Spatial Structure and Genetic Variation of a Mangrove Species (Avicennia marina (Forssk.) Vierh) in the Farasan Archipelago
Abstract
1. Introduction
2. Material and Methods
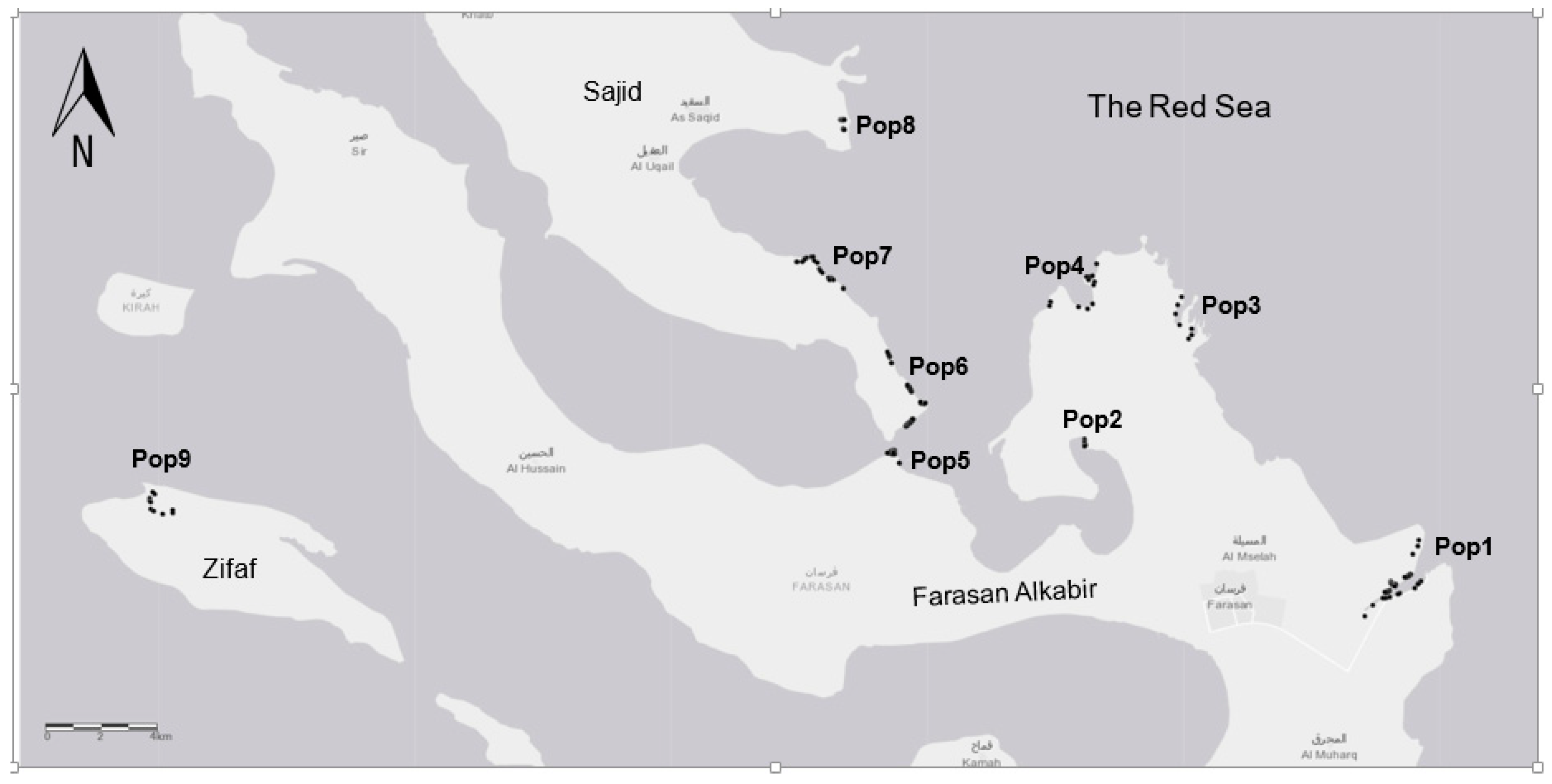
2.1. Study Area
2.2. DNA Extraction
2.3. DNA Amplification by PCR
2.4. Basic Genetic Parameters: Diversity and Differentiation
2.5. Genetic Structure Across the Farasan Islands
2.6. The Red Sea Movement
3. Results
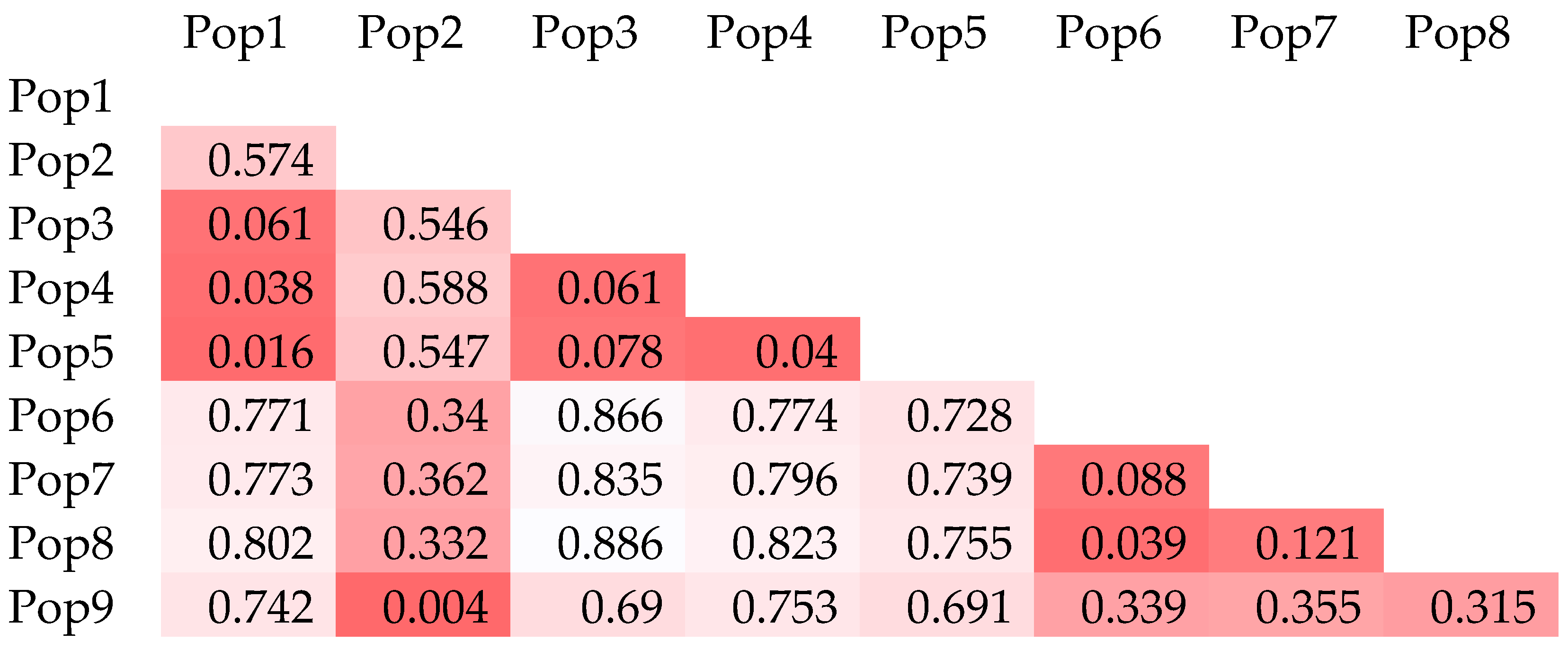
3.1. Basic Parameters: Genetic Diversity and Variation
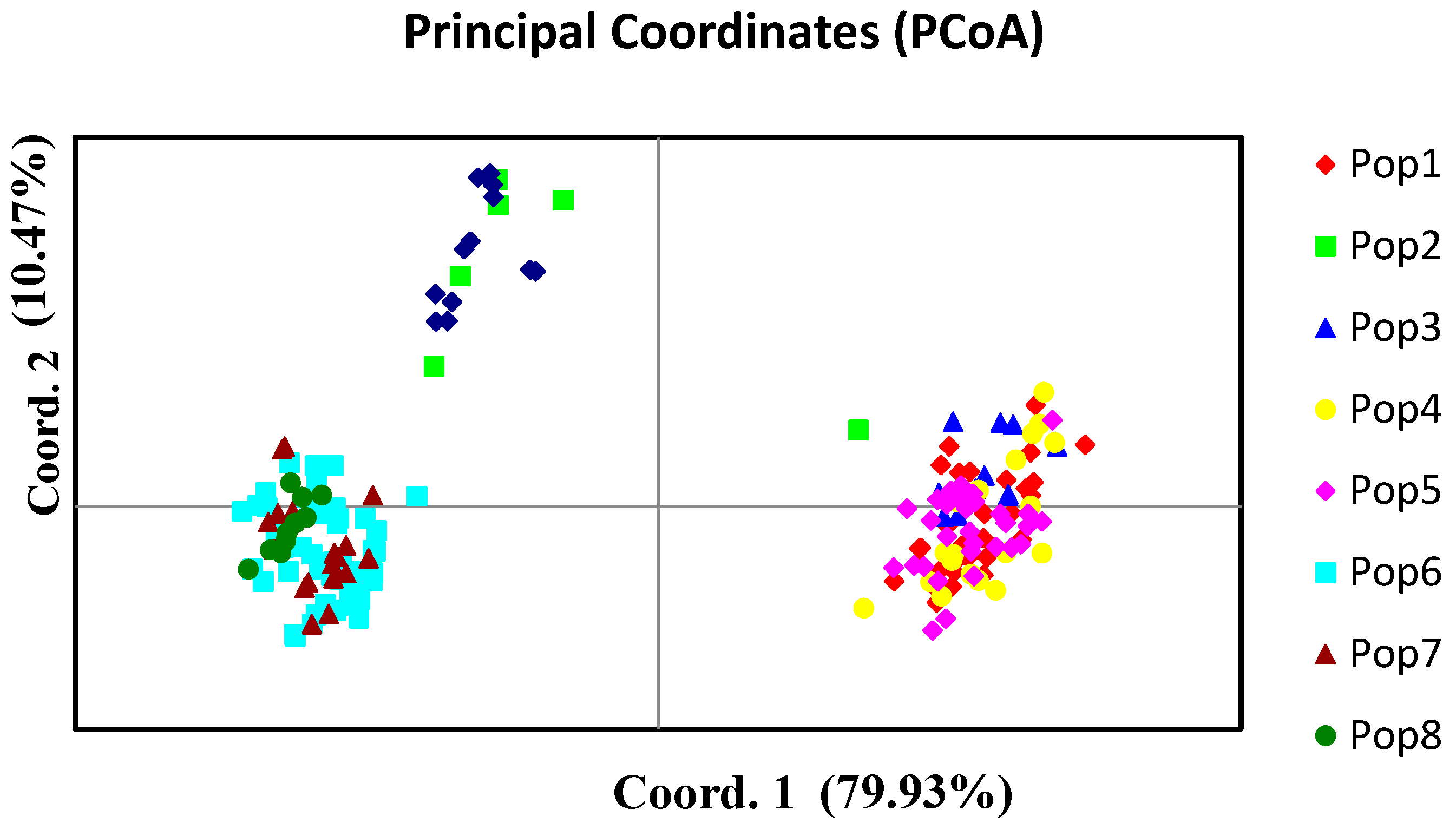
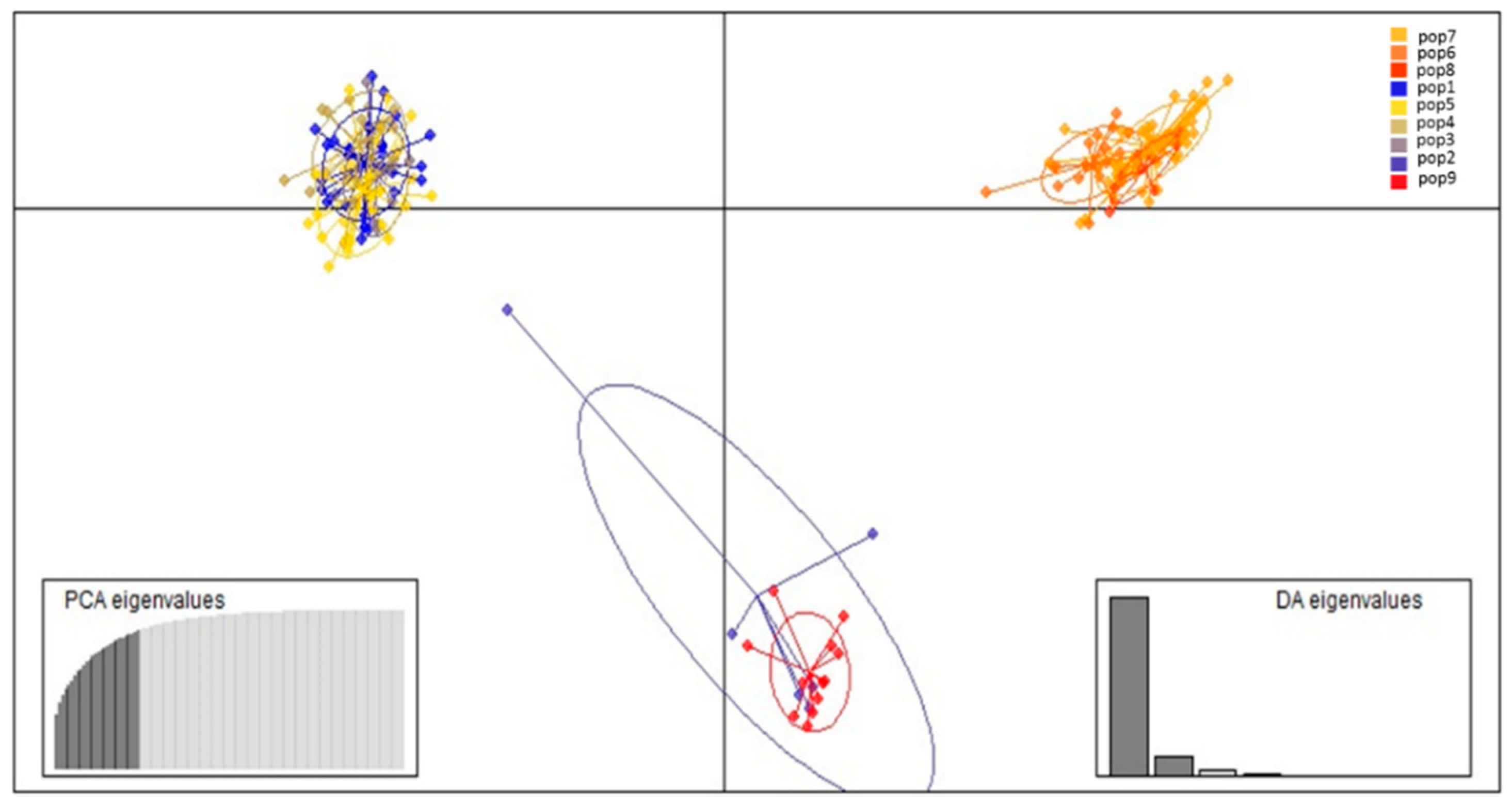
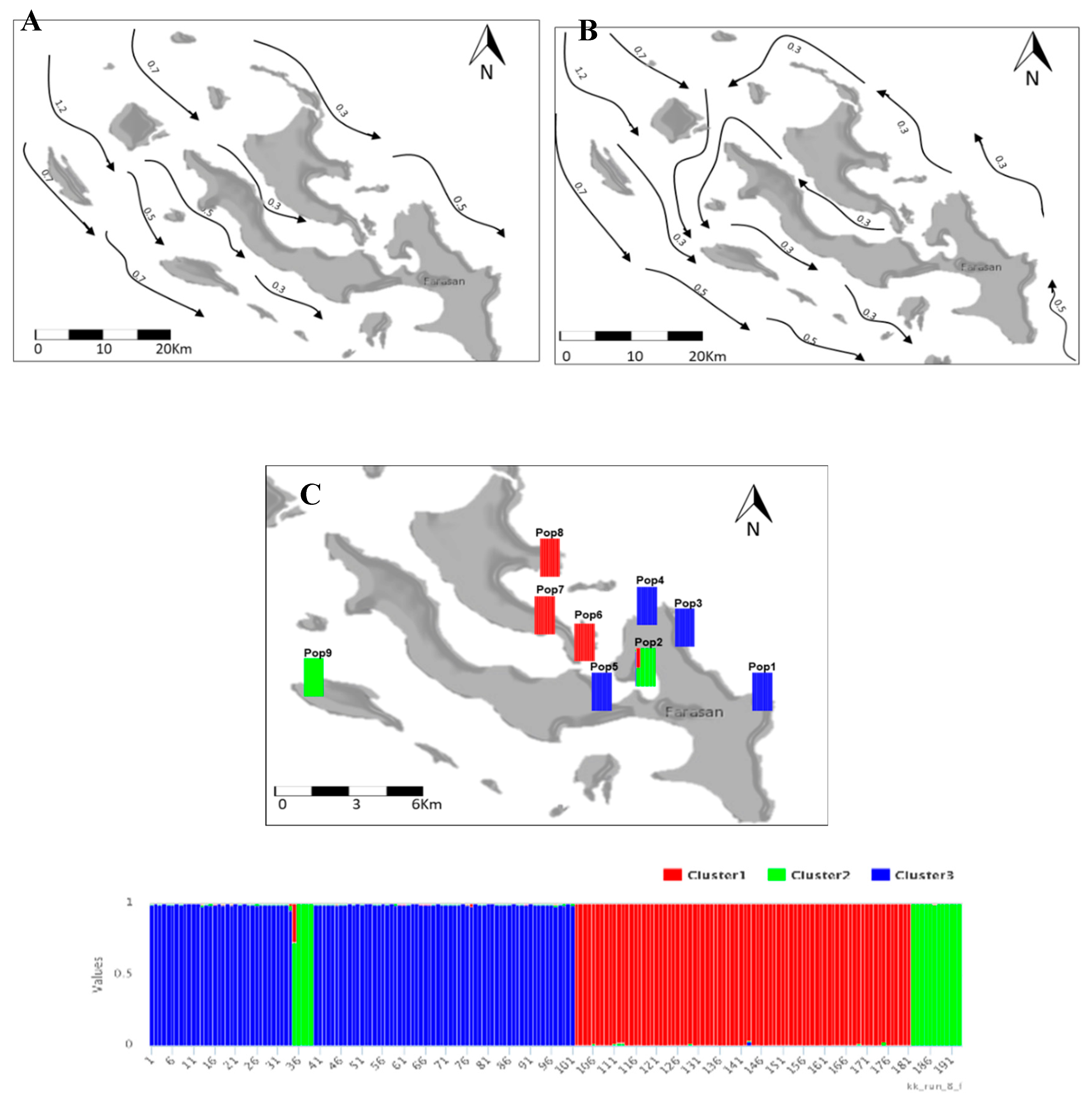
3.2. Population Structure and Cluster Analysis Across the Farasan Islands
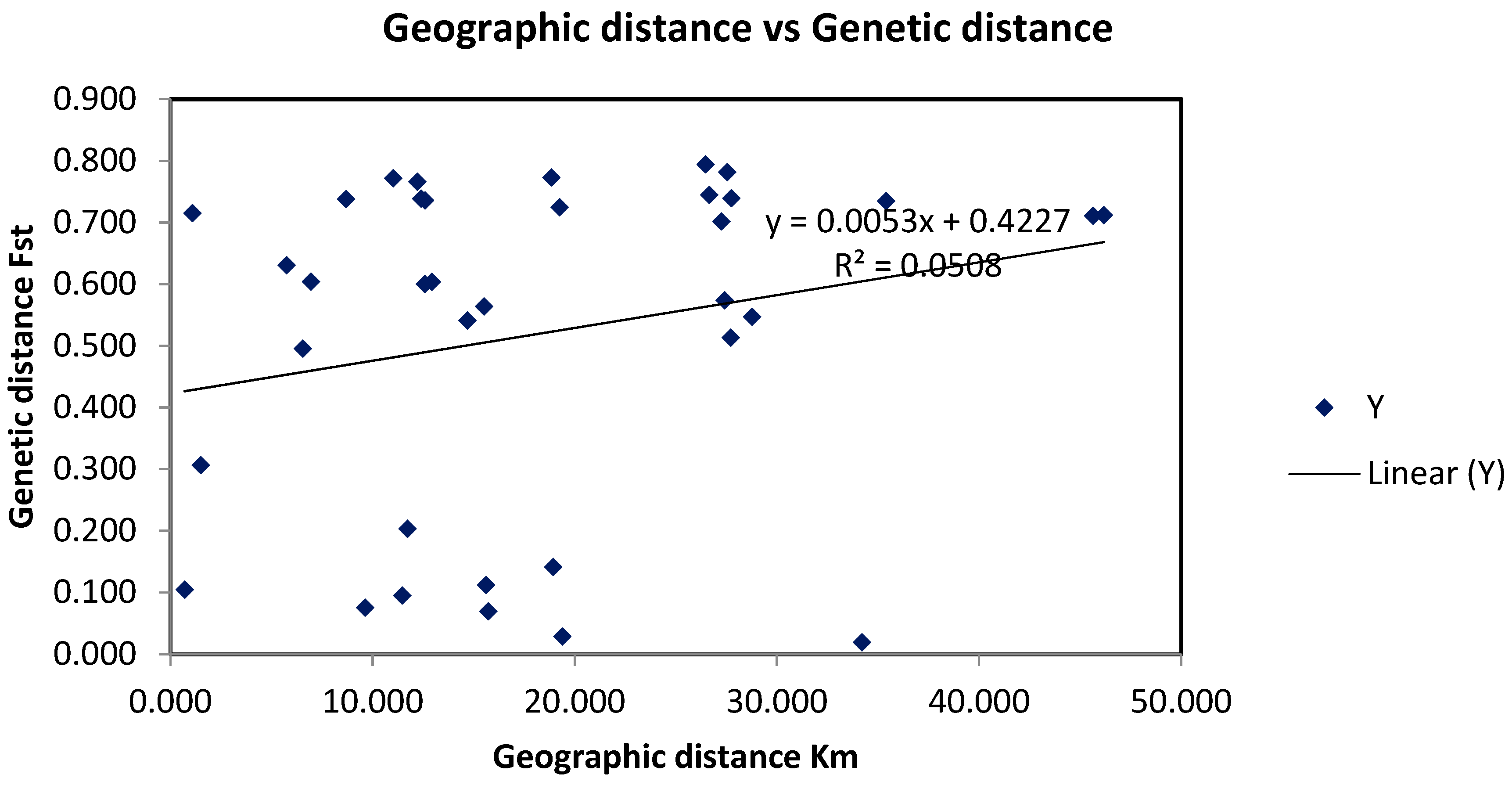
3.3. Red Sea Current Surface Simulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Duke, N.C. Mangrove floristics and biogeography. Trop. Mangrove Ecosyst. 1992, 41, 63–100. [Google Scholar]
- Chen, R.; Twilley, R.R. A simulation model of organic matter and nutrient accumulation in mangrove wetland soils. Biogeochemistry 1999, 44, 93–118. [Google Scholar] [CrossRef]
- Kathiresan, K.; Bingham, B.L. Biology of mangroves and mangrove ecosystems. Adv. Mar. Biol. 2001, 40, 81–251. [Google Scholar]
- Giri, C.; Ochieng, E.; Tieszen, L.L.; Zhu, Z.; Singh, A.; Loveland, T.; Masek, J.; Duke, N. Status and distribution of mangrove forests of the world using earth observation satellite data. Glob. Ecol. Biogeogr. 2011, 20, 154–159. [Google Scholar] [CrossRef]
- Bosire, J.O.; Dahdouh-Guebas, F.; Kairo, J.G.; Cannicci, S.; Koedam, N. Spatial variations in macrobenthic fauna recolonisation in a tropical mangrove bay. Biodivers. Conserv. 2004, 13, 1059–1074. [Google Scholar] [CrossRef]
- Barbier, E.B.; Hacker, S.D.; Kennedy, C.; Koch, E.W.; Stier, A.C.; Silliman, B.R. The value of estuarine and coastal ecosystem services. Ecol. Monogr. 2011, 81, 169–193. [Google Scholar] [CrossRef]
- Mastaller, M. Mangroves: The Forgotten Forest between Land and Sea; Tropical Press: Kuala Lumpur, Malaysia, 1997. [Google Scholar]
- Duke, N.C.; Meynecke, J.O.; Dittmann, S.; Ellison, A.M.; Anger, K.; Berger, U.; Cannicci, S.; Diele, K.; Ewel, K.C.; Field, C.D.; et al. A World without Mangroves? Science 2007, 317, 41–42. [Google Scholar] [CrossRef] [PubMed]
- Tawfiq, N.; Olsen, D.A. Saudi Arabia’s response to the 1991 Gulf oil spill. Mar. Pollut. Bull. 1993, 27, 333–345. [Google Scholar] [CrossRef]
- Macintosh, D.J.; Ashton, E.C. A Review of Mangrove Biodiversity Conservation and Management; Centre for Tropical Ecosystems Research, University of Aarhus: Aarhus, Denmark, 2002. [Google Scholar]
- PERSGA. Strategic Action Programme for the Red Sea and Gulf of Aden, (Regional Organization for the Conservation of the Environment of the Red Sea and Gulf of Aden); The World Bank: Washington, DC, USA, 2004. [Google Scholar]
- Kotb, M.; Abdulaziz, M.; Al-Agwan, Z.; Alshaikh, K.; Al-Yami, H.; Banajah, A.; Devantier, L.; Eisinger, M.; Eltayeb, M.; Hassan, M. Status of coral reefs in the Red Sea and Gulf of Aden in 2004. Wilkinson 2004, 70, 137–139. [Google Scholar]
