Monitoring and Management of the Pine Processionary Moth in the North-Western Italian Alps
Abstract
1. Introduction
2. Materials and Methods
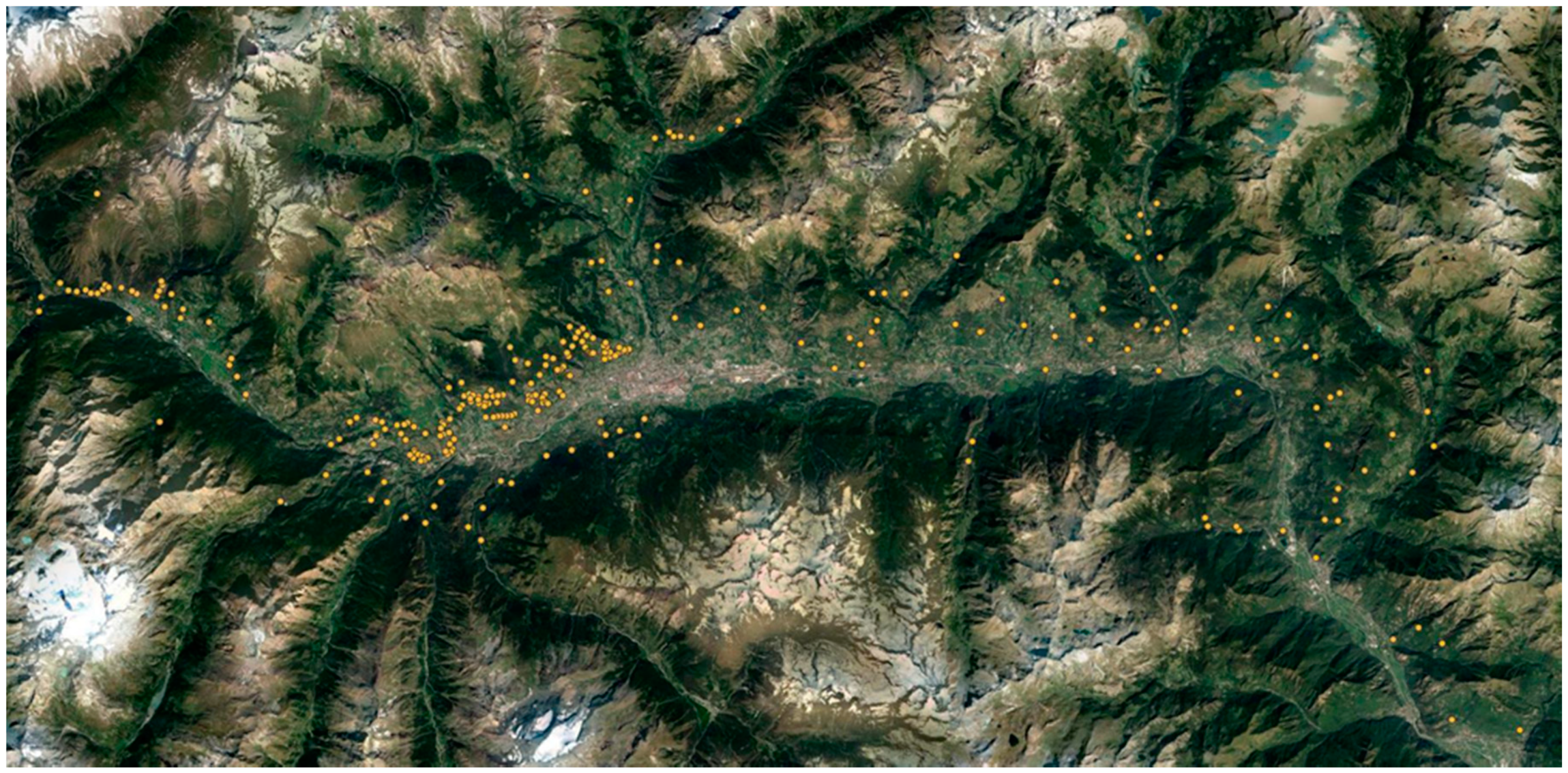
2.1. Study Area
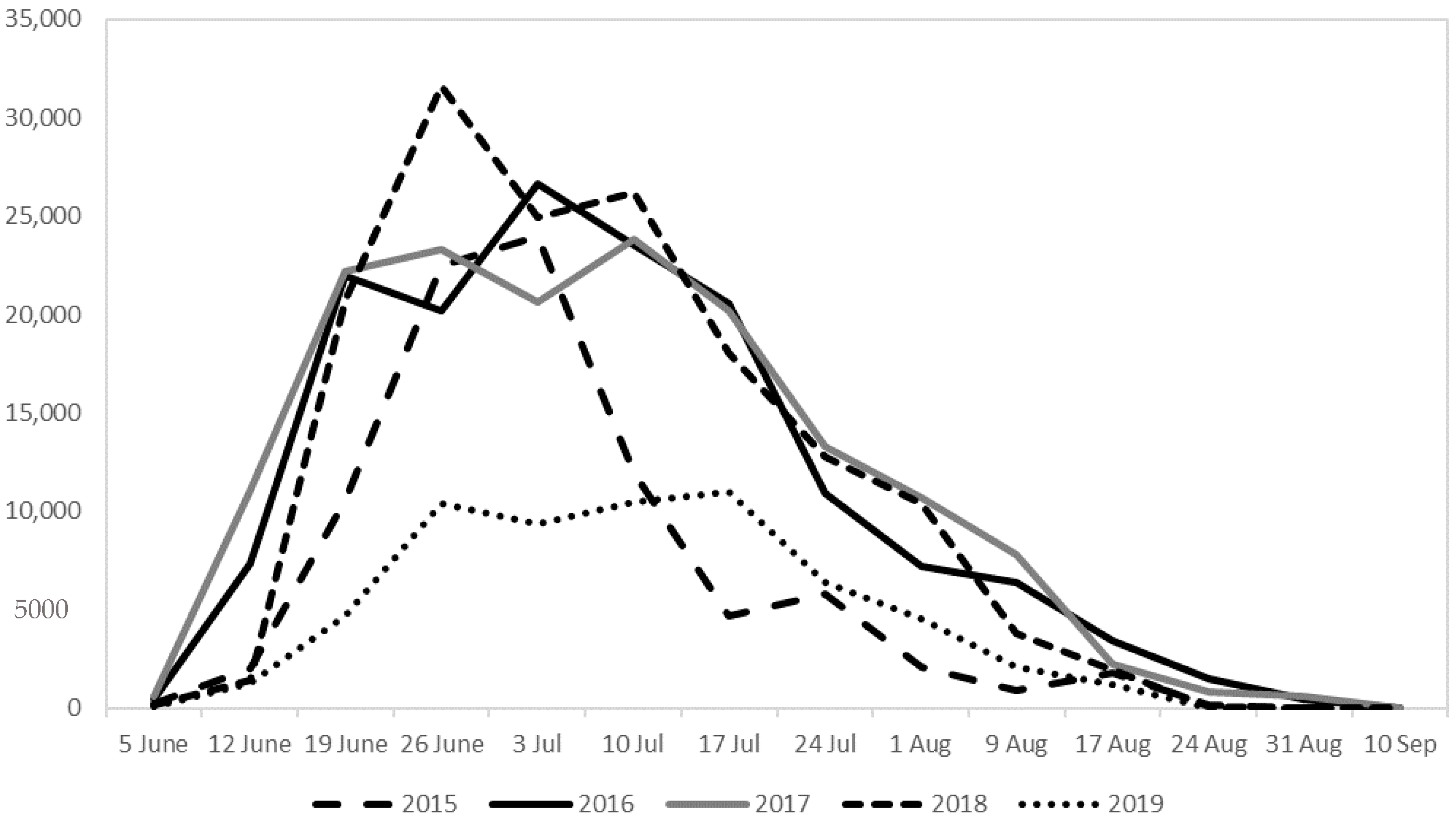
2.2. Seasonal Flight Activity
2.3. Infestation Index
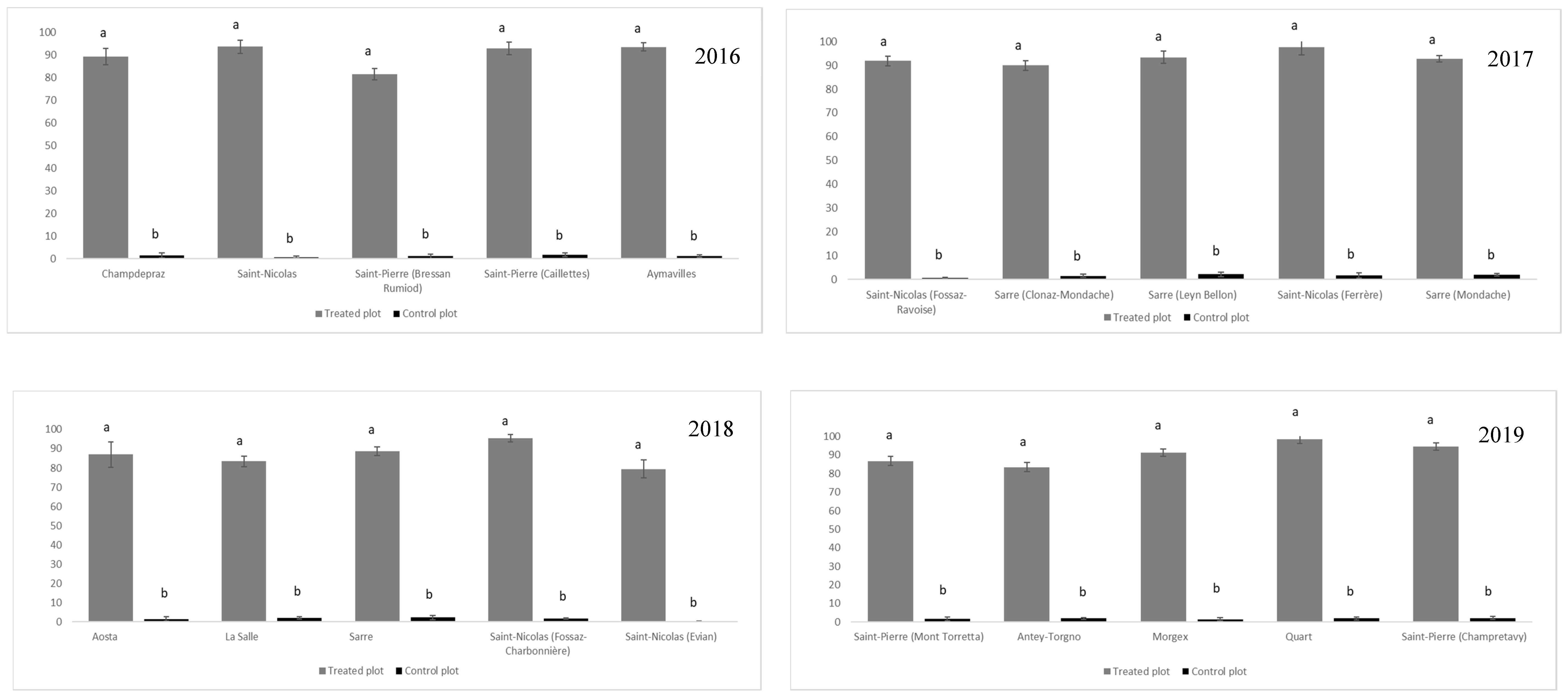
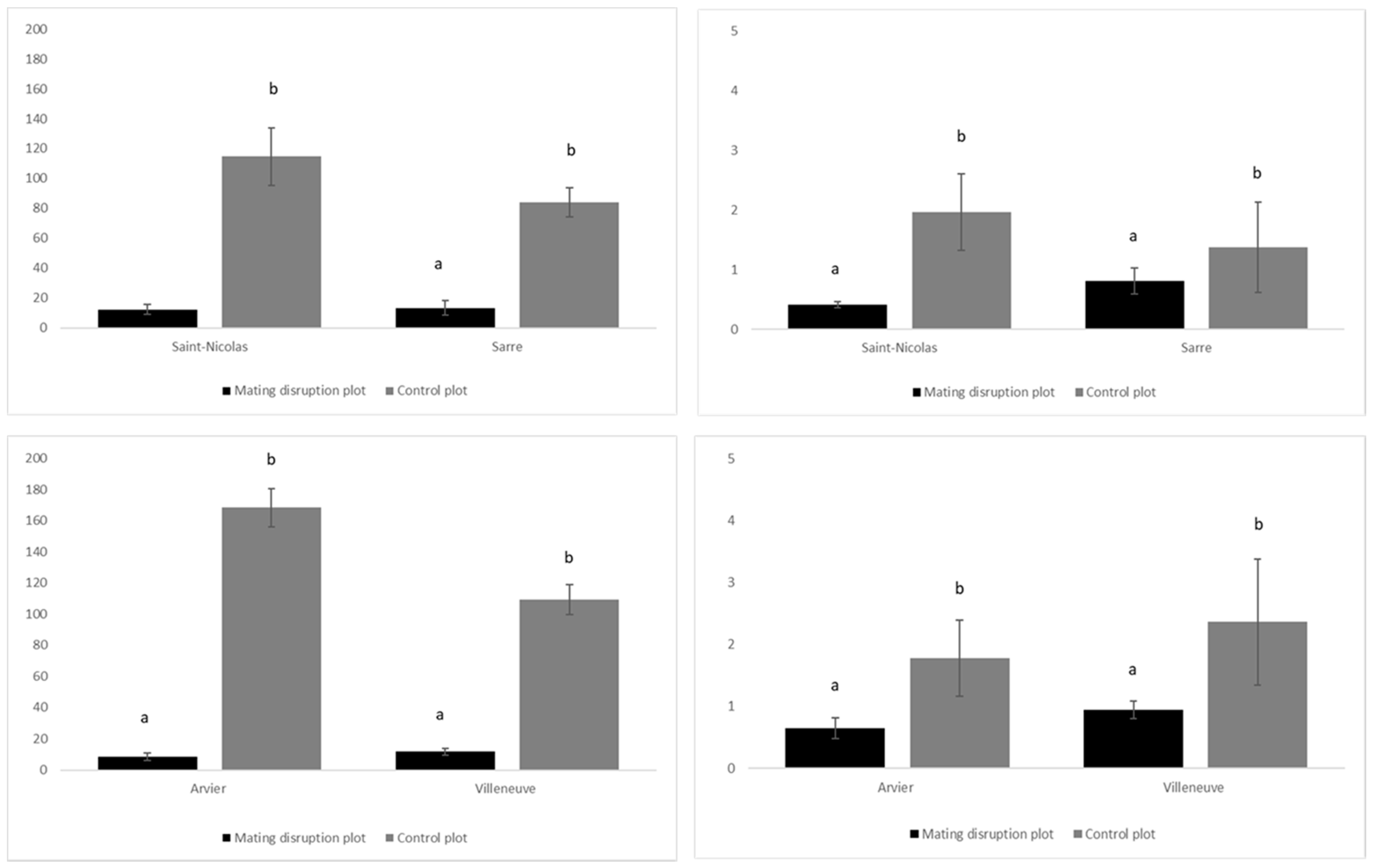
2.4. Application of Control Strategies
2.5. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chenchouni, H.; Zanati, K.; Rezougui, A.; Briki, A.; Arar, A. Population monitoring of Pine Processionary Moth (Thaumetopoea pityocampa) by pheromone trapping at the southern limit of distribution of Pinus halepensis in Eastern Algeria. For. Sci. Technol. 2010, 6, 67–79. [Google Scholar] [CrossRef]
- Devkota, B.; Schmidt, G.H. Larval development of Thaumetopoea pityocampa (Den. & Schiff.) (Lep. Thaumetopoeidae) from Greece as influenced by different host plants under laboratory conditions. J. Appl. Èntomol. 1990, 109, 321–330. [Google Scholar] [CrossRef]
- Battisti, A.; Stastny, M.; Netherer, S.; Robinet, C.; Schopf, A.; Roques, A.; Larsson, S. Expansion of geographic range in the pine processionary moth caused by increased winter temperatures. Ecol. Appl. 2005, 15, 2084–2096. [Google Scholar] [CrossRef]
- Buffo, E.; Battisti, A.; Stastny, M.; Larsson, S. Temperature as a predictor of survival of the pine processionary moth in the Italian Alps. Agric. For. Èntomol. 2007, 9, 65–72. [Google Scholar] [CrossRef]
- Arnaldo, P.S.; Torres, L.M. Effect of different hosts on Thaumetopoea pityocampa populations in northeast Portugal. Phytoparasitica 2006, 34, 523–530. [Google Scholar] [CrossRef]
