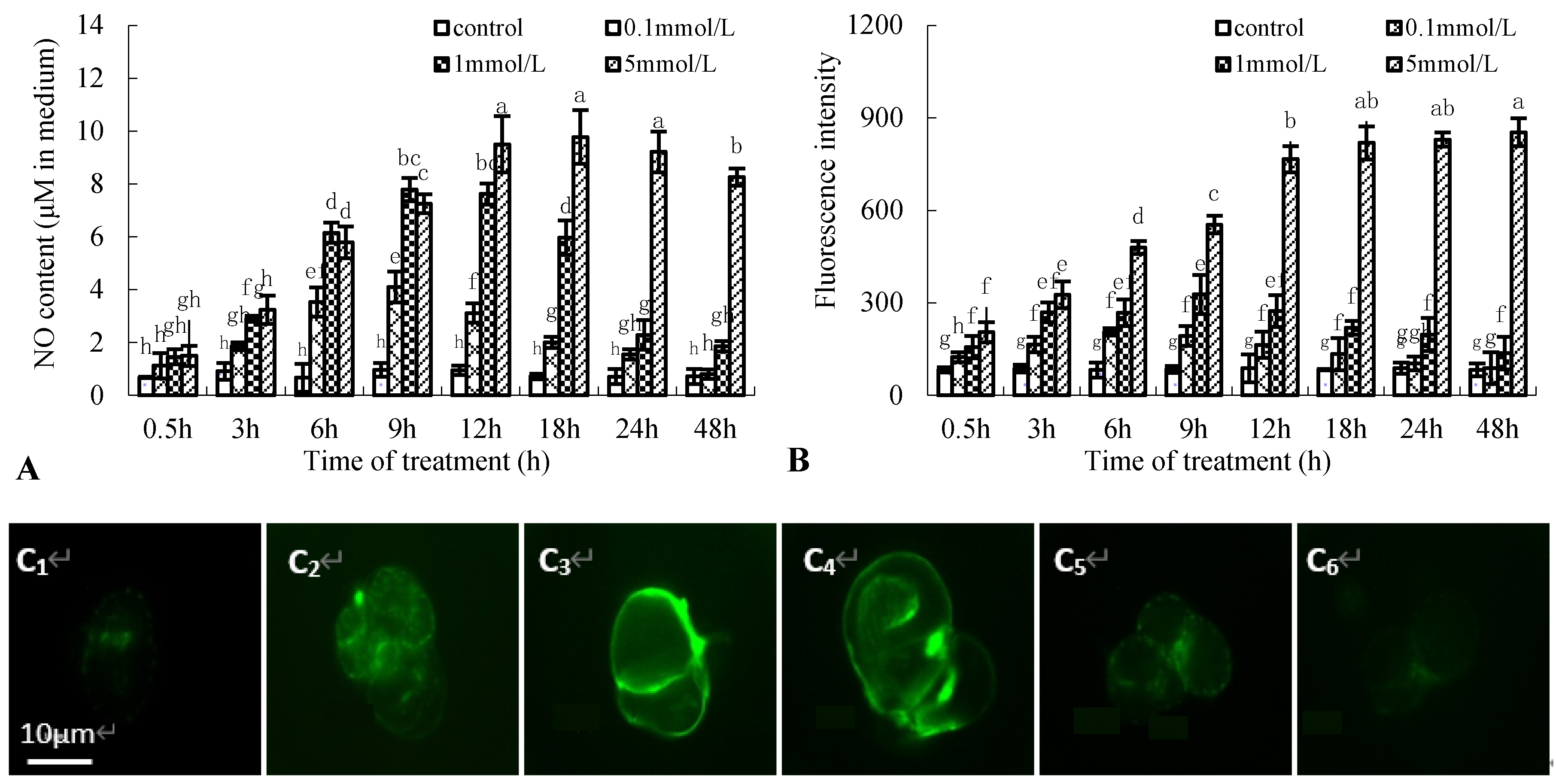
NO production in birch suspension cells was examined using the Greiss reagent assay kit and a fluorometric method. Figure 2A shows the time course of NO generation in a medium of birch suspension cells treated with 0.1, 1, and 5 mmol·L−1 Put. NO content peaked at 9 h after 0.1 or 1 mmol·L−1 Put treatment, 18 h after 5 mmol·L−1 Put treatment, approximately 4-, 8-, and 13-times that in the control, respectively. NO fluorescence intensity in suspension cells after Put treatment had a similar change tendency to NO content in medium of birch suspension cells (Figure 2B), and the maximum fluorescence intensity was also at 9 h after 1 mmol·L−1 Put treatment (Figure 2C).
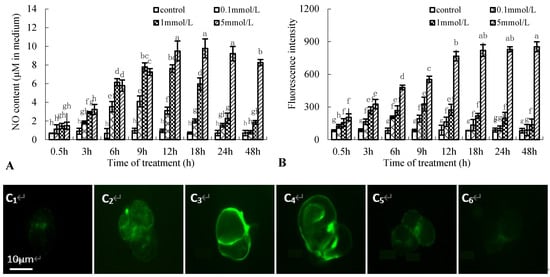
Figure 2.
Effect of Put on nitric oxide (NO) generation in suspension cells of birch. (A), NO content in culture medium of birch; (B), NO fluorescence intensity in suspension cells of birch; (C), NO fluorescence intensity in suspension cells after 1 mmol·L−1 Put treatment (C1, Control; C2, 3 h after Put treatment; C3, 9 h after Put treatment; C4, 12 h after Put treatment; C5, 24 h after Put treatment; C6, 48 h after Put treatment).
