Moderate- to High-Severity Disturbances Shaped the Structure of Primary Picea Abies (L.) Karst. Forest in the Southern Carpathians
Abstract
1. Introduction
- What was the range of past natural disturbance variability of montane spruce forests in the Southern Carpathians?
- Is there a relationship between past disturbance regimes and present structural characteristics?
2. Materials and Methods
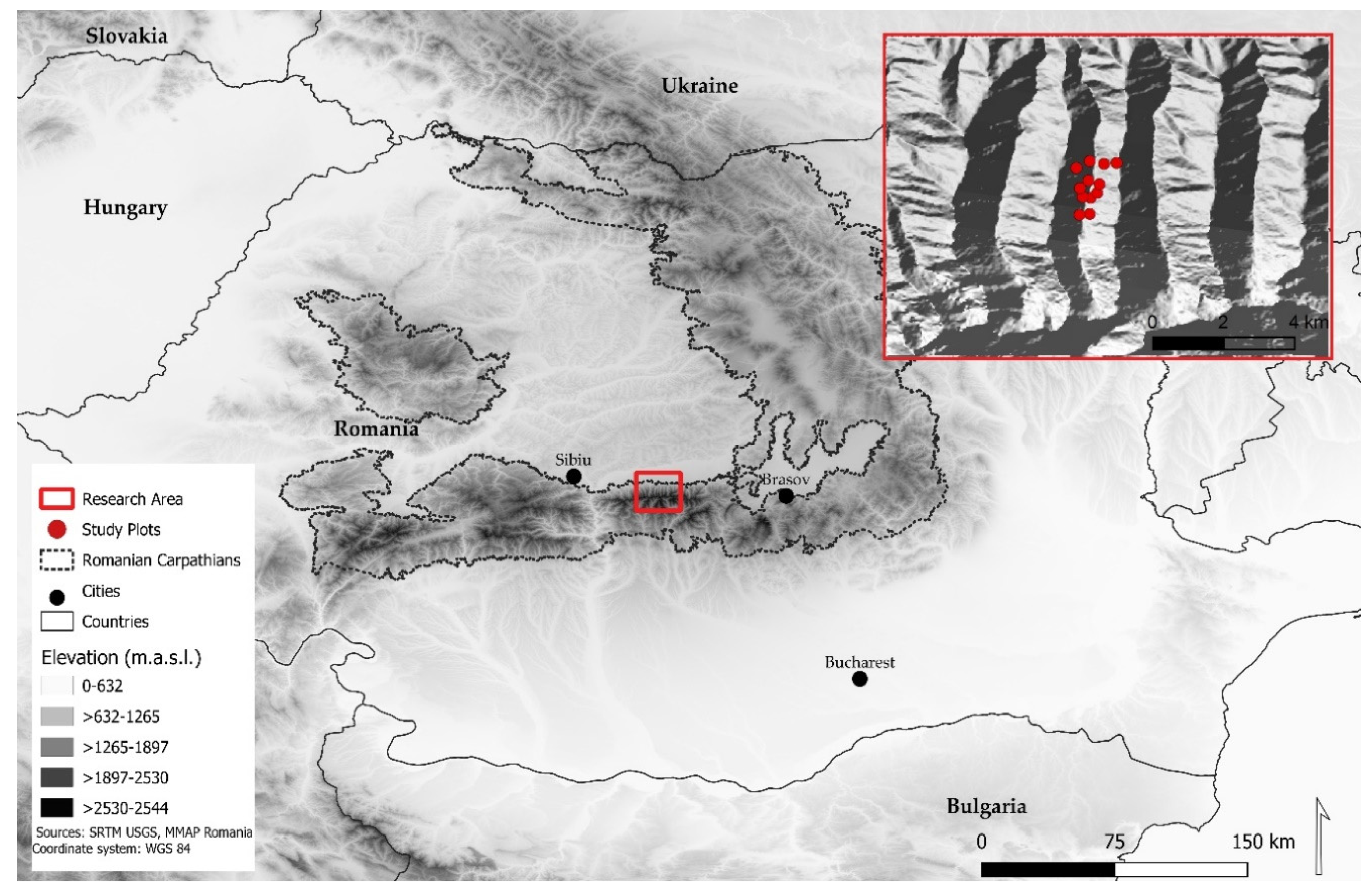
2.1. Study Area
2.2. Experimental Design
2.3. Data Measurements
2.4. Data Analysis
2.4.1. Structure Analysis
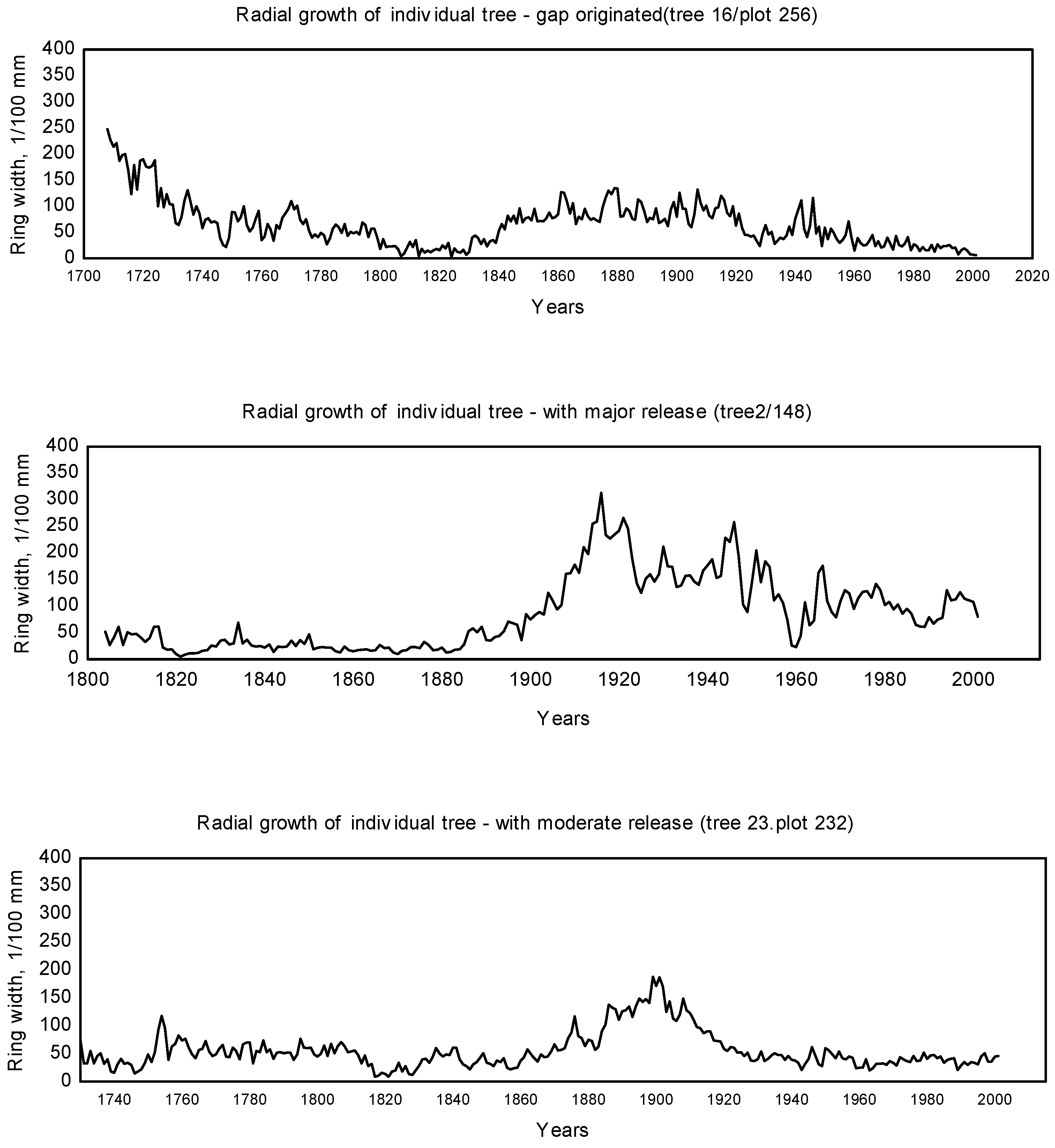
2.4.2. Dendrochronological Analysis
3. Results
3.1. Disturbance Patterns
3.2. Structure
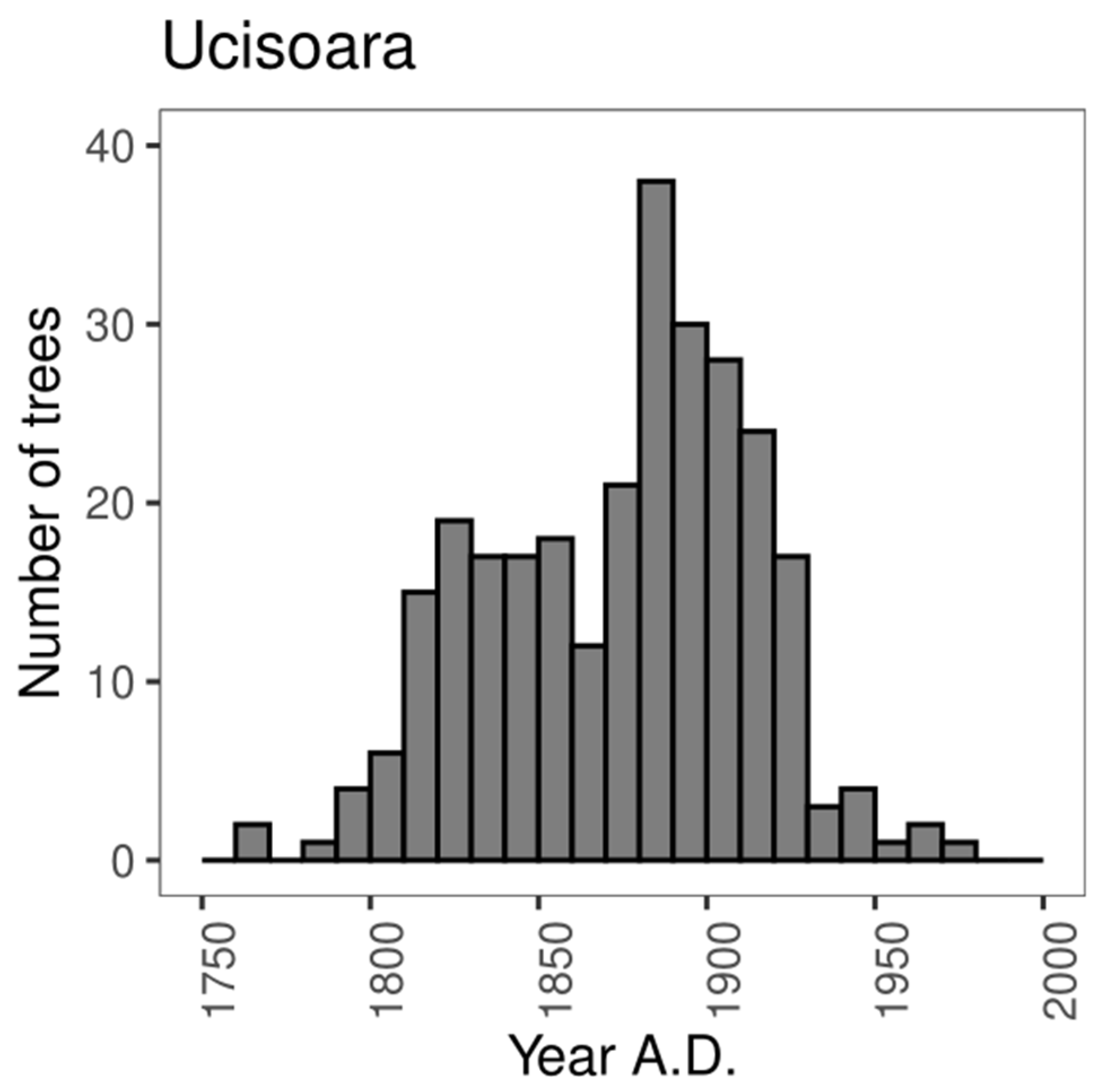
3.2.1. Age Structure
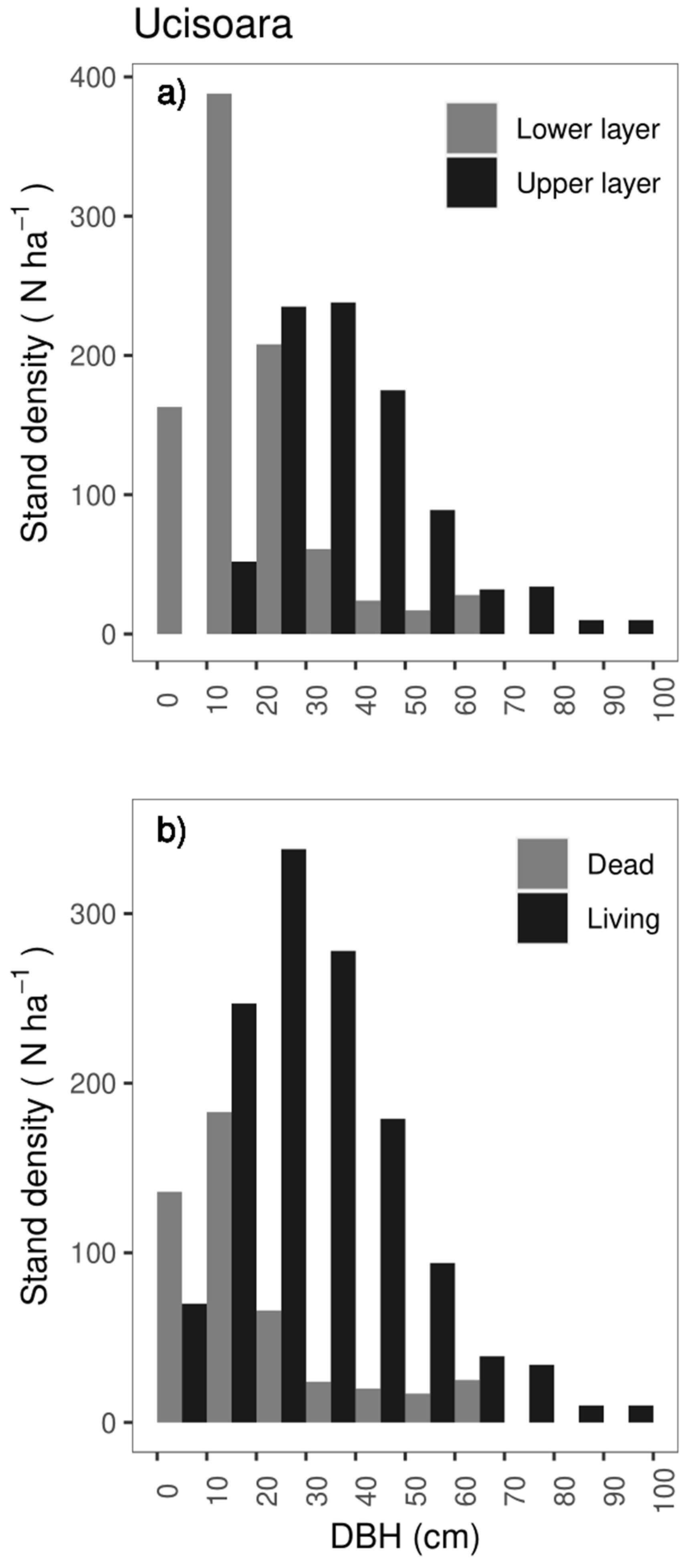
3.2.2. Tree Characteristics
3.2.3. Deadwood Structure
3.2.4. Regeneration
4. Discussion
4.1. Disturbance Regime
4.2. Stand Structure
4.3. Management Implications
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Plots | Mean Elevation | Mean Slope | Mean Tree Density | DBH | Mean Height | Mean Basal Area | MEAN Age | Max Age |
|---|---|---|---|---|---|---|---|---|
| m.a.s.l. | ° | N·ha−1 | cm | m | m2·ha−1 | years | years | |
| P1 | 1269 | 34.6 | 420 | 42.5 | 26.3 | 68.6 | 126 | 160 |
| P20 | 1519 | 36.1 | 720 | 30.9 | 22.4 | 62.1 | 118 | 177 |
| P23 | 1506 | 36.4 | 450 | 43.7 | 25.6 | 70.9 | 161 | 283 |
| P91 | 1426 | 32.3 | 760 | 32.3 | 23.1 | 66.6 | 185 | 216 |
| P94 | 1369 | 37.5 | 1320 | 21.2 | 18.2 | 50.5 | 90 | 120 |
| P123 | 1417 | 38.2 | 230 | 38.4 | 18.5 | 31.6 | 131 | 220 |
| P139 | 1457 | 43.2 | 450 | 38.8 | 26.4 | 61.5 | 142 | 219 |
| P148 | 1361 | 38.8 | 360 | 35.4 | 23.4 | 42.5 | 167 | 319 |
| P194 | 1316 | 38.1 | 430 | 37.0 | 22.5 | 54.0 | 168 | 218 |
| P232 | 1498 | 35.6 | 750 | 28.4 | 21.5 | 50.4 | 154 | 343 |
| P256 | 1307 | 40.5 | 520 | 36.6 | 23.8 | 61.1 | 131 | 305 |
| P262 | 1499 | 37.5 | 830 | 28.6 | 20.7 | 59.8 | 99 | 115 |