- Kumar, A. Reclaimed islands and new offshore townships in the Arabian Gulf: Potential natural hazards. Curr. Sci. (00113891) 2009, 96, 34–44. [Google Scholar]
- Kumar, A.; Khan, M.A.; Muqtadir, A. Distribution of mangroves along the Red Sea coast of the Arabian Peninsula: Part-I: The northern coast of western Saudi Arabia. Earth Sci. India 2010, 3, 1–22. [Google Scholar]
- Spalding, M.D.; Blasco, F.; Field, C.D. World Mangrove Atlas; The International Society for Mangrove Ecosystems: Okinawa, Japan, 1997; p. 178. [Google Scholar]
- Ellison, J.C.; Stoddart, D.R. Mangrove ecosystem collapse during predicted sea-level rise: Holocene analogues and implications. J. Coast. Res. 1991, 7, 151–165. [Google Scholar]
- Schneider, P. The discovery of tropical mangroves in Graeco-Roman antiquity: Science and wonder. J. Hakluyt Soc. 2011, 3, 1–16. [Google Scholar]
- Flenley, J.R. Tropical Forests under the Climates of the Last 30,000 Years. In Potential Impacts of Climate Change on Tropical Forest Ecosystems; Markham, A., Ed.; Springer: Dordrecht, The Netherlands, 1998; pp. 37–57. [Google Scholar]
- Mandura, A.S.; Khafaji, A.K.; Saifullah, S.M. Mangrove ecosystem of southern Red Sea coast of Saudi Arabia. Proc. Saudi Biol. Soc. 1987, 10, 165–193. [Google Scholar]
- Saifullah, S. Mangrove ecosystem of Red Sea coast (Saudi Arabia). Pak. J. Mar. Sci. 1997, 6, 115–124. [Google Scholar]
- Bailey, G. The Red Sea, Coastal Landscapes, and Hominin Dispersals. In The Evolution of Human Populations in Arabia. Vertebrate Palaeobiology and Palaeoanthropology; Petraglia, M.D., Rose, J.I., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 15–37. [Google Scholar]
- Rohling, E.J.J.P. Glacial conditions in the Red Sea. Paleoceanography 1994, 9, 653–660. [Google Scholar] [CrossRef]
- Khan, M.A.; Kumar, A.; Muqtadir, A. Distribution of Mangroves along the Red Sea Coast of the Arabian Peninsula: Part 2. The Southern Coast of Western Saudi Arabia. Earth Sci. India 2010, 3, 154–162. [Google Scholar]
- Bailey, G.N.; Sakellariou, D.; Alsharekh, A.; Al Nomani, S.; Devès, M.; Georgiou, P.; Kallergis, M.; Kalogirou, S.; Manousakis, L.; Mantopoulos, P. Africa-Arabia connections and geo-archaeological exploration in the southern Red Sea: Preliminary results and wider significance. In Under the Sea: Archaeology and Palaeolandscapes of the Continental Shelf; Springer: Dordrecht, The Netherlands, 2017; pp. 361–373. [Google Scholar]
- Almahasheer, H.; Aljowair, A.; Duarte, C.M.; Irigoien, X. Decadal stability of Red Sea mangroves. Estuar. Coast. Shelf Sci. 2016, 169, 164–172. [Google Scholar] [CrossRef]
- Hall, M.; Llewellyn, O.; Miller, A.; Al-Abbasi, T.; Al-Wetaid, A.; Al-Harbi, R.; Al-Shammari, K. Important Plant Areas in the Arabian Peninsula: 2. Farasan Archipelago. Edinb. J. Bot. 2010, 67, 189–208. [Google Scholar] [CrossRef]
- El-Demerdash, M. The vegetation of the farasān islands, Red Sea, Saudi Arabia. J. Veg. Sci. 1996, 7, 81–88. [Google Scholar] [CrossRef]
- Farooqui, N.U.; Al Zahrani, D.A.; El Metwally, M.; Dangi, C. A Review on the Impact of Exotoxicology and Oil Spills in Mangrove of Saudi Arabia. J. Pure Appl. Microbiol. 2015, 9, 549–556. [Google Scholar]