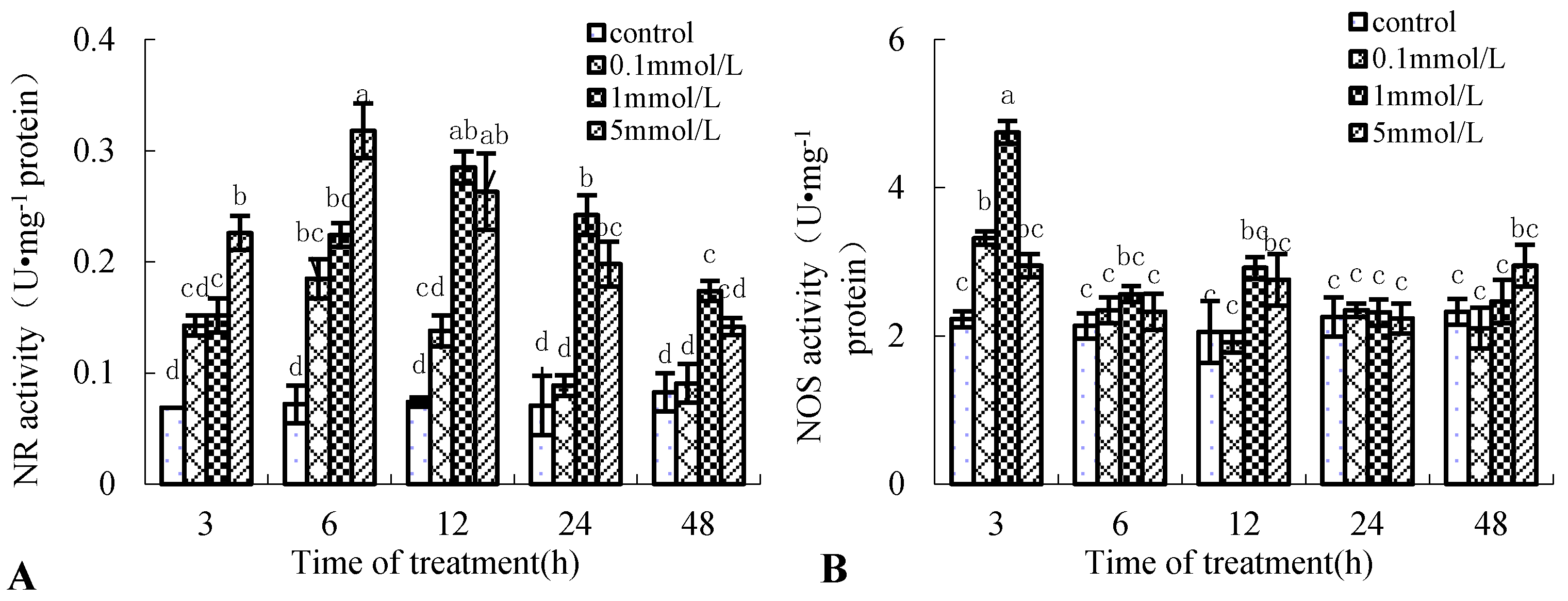
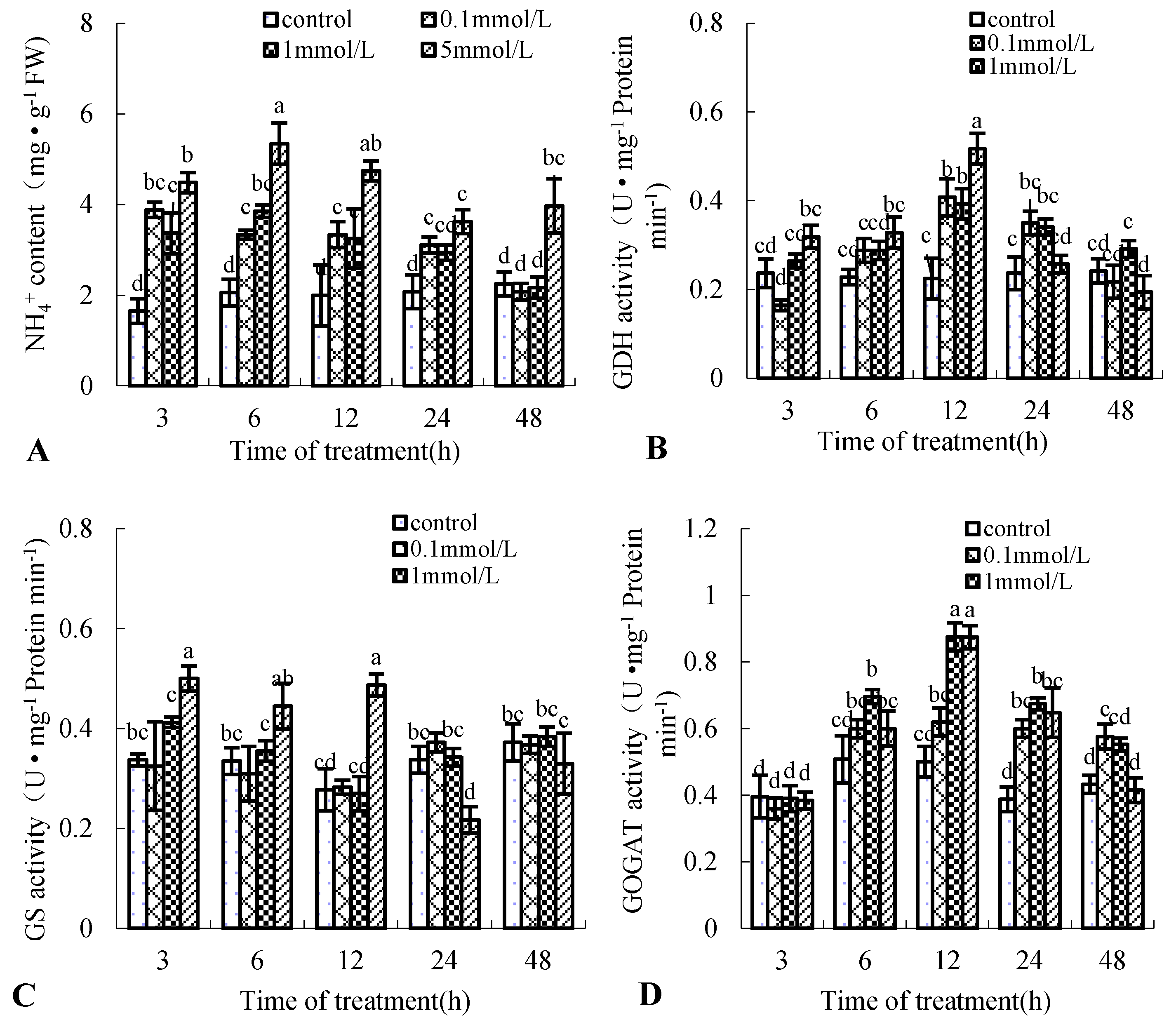
NR and NOS are the two NO-generating key enzymes in plants [10]. Figure 3 shows that the enzyme activity of NR and NOS of the birch suspension cells induced by Put was also time-dependent and dose-dependent. Put treatment increased the enzyme activity of NR and NOS. NR enzyme activity peaked at 6 and 12 h after 5 and 1 mmol·L−1 Put treatment, which was 4.4-fold and 3.8-fold that of the control, respectively (Figure 3A). NOS enzyme activity peaked at 3 h after 0.1 and 1 mmol·L−1 Put treatment, which was 2.3-fold and 2.9-fold that of the control, respectively (Figure 3B). These results had the same change tendency as NO content in the medium of birch suspension cells.