Appendix B
References
- Seidl, R.; Schelhaas, M.-J.; Rammer, W.; Verkerk, P.J. Increasing forest disturbances in Europe and their impact on carbon storage. Nat. Clim. Chang. 2014, 4, 806–810. [Google Scholar] [CrossRef]
- Navarro, L.; Morin, H.; Bergeron, Y.; Girona, M.M. Changes in Spatiotemporal Patterns of 20th Century Spruce Budworm Outbreaks in Eastern Canadian Boreal Forests. Front. Plant Sci. 2018, 9, 1905. [Google Scholar] [CrossRef] [PubMed]
- Diaci, J. Virgin Forests and Forest Reserves in Central and East European Countries; Department of Forestry and Renewable Forest Resources, Biotechnology Faculty, University of Ljubljana: Ljubljana, Slovenia, 1998; ISBN 9616020218. [Google Scholar]
- Knorn, J.; Kuemmerle, T.; Radeloff, V.C.; Keeton, W.S.; Gancz, V.; Biriş, I.-A.; Svoboda, M.; Griffiths, P.; Hagatis, A.; Hostert, P. Continued loss of temperate old-growth forests in the Romanian Carpathians despite an increasing protected area network. Environ. Conserv. 2012, 40, 182–193. [Google Scholar] [CrossRef]
- Paillet, Y.; Bergès, L.; Hjältén, J.; Ódor, P.; Avon, C.; Bernhardt-Römermann, M.; Bijlsma, R.-J.; De Bruyn, L.; Fuhr, M.; Grandin, U.; et al. Biodiversity Differences between Managed and Unmanaged Forests: Meta-Analysis of Species Richness in Europe. Conserv. Biol. 2010, 24, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Veen, P.; Fanta, J.; Raev, I.; Biriş, I.-A.; De Smidt, J.; Maes, B. Virgin forests in Romania and Bulgaria: Results of two national inventory projects and their implications for protection. Biodivers. Conserv. 2010, 19, 1805–1819. [Google Scholar] [CrossRef]
- Angelstam, P.K. Maintaining and restoring biodiversity in European boreal forests by developing natural disturbance regimes. J. Veg. Sci. 1998, 9, 593–602. [Google Scholar] [CrossRef]
- Angermeier, P.L. The Natural Imperative for Biological Conservation. Conserv. Biol. 2000, 14, 373–381. [Google Scholar] [CrossRef]