- Khafaji, A.K.; Manfura, A.S.; Saifullah, S.M.; Sambas, A.Z. Litter production in two mangrove stands in southern Red Sea coast of Saudi Arabia (Jizan). J. King Abdulaziz Univ. Mar. Sci. 1991, 2, 93–100. [Google Scholar] [CrossRef]
- Migahid, A.M. Flora of Saudi Arabia; Riyadh University Publications: Riyadh, Saudi Arabia, 1978. [Google Scholar]
- Dodd, R.S.; Blasco, F.; Rafii, Z.A.; Torquebiau, E. Mangroves of the United Arab Emirates: Ecotypic diversity in cuticular waxes at the bioclimatic extreme. Aquat. Bot. 1999, 63, 291–304. [Google Scholar] [CrossRef]
- Mutairi, K.A.; El-Bana, M.; Mansor, M.; Al-Rowaily, S.; Mansor, A. Floristic diversity, composition, and environmental correlates on the arid, coralline islands of the Farasan Archipelago, Red Sea, Saudi Arabia. Arid Land Res. Manag. 2012, 26, 137–150. [Google Scholar] [CrossRef]
- Rasul, N.M.A.; Stewart, I.C.F. The Red Sea: The Formation, Morphology, Oceanography and Environment of a Young Ocean Basin; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Mandura, A.S.; Khafaji, A.K. Human impact on the mangrove of Khor Farasan Island, southern Red Sea coast of Saudi Arabia. In Towards the Rational Use of High Salinity Tolerant Plants; Springer: Dordrecht, The Netherlands, 1993; pp. 353–361. [Google Scholar]
- Hariri, K.; Gladstone, W.; Facey, C. State of the Marine Environment-Report for the Red Sea and Gulf of Aden: 2006; PERSGA: Jeddah, Saudi Arabia, 2014. [Google Scholar]
- Al-Wetaid, A.; Khalil, A. Status of Red Sea Mangrove in Kingdom of Saudi Arabia; Draft Report; PERSGA: Jeddah, Saudi Arabia, 2003. [Google Scholar]
- Gladstone, W. The ecological and social basis for management of a Red Sea marine-protected area. Ocean Coast. Manag. 2000, 43, 1015–1032. [Google Scholar] [CrossRef][Green Version]
- Allendorf, F.W.; Luikart, G. Conservation and the Genetics of Populations; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Duke, N.C.; Benzie, J.A.; Goodall, J.A.; Ballment, E.R. Genetic structure and evolution of species in the mangrove genus Avicennia (Avicenniaceae) in the Indo-West Pacific. Evolution 1998, 52, 1612–1626. [Google Scholar] [CrossRef]
- Parani, M.; Lakshmi, M.; Elango, S.; Ram, N.; Anuratha, C.; Parida, A. Molecular phylogeny of mangroves II. Intra-and inter-specific variation in Avicennia revealed by RAPD and RFLP markers. Genome 1997, 40, 487–495. [Google Scholar] [CrossRef]
- Parida, A.; Parani, M.; Lakshmi, M.; Elango, S.; Ram, N.; Anuratha, C. Molecular Phylogeny of Mangroves IV. Nature and Extent of Intra-Specific Genetic Variation and Species Diversity in Mangroves; M.S. Swaminathan Research Foundation: Madras, India, 1998; pp. 95–105. [Google Scholar]
- Hazarika, D.; Thangaraj, M.; Sahu, S.K.; Kathiresan, K. Genetic diversity in three populations of Avicennia marina along the eastcoast of India by RAPD markers. J. Environ. Biol. 2013, 34, 663. [Google Scholar]
- Maguire, T.; Peakall, R.; Saenger, P. Comparative analysis of genetic diversity in the mangrove species Avicennia marina (Forsk.) Vierh. (Avicenniaceae) detected by AFLPs and SSRs. Theor. Appl. Genet. 2002, 104, 388–398. [Google Scholar] [CrossRef]