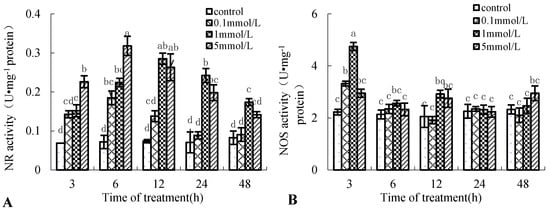
Figure 3.
Effect of Put on enzyme activity of nitrate reductase (NR) and NO synthase (NOS) in suspension cells of birch. (A), NR activity in suspension cells of birch; (B), NOS activity in suspension cells of birch.
2.3. NO-Mediated Put Induced Betulin Accumulation
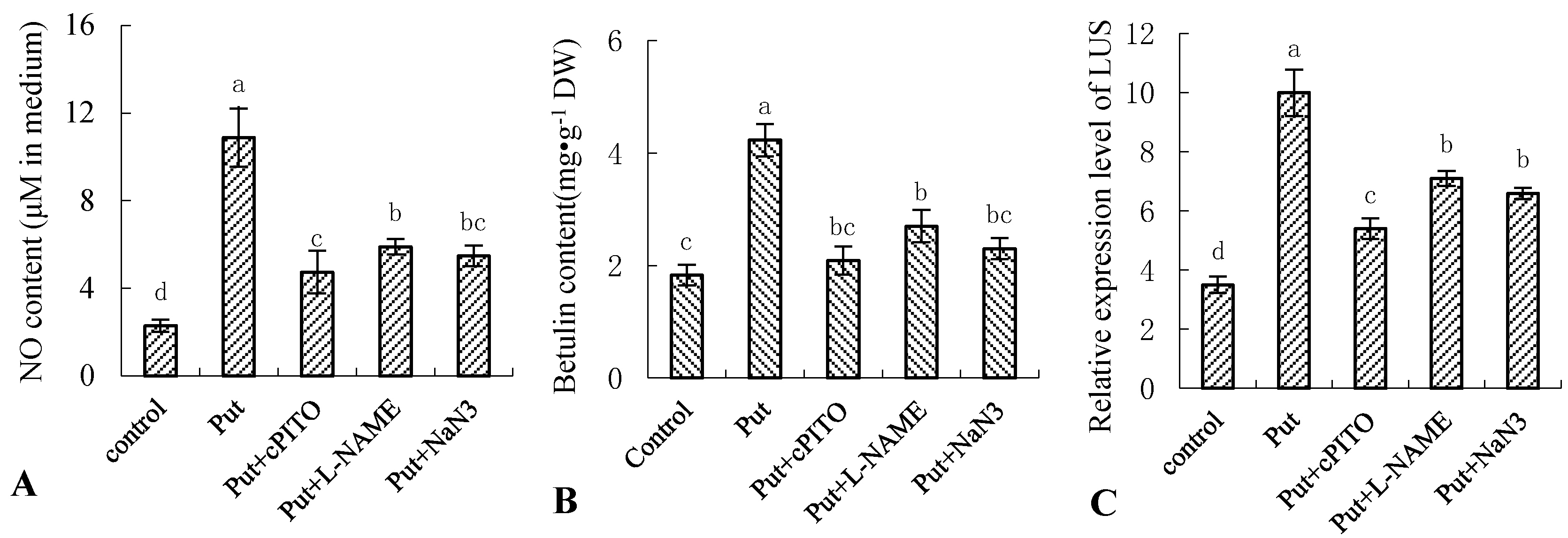
To investigate the role of NO in Put-induced betulin production, we examined the effects of NO scavenger cPTIO, NOS inhibitor L-NAME, and NR inhibitor NaN3 on Put-induced NO burst, betulin production, and gene expression of LUS in the birch suspension cells (Figure 4). As shown, pretreatment of the suspension cells with cPTIO, L-NAME, and NaN3 abolished the Put-triggered NO generation (Figure 4A) and blocked the Put-induced betulin production and gene expression of LUS (Figure 4B,C). Thus, the data indicated that Put-induced NO was essential for betulin production, and it may have been mainly derived from NR and NOS biosynthetic pathways in birch suspension cell cultures.
