Why Do Beavers Leave Home? Lodge Abandonment in an Invasive Population in Patagonia
Abstract
:1. Introduction
2. Material and Methods
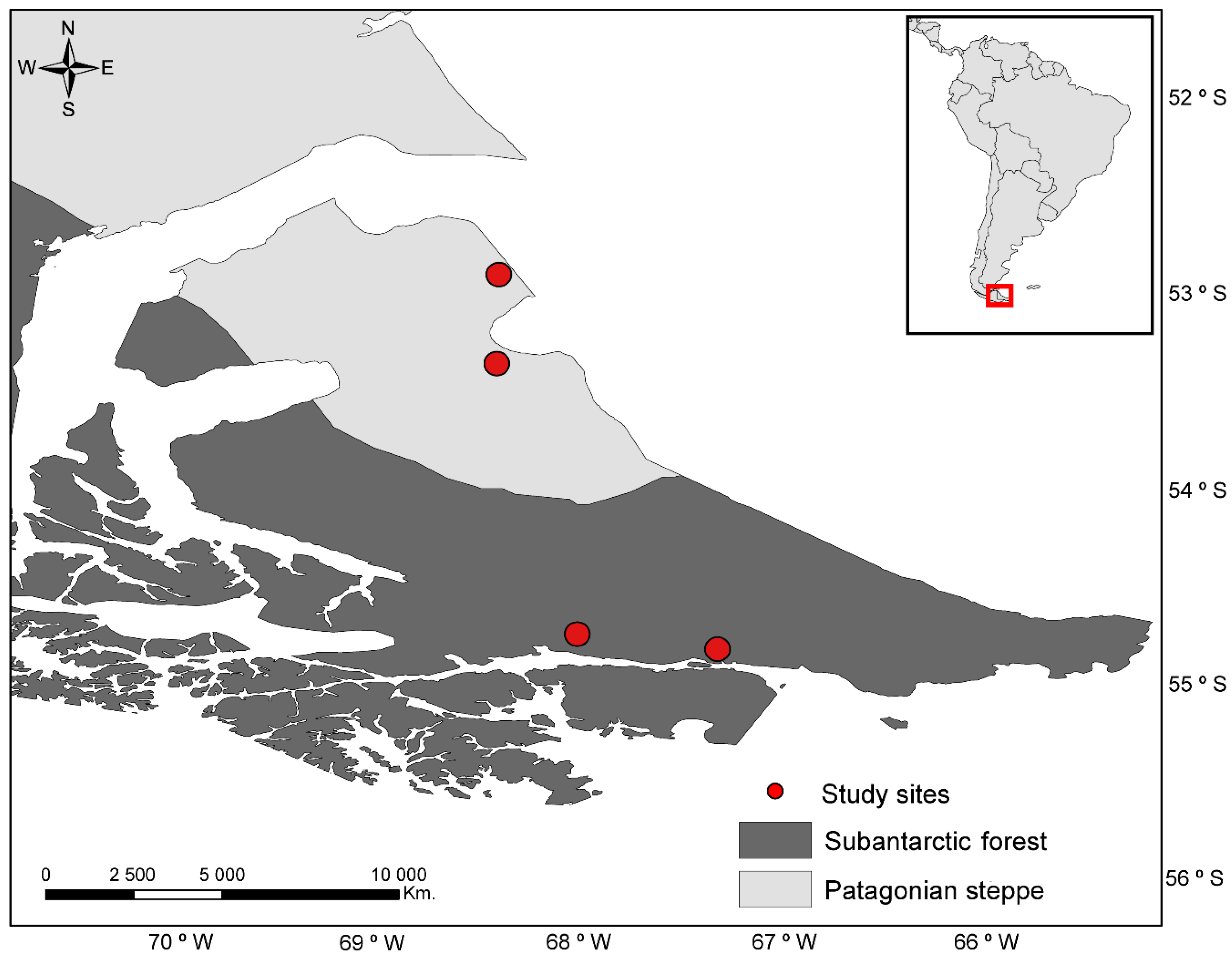
2.1. Study Area
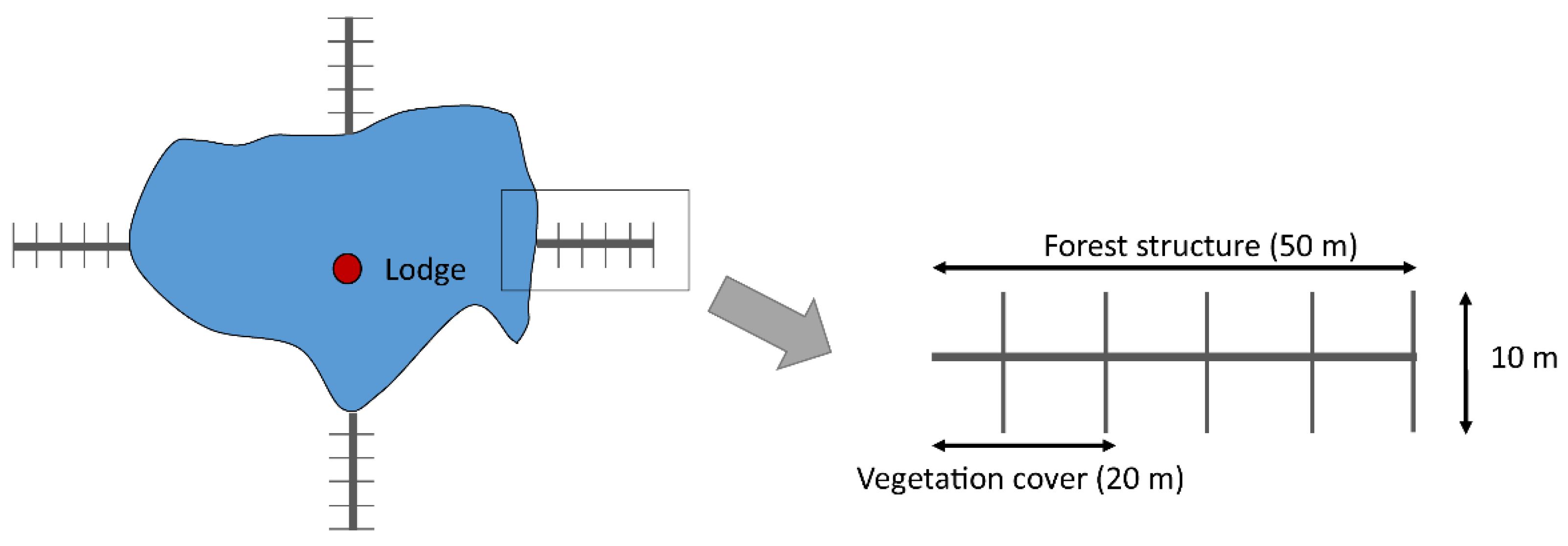
2.2. Experimental Design and Site Selection
2.3. Data Compilation and Measurements
2.3.1. Water Level Variation
2.3.2. Stream Gradient
2.3.3. Shrub Cover in the Steppe and Understory Cover in the Forest
2.3.4. Forest Structure
2.4. Data Analysis
3. Results
3.1. General Abandonment Pattern
3.2. Between-Habitat Models
3.3. Within-Habitat Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bergeron, Y.; Gauthier, S.; Kafka, V.; Lefort, P.; Lesieur, D. Natural fire frequency for the eastern Canadian boreal forest: Consequences for sustainable forestry. Can. J. For. Res. 2001, 31, 384–391. [Google Scholar] [CrossRef]
- Pickett, S.T.; White, P.S. The Ecology of Natural Disturbance and Patch Dynamics; Academic Press: Cambridge, MA, USA, 1985. [Google Scholar]
- Mitchell, S.J. Wind as a natural disturbance agent in forests: A synthesis. Forestry 2013, 86, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Wright, J.P.; Jones, C.G.; Flecker, A.S. An ecosystem engineer, the beaver, increases species richness at the landscape scale. Oecologia 2002, 132, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Badano, E.I.; Marquet, P.A. Ecosystem engineering affects ecosystem functioning in high-Andean landscapes. Oecologia 2008, 155, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Fox-Dobbs, K.; Doak, D.F.; Brody, A.K.; Palmer, T.M. Termites create spatial structure and govern ecosystem function by affecting N2 fixation in an East African savanna. Ecology 2010, 91, 1296–1307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, C.B.; Rosemond, A.D. Ecosystem engineering by invasive exotic beavers reduces in-stream diversity and enhances ecosystem function in Cape Horn, Chile. Oecologia 2007, 154, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Tait, L.W.; Lohrer, A.M.; Townsend, M.; Atalah, J.; Floerl, O.; Inglis, G.J. Invasive ecosystem engineers threaten benthic nitrogen cycling by altering native infaunal and biofouling communities. Sci. Rep. 2020, 10, 1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrios-Garcia, M.N.; Ballari, S.A. Impact of wild boar (Sus scrofa) in its introduced and native range: A review. Biol. Invasions 2012, 14, 2283–2300. [Google Scholar] [CrossRef]
- Cuddington, K.; Hastings, A. Invasive engineers. Ecol. Model. 2004, 178, 335–347. [Google Scholar] [CrossRef]
- Pietrek, A.G.; Fasola, L. Origin and history of the beaver introduction in South America. Mastozool. Neotrop. 2014, 21, 355–359. [Google Scholar]