- Le, H.G.; Hong, P.N.; Tuan, M.S.; Harada, K. Genetic variation of Avicennia marina (Forsk.) Vierh. (Avicenniaceae) in Vietnam revealed by microsatellite and AFLP markers. Genes Genet. Syst. 2003, 78, 399–407. [Google Scholar]
- Allendorf, F.W.; Luikart, G. Conservation and the Genetics of Populations; Blackwell Pub.: Malden, MA, USA, 2013. [Google Scholar]
- Maguire, T.L.; Saenger, P.; Baverstock, P.; Henry, R. Microsatellite analysis of genetic structure in the mangrove species Avicennia marina (Forsk.) Vierh. (Avicenniaceae). Mol. Ecol. 2000, 9, 1853–1862. [Google Scholar] [CrossRef] [PubMed]
- Yoshimori, I.; Seo, A.; Nawata, H.; Fouda, M.M.; Yoshikawa, K. New Microsatellite Markers to Analyze Genetic Structure of Gray Mangrove, Avicennia marina. J. Arid Land Stud. 2015, 25, 11–16. [Google Scholar]
- Zolgharnien, H.; Kamyab, M.; Keyvanshokooh, S. Genetic diversity of Avicennia marina populatians in the persian Gulf by Microsatellite Markers. J. Fish. Aquat. Sci. 2010, 223–229. [Google Scholar]
- Manurung, J.; Siregar, I.Z.; Kusmana, C.; Dwiyanti, F.G. Genetic variation of the mangrove species Avicennia marina in heavy metal polluted estuaries of Cilegon Industrial Area, Indonesia. Biodiversitas J. Biol. Divers. 2017, 18, 1109–1115. [Google Scholar] [CrossRef]
- Duke, N.C. Mangrove taxonomy, biogeography and evolution—An Indo West Pacific perspective of implications for conservation and management. Perm. Agric. Resour. 2006, 77, 641–666. [Google Scholar]
- De Ryck, D.J.; Koedam, N.; Van Der Stocken, T.; Van Der Ven, R.M.; Adams, J.; Triest, L. Dispersal limitation of the mangrove Avicennia marina at its South African range limit in strong contrast to connectivity in its core East African region. Mar. Ecol. Prog. Ser. 2016, 545, 123–134. [Google Scholar] [CrossRef]
- Leonardi, S.; Menozzi, P. Spatial structure of genetic variability in natural stands of Fagus sylvatica L. (beech) in Italy. Heredity 1996, 77, 359. [Google Scholar] [CrossRef]
- Rossetto, M.; Slade, R.; Baverstock, P.R.; Henry, R.J.; Lee, L.S. Microsatellite variation and assessment of genetic structure in tea tree (Melaleuca alternifolia–Myrtaceae). Mol. Ecol. 1999, 8, 633–643. [Google Scholar] [CrossRef]
- Maguire, T.L.; Edwards, K.J.; Saenger, P.; Henry, R. Characterisation and analysis of microsatellite loci in a mangrove species, Avicennia marina (Forsk.) Vierh. (Avicenniaceae). Theor. Appl. Genet. 2000, 101, 279–285. [Google Scholar] [CrossRef]
- Arnaud-Haond, S.; Teixeira, S.; Massa, S.I.; Billot, C.; Saenger, P.; Coupland, G.; Duarte, C.M.; Serrao, E. Genetic structure at range edge: Low diversity and high inbreeding in Southeast Asian mangrove (Avicennia marina) populations. Mol. Ecol. 2006, 15, 3515–3525. [Google Scholar] [CrossRef]
- Melville, F.; Burchett, M. Genetic variation in Avicennia marina in three estuaries of Sydney (Australia) and implications for rehabilitation and management. Mar. Pollut. Bull. 2002, 44, 469–479. [Google Scholar] [CrossRef]
- Kahrood, H.V.; Korori, S.A.A.; Pirseyedi, M.; Shirvany, A.; Danehkar, A. Genetic variation of mangrove species Avicennia marina in Iran revealed by microsatellite markers. Afr. J. Biotechnol. 2008, 7, 1–16. [Google Scholar]