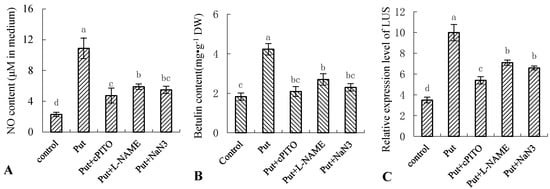
Figure 4.
Effect of Put and NO inhibitor on NO production, betulin content, and gene expression of LUS in birch suspension cells. Eight-day-old suspension cells pretreated for 0.5 h with 150 μmol·L−1 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO; a NO-specific scavenger), 0.5 mmol·L−1 NR inhibitor sodium azide (NaN3), and 3 mmol·L−1 NOS inhibitor NG-nitro-L-Arg methyl ester (L-NAME) were treated with 1 mmol·L−1 for 12 h, respectively. (A), NO content in culture medium of birch; (B), betulin content in suspension cells of birch; (C), gene expression of LUS in suspension cells of birch.
2.4. Put Induced NH4+ Production
NO and NH4+ are all products of NR. Put treatment significantly increased the enzyme activity of NR (Figure 3A); the effect of Put treatment on NH4+ production was unknown. In this study, we examined the effect of Put treatment on NH4+ production in birch suspension cells. Figure 5A shows that Put treatment significantly increased NH4+ production except after 48 h. NH4+ content peaked at 3 h after 0.1, 1, and 5 mmol·L−1 Put, approximately 2.35-, 2.04-, and 2.72-fold that in the control, respectively. GS, GOGAT, and GDH are involved in ammonium assimilation [20]; the activities of GOGAT and GDH had the same change tendency as NH4+ content (Figure 5B,D), but the activity of GS was different in birch suspension cells under Put treatment (Figure 5C).
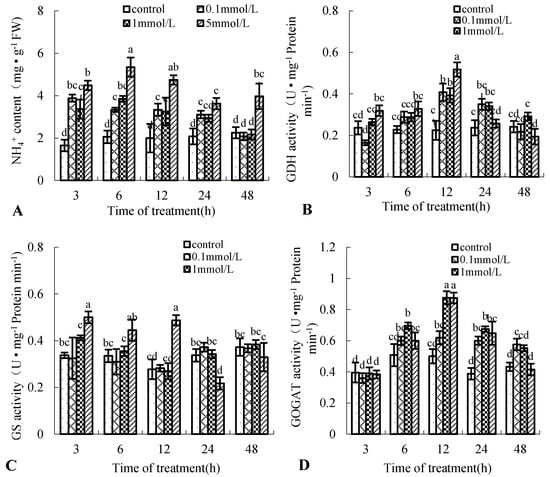
Figure 5.
Effect of Put on the content of NH4+ and its assimilation enzyme activities in suspension cells of birch. (A), NH4+ content in culture medium of birch; (B), glutamate dehydrogenase (GDH) activity in suspension cells of birch; (C), glutamine synthase (GS) activity in suspension cells of birch; (D), glutamine-2-oxoglutarate aminotransferase (GOGAT) activity in suspension cells of birch.
2.5. NH4+ Induced NO and Betulin Production
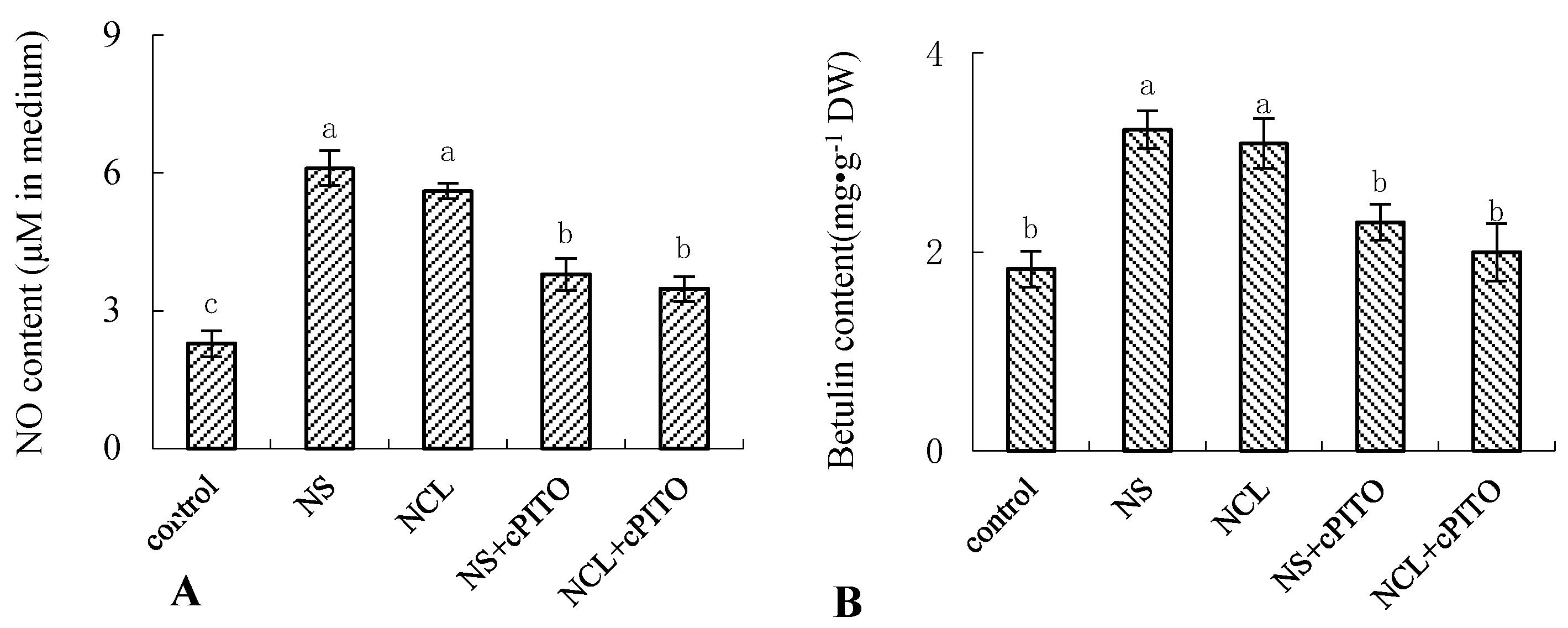
To further investigate the roles of NH4+-induced NO on betulin production, we examined the effect of the NO-specific scavenger cPTIO on NH4+-induced betulin accumulation in birch suspension cells. Figure 6 shows that 10 mmol·L−1 (NH4)2SO4 (NS) and NH4Cl (NCL) enhanced NO and betulin production, respectively. Pretreatment of birch suspension cells with cPTIO reduced NH4+-triggered NO generation (Figure 6A) and blocked NH4+-induced betulin production (Figure 6B).