- Choi, C. Tierra del Fuego: The beavers must die. Nature 2008, 453, 968. [Google Scholar] [CrossRef]
- Pietrek, A.G.; González-Roglich, M. Post-establishment changes in habitat selection by an invasive species: Beavers in the Patagonian steppe. Biol. Invasions 2015, 17, 3225–3235. [Google Scholar] [CrossRef]
- Skewes, O.; Gonzalez, F.; Olave, R.; Avila, A.; Vargas, V.; Paulsen, P.; Konig, H.E. Abundance and distribution of American beaver, Castor canadensis (Kuhl 1820), in Tierra del Fuego and Navarino islands, Chile. Eur. J. Wildl. Res. 2006, 52, 292–296. [Google Scholar] [CrossRef]
- Wallem, P.K.; Jones, C.G.; Marquet, P.A.; Jaksic, F.M. Identifying the mechanisms underlying the invasion of Castor canadensis (Rodentia) into Tierra del Fuego archipelago, Chile. Rev. Chil. Hist. Nat. 2007, 80, 309–325. [Google Scholar] [CrossRef]
- Parkes, J.; Paulson, J.; Donlan, C.; Campbell, K. Control of North American Beavers in Tierra del Fuego: Feasibility of Eradication and Alternative Management Options; Landcare Research Contract Report LC0708/084; Landcare Research New Zealand Ltd.: Lincoln, New Zealand, 2008. [Google Scholar]
- Anderson, C.B.; Griffith, C.R.; Rosemond, A.D.; Rozzi, R.; Dollenz, O. The effects of invasive North American beavers on riparian plant communities in Cape Horn, Chile: Do exotic beavers engineer differently in sub-Antarctic ecosystems? Biol. Conserv. 2006, 128, 467–474. [Google Scholar] [CrossRef]
- Naiman, R.J.; Melillo, J.M.; Hobbie, J.E. Ecosystem alteation of boreal forest streams by beaver (Castor canadensis). Ecology 1986, 67, 1254–1269. [Google Scholar] [CrossRef]
- Martell, K.A.; Foote, A.L.; Cumming, S.G. Riparian disturbance due to beavers (Castor canadensis) in Alberta’s boreal mixedwood forests: Implications for forest management. Ecoscience 2006, 13, 164–171. [Google Scholar] [CrossRef]
- Touihri, M.; Labbé, J.; Imbeau, L.; Darveau, M. North American beaver (Castor canadensis Kuhl) key habitat characteristics: Review of the relative effects of geomorphology, food availability and anthropogenic infrastructure. Ecoscience 2018, 25, 9–23. [Google Scholar] [CrossRef]
- Cunningham, J.M.; Calhoun, A.J.; Glanz, W.E. Patterns of beaver colonization and wetland change in Acadia National Park. Northeast. Nat. 2006, 13, 583–596. [Google Scholar] [CrossRef]
- Westbrook, C.J.; Cooper, D.J.; Baker, B.W. Beaver assisted river valley formation. River Res. Appl. 2011, 27, 247–256. [Google Scholar] [CrossRef]
- Nummi, P.; Kuuluvaine, T. Forest disturbance by an ecosystem engineer: Beaver in boreal forest landscapes. Boreal Environ. Res. 2013, 18, 13–24. [Google Scholar]