- Raju, A.J.S.; Rao, P.V.S.; Kumar, R.; Mohan, S.R. Pollination biology of the crypto-viviparous Avicennia species (Avicenniaceae). J. Threat. Taxa 2012, 4, 3377–3389. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: Dordrecht, The Netherlands, 2016. [Google Scholar]
- Van Der Stocken, T.; Vanschoenwinkel, B.; De Ryck, D.J.; Bouma, T.J.; Dahdouh-Guebas, F.; Koedam, N. Interaction between water and wind as a driver of passive dispersal in mangroves. PLoS ONE 2015, 10, e0121593. [Google Scholar] [CrossRef] [PubMed]
- Giesen, W.; Wulffraat, S.; Zieren, M.; Scholten, L. Mangrove guidebook for Southeast Asia. In Mangrove Guidebook for Southeast Asia; Wetlands International: Wageningen, The Netherlands, 2007; 769p. [Google Scholar]
- Alothman, A.O.; Bos, M.S.; Fernandes, R.M.S.; Ayhan, M.E. Sea level rise in the north-western part of the Arabian Gulf. J. Geodyn. 2014, 81, 105–110. [Google Scholar] [CrossRef]
- Blankespoor, B.; Dasgupta, S.; Laplante, B. Sea-level rise and coastal wetlands. Ambio 2014, 43, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Maguire, T.L.; Collins, G.G.; Sedgley, M. A modified CTAB DNA extraction procedure for plants belonging to the family Proteaceae. Plant Mol. Biol. Report. 1994, 12, 106–109. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Yeh, F.C.; Yang, R.C.; Boyle, T.B.J.; Ye, Z.; Xiyan, J.M. PopGene32, Microsoft Windows-Based Freeware for Population Genetic Analysis; Version 1.32; Molecular Biology and Biotechnology Centre, University of Alberta: Edmonton, AB, Canada, 2000. [Google Scholar]
- Kamvar, Z.N.; Tabima, J.F.; Grünwald, N.J. Poppr: An R package for genetic analysis of populations with clonal, partially clonal, and/or sexual reproduction. PeerJ 2014, 2, e281. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GENALEX 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Weir, B.S.; Cockerham, C.C. Estimating F-statistics for the analysis of population structure. Evolution 1984, 38, 1358–1370. [Google Scholar] [PubMed]
- Excoffier, L.; Lischer, H.E. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Jombart, T. Adegenet: A R package for the multivariate analysis of genetic markers. Bioinformatics 2008, 24, 1403–1405. [Google Scholar] [CrossRef] [PubMed]
- Jombart, T.; Devillard, S.; Balloux, F. Discriminant analysis of principal components: A new method for the analysis of genetically structured populations. BMC Genet. 2010, 11, 94. [Google Scholar] [CrossRef]
- Earl, D.A. Structure Harvester: A website and program for visualizing structure output and implementing the Evanno method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Murtagh, F.; Legendre, P. Ward’s hierarchical agglomerative clustering method: Which algorithms implement Ward’s criterion? J. Classif. 2014, 31, 274–295. [Google Scholar] [CrossRef]
- Sofiaos, S.S. An Oceanic General Circulation Model (OGCM) investigation of the Red Sea circulation, 1. Exchange between the Red Sea and the Indian Ocean. J. Geophys. Res. 2002, 107, 1–17. [Google Scholar]
- Sofiaos, S.S. An Oceanic General Circulation Model (OGCM) investigation of the Red Sea circulation: 2. Three-dimensional circulation in the Red Sea. J. Geophys. Res. 2003, 108. [Google Scholar] [CrossRef]