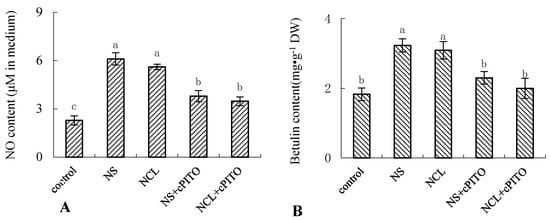
Figure 6.
Effect of NH4+ on the content of NO and betulin in suspension cells of birch. Eight-day-old suspension cells pretreated for 0.5 h with 150 μmol·L−1 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimi-dazoline-1-oxyl-3-oxide (cPTIO) (NO-specific scavenger) were treated with 10 mmol·L−1 (NH4)2SO4 (NS) and 10 mmol·L−1 NH4Cl (NCL) for 12 h, respectively. (A), NO content in culture medium of birch; (B), betulin content in suspension cells of birch.
3. Discussion
Plants do not appear to have specific receptors for PAs; thus, it is particularly interesting to investigate how PAs perform diverse functions in plant cells [21]. Recently, some experiments have shown that Put can induce the production of NO in plants, but the roles and origins of NO induced by Put in plants remain poorly understood.
In our previous report, exogenous Put treatment increased the production of triterpenoids and flavonoids in suspension cells of birch, and exogenous NO donor sodium nitroprussiate also enhanced the accumulation of triterpenoids and the gene expression of LUS, one of the key enzymes in the triterpenoid biosynthesis pathway [18,22]. Betulin is one of the main triterpenoids in the bark of birch trees. In this study, we further verified that Put treatment enhanced betulin production at the metabolic and gene expression levels and confirmed Put promoted NO production. To further clarify the function of NO-induced by Put, 8-day-old suspension cells were treated with Put and cPTIO, a specific scavenger of NO. The results showed that the Put and cPTIO treatment abolished Put-triggered NO generation and blocked Put-induced betulin production and gene expression of LUS. Thus, the above data suggest a crucial role of NO in Put-induced betulin accumulation in birch suspension cells.
NO is produced from a range of enzymatic and nonenzymatic sources in plants [10]. NR and NOS have been known as major enzymatic sources of NO production, although the existence of NOS in plants remains controversial [8,9]. To understand the effect of Put on NO biosynthesis, we analyzed the role of NOS and NR in Put-induced NO biosynthesis. The results showed that Put treatment enhanced the activity of NR and NOS; NR inhibitor NaN3 or NOS inhibitor L-NAME reduced NO and betulin production in birch suspension cells induced by Put. Similar results were also reported in our previous research: NR-dependent NO and NOS-dependent NO mediated betulin production in birch suspension cells induced by a fungal elicitor or hydrogen sulfide [12,18]. Thus, our recent research suggests that NR-dependent NO and NOS-dependent NO mediate betulin production in birch suspension cells.
NO can be induced by NH4+ in plants [23], and NH4+ supply enhanced secondary metabolite biosynthesis, such as catechin and epicatechin in tea plant leaves [24,25]. NO3− and NH4+ are the two primary N sources for plants, and NR is necessary for both the first step of the reduction of NO3− to NH4+ and for the formation of NO in plants [9]. However, the role of NH4+-induced NO and Put -induced NH4+ on secondary metabolite production is unclear. Our results showed that NO scavenger cPTIO reduced NH4+-triggered NO generation and decreased NH4+-induced betulin production; Put treatment enhanced NH4+ production and the activities of GOGAT and GDH, which are the NH4+ assimilation enzymes. Awasthi et al. (2013) also found that Put treatment increased the activities of GOGAT and GDH in mice [26]. Thus, the above data first suggests that NH4+ or NH4+-triggered NO participates in Put-induced betulin accumulation in birch suspension cells, and this suggestion should be further confirmed.
The results of this work demonstrated that NO signaling was involved in Put-induced betulin production of birch suspension cells. However, the data also showed that Put could still stimulate betulin production in the birch suspension cells even though Put-triggered NO accumulation was abolished by cPTIO, which implied that Put-induced betulin production was not completely dependent on NO signaling. In addition to NO, many other signal molecules such as hydrogen peroxide (H2O2), jasmonic acid (JA), and salicylic acid (SA) have also been reported to play roles in secondary metabolite production [16,27,28]. Whether these signal molecules are involved in Put-induced betulin production still needs to be further investigated.
The current study allowed us to conclude that Put-induced NO is essential to betulin production in birch suspension cell cultures, and it may have been derived from NR and NOS biosynthetic pathways and nonenzymatic pathways in NH4+. This may be beneficial to further understanding of the role of Put-elicited betulin biosynthesis in plant cell cultures and in the rational manipulation of secondary metabolism.