- Wesołowski, T. Lessons from long-term hole-nester studies in a primeval temperate forest. J. Ornithol. 2007, 148, 395–405. [Google Scholar] [CrossRef]
- Franklin, J.F.; Spies, T.; Van Pelt, R.; Carey, A.B.; Thornburgh, D.; Berg, D.R.; Lindenmayer, D.B.; Harmon, M.; Keeton, W.S.; Shaw, D.C.; et al. Disturbances and structural development of natural forest ecosystems with silvicultural implications, using Douglas-fir forests as an example. For. Ecol. Manag. 2002, 155, 399–423. [Google Scholar] [CrossRef]
- Oliver, C.D.; Larson, B.C. Forest Stand Dynamics: Updated Edition; John Wiley and Sons: New York, NY, USA, 1996; ISBN 0471138339. [Google Scholar]
- Martin, M.; Girona, M.M.; Morin, H. Driving factors of conifer regeneration dynamics in eastern Canadian boreal old-growth forests. PLoS ONE 2020, 15, e0230221. [Google Scholar] [CrossRef]
- Raffa, K.F.; Aukema, B.H.; Bentz, B.J.; Carroll, A.L.; Hicke, J.A.; Turner, M.G.; Romme, W.H. Cross-scale Drivers of Natural Disturbances Prone to Anthropogenic Amplification: The Dynamics of Bark Beetle Eruptions. Bioscience 2008, 58, 501–517. [Google Scholar] [CrossRef]
- Schelhaas, M.-J.; Nabuurs, G.-J.; Schuck, A. Natural Disturbances in the European Forests in the 19th and 20th Centuries. Glob. Chang. Biol. 2003, 9, 1620–1633. [Google Scholar] [CrossRef]
- Svoboda, M.; Janda, P.; Bače, R.; Fraver, S.; Nagel, T.A.; Rejzek, J.; Mikoláš, M.; Douda, J.; Boublík, K.; Šamonil, P.; et al. Landscape-level variability in historical disturbance in primary Picea abies mountain forests of the Eastern Carpathians, Romania. J. Veg. Sci. 2014, 25, 386–401. [Google Scholar] [CrossRef]
- Bergeron, O.; Margolis, H.A.; Coursolle, C. Forest floor carbon exchange of a boreal black spruce forest in eastern North America. Biogeosciences 2009, 6, 1849–1864. [Google Scholar] [CrossRef]