- Yao, F.; Hoteit, I.; Pratt, L.J.; Bower, A.S.; Köhl, A.; Gopalakrishnan, G.; Rivas, D. Rivas, Seasonal overturning circulation in the Red Sea: 2. Winter circulation. J. Geophys. Res. Ocean. 2014, 119, 2263–2289. [Google Scholar] [CrossRef]
- Yao, F.; Hoteit, I.; Pratt, L.J.; Bower, A.S.; Zhai, P.; Köhl, A.; Gopalakrishnan, G. Seasonal overturning circulation in the Red Sea: 1. Model validation and summer circulation. J. Geophys. Res. Ocean. 2014, 119, 2238–2262. [Google Scholar] [CrossRef]
- Neteler, M.; Mitasova, H. Open Source GIS: A GRASS GIS Approach; Springer Science & Business Media: Berlin, Germany, 2013. [Google Scholar]
- Biswas, M.K.; Xu, Q.; Mayer, C.; Deng, X. Genome wide characterization of short tandem repeat markers in sweet orange (Citrus sinensis). PLoS ONE 2014, 9, e104182. [Google Scholar] [CrossRef] [PubMed]
- Cerón-Souza, I.; Bermingham, E.; McMillan, W.O.; Jones, F.A. Comparative genetic structure of two mangrove species in Caribbean and Pacific estuaries of Panama. BMC Evol. Biol. 2012, 12, 205. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Lian, C.; Kameyama, N.; Hogetsu, T. Low genetic diversity and limited gene flow in a dominant mangrove tree species (Rhizophora stylosa) at its northern biogeographical limit across the chain of three Sakishima islands of the Japanese archipelago as revealed by chloroplast and nuclear SSR analysis. Plant Syst. Evol. 2013, 300, 1123–1136. [Google Scholar]
- Yang, Y.; Li, J.; Yang, S.; Li, X.; Fang, L.; Zhong, C.; Duke, N.C.; Zhou, R.; Shi, S. Effects of Pleistocene sea-level fluctuations on mangrove population dynamics: A lesson from Sonneratia alba. BMC Evol. Biol. 2017, 17, 22. [Google Scholar] [CrossRef]
- Saad, A.M.E. Wave and Wind Conditions in the Red Sea. A Numerical Study Using a Third Generation Wave Model; The University of Bergen: Bergen, Norway, 2010. [Google Scholar]
- Pavlopoulos, K.; Koukousioura, O.; Triantaphyllou, M.; Vandarakis, D.; De Procé, S.M.; Chondraki, V.; Fouache, E.; Kapsimalis, V. Geomorphological changes in the coastal area of Farasan Alkabir Island (Saudi Arabia) since mid Holocene based on a multi-proxy approach. Quat. Int. 2018, 493, 198–211. [Google Scholar] [CrossRef]
- Dodd, R.S.; Rafii, Z.A. Evolutionary genetics of mangroves: Continental drift to recent climate change. Trees 2002, 16, 80–86. [Google Scholar] [CrossRef]

| Study Area | No. of Pop | Marker | No. Loci | No. Alleles | Genetic Diversity | Fst | Genetic Structure | Data Analysis | Study Source |
|---|---|---|---|---|---|---|---|---|---|
| Pichavaram, Bhitarkanika Goa, Calicut | 10 | RAPD | 14 | 132 | 27.47% | 26.5% | Discrete subpopulations, 77% differential among populations. | Multivariate statistics package | [40] |
| Worldwide | 18 | Allozymes | 8 | 26 | High level 0–0.217 | Low level 0.384 | Discrete subpopulations, gene flow among populations was relatively low, except where populations were geographically continuous. | Biosys-l | [39] |
| India | 10 | RAPD | 14 | 172 | 76.7% | - | - | Nei’s index, UPGMA tree | [41] |