- Arnaldo, P.; Chacim, S.; Lopes, D.; Arnaldo, P.S. Effects of defoliation by the pine processionary moth Thaumetopoea pityocampa on biomass growth of young stands of Pinus pinaster in northern Portugal. iFor. BiogeoSci. For. 2010, 3, 159–162. [Google Scholar] [CrossRef]
- Buxton, R.D. Forest management and the Pine Processionary Moth. Outlook Agric. 1983, 12, 34–39. [Google Scholar] [CrossRef]
- Çatal, Y. The effects of pine processionary moth (PPM) defoliation degree on radial growth of brutian pine (Pinus brutia). Afr. J. Agric. Res. 2011, 6, 4931–4936. [Google Scholar] [CrossRef]
- Gatto, P.; Zocca, A.; Battisti, A.; Barrento, M.J.; Branco, M.; Paiva, M.R. Economic assessment of managing processionary moth in pine forests: A case-study in Portugal. J. Environ. Manag. 2009, 90, 683–691. [Google Scholar] [CrossRef]
- Kanat, M.; Alma, M.H.; Sivrikaya, F. Effect of defoliation by Thaumetopoea pityocampa (Den. & Schiff.) (Lepidoptera: Thaumetopoeidae) on annual diameter increment of Pinus brutia Ten. in Turkey. Ann. For. Sci. 2005, 62, 91–94. [Google Scholar] [CrossRef]
- Morr, I. Diagnostic protocols for regulated pests. Protocoles de diagnostic pour les organismes réglementés. EPPO Bull. 2004, 34, 271–279. [Google Scholar]
- Colacci, M.; Kavallieratos, N.G.; Athanassiou, C.G.; Boukouvala, M.C.; Rumbos, C.I.; Kontodimas, D.C.; Pardo, D.; Sancho, J.; Benavent-Fernández, E.; Gálvez-Settier, S.; et al. Management of the Pine Processionary Moth, Thaumetopoea pityocampa (Lepidoptera: Thaumetopoeidae), in urban and suburban areas: Trials with trunk barrier and adhesive barrier trap devices. J. Econ. Èntomol. 