Relating Climate, Drought and Radial Growth in Broadleaf Mediterranean Tree and Shrub Species: A New Approach to Quantify Climate-Growth Relationships
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Tree Species
2.2. Climate Data and Drought Index
2.3. Field Sampling and Processing of Tree-Ring-Width Data
2.4. Climate– and Drought–Growth Relationships
2.5. Using Climwin to Define the Climate Window Most Tightly Related to Growth
3. Results
3.1. Tree-Ring Statistics of Species’ Mean Chronologies
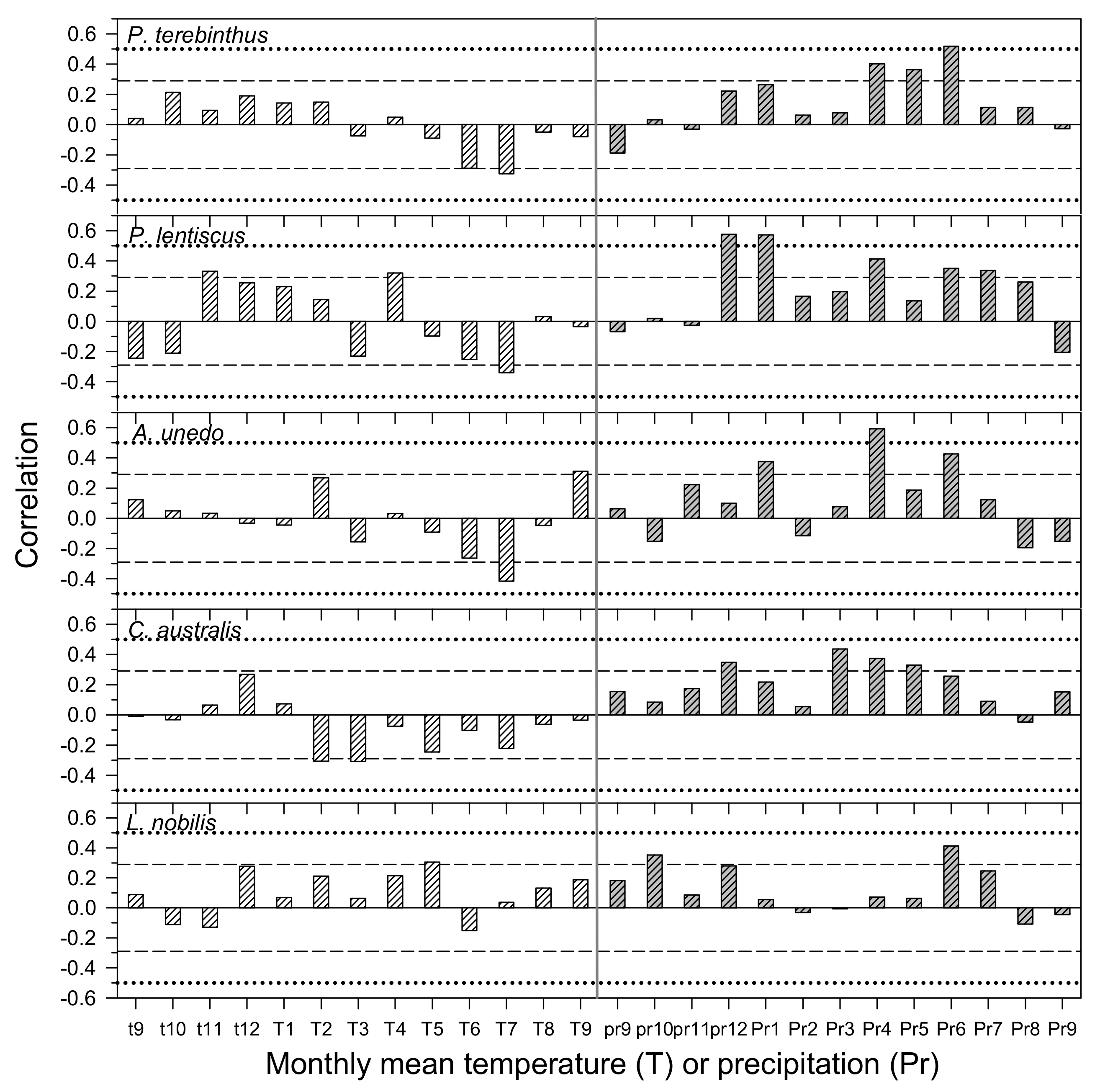
3.2. Climate– and Drought–Growth Relationships Based on Pearson Correlations
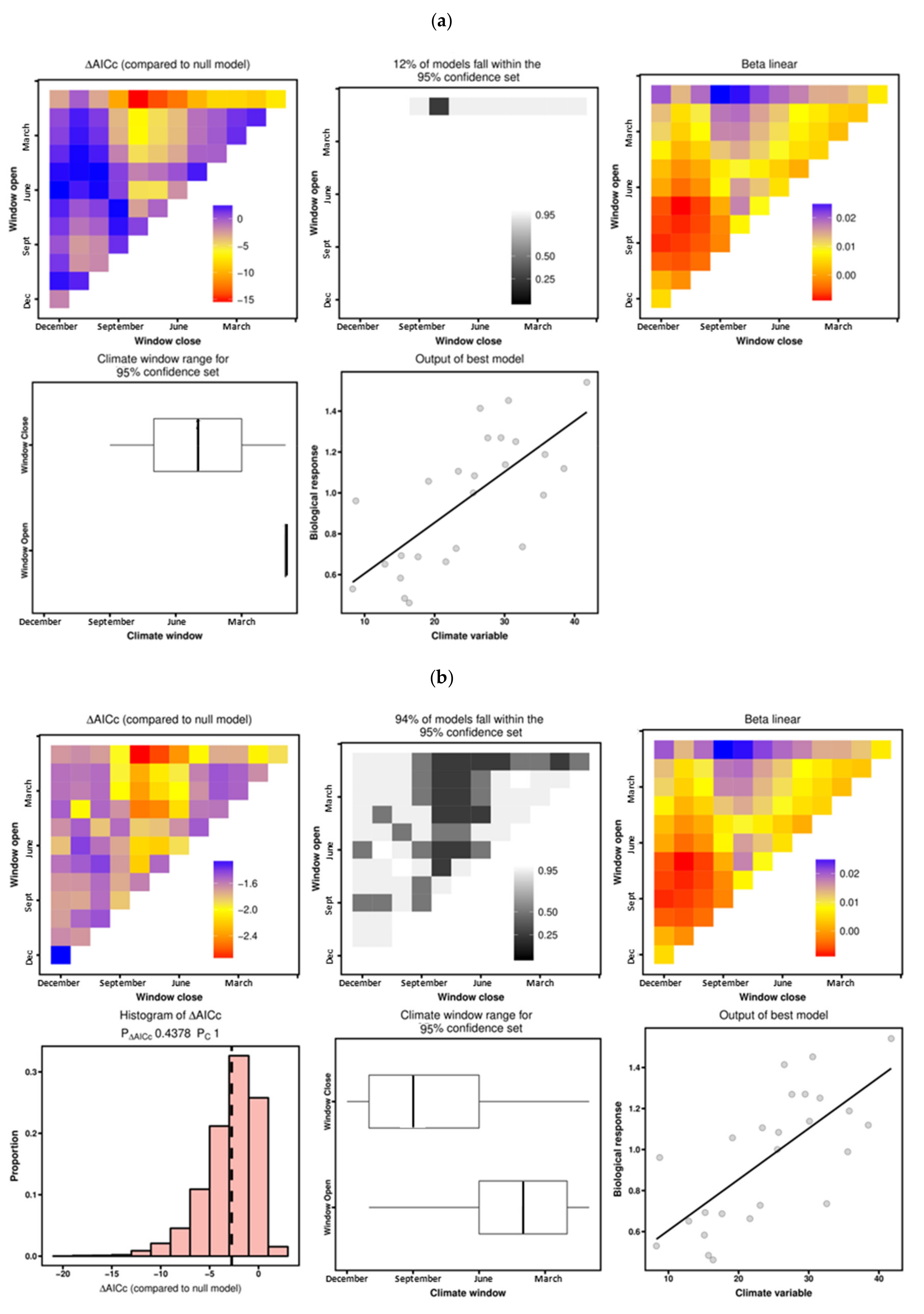