- Arismendi, I.; Penaluna, B.E.; Jara, C.G. Introduced beaver improve growth of non-native trout in Tierra del Fuego, South America. Ecol. Evol. 2020, 10, 9454–9465. [Google Scholar] [CrossRef] [PubMed]
- Lizarralde, M.S.; Escobar, J.M.; Deferrari, G. Invader species in Argentina: A review about the beaver (Castor canadensis) population situation on Tierra del Fuego ecosystem. Interciencia 2004, 29, 352–356. [Google Scholar]
- Pastur, G.M.; Lencinas, M.V.; Escobar, J.M.; Quiroga, P.; Malmierca, L.; Lizarralde, M.S. Understorey succession in Nothofagus forests in Tierra del Fuego (Argentina) affected by Castor canadensis. Appl. Veg. Sci. 2006, 9, 143–154. [Google Scholar] [CrossRef]
- Anderson, C.B.; Pastur, G.M.; Lencinas, M.V.; Wallem, P.K.; Moorman, M.C.; Rosemond, A.D. Do introduced North American beavers Castor canadensis engineer differently in southern South America? An overview with implications for restoration. Mammal Rev. 2009, 39, 33–52. [Google Scholar] [CrossRef]
- Fryxell, J.M. Habitat suitability and source–sink dynamics of beavers. J. Anim. Ecol. 2001, 70, 310–316. [Google Scholar]
- Barnes, D.M.; Mallik, A.U. Habitat factors influencing beaver dam establishment in a northern Ontario watershed. J. Wildl. Manag. 1997, 61, 1371–1377. [Google Scholar] [CrossRef]
- Jenkins, S.H. A size-distance relation in food selection by beavers. Ecology 1980, 61, 740–746. [Google Scholar] [CrossRef] [Green Version]
- Townsend, J.E. Beaver ecology in western Montana with special reference to movements. J. Mammal. 1953, 34, 459–479. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, S.H.; Busher, P.E. Castor canadensis. Mamm. Species 1979, 120, 1–8. [Google Scholar] [CrossRef]
- Müller-Schwarze, D.; Sun, L. The Beaver: Natural History of A Wetlands Engineer; Cornell University Press: Ithaca, NY, USA, 2003. [Google Scholar]
- Svendsen, G.E. Pair formation, duration of pair-bonds, and mate replacement in a population of beavers (Castor canadensis). Can. J. Zool. 1989, 67, 336–340. [Google Scholar] [CrossRef]
- Smith, D.W.; Peterson, R.O. Behavior of beaver in lakes with varying water levels in northern Minnesota. Environ. Manag. 1991, 15, 395. [Google Scholar] [CrossRef]
- Dieter, C.D.; McCabe, T.R. Factors influencing beaver lodge-site selection on a prairie river. Am. Midl. Nat. 1989, 122, 408–411. [Google Scholar] [CrossRef] [Green Version]
- Gurnell, A.M. The hydrogeomorphological effects of beaver dam-building activity. Prog. Phys. Geogr. 1998, 22, 167–189. [Google Scholar] [CrossRef]
- Beier, P.; Barrett, R.H. Beaver habitat use and impact in Truckee River basin, California. J. Wildl. Manag. 1987, 51, 794–799. [Google Scholar] [CrossRef] [Green Version]