2017, 111, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Rossi, J.-P.; Imbault, V.; Lamant, T.; Rousselet, J. A citywide survey of the pine processionary moth Thaumetopoea pityocampa spatial distribution in Orléans (France). Urban For. Urban Green. 2016, 20, 71–80. [Google Scholar] [CrossRef]
- Vega, J.M.; García-Ortiz, J.C.; Palla, P.S.; Sanchís, M.E.; Vega, J.; González-Muñoz, M.; Moneo, I.; Battisti, A.; Roques, A. Prevalence of cutaneous reactions to the pine processionary moth (Thaumetopoea pityocampa) in an adult population. Contact Dermat. 2011, 64, 220–228. [Google Scholar] [CrossRef]
- Cayuela, L.; Hódar, J.A.; Zamora, R. Is insecticide spraying a viable and cost-efficient management practice to control pine processionary moth in Mediterranean woodlands? For. Ecol. Manag. 2011, 261, 1732–1737. [Google Scholar] [CrossRef]
- Battisti, A.; Longo, S.; Tiberi, R.; Triggiani, O. Results and perspectives in the use of Bacillus thuringiensis Berl. var. kurstaki and other pathogens against Thaumetopoea pityocampa (Den. et Schiff.) in Italy (Lep., Thaumetopoeidae). Anz. Schadl. 1998, 71, 72–76. [Google Scholar]
- Shevelev, A.B.; Battisti, A.; Volynskaya, A.M.; Inovikova, S.; Kostina, L.A.; Zalunin, I. Susceptibility of the pine processionary caterpillar Thaumetopoea pityocampa (Lepidoptera: Thaumetopoeidae) toward delta-endotoxins of Bacillus thuringiensis under laboratory conditions. Ann. Appl. Biol. 2001, 138, 255–261. [Google Scholar] [CrossRef]