- Lavoie, J.; Girona, M.M.; Morin, H. Vulnerability of Conifer Regeneration to Spruce Budworm Outbreaks in the Eastern Canadian Boreal Forest. Forests 2019, 10, 850. [Google Scholar] [CrossRef]
- Girona, M.M.; Morin, H.; Lussier, J.-M.; Ruel, J.-C. Post-cutting Mortality Following Experimental Silvicultural Treatments in Unmanaged Boreal Forest Stands. Front. For. Glob. Chang. 2019, 2, 2. [Google Scholar] [CrossRef]
- Kozák, D.; Mikoláš, M.; Svitok, M.; Bače, R.; Paillet, Y.; Larrieu, L.; Nagel, T.A.; Begović, K.; Čada, V.; Diku, A.; et al. Profile of tree-related microhabitats in European primary beech-dominated forests. For. Ecol. Manag. 2018, 429, 363–374. [Google Scholar] [CrossRef]
- Kozák, D.; Svitok, M.; Wiezik, M.; Mikoláš, M.; Thorn, S.; Buechling, A.; Hofmeister, J.; Matula, R.; Trotsiuk, V.; Bače, R.; et al. Historical Disturbances Determine Current Taxonomic, Functional and Phylogenetic Diversity of Saproxylic Beetle Communities in Temperate Primary Forests. Ecosystems 2020, 1–19. [Google Scholar] [CrossRef]
- Sabatini, F.M.; Burrascano, S.; Keeton, W.S.; Levers, C.; Lindner, M.; Pötzschner, F.; Verkerk, P.J.; Bauhus, J.; Buchwald, E.; Chaskovsky, O.; et al. Where are Europe’s last primary forests? Divers. Distrib. 2018, 24, 1426–1439. [Google Scholar] [CrossRef]
- Mikoláš, M.; Svitok, M.; Bollmann, K.; Reif, J.; Bače, R.; Janda, P.; Trotsiuk, V.; Čada, V.; Vítková, L.; Teodosiu, M.; et al. Mixed-severity natural disturbances promote the occurrence of an endangered umbrella species in primary forests. For. Ecol. Manag. 2017, 405, 210–218. [Google Scholar] [CrossRef]
- Ministerul Apelor și Pădurilor, România. Study on Virgin Forests. Available online: http://apepaduri.gov.ro/paduri-virgine/ (accessed on 8 December 2020).
- Timber Regulation, Romania. Available online: https://lemncontrolat.ro/interactive-maps/map-of-protected-natural-areas-in-romania/ (accessed on 8 December 2020).