4. Materials and Methods
4.1. Plant Cell Culture
The cell line used in the study was developed from the axillary buds of a 30-year-old B. platyphylla Suk tree. Birch suspension cell cultures were cultivated on Nagata–Takebe medium supplemented with 0.1 mg·L−1 6-benzyladenine, 0.01 mg·L−1 thidiazuron, and 20 g·L−1 sucrose [29]. The medium was adjusted to pH 5.6 and then sterilized by autoclaving at 121 °C for 20 min. The suspension culture was maintained in 250 mL Erlenmeyer flasks, with a liquid volume of 100 mL in each flask, and inoculated with 5.0 g fresh weight of 8-day-old cell suspension cultures. The Erlenmeyer flasks were incubated on a rotary shaker (110 rpm) at 25 °C. Illumination was regulated to give 14 h of light [12].
4.2. Chemical Reagents and Treatment
The chemical reagents and their concentrations used in these experiments were as follows: 0.1, 1, and 5 mmol·L−1 putrescine (Put), 10 mmol·L−1 (NH4)2SO4, 10 mmol·L−1 NH4Cl, 150 μmol·L−1 2- (4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline- 1-oxyl-3-oxide (cPTIO; a NO-specific scavenger), 0.5 mmol·L−1 NR inhibitor sodium azide (NaN3), and 3 mmol·L−1 NOS inhibitor NG-nitro-L-Arg methyl ester (L-NAME). Among them, cPTIO was purchased from Sigma Corporation (St Louis, MO, USA); the other chemicals were purchased from Beijing Huagong Biotechnology Institute, China.
Eight-day-old suspension cells were used for the above chemical treatment. The controls were treated with the same volume of distilled water.
4.3. Betulin Estimation
Betulin was extracted from birch suspension cells using a procedure reported by Fan et al. (2014) [18]. Briefly, 0.50 g powder of dried suspension cells was soaked in 20 mL hydrochloric acid:ethanol solution (2:8 by volume). The solution was refluxed for 3 h in a water bath at 90 °C. The filtrate was extracted three times with 15 mL of ether. The combined extracts were evaporated to dryness at 40 °C and redissolved in 2 mL ethanol for HPLC analysis. Betulin content was analyzed by reversed-phase HPLC with ultraviolet detection at 210 nm. The mobile phase consisted of 80:20 (v/v) acetonitrile:water. The flow rate was 1 mL∙min−1. The betulin standard was obtained from Sigma–Aldrich Chemical Corporation (St Louis, MO, USA).
4.4. Detection of NO
Briefly, 2 mL suspension culture medium was used to determine the content of NO by the Greiss reagent method using an assay kit (Nanjing Jiangcheng Biotechnology Institute, China). The absorbance values were determined at the wavelength of 540 nm. The images of NO-induced fluorescence were examined with a confocal laser scanning microscope (Zeiss LSM 510, Mannheim, Germany) using standard filters and collection modalities for visualizing DAF-FM green fluorescence (excitation 495 nm; emission 515 nm) [30]. Image Pro Plus 6.0 software (Media Cybernetics, Bethesda, Rockville, MD, USA) was applied to quantify the fluorescence intensity of NO; mean density = IOD SUM/area sum.
4.5. Detection of NH4+
Fresh suspension cells (2 g) were used to detect concentrations of NH4+, according to the manufacturer’s instructions of commercially available assay kits (Nanjing Jiangcheng Biotechnology Institute, China). NH4+ was extracted in double-distilled water. The absorbance values were determined at the wavelength of 580 nm for NH4+ by a spectrophotometer.
4.6. Determination of Enzyme Activities
First, 2 g of fresh suspension cells was ground with liquid N2 and then resuspended in extraction buffer containing 100 mM HEPES-KOH (pH 7.5), 1 mmol·L−1 EDTA, 10% (v/v) glycerol, 5 mmol·L−1 dithiothreitol, 0.1% TritonX-100, 0.5 mmol·L−1 phenylmethylsulfonyl fluoride, 20 mmol·L−1 FAD, 1 mmol·L−1 leupeptin, 5 mmol·L−1 Na2MoO4, and 1% polyvinylpyrrolidone. Then, after centrifugation at 10,000×g for 20 min at 4 °C, the supernatant was used to determine enzyme activity.
Enzyme activities of glutamine synthase (GS), glutamine-2-oxoglutarate aminotransferase (GOGAT), glutamate dehydrogenase (GDH), and NOS were determined using commercially available assay kits (Nanjing Jiangcheng Biotechnology Institute, Nanjing, China and Beijing Solarbio Science & Technology Co., Ltd., Beijing, China). The absorbance values were recorded at the wavelength of 540, 340, 340, and 530 nm for GS, GOGAT, GDH, and NOS by spectrophotometer, respectively. Enzyme activity was expressed as units per mg of protein.
NR activity was measured by mixing 1 volume of the extract with 5 volumes of prewarmed (25 °C) assay buffer (100 mmol·L−1 HEPES-KOH, pH 7.5, 5 mmol·L−1 KNO3, and 0.25 mmol·L−1 NADH). The reaction was started by the addition of an assay buffer, incubated at 25 °C for 30 min, and then stopped by adding 0.1 mol·L−1 zinc acetate. After 15 min, the tubes were centrifuged at 13,000× g for 5 min. The nitrite produced was measured colorimetrically at 520 nm by adding 1 mL of 1% (w/v) sulfanilamide in 3 mol·L−1 HCl plus 1 mL of 0.02% (v/v) N-(1-naphthyl)-ethylenediamine in distilled water [31]. The protein content of the supernatant was determined according to the method described by Bradford (1976). Bovine serum albumin was used as a standard [32].