- Parlak, S.; Özçankaya, I.M.; Batur, M.; Akkaş, M.E.; Boza, Z.; Toprak, Ö. Efficiency of funnel traps in controlling pine processionary moth. J. Plant Dis. Prot. 2018, 125, 539–548. [Google Scholar] [CrossRef]
- Guerrero, A.; Camps, F.; Coll, J.; Riba, M.; Einhorn, J.; Descoins, C.; Lallemand, J. Identification of a potential sex pheromone of the processionary moth, Thaumetopea pityocampa (Lepidoptera, Notodontidae). Tetrahedron Lett. 1981, 22, 2013–2016. [Google Scholar] [CrossRef]
- Bonsignore, C.P.; Manti, F. Influence of habitat and climate on the capture of male pine processionary moths. Bull. Insectology 2013, 66, 27–34. [Google Scholar]
- Geri, C. Repartition et evolution des populations de la processionnaire du pin, Thaumetopoea pityocampa Schiff (Lep, Thaumetopoeidae) dans les montagnes. Acta Oecologica Oecologia 1983, 5, 3–22. [Google Scholar]
- Jactel, H.; Birgersson, G.; Andersson, S.; Schlyter, F. Non-host volatiles mediate associational resistance to the pine processionary moth. Oecologia 2011, 166, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Demolin, G. Comportement des adultes de Thaumetopoea pityocampa Schiff. Dispersion spatiale, importance écologique. Ann. Sci. For. 1969, 26, 81–102. [Google Scholar] [CrossRef]
- Masutti, L.; Battisti, A. Thaumetopoea pityocampa (Den. & Schiff.) in Italy Bionomics and perspectives of integrated control. J. Appl. Èntomol. 1990, 110, 229–234. [Google Scholar] [CrossRef]
- Robinet, C.; Rousselet, J.; Pineau, P.; Miard, F.; Roques, A. Are heat waves susceptible to mitigate the expansion of a species progressing with global warming? Ecol. Evol. 2013, 3, 2947–2957. [Google Scholar] [CrossRef]