- Collen, P.; Gibson, R. The general ecology of beavers (Castor spp.), as related to their influence on stream ecosystems and riparian habitats, and the subsequent effects on fish–a review. Rev. Fish Biol. Fish. 2001, 10, 439–461. [Google Scholar] [CrossRef]
- Bloomquist, C.K.; Nielsen, C.K.; Shew, J.J. Spatial organization of unexploited beavers (Castor canadensis) in southern Illinois. Am. Midl. Nat. 2012, 167, 188–197. [Google Scholar] [CrossRef]
- Slough, B.G.; Sadleir, R. A land capability classification system for beaver (Castor canadensis Kuhl). Can. J. Zool. 1977, 55, 1324–1335. [Google Scholar] [CrossRef]
- Howard, R.J.; Larson, J.S. A stream habitat classification system for beaver. J. Wildl. Manag. 1985, 49, 19–25. [Google Scholar] [CrossRef]
- Coronato, A.; Escobar, J.; Mallea, C.; Roig, C.; Lizarralde, M. Características geomorfológicas de ríos de montaña colonizados por Castor canadensis en Tierra del Fuego, Argentina. Ecol. Austral 2003, 13, 15–26. [Google Scholar]
- Suzuki, N.; McComb, W.C. Habitat classification models for beaver (Castor canadensis) in the streams of the central Oregon Coast Range. Nofihwest Sci. 1998, 72, 102–110. [Google Scholar]
- Labrecque-Foy, J.P.; Morin, H.; Girona, M.M. Dynamics of Territorial Occupation by North American Beavers in Canadian Boreal Forests: A Novel Dendroecological Approach. Forests 2020, 11, 221. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, M.; Pérez-Alberti, A.; Crujeiras, R.M.; Rodríguez-Casal, A.; Castillo-Rodríguez, F. A new method for analysing and representing ground temperature variations in cold environments. The Fuegian Andes, Tierra del Fuego, Argentina. Cuad. Investig. Geogr. 2018, 44, 293–320. [Google Scholar] [CrossRef] [Green Version]
- Oliva, G.; González, L.; Rial, P.; Livraghi, E. El ambiente en la Patagonia Austral. In Ganadería ovina Sustentable en la Patagonia Austral. Tecnología de Manejo Extensivo; Borelli, P., Oliva, G., Eds.; INTA: Santa Cruz, Argentina, 2001; pp. 17–80. [Google Scholar]
- Frederiksen, P. Soils of Tierra del Fuego: A satellite-based land survey approach. Folia Geogr. Danica 1988, 18, 159. [Google Scholar]
- Pastur, G.M.; Peri, P.L.; Fernández, M.C.; Staffieri, G.; Lencinas, M.V. Changes in understory species diversity during the Nothofagus pumilio forest management cycle. J. For. Res. 2002, 7, 165–174. [Google Scholar] [CrossRef]
- Peri, P.L.; Lencinas, M.V.; Martínez Pastur, G.; Wardell-Johnson, G.W.; Lasagno, R. Diversity patterns in the steppe of Argentinean southern Patagonia: Environmental drivers and impact of grazing. In Steppe Ecosystems: Biological Diversity, Management and Restoration; NOVA Science Publishers Inc.: New York, NY, USA, 2013; p. 346. [Google Scholar]