4.7. RNA Isolation and Real-Time Quantitative RT-PCR
Total RNA was isolated using the CTAB-based method [33]. The b-tubulin (Tu) and ubiquitin (UbQ) genes were used as references [18]. The TaqMan probes and primers are presented in Table 1. PCR amplification was performed under standard cycling conditions, namely, 94 °C for 30 s (initial polymerase activation), followed by 45 cycles of 94 °C for 12 s (denaturation), 58 °C for 30 s (annealing), and 72 °C for 40 s. Gene expression data were calculated relative to the reference gene following the 2−ΔΔCt method [34].

Table 1.
Sequences of primer pairs for quantitative real-time RT-PCR (qRT-PCR) assay.
4.8. Statistical Analysis
All experiments were conducted in triplicate. Data (mean ± standard error) were statistically analyzed using SPSS version 15.0. Tukey’s test was used for multiple comparisons among means, and different letters show significant differences among means (p < 0.05).
5. Conclusions
In this study, the application of Put significantly enhanced betulin production and gene expression of LUS, one of its synthetic key enzymes in birch suspension cells. Put treatment promoted NO bursts and increased the activity of NOS and NR. However, the above positive inductive effects can be blocked with NO scavenger cPTIO, NR inhibitor NaN3, and NOS inhibitor L-NAME. Put treatment improved the content of NH4+ and its assimilation enzyme activity of GOGAT and GDH. NH4+ supplementation also promoted NO and betulin production. Thus, the data indicate that Put-induced NO is essential to betulin production, and NO may have been derived from NR, NOS, and NH4+ in birch suspension cell cultures induced by Put.
Author Contributions
G.F. and Y.Z. conceived and designed the experiments. Y.L. and T.Z. performed the research. G.F., Y.Z. and B.Z. analyzed the data and wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Fundamental Research Funds for the Central Universities (2572020DY17), the Foundation of State Key Laboratory of Desert and Oasis Ecology, the Xinjiang Institute of Ecology and Geography, the Chinese Academy of Sciences (G2018-02-07), and the Heilongjiang Touyan Innovation Team Program (Tree Genetics and Breeding Innovation Team).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| Put | putrescine |
| LUS | lupeol synthase |
| NO | nitric oxide |
| NR | nitrate reductase |
| NOS | NO synthase |
| cPTIO | 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline- 1-oxyl-3-oxide |
| NaN3 | sodium azide |
| L-NAME | NG-nitro-L-Arg methyl ester |
| GOGAT | glutamine-2-oxoglutarate aminotransferase |
| GDH | glutamate dehydrogenase |
| NS | (NH4)2SO4 |
| NCL | NH4Cl |
References
- Hussaina, S.S.; Ali, M.; Ahmad, M.; Siddique, K.H.M. Polyamines: Natural and engineered abiotic and biotic stress tolerance in plants. Biotechnol. Adv. 2011, 29, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Silveira, V.; Santa-Catarina, C.; Tun, N.N.; Scherer, G.F.E.; Handro, W.; Guerra, M.P.; Floh, E.I.S. Polyamine effects on the endogenous polyamine contents, nitric oxide release, growth and differentiation of embryogenic suspension cultures of Araucaria angustifolia (Bert.) O. Ktze. Plant Sci. 2006, 171, 91–98. [Google Scholar] [CrossRef]
- Handa, A.K.; Mattoo, A.K. Differential and functional interactions emphasize the multiple roles of polyamines in plants. Plant Physiol. Bioch. 2010, 48, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Pál, M.; Ivanovska, B.; Oláh, T.; Tajti, J.; Hamow, K.Á.; Szalai, G.; Khalil, R.; Vanková, R.; Dobrev, P.; Misheva, S.P.; et al. Role of polyamines in plant growth regulation of Rht wheat mutants. Plant Physiol. Bioch. 2019, 137, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Pál, M.; Szalai, G.; Janda, T. Speculation: Polyamines are important in abiotic stress signaling. Plant Sci. 2015, 237, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Q.; Shu, S.; Li, C.C.; Sun, J.; Guo, S.R. Spermidine-mediated hydrogen peroxide signaling enhances the antioxidant capacity of salt-stressed cucumber roots. Plant Physiol. Bioch. 2018, 128, 152–162. [Google Scholar] [CrossRef]
- Benkő, P.; Jee, S.; Kaszler, N.; Fehér, A.; Gémes, K. Polyamines treatment during pollen germination and pollen tube elongation in tobacco modulate reactive oxygen species and nitric oxide homeostasis. J. Plant Physiol. 2020, 244, 153085. [Google Scholar] [CrossRef]
- Domingos, P.; Prado, A.M.; Wong, A.; Gehring, C.; Feijo, J.A. Nitric oxide: A multitasked signaling gas in plants. Mol. Plant 2015, 8, 506–520. [Google Scholar] [CrossRef]
- Castello, F.D.; Nejamkin, A.; Cassia, R.; Correa-Aragunde, N.; Fernández, B.; Foresi, N.; Lombardo, C.; Ramirez, L.; Lamattina, L. The era of nitric oxide in plant biology: Twenty years tying up loose ends. Nitric Oxide 2019, 85, 17–27. [Google Scholar] [CrossRef]
- Santolini, J.; André, F.; Jeandroz, S.; Wendehenne, D. Nitric oxide synthase in plants: Where do we stand? Nitric Oxide 2017, 63, 30–38. [Google Scholar] [CrossRef]