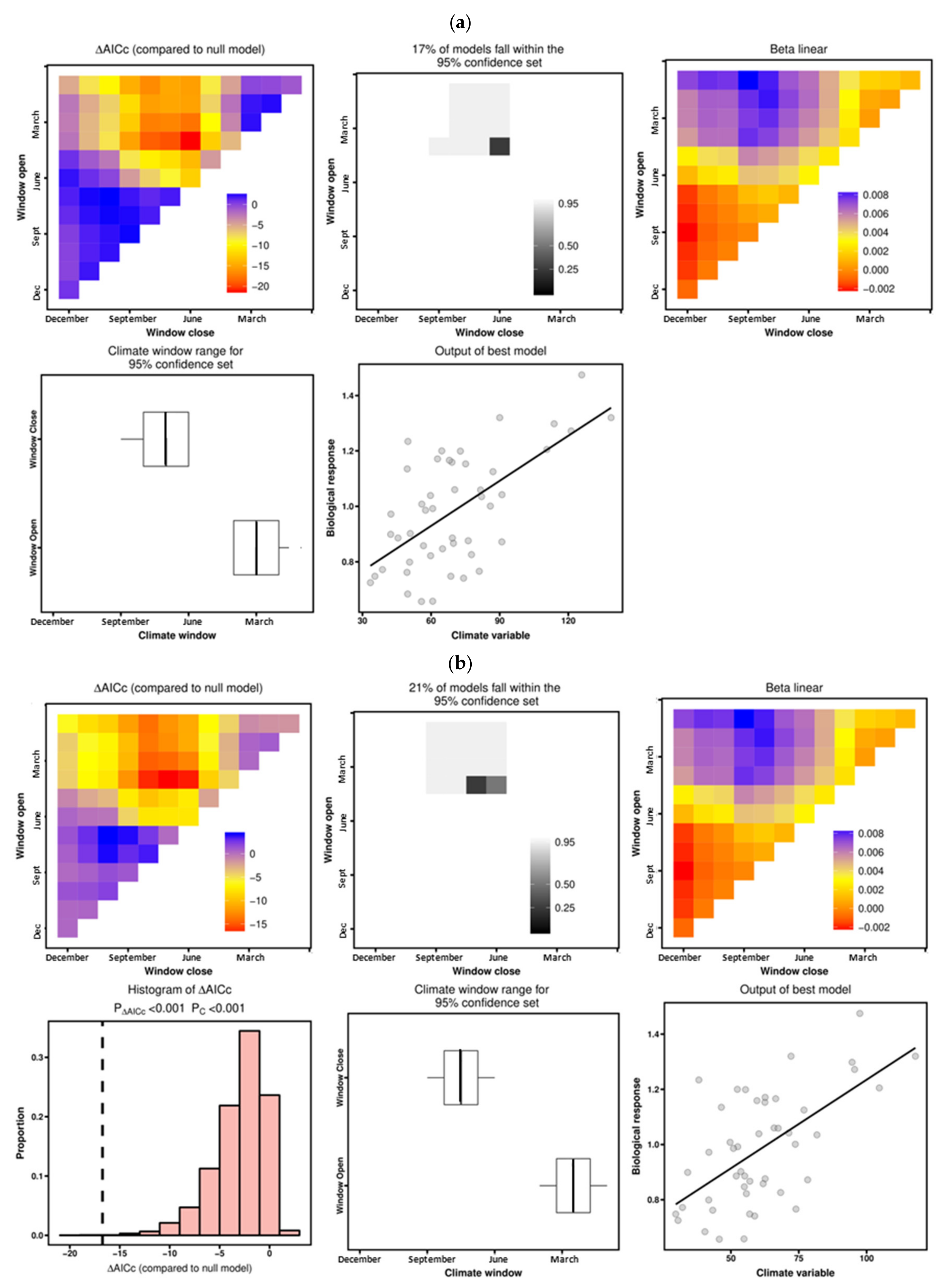
3.3. Climate–Growth Relationships According to “Climwin” Analyses
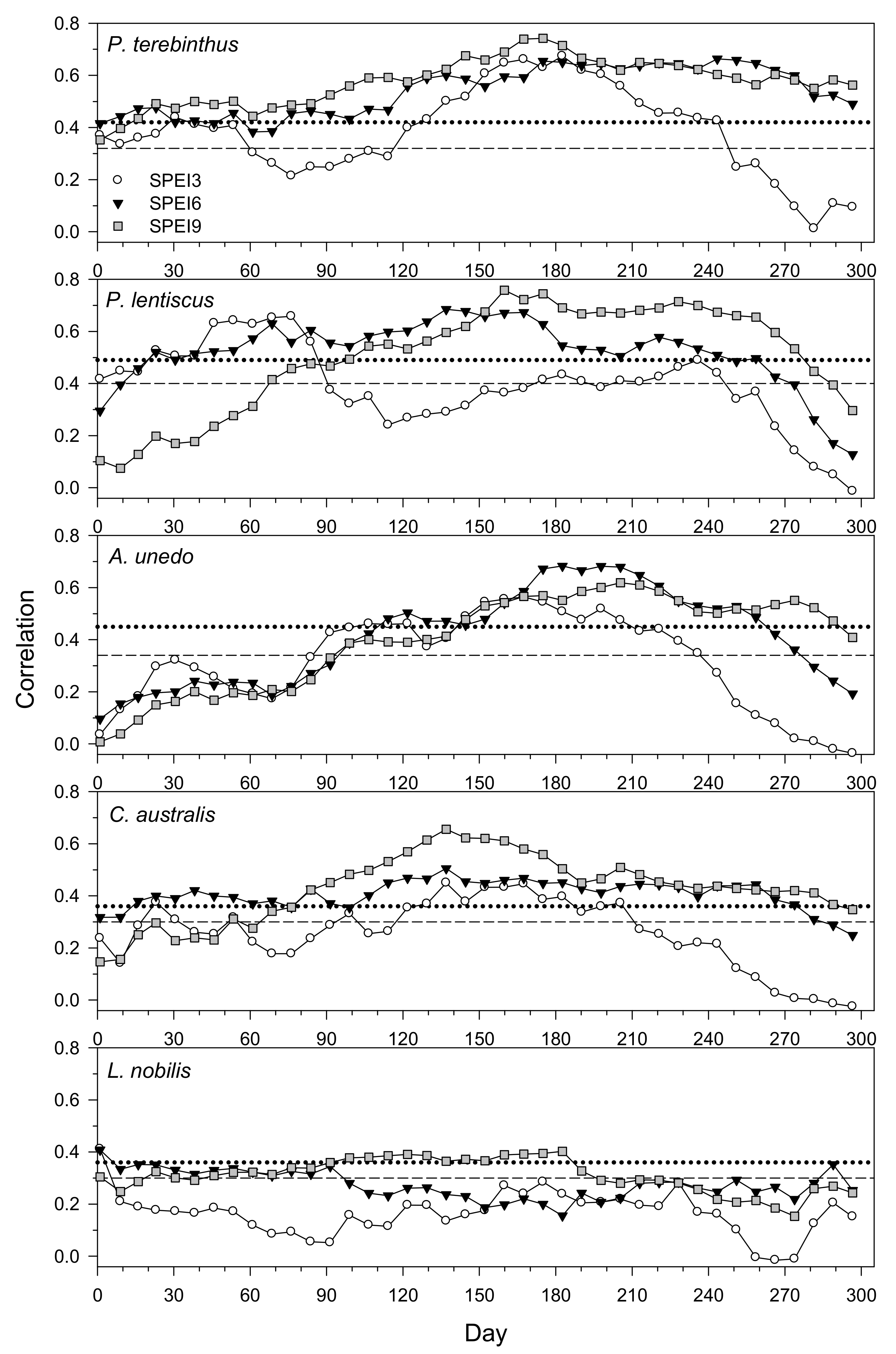
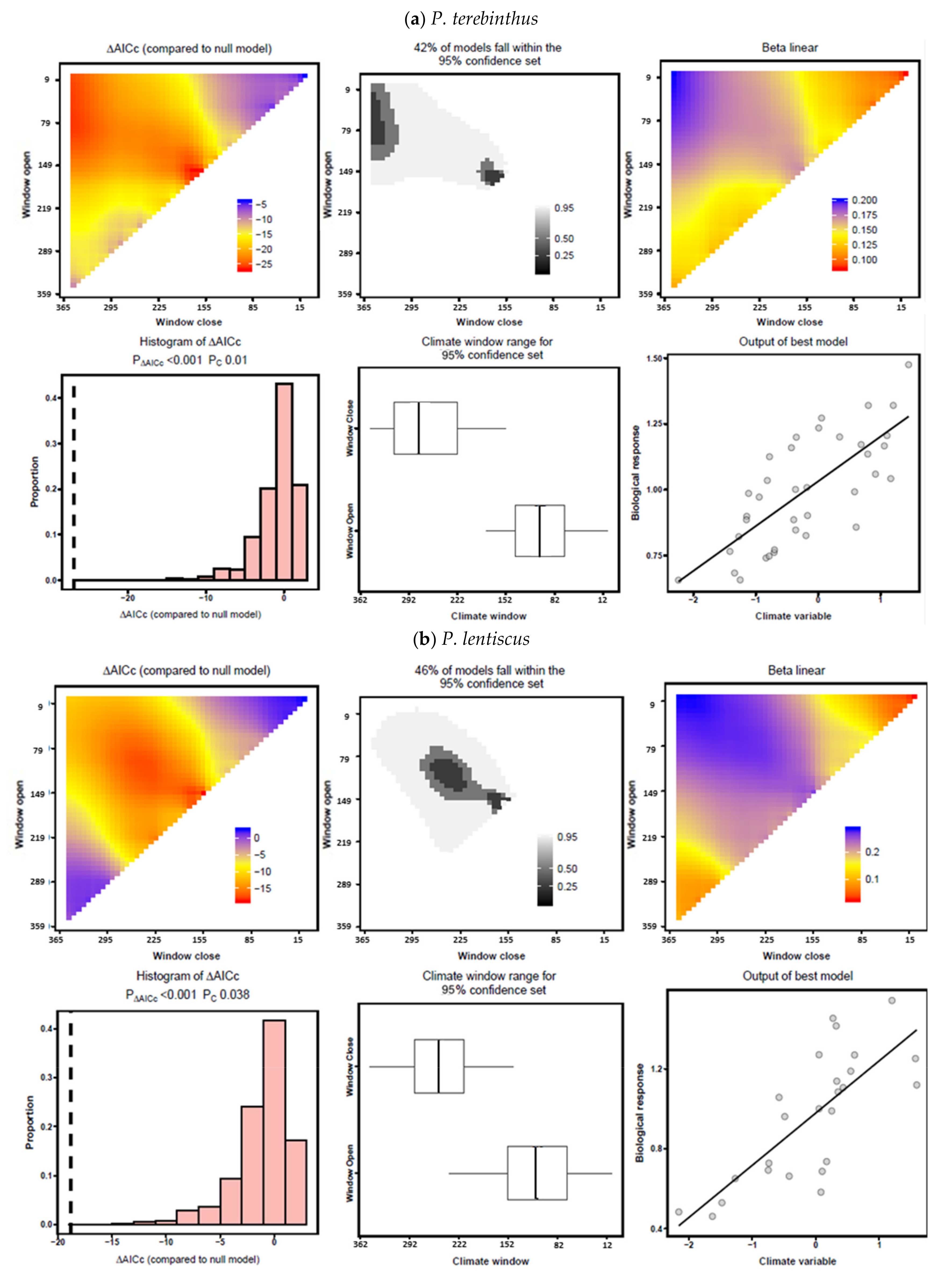