- Battisti, A.; Stastny, M.; Buffo, E.; Larsson, S. A rapid altitudinal range expansion in the pine processionary moth produced by the 2003 climatic anomaly. Glob. Chang. Biol. 2006, 12, 662–671. [Google Scholar] [CrossRef]
- Mirchev, P.; Georgiev, G.; Geshev, G. Dispersal of male butterflies of pine processionary moth (Thaumetopoea pityocampa). Silva Balc. 2013, 14, 102–108. [Google Scholar]
- Peter, B.; Manti, F.; Castiglione, E.; Battisti, A. Pupal traits and adult emergence in the pine processionary moth Thaumetopoea pityocampa (Lepidoptera: Notodontidae) are affected by pupal density. Eur. J. Èntomol. 2019, 116, 320–329. [Google Scholar] [CrossRef]
- Van Frankenhuyzen, K. Development and current status of Bacillus thuringiensis for control of defoliating forest insects. For. Chron. 1990, 66, 498–507. [Google Scholar] [CrossRef][Green Version]
- Cunningham, J.C.; van Frankenhuyzen, K. Microbial insecticides in forestry. For. Chron. 1991, 67, 473–480. [Google Scholar] [CrossRef][Green Version]
- Avtzis, N.D. The use of Bacillus thuringiensis against Thaumetopoea pityocampa Schiff. (Lepidoptera: Thaumetopoeidae) in Greece. In Proceedings of the Population Dynamics, Impacts and Integrated Management of Forest Defoliating Insects, USDA Forest Service, General Technical Report NE-247, Banská Štiavnica, Slovakia, 18–23 August 1998; pp. 311–316. [Google Scholar]
- Cebeci, H.; Öymen, R.; Acer, S. Control of pine processionary moth, Thaumetopoea pityocampa with Bacillus thuringiensis in Antalya, Turkey. J. Environ. Biol. 2010, 31, 357–361. [Google Scholar]