- Collado, L. Los bosques de Tierra del Fuego. Análisis de su estratificación mediante imágenes satelitales para el inventario forestal de la provincia. Multequina 2001, 10, 1–15. [Google Scholar]
- Allué, C.; Arranz, J.A.; Bava, J.O.; Beneitez, J.M.; Collado, L.; López, J.G. Caracterización y cartografía fitoclimáticas del bosque nativo subantártico en la Isla Grande de Tierra del Fuego (Patagonia, Argentina). For. Syst. 2010, 19, 189–207. [Google Scholar]
- Barrera, M.D.; Frangi, J.L.; Richter, L.L.; Perdomo, M.H.; Pinedo, L.B. Structural and functional changes in Nothofagus pumilio forests along an altitudinal gradient in Tierra del Fuego, Argentina. J. Veg. Sci. 2000, 11, 179–188. [Google Scholar] [CrossRef]
- Moore, D.M. Flora of Tierra del Fuego. In Missouri Botanical Garden; Anthony Nelson: Oswestry, UK, 1983. [Google Scholar]
- Slough, B.G. Beaver food cache structure and utilization. J. Wildl. Manag. 1978, 42, 644–646. [Google Scholar] [CrossRef]
- Pietrek, A.G.; Escobar, J.M.; Fasola, L.; Roesler, I.; Schiavini, A. Why invasive Patagonian beavers thrive in unlikely habitats: A demographic perspective. J. Mammal. 2017, 98, 283–292. [Google Scholar] [CrossRef]
- Fryxell, J.; Doucet, C. Diet choice and the funcional response of beavers. Ecology 1993, 74, 1297–1306. [Google Scholar] [CrossRef]
- Pinheiro, J.C.; Bates, D.M. Mixed-Effects Models in S and S-PLUS; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Bumham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach; Springer: New York, NY, USA, 2002. [Google Scholar]
- Anderson, D.R.; Burnham, K.P. Avoiding pitfalls when using information-theoretic methods. J. Wildl. Manag. 2002, 66, 912–918. [Google Scholar] [CrossRef]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. lme4: Linear mixed-effects models using Eigen and S4. R package version 1.1-7. J. Stat. Softw. 2014, 67, 1–48. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing [Computer Software]; R foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Arnold, T.W. Uninformative Parameters and Model Selection Using Akaike’s Information Criterion. J. Wildl. Manag. 2010, 74, 1175–1178. [Google Scholar] [CrossRef]
- Leroux, S.J. On the prevalence of uninformative parameters in statistical models applying model selection in applied ecology. PLoS ONE 2019, 14, e0206711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nixon, C.M.; Ely, J. Foods eaten by a beaver colony in southeast Ohio. Ohio J. Sci. 1969, 69, 313–319. [Google Scholar]