- Cao, X.C.; Zhu, C.Q.; Zhong, C.; Zhang, J.H.; Wu, L.H.; Jin, Q.Y.; Ma, Q.X. Nitric oxide synthase-mediated early nitric oxide burst alleviates water stress-induced oxidative damage in ammonium-supplied rice roots. BMC Plant Biol. 2019, 19, 108. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Jiang, Y.; Yu, Z.Y.; Huang, Y.T.; Zhan, Y.G.; Fan, G.Z. H2S-induced NO/SNO positively promotes betulin production in Betula Platyphylla. Ind. Crops Prod. 2019, 140, 111608. [Google Scholar] [CrossRef]
- Krasuska, U.; Ciacka, K.; Gniazdowska, A. Nitric oxide-polyamines cross-talk during dormancy release and germination of apple embryos. Nitric Oxide 2017, 68, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Chamizo-Ampudia, A.; Sanz-Luque, E.; Llamas, A.; Galvan, A.; Fernandez, E. Nitrate reductase regulates plant nitric oxide homeostasis. Trends Plant Sci. 2017, 22, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Chandran, H.; Meena, M.; Barupal, T.; Sharma, K. Plant tissue culture as a perpetual source for production of industrially important bioactive compounds. Biotech. Rep. 2020, 26, e00450. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Bhattacharya, S.; Khosla, P.K.; Puri, S. Improving production of plant secondary metabolites through biotic and abiotic elicitation. J. Appl. Res. Med. Aroma. 2019, 12, 1–12. [Google Scholar] [CrossRef]
- Zhao, F.Q.; Mai, Q.Q.; Ma, J.H.; Xu, M.; Wang, X.; Cui, T.T.; Qiu, F.; Han, G. Triterpenoids from Inonotus obliquus and their antitumor activities. Fitoterapia 2015, 101, 34–40. [Google Scholar] [CrossRef]
- Fan, G.Z.; Liu, Y.T.; Wang, X.D.; Zhan, Y.G. Cross-talk of polyamines and nitric oxide in endophytic fungus-induced betulin production in Betula platyphylla plantlets. Trees-Struct. Funct. 2014, 28, 635–641. [Google Scholar] [CrossRef]
- Fan, G.Z.; Zhai, Q.L.; Zhan, Y.G. Gene expression of lupeol synthase and biosynthesis of nitric oxide in cell suspension cultures of Betula platyphylla in response to a Phomopsis elicitor. Plant Mol. Biol. Rep. 2013, 31, 296–302. [Google Scholar] [CrossRef]
- Navarro-León, E.; Barrameda-Medina, Y.; Lentini, M.; Esposito, S. Comparative study of Zn deficiency in L. sativa and B. oleracea plants NH4+ assimilation and nitrogen derived protective compounds. Plant Sci. 2016, 248, 8–16. [Google Scholar] [CrossRef]
- Yang, B.N.; Wu, J.Z.; Gao, F.M.; Wang, J.; Su, G.X. Polyamine-induced nitric oxide generation and its potential requirement for peroxide in suspension cells of soybean cotyledon node callus. Plant Physiol. Bioch. 2014, 79, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C.; Zhang, Y.G.; Wang, X.D.; Liu, Y.T.; Li, D.; Rong, L.Z.; Fan, G.Z. Effect of polyamine on growth of Betula platyphylla suspension cells and triterpenoid accumulation. Chin. Tradit. Herbal Drugs 2013, 44, 463–467. [Google Scholar]
- Zhu, X.F.; Dong, X.Y.; Wu, Q.; Shen, R.F. Ammonium regulates Fe deficiency responses by enhancing nitric oxide signaling in Arabidopsis thaliana. Planta 2019, 250, 1089–1102. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.Y.; Burgos, A.; Zhang, Q.F.; Tang, D.D.; Shi, Y.Z.; Ma, L.F.; Yi, X.Y.; Ruan, J.Y. Analyses of transcriptome profiles and selected metabolites unravel the metabolic response to NH4+ and NO3− as signaling molecules in tea plant (Camellia sinensis L.). Sci. Hortic. 2017, 218, 293–303. [Google Scholar] [CrossRef]
- Radušienė, J.; Marksa, M.; Ivanauskas, L.; Jakštas, V.; Çalişkan, Ö.; Kurt, D.; Odabaş, M.S.; Çirak, C. Effect of nitrogen on herb production, secondary metabolites and antioxidant activities of Hypericum pruinatum under nitrogen application. Ind. Crops. Prod. 2019, 139, 111519. [Google Scholar] [CrossRef]
- Awasthi, V.; Gautam, I.K.; Sengar, R.S.; Garg, S.K. Influence of putrescine on enzymes of ammonium assimilation in maize seedling. Am. J. Plant Sci. 2013, 4, 297–301. [Google Scholar] [CrossRef]
- Zhao, J.; Davis, L.C.; Verpoorte, R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv. 2005, 23, 283–333. [Google Scholar] [CrossRef]
- Li, Y.Q.; Kong, D.X.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Bioch. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Nagata, T.; Takebe, I. Cell Wall Regeneration and Cell Division in Isolated Tobacco Mesophyll Protoplasts. Planta 1970, 92, 301–308. [Google Scholar] [CrossRef]
- Hong, W.Y.; Yang, T.W.; Wang, C.M.; Syu, J.H.; Lin, Y.C.; Meng, H.F.; Tsai, M.J.; Cheng, H.; Zan, H.W.; Horng, S.F. Conversion of absorption to fluorescence probe in solid-state sensor for nitric oxide and nitrite. Org. Electron. 2013, 14, 1136–1141. [Google Scholar] [CrossRef]
- Zhao, M.G.; Chen, L.; Zhang, L.L.; Zhang, W.H. Nitric reductase-dependent nitric oxide production is involved in cold acclimation and freezing tolerance in Arabidopsis. Plant Physiol. 2009, 151, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Zeng, F.S.; Nan, N.; Zhan, Y.G. Extraction of total RNA from mature leaves rich in polysaccharides and secondary metabolites of Betula platyphylla Suk. Plant Physiol. Commun. 2007, 43, 913–916. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆Ct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).