- Dubois, N.R.; Reardon, R.C.; Kolodny-Hirsch, D.M. Field efficacy of the NRD-12 Strain of Bacillus thuringiensis against gypsy moth (Lepidoptera: Lymantriidae). J. Econ. Èntomol. 1988, 81, 1672–1677. [Google Scholar] [CrossRef]
- Roversi, P.F. Aerial Spraying of Bacillus thuringiensis var. kurstaki for the control of Thaumetopoea processionea in Turkey oak woods. Phytoparasitica 2008, 36, 175–186. [Google Scholar] [CrossRef]
- Hajek, A.; Van Frankenhuyzen, K. Use of Entomopathogens against forest pests. In Microbial Control of Insect and Mite Pests; Elsevier BV: Amsterdam, The Netherlands, 2017; Volume 21, pp. 313–330. [Google Scholar]
- Trematerra, P.; Colacci, M.; Athanassiou, C.G.; Kavallieratos, N.G.; Rumbos, C.; Boukouvala, M.C.; Nikolaidou, A.J.; Kontodimas, D.C.; Benavent-Fernández, E.; Gálvez-Settier, S. Evaluation of Mating disruption for the control of Thaumetopoea pityocampa (Lepidoptera: Thaumetopoeidae) in suburban recreational areas in Italy and Greece. J. Econ. Èntomol. 2019, 112, 2229–2235. [Google Scholar] [CrossRef]
- Carde, R.T.; Minks, A.K. Control of moth pests by mating disruption: Successes and constraints. Annu. Rev. Èntomol. 1995, 40, 559–585. [Google Scholar] [CrossRef]
- Robinet, C.; Baier, P.; Pennerstorfer, J.; Schopf, A.; Roques, A. Modelling the effects of climate change on the potential feeding activity of Thaumetopoea pityocampa (Den. & Schiff.) (Lep. Notodontidae) in France. Glob. Ecol. Biogeogr. 2007, 16, 460–471. [Google Scholar] [CrossRef]
- Geri, C.; Miller, C.; Xeuxet, D. Mesure des populations de processionnaire du pin (Thaumetopoea pityocampa Schiff—Lepidoptere Thaumetopoeidae) au Mont-Ventoux. Ann. Sci. For. 1985, 42, 143–184. [Google Scholar] [CrossRef]