- Henry, D.B.; Bookhout, T.A. Utilization of woody plants by beavers in northeastern Ohio. Ohio J. Sci. 1970, 70, 123–127. [Google Scholar]
- Allers, D.; Culik, B.M. Energy requirements of beavers (Castor canadensis) swimming underwater. Physiol. Zool. 1997, 70, 456–463. [Google Scholar] [CrossRef]
- Hood, G.A. Not all ponds are created equal: Long-term beaver (Castor canadensis) lodge occupancy in a heterogeneous landscape. Can. J. Zool. 2020, 98, 210–218. [Google Scholar] [CrossRef]
- Basey, J.M.; Jenkins, S.H. Influences off predation risk and energy maximization on food selection by beavers (Castor canadensis). Can. J. Zool. 1995, 73, 2197–2208. [Google Scholar] [CrossRef]
- Johnson-Bice, S.M.; Ferguson, J.M.; Erb, J.D.; Gable, T.D.; Windels, S.K. Ecological forecasts reveal limitations of common model selection methods: Predicting changes in beaver colony densities. Ecol. Appl. 2020. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; McClintic, L.F.; Taylor, J.D. Habitat selection by American beaver at multiple spatial scales. Anim. Biotelem. 2019, 7, 10. [Google Scholar] [CrossRef] [Green Version]
- Milligan, H.E.; Humphries, M.M. The importance of aquatic vegetation in beaver diets and the seasonal and habitat specificity of aquatic-terrestrial ecosystem linkages in a subarctic environment. Oikos 2010, 119, 1877–1886. [Google Scholar] [CrossRef]
- González-Suárez, M.; Bacher, S.; Jeschke, J.M. Intraspecific Trait Variation Is Correlated with Establishment Success of Alien Mammals. Am. Nat. 2015, 185, 737–746. [Google Scholar] [CrossRef] [Green Version]
- Sakai, A.K.; Allendorf, F.W.; Holt, J.S.; Lodge, D.M.; Molofsky, J.; With, K.A.; Baughman, S.; Cabin, R.J.; Cohen, J.E.; Ellstrand, N.C. The population biology of invasive species. Annu. Rev. Ecol. Syst. 2001, 32, 305–332. [Google Scholar] [CrossRef] [Green Version]
- Piscart, C.; Rousel, J.M.; Dick, J.T.; Grosbois, G.; Marmonier, P. Effects of coexistence on habitat use and trophic ecology of interacting native and invasive amphipods. Freshw. Biol. 2011, 56, 325–334. [Google Scholar] [CrossRef]
- Curtis, P.D.; Jensen, P.G. Habitat features affecting beaver occupancy along rfoadsides in New York state. J. Wildl. Manag. 2004, 68, 278–287. [Google Scholar] [CrossRef]

| Habitat Variables | Description | Type of Variable and Units |
|---|---|---|
| HAB | Habitat where the lodge was identified | Categorical |
| WLV | Difference of the water level of the lodge entrance between the year of first recording and the following abandonment year | Continuous (cm) |
| GRA | Slope of the watercourse 50 m from the lodge downstream | Continuous (°) |