- Mikoláš, M.; Ujházy, K.; Jasík, M.; Wiezik, M.; Gallay, I.; Polák, P.; Vysoký, J.; Čiliak, M.; Meigs, G.W.; Svoboda, M.; et al. Primary forest distribution and representation in a Central European landscape: Results of a large-scale field-based census. For. Ecol. Manag. 2019, 449, 117466. [Google Scholar] [CrossRef]
- Damato, A.W.; Orwig, D.A. Stand and landscape-level disturbance dynamics in old-growth forests in western massachusetts. Ecol. Monogr. 2008, 78, 507–522. [Google Scholar] [CrossRef]
- Čada, V.; Svoboda, M.; Janda, P. Dendrochronological reconstruction of the disturbance history and past development of the mountain Norway spruce in the Bohemian Forest, central Europe. For. Ecol. Manag. 2013, 295, 59–68. [Google Scholar] [CrossRef]
- Janda, P.; Trotsiuk, V.; Mikoláš, M.; Bače, R.; Nagel, T.A.; Seidl, R.; Seedre, M.; Morrissey, R.C.; Kucbel, S.; Jaloviar, P.; et al. The historical disturbance regime of mountain Norway spruce forests in the Western Carpathians and its influence on current forest structure and composition. For. Ecol. Manag. 2017, 388, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Schurman, J.S.; Trotsiuk, V.; Bače, R.; Čada, V.; Fraver, S.; Janda, P.; Kulakowski, D.; Labusova, J.; Mikoláš, M.; Nagel, T.A.; et al. Large-scale disturbance legacies and the climate sensitivity of primary Picea abies forests. Glob. Chang. Biol. 2018, 24, 2169–2181. [Google Scholar] [CrossRef] [PubMed]
- Čada, V.; Trotsiuk, V.; Janda, P.; Mikoláš, M.; Bače, R.; Nagel, T.A.; Morrissey, R.C.; Tepley, A.J.; Vostarek, O.; Begović, K.; et al. Quantifying natural disturbances using a large-scale dendrochronological reconstruction to guide forest management. Ecol. Appl. 2020, 30. [Google Scholar] [CrossRef] [PubMed]
- Șofletea, N.; Curtu, L. Dendrologie; Editura Universității Transilvania: Brașov, Romania, 2007; pp. 40–58. ISBN 978-973-635-885-2. [Google Scholar]
- NASA Portal. Shuttle Radar Topography Mission. Available online: https://www2.jpl.nasa.gov/srtm/ (accessed on 5 September 2020).