- Li, S.; Daudin, J.-J.; Piou, D.; Robinet, C.; Jactel, H. Periodicity and synchrony of pine processionary moth outbreaks in France. For. Ecol. Manag. 2015, 354, 309–317. [Google Scholar] [CrossRef]
- Tamburini, G.; Marini, L.; Hellrigl, K.G.; Salvadori, C.; Battisti, A. Effects of climate and density-dependent factors on population dynamics of the pine processionary moth in the Southern Alps. Clim. Chang. 2013, 121, 701–712. [Google Scholar] [CrossRef]
- Bonsignore, C.; Manti, F.; Castiglione, E. Interactions between pupae of the pine processionary moth (Thaumetopoea pityocampa) and parasitoids in a Pinus forest. Bull. Èntomol. Res. 2015, 105, 621–628. [Google Scholar] [CrossRef]
- Tarasco, E.; Triggiani, O.; Zamoum, M.; Oreste, M. Natural enemies emerged from Thaumetopoea pityocampa (Denis & Sciffermüller) (Lepidoptera Notodontidae) pupae in Southern Italy. Redia 2015, 98, 103–108. [Google Scholar]
- De Boer, J.G.; Harvey, J.A. Range-Expansion in processionary moths and biological control. Insects 2020, 11, 267. [Google Scholar] [CrossRef] [PubMed]
- Houri, A.; Doughan, D. Behaviour Patterns of the Pine Processionary Moth (Thaumetopoea wilkinsoni Tams; Lepidoptera: Thaumetopoeidae). Am. J. Agric. Biol. Sci. 2006, 1, 1–5. [Google Scholar] [CrossRef][Green Version]
| 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. Municipality | ha | No. Municipality | ha | No. Municipality | ha | No. Municipality | ha | No. Municipality | ha | No. Municipality | ha | ||