| Worldwide closet site = 500 km | 14 | SSRs | 3 | 70 | 0.407 | 0.384 | Discrete sub-populations with inbreeding at the edge. | AMOVA- Mantel tests- Genepop | [54] |
| Australia | 6 | SSRS AFLPs | 3 3 | 52 918 b | 0.78 0.193 | - | GeeneAlEX (AMOVA-Mantel test) | [43] | |
| Local scale: Sydney | 9 | Isozyme | 22 | 83 | - | - | Isolation by distance with inbreeding in each estuary. | AMOVA, TWINSPAN | [56] |
| Vietnam | 6 | AFLAP SSRS | 3 5 | 232 21 | 0.086 0.210 | 0.262 0.338 | Discrete sub-populations with inbreeding at all sites. | Popgene 3.2, PHYLP | [44] |
| Southeast Asia | 12 | SSRs | 7 | 118 | 0.15–0.79 | 0.01–0.67 | Discrete sub-populations with reduced diversity at range edges. | Bottleneck 1.2.02, Genetix. | [55] |
| Iran | 3 | SSRs | 3 | 14 | 0.451 to 0.667 | 0.03–0.05 | Reduced level of genetic variation was found in the central population, indicating a strong genetic structure among the other population with a large area and less exploitation. | Popgene 3.2, UPGMA tree | [57] |
| Arabian Gulf | 4 | SSRs | 5 | 4 to 4.6 | 0.782 to 0.960 | 0.044 | Low genetic differentiation among the populations. | FSTAT, UPGMA tree by TFPGA Ver. 1.3 | [48] |
| East coast of India | 3 | RAPD | 10 | 388 | 0.2274 ± 0.1122 | - | - | PopGene | [42] |
| North Red Sea coast | 3 | Est SSRs | 5 | 3–6 | 0.5–0.7 | - | - | GeeneAlEX Version 6.4 | [47] |
| Indonesia | 3 | SSRs | 4 | 14 | 0.54–0.6 | 0.002–0.09 | Low level of genetic variation in heavily polluted area. | PopGene, AMOVA by GeneAlEX Ver. 6.4,UPGMA tree by NTSys | [49] |
| Island’s Populations | Habitat | Inhabited Area | Code | Latitude | Longitude |
|---|---|---|---|---|---|
| Farasan Al-Kabir | Sand | Yes | Pop1 | 16.708608 | 42.177948 |
| Farasan Al-Kabir | Sand | Yes | Pop2 | 16.752721 | 42.065627 |
| Farasan Al-Kabir | Rocky | Yes | Pop3 | 16.704969 | 42.172678 |
| Farasan Al-Kabir | Rocky | Yes | Pop4 | 16.804166 | 42.069108 |
| Farasan Al-Kabir | Sand | Yes | Pop5 | 16.747602 | 42.000543 |
| Sajid | Sand | Yes | Pop6 | 16.756755 | 42.00426 |
| Sajid | Sand | Yes | Pop7 | 16.856417 | 41.968098 |
| Sajid | Sand | Yes | Pop8 | 16.857648 | 41.981981 |
| Zifaf | Rocky | No | Pop9 | 16.733156 | 41.745078 |
| Locus | Primer Sequence | Tm (°C) | Repetitive Sequence | Range Size (bp) | A | Ho | He | Nm | Fis | Fit | Fst |
|---|---|---|---|---|---|---|---|---|---|---|---|
| M13 | F:CAATGGTGATTCTCCAAAATTGCTTTG R:TGGTGAATAGATGACAGTAAGGATCAGCC | 56 | (AT)10(GT)12 | 175–205 | 12 | 0.777 | 0.627 | 0.889 | −0.238 | 0.033 | 0.219 |
| M3 | F:GGTTCCTGCAAGTATGTCAACACCCTC R:ACCTCGATTCCTCCCCGAATGC | 60 | (TG)15 | 183–200 | 16 | 0.694 | 0.709 | 0.965 | 0.021 | 0.222 | 0.206 |
| M27 | F:GGTGGAGTTTCAGTTCATCGTTCG R:CCGCAGTGGGGTTCATCAAAC | 56 | (CCG)8 | 103–108 | 5 | 0.383 | 0.280 | 1.666 | −0.370 | −0.191 | 0.131 |
| M32 | F:TGTGAACTTTGCTTCAGAGTCTCGAAGATG R:AGTCAAATGGAGCCTCATTCCTCCG | 60 | (AC)14 | 156–177 | 11 | 0.417 | 0.370 | 2.252 | −0.126 | −0.014 | 0.100 |
| M34 | F:TCTGCTGTTGCTGTTGTTGTTGATGC R:TGGTGTTGAAGACTAATCATGTGTTTCGC | 60 | (GCT)14 | 189–194 | 4 | 0.262 | 0.215 | 0.147 | −0.218 | 0.550 | 0.630 |
| M40 | F:CCCATAGATGACGGCAATCTTATGATCC R:ACCATCCAAAATAAAATAAATCTCCCTCCC | 60 | (AG)32 | 140–168 | 14 | 0.609 | 0.682 | 1.284 | 0.106 | 0.252 | 0.163 |