- WWF. WWF National Catalogue of Virgin Forests. Available online: https://wwf-ro.maps.arcgis.com/apps/webappviewer/index.html?id=31831d9db12c4e32ab5456c13952bfef& (accessed on 5 January 2020).
- Field Map Data Collector. Available online: https://www.fieldmap.cz/?page=FMDC2 (accessed on 15 August 2020).
- Van Wagner, C.E. The line intersect method in forest fuel sampling. For. Sci. 1968, 14, 20–26. [Google Scholar]
- Marshall, P.L.; Davis, G.; LeMay, V.M. Using Line Intersect Sampling for Coarse Woody Debris; Research Section; Vancouver Forest Region: Nanaimo, BC, Canada, 2000. [Google Scholar]
- Rubino, D.; McCarthyz, B. Comparative analysis of dendroecological methods used to assess disturbance events. Dendrochronologia 2004, 21, 97–115. [Google Scholar] [CrossRef]
- Girona, M.M.; Morin, H.; Lussier, J.-M.; Walsh, D. Radial Growth Response of Black Spruce Stands Ten Years after Experimental Shelterwoods and Seed-Tree Cuttings in Boreal Forest. Forests 2016, 7, 240. [Google Scholar] [CrossRef]
- Frelich, L.E.; Lorimer, C.G. Natural Disturbance Regimes in Hemlock-Hardwood Forests of the Upper Great Lakes Region. Ecol. Monogr. 1991, 61, 145–164. [Google Scholar] [CrossRef]
- Lorimer, C.G.; Frelich, L.E. A methodology for estimating canopy disturbance frequency and intensity in dense temperate forests. Can. J. For. Res. 1989, 19, 651–663. [Google Scholar] [CrossRef]
- Janda, P.; Tepley, A.J.; Schurman, J.S.; Brabec, M.; Nagel, T.A.; Bače, R.; Begović, K.; Chaskovskyy, O.; Čada, V.; Dušátko, M.; et al. Drivers of basal area variation across primary late-successional Picea abies forests of the Carpathian Mountains. For. Ecol. Manag. 2019, 435, 196–204. [Google Scholar] [CrossRef]
- Duncan, R.P. An Evaluation of Errors in Tree Age Estimates Based on Increment Cores in kahikatea (Dacrycarpus dacrydioides). N. Z. Nat. Sci. 1989, 16, 31–37. [Google Scholar]
- Rinntech, Technology for Tree and Wood Analysis. Available online: http://www.rinntech.de/ (accessed on 8 October 2020).
- Yamaguchi, D.K. A simple method for cross-dating increment cores from living trees. Can. J. For. Res. 1991, 21, 414–416. [Google Scholar] [CrossRef]
- Holmes, R.L. Computer-Assisted Quality Control in Tree-Ring Dating and Measurement. Tree Ring Bull. 1983, 43, 51–57. [Google Scholar]
- Giurgiu, V.; Decei, I.; Drăghiciu, D. Dendrometrics Methods and Tables; Ceres Publishing House: Bucharest, Romania, 2004. [Google Scholar]
- Van Wagner, C.E. Practical Aspects of the Line Intersect Method; Petawawa National Forestry Institute: Chalk River, ON, Canada, 1982; Volume 12, ISBN 0-662-11816-2. [Google Scholar]
- IBM SPSS Statistics for Windows, version 24.0; IBM Corp: Armonk, NY, USA, 2016.
- Trotsiuk, V.; Svoboda, M.; Janda, P.; Mikolas, M.; Bace, R.; Rejzek, J.; Samonil, P.; Chaskovskyy, O.; Korol, M.; Myklush, S. A mixed severity disturbance regime in the primary Picea abies (L.) Karst. forests of the Ukrainian Carpathians. For. Ecol. Manag. 2014, 334, 144–153. [Google Scholar] [CrossRef]
- Hosmer, D.W., Jr.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression; John Wiley & Sons: Hoboken, NJ, USA, 2013; Volume 398, ISBN 0-470-58247-2. [Google Scholar]
- Fraver, S.; White, A.S. Identifying growth releases in dendrochronological studies of forest disturbance. Can. J. For. Res. 2005, 35, 1648–1656. [Google Scholar] [CrossRef]
- Tjoelker, M.G.; Boratynski, A.; Bugala, W. Biology and Ecology of Norway Spruce; Springer Science & Business Media: Dordrecht, The Netherlands, 2007; Volume 78, ISBN 1-4020-4840-8. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015; Available online: http://www.R-project.org (accessed on 5 June 2020).
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. Nlme: Linear and Nonlinear Mixed Effects Models, R Package Version 3.1-120; 2015. Available online: https://cran.r-project.org/web/packages/nlme/ (accessed on 10 July 2020).