| 42 | 42 | 42 | 42 | 42 | 42 | ||||||||
| Infestation index | 0 | 7598 | 7310 | 5482 | 4809 | 4713 | 4520 | ||||||
| 1 | 1058 | 1154 | 1539 | 2597 | 2212 | 4040 | |||||||
| 2 | 481 | 385 | 769 | 673 | 866 | 673 | |||||||
| 3 | 385 | 481 | 866 | 673 | 614 | 289 | |||||||
| 4 | 96 | 192 | 673 | 577 | 439 | 96 | |||||||
| 5 | 0 | 96 | 289 | 289 | 197 | 0 | |||||||
| Total treated (ha) | - | - | 367 | 333 | 208 | 253 | |||||||
| Bacillus thuringiensis var. kustaki | - | - | - | - | 14 | 344 | 9 | 314 | 12 | 208 | 250 | ||
| Mating disruption | - | - | - | - | 2 | 23 | 2 | 19 | - | - | - | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferracini, C.; Saitta, V.; Pogolotti, C.; Rollet, I.; Vertui, F.; Dovigo, L. Monitoring and Management of the Pine Processionary Moth in the North-Western Italian Alps. Forests 2020, 11, 1253. https://doi.org/10.3390/f11121253
Ferracini C, Saitta V, Pogolotti C, Rollet I, Vertui F, Dovigo L. Monitoring and Management of the Pine Processionary Moth in the North-Western Italian Alps. Forests. 2020; 11(12):1253. https://doi.org/10.3390/f11121253
Chicago/Turabian StyleFerracini, Chiara, Valerio Saitta, Cristina Pogolotti, Ivan Rollet, Flavio Vertui, and Luca Dovigo. 2020. "Monitoring and Management of the Pine Processionary Moth in the North-Western Italian Alps" Forests 11, no. 12: 1253. https://doi.org/10.3390/f11121253
APA StyleFerracini, C., Saitta, V., Pogolotti, C., Rollet, I., Vertui, F., & Dovigo, L. (2020). Monitoring and Management of the Pine Processionary Moth in the North-Western Italian Alps. Forests, 11(12), 1253. https://doi.org/10.3390/f11121253