| M47 | F:TGACACCAAGGGAAATCAACATGCC R:GAACCTAGCGACCAATAGATCATCCTGG | 60 | (CA)13 | 180–210 | 16 | 0.333 | 0.665 | 0.813 | 0.499 | 0.617 | 0.235 |
| M49 | F:TTTCCTCACGACAGACTAGAAACCACC R:CAATAAACTTGGATAAAGGCAACTCCGAC | 56 | (TG)16 | 176–180 | 5 | 0.098 | 0.297 | 0.572 | 0.671 | 0.771 | 0.304 |
| M62 | F:TTGAGGAAAACATGGGACTTTCACTCG R:GTGGGAGTAGCCGCATAGAGTCACG | 60 | (CGC)8 | 227–229 | 2 | 0.000 | 0.031 | 0.017 | 1.000 | 1.000 | 0.938 |
| M64 | F:CAAACCCTACCAATCAGAACACTTCAAGC R:CGATATTTGGCTAATCCACTCTGCTGACTG | 60 | (CAG)8 | 148–158 | 7 | 0.395 | 0.330 | 0.192 | −0.199 | 0.479 | 0.565 |
| M73 | F:TTCCACAATCACTTGACCCTCGTCC R:TCTTCACAGGTCCTCTCCTGCCCTG | 60 | (TG)15 | 167–172 | 12 | 0.184 | 0.276 | 0.180 | 0.334 | 0.721 | 0.582 |
| M75 | F:TCCATAATCAAACAACTCGACAACGAAATC R:TCTTCTCTCCCTATTCCAAACTGGCTTG | 60 | (TG)14 | 208–210 | 9 | 0.412 | 0.574 | 1.688 | 0.281 | 0.374 | 0.129 |
| M81 | F:GAATGATGATCGGATGTTGCTACTCCTG R:CAATCCCAAAGCCCCAAAAATAATCC | 60 | (CA)9(CT)1 | 154–167 | 16 | 0.292 | 0.429 | 0.351 | 0.318 | 0.602 | 0.416 |
| M85 | F:TGACAGAGGTTTAGAGACATGGAGGGTGAG R:TGCCTCCCACATTCACCACACTGC | 60 | (GGC)8 | 105–118 | 9 | 0.201 | 0.224 | 2.251 | 0.104 | 0.193 | 0.100 |
| M98 | F:CCCAAACTCGTTACGATGGATGACTTC R:CTTACAGTTGCGGTAAAATGAGACGTGC | 60 | (CGG)8 | 211–220 | 4 | 0.212 | 0.186 | 2.077 | −0.139 | −0.017 | 0.107 |
| Population | N | A | Na | Pic | Ne | H0 | HE | I | F |
|---|---|---|---|---|---|---|---|---|---|
| Pop1 | 33 | 86 | 5.733 | 93.33% | 2.759 | 0.434 | 0.500 | 1.040 | 0.114 |
| Pop2 | 6 | 48 | 3.200 | 86.67% | 2.236 | 0.356 | 0.421 | 0.791 | 0.190 |
| Pop3 | 11 | 55 | 3.667 | 93.33% | 2.412 | 0.461 | 0.459 | 0.878 | −0.047 |
| Pop4 | 19 | 58 | 3.867 | 86.67% | 2.398 | 0.344 | 0.437 | 0.841 | 0.169 |
| Pop5 | 32 | 61 | 4.067 | 93.33% | 2.402 | 0.404 | 0.462 | 0.888 | 0.084 |
| Pop6 | 50 | 54 | 3.600 | 86.67% | 2.045 | 0.320 | 0.389 | 0.740 | 0.098 |
| Pop7 | 20 | 40 | 2.667 | 80.00% | 1.679 | 0.273 | 0.292 | 0.535 | 0.028 |
| Pop8 | 10 | 31 | 2.067 | 73.33% | 1.470 | 0.253 | 0.252 | 0.422 | 0.005 |
| Pop9 | 12 | 35 | 2.333 | 73.33% | 1.820 | 0.317 | 0.325 | 0.557 | −0.041 |
| Total/Mean | 193 | 52 | 3.467 | 85.19% | 2.136 | 0.351 | 0.393 | 0.743 | 0.070 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Qthanin, R.N.; Alharbi, S.A. Spatial Structure and Genetic Variation of a Mangrove Species (Avicennia marina (Forssk.) Vierh) in the Farasan Archipelago. Forests 2020, 11, 1287. https://doi.org/10.3390/f11121287
Al-Qthanin RN, Alharbi SA. Spatial Structure and Genetic Variation of a Mangrove Species (Avicennia marina (Forssk.) Vierh) in the Farasan Archipelago. Forests. 2020; 11(12):1287. https://doi.org/10.3390/f11121287
Chicago/Turabian StyleAl-Qthanin, Rahmah N., and Samah A. Alharbi. 2020. "Spatial Structure and Genetic Variation of a Mangrove Species (Avicennia marina (Forssk.) Vierh) in the Farasan Archipelago" Forests 11, no. 12: 1287. https://doi.org/10.3390/f11121287
APA StyleAl-Qthanin, R. N., & Alharbi, S. A. (2020). Spatial Structure and Genetic Variation of a Mangrove Species (Avicennia marina (Forssk.) Vierh) in the Farasan Archipelago. Forests, 11(12), 1287. https://doi.org/10.3390/f11121287