- Kulakowski, D.; Bebi, P. Range of Variability of unmanaged subalpine forests. Forum Sci. For. Prot. Nat. Hazards 2004, 2004, 47–54. [Google Scholar]
- Rammig, A.; Fahse, L.; Bugmann, H.; Bebi, P. Forest regeneration after disturbance: A modelling study for the Swiss Alps. For. Ecol. Manag. 2006, 222, 123–136. [Google Scholar] [CrossRef]
- Zielonka, T.; Holeksa, J.; Fleischer, P.; Kapusta, P. A tree-ring reconstruction of wind disturbances in a forest of the Slovakian Tatra Mountains, Western Carpathians. J. Veg. Sci. 2010, 21, 31–42. [Google Scholar] [CrossRef]
- Bauhus, J.; Puettmann, K.; Messier, C. Silviculture for old-growth attributes. For. Ecol. Manag. 2009, 258, 525–537. [Google Scholar] [CrossRef]
- Holeksa, J.; Saniga, M.; Szwagrzyk, J.; Dziedzic, T.; Ferenc, S.; Wodka, M. Altitudinal variability of stand structure and regeneration in the subalpine spruce forests of the Pol’ana biosphere reserve, Central Slovakia. Eur. J. For. Res. 2007, 126, 303–313. [Google Scholar] [CrossRef]
- Meigs, G.W.; Morrissey, R.C.; Bače, R.; Chaskovskyy, O.; Čada, V.; Després, T.; Donato, D.C.; Janda, P.; Lábusová, J.; Després, T.; et al. More ways than one: Mixed-severity disturbance regimes foster structural complexity via multiple developmental pathways. For. Ecol. Manag. 2017, 406, 410–426. [Google Scholar] [CrossRef]
- LaMedica, S.; Lingua, E.; Popa, I.; Motta, R.; Carrer, M. Spatial structure in four Norway spruce stands with different management history in the Alps and Carpathians. Silva Fenn. 2011, 45, 865–873. [Google Scholar] [CrossRef]
- Trotsiuk, V.; Hobi, M.L.; Commarmot, B. Age structure and disturbance dynamics of the relic virgin beech forest Uholka (Ukrainian Carpathians). For. Ecol. Manag. 2012, 265, 181–190. [Google Scholar] [CrossRef]
- Brang, P. Virgin Forests as a Knowledge Source for Central European Silviculture: Reality or Myth? Forest Snow Landsc. Res. 2005, 79, 19–32. [Google Scholar]
- Merganičová, K.; Merganič, J.; Svoboda, M.; Bače, R.; Šebeň, V. Deadwood in Forest Ecosystems. In Forest Ecosystems—More Than Just Trees; InTech: Rijeka, Croatia, 2012; pp. 81–108. [Google Scholar] [CrossRef]
- Lombardi, F.; Lasserre, B.; Tognetti, R.; Marchetti, M. Deadwood in Relation to Stand Management and Forest Type in Central Apennines (Molise, Italy). Ecosystems 2008, 11, 882–894. [Google Scholar] [CrossRef]
- Nichiforel, L. Silvicultură Pentru Învăţământ la Distanţă; Facultatea de Silvicultură, Universiatatea Ștefan cel Mare: Suceava, Romania, 2015; pp. 2014–2015. [Google Scholar]
- Michalová, Z.; Morrissey, R.C.; Wohlgemuth, T.; Bače, R.; Fleischer, P.; Svoboda, M. Salvage-Logging after Windstorm Leads to Structural and Functional Homogenization of Understory Layer and Delayed Spruce Tree Recovery in Tatra Mts., Slovakia. Forests 2017, 8, 88. [Google Scholar] [CrossRef]
- Thorn, S.; Bässler, C.; Brandl, R.; Burton, P.J.; Cahall, R.; Campbell, J.L.; Castro, J.; Choi, C.-Y.; Cobb, T.; Donato, D.C.; et al. Impacts of salvage logging on biodiversity: A meta-analysis. J. Appl. Ecol. 2018, 55, 279–289. [Google Scholar] [CrossRef]
- Thorn, S.; Chao, A.; Georgiev, K.B.; Müller, J.; Bässler, C.; Campbell, J.L.; Castro, J.; Chen, Y.-H.; Choi, C.-Y.; Cobb, T.P.; et al. Estimating retention benchmarks for salvage logging to protect biodiversity. Nat. Commun. 2020, 11, 1–8. [Google Scholar] [CrossRef]
- Svoboda, M.; Fraver, S.; Janda, P.; Bače, R.; Zenáhlíková, J. Natural development and regeneration of a Central European montane spruce forest. For. Ecol. Manag. 2010, 260, 707–714. [Google Scholar] [CrossRef]
- Kulakowski, D.; Seidl, R.; Holeksa, J.; Kuuluvainen, T.; Nagel, T.A.; Panayotov, M.; Svoboda, M.; Thorn, S.; Vacchiano, G.; Whitlock, C.; et al. A walk on the wild side: Disturbance dynamics and the conservation and management of European mountain forest ecosystems. For. Ecol. Manag. 2017, 388, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Kuuluvainen, T.; Aakala, T. Natural forest dynamics in boreal Fennoscandia: A review and classification. Silva Fenn. 2011, 45, 823–841. [Google Scholar] [CrossRef]
- Bengtsson, J.; Nilsson, S.G.; Franc, A.; Menozzi, P. Biodiversity, disturbances, ecosystem function and management of European forests. For. Ecol. Manag. 2000, 132, 39–50. [Google Scholar] [CrossRef]