| COV | Proportion of the total length of the transect covered by shrub cover in the steppe and understory cover in the forest. | Continuous (%) |
| FSTR | Median diameter of standing trees registered up to 50 m from the edge of the pond | Continuous (cm) |
| Habitat | State | 2012/2013 | 2013/2014 | Total |
|---|---|---|---|---|
| Forest | Occupied | 12 | 9 | 21 |
| Abandoned | 5 | 6 | 11 | |
| Steppe | Occupied | 10 | 12 | 22 |
| Abandoned | 8 | 5 | 13 | |
| Total | 35 | 32 | 67 |
| Candidate Model | K | AICc | ∆ AICC | Akaike Weight (wi) | Log-Likelihood |
|---|---|---|---|---|---|
| WLV | 3 | 78.41 | 0.00 | 0.45 | −35.99 |
| WLV + GRA | 4 | 79.55 | 1.14 | 0.25 | −35.42 |
| WLV + HAB | 4 | 80.38 | 1.97 | 0.17 | −35.83 |
| WLV + GRA + HAB | 5 | 81.92 | 3.51 | 0.08 | −35.42 |
| Habitat | Candidate Model | K | AICc | ∆ AICC | wi | Log-Likelihood |
|---|---|---|---|---|---|---|
| Forest | WLV + GRA + COV | 5 | 29.21 | 0.00 | 0.40 | −8.02 |
| WLV + GRA | 4 | 30.19 | 0.99 | 0.24 | −10.10 | |
| WLV + COV | 4 | 31.37 | 2.16 | 0.14 | −10.68 | |
| WLV + GRA + FSTR | 5 | 33.09 | 3.88 | 0.06 | −9.96 | |
| Steppe | WLV | 3 | 49.04 | 0.00 | 0.35 | −21.13 |
| NULL | 2 | 50.48 | 1.44 | 0.17 | −23.05 | |
| WLV + GRA | 4 | 50.72 | 1.68 | 0.15 | −20.69 | |
| WLV + COV | 4 | 51.39 | 2.35 | 0.11 | −21.03 | |
| WLV + GRA | 4 | 51.78 | 2.74 | 0.09 | −22.50 | |
| COV | 3 | 52.72 | 3.68 | 0.06 | −22.97 |
| Habitat | Coefficients | Slope | 95% CI | p Value | RVI |
|---|---|---|---|---|---|
| Forest | Water level variation (WLV) | −0.13 | −0.3; −0.04 | 0.08 | 0.84 |
| Stream gradient (GRA) | −0.55 | −1.46; −0.11 | 0.13 | 0.70 | |
| Understory cover (COV) | −0.15 | −0.49; −0.02 | 0.23 | 0.54 | |
| Steppe | Water level variation (WLV) | −0.04 | −0.09; −0.007 | 0.07 | 0.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feldman, M.J.; Girona, M.M.; Grosbois, G.; Pietrek, A.G. Why Do Beavers Leave Home? Lodge Abandonment in an Invasive Population in Patagonia. Forests 2020, 11, 1161. https://doi.org/10.3390/f11111161
Feldman MJ, Girona MM, Grosbois G, Pietrek AG. Why Do Beavers Leave Home? Lodge Abandonment in an Invasive Population in Patagonia. Forests. 2020; 11(11):1161. https://doi.org/10.3390/f11111161
Chicago/Turabian StyleFeldman, Mariano J., Miguel Montoro Girona, Guillaume Grosbois, and Alejandro G. Pietrek. 2020. "Why Do Beavers Leave Home? Lodge Abandonment in an Invasive Population in Patagonia" Forests 11, no. 11: 1161. https://doi.org/10.3390/f11111161
APA StyleFeldman, M. J., Girona, M. M., Grosbois, G., & Pietrek, A. G. (2020). Why Do Beavers Leave Home? Lodge Abandonment in an Invasive Population in Patagonia. Forests, 11(11), 1161. https://doi.org/10.3390/f11111161