- North, M.; Keeton, W.S. Emulating Natural Disturbance Regimes: An Emerging Approach for Sustainable Forest Management. In Patterns and Processes in Forest Landscapes; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2008; pp. 341–372. [Google Scholar]
- Gustafsson, L.; Bauhus, J.; Asbeck, T.; Augustynczik, A.L.D.; Basile, M.; Frey, J.; Gutzat, F.; Hanewinkel, M.; Helbach, J.; Jonker, M.; et al. Retention as an integrated biodiversity conservation approach for continuous-cover forestry in Europe. Ambio 2020, 49, 85–97. [Google Scholar] [CrossRef]
- Vuidot, A.; Paillet, Y.; Archaux, F.; Gosselin, F. Influence of tree characteristics and forest management on tree microhabitats. Biol. Conserv. 2011, 144, 441–450. [Google Scholar] [CrossRef]
- Sabatini, F.M.; Keeton, W.S.; Lindner, M.; Svoboda, M.; Verkerk, P.J.; Bauhus, J.; Bruelheide, H.; Burrascano, S.; Debaive, N.; Duarte, I.; et al. Protection gaps and restoration opportunities for primary forests in Europe. Divers. Distrib. 2020, 26, 1646–1662. [Google Scholar] [CrossRef]


| Decay Classes | Volume of Deadwood (m3·ha−1) | ||
|---|---|---|---|
| Downed Deadwood | Standing Deadwood | Total | |
| 1. hard wood, completely covered with bark, fresh phloem sometimes present | 6.92 | 0 | 6.92 |
| 2. wood mostly hard, most of the bark left, but no fresh phloem present. | 14.10 | 13.66 | 27.76 |
| 3. wood partly decayed on the surface or in the center, large piece of bark usually loosened or detached, branches still present. | 21.90 | 8.08 | 29.98 |
| 4. most of the wood soft, the central parts can remain hard, while the surface layers of the wood can be missing. | 16.92 | 1.05 | 17.97 |
| 5. wood very soft, usually covered by field-layer vegetation. | 1.47 | 1.23 | 2.69 |
| Total (mean ± SE) max.–min. | 61.31 ± 14.03 (17.54–163.85) | 24.01 ± 4.75 (5.72–61.2) | 85.32 ± 12.55 (38.99–168.57) |
| Layer | Basal Area (m2·ha−1) | Density (Trees Per ha) | DBH (cm) | Height (m) | |
|---|---|---|---|---|---|
| Upper | mean (± SE) | 48.5 ± 3.46 | 389.2 ± 55.47 | 41.25 ± 2.38 | 26.29 ± 1.3 |
| min.–max. | (25.1–65.3) | (110–830) | (24.8–52.4) | (19.73–34.4) | |
| Lower | mean (± SE) | 8.13 ± 0.73 | 214.2 ± 35.03 | 22.26 ± 1.15 | 12.86 ± 1.97 |
| min.–max. | 3.3–13.2 | 70–490 | 14.82–31.15 | 8.5–19.4 | |
| Total | mean (± SE) | 65.54 ± 3.3 | 603.3 ± 84.4 | 31.75 ± 1.62 | 19.57 ± 1.43 |
| 31.6–70.95 | 230–1320 | 19.8–39.2 | 11.21–27.7 | ||
| Region | Altitude | Mean Temperature | Mean Precipitation | Basal Area | Stem Density | DBH | Coarse Woody Debris |
|---|---|---|---|---|---|---|---|
| m.a.s.l. | °C | mm | m2 ha−1 | N ha−1 | cm | m3 ha−1 | |
| Slovakia [59] | 1350 | 2.8 | 1105 | 41 | 290 | 42.4 | 144 |
| Romania [61] | 1250 | 4.9 | 755 | 42.1 | 348 | 39.2 | - |
| Romania (Călimani) [15] | 1484–1626 | 2.4–4.0 | 1100–1650 | 55.1 | 565 | 24.3–39.7 | - |
| Romania (Giumalău) [15] | 1430 | 2.4–4.0 | 1100–1650 | 47.3 | 518 | 32.3 | - |
| Valea Ucișoarei Present study | 1269–1519 | 4.5 | 1000 | 65.54 | 603 | 31.8 | 85.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spînu, A.P.; Petrițan, I.C.; Mikoláš, M.; Janda, P.; Vostarek, O.; Čada, V.; Svoboda, M. Moderate- to High-Severity Disturbances Shaped the Structure of Primary Picea Abies (L.) Karst. Forest in the Southern Carpathians. Forests 2020, 11, 1315. https://doi.org/10.3390/f11121315
Spînu AP, Petrițan IC, Mikoláš M, Janda P, Vostarek O, Čada V, Svoboda M. Moderate- to High-Severity Disturbances Shaped the Structure of Primary Picea Abies (L.) Karst. Forest in the Southern Carpathians. Forests. 2020; 11(12):1315. https://doi.org/10.3390/f11121315
Chicago/Turabian StyleSpînu, Andreea Petronela, Ion Catălin Petrițan, Martin Mikoláš, Pavel Janda, Ondřej Vostarek, Vojtěch Čada, and Miroslav Svoboda. 2020. "Moderate- to High-Severity Disturbances Shaped the Structure of Primary Picea Abies (L.) Karst. Forest in the Southern Carpathians" Forests 11, no. 12: 1315. https://doi.org/10.3390/f11121315
APA StyleSpînu, A. P., Petrițan, I. C., Mikoláš, M., Janda, P., Vostarek, O., Čada, V., & Svoboda, M. (2020). Moderate- to High-Severity Disturbances Shaped the Structure of Primary Picea Abies (L.) Karst. Forest in the Southern Carpathians. Forests, 11(12), 1315. https://doi.org/10.3390/f11121315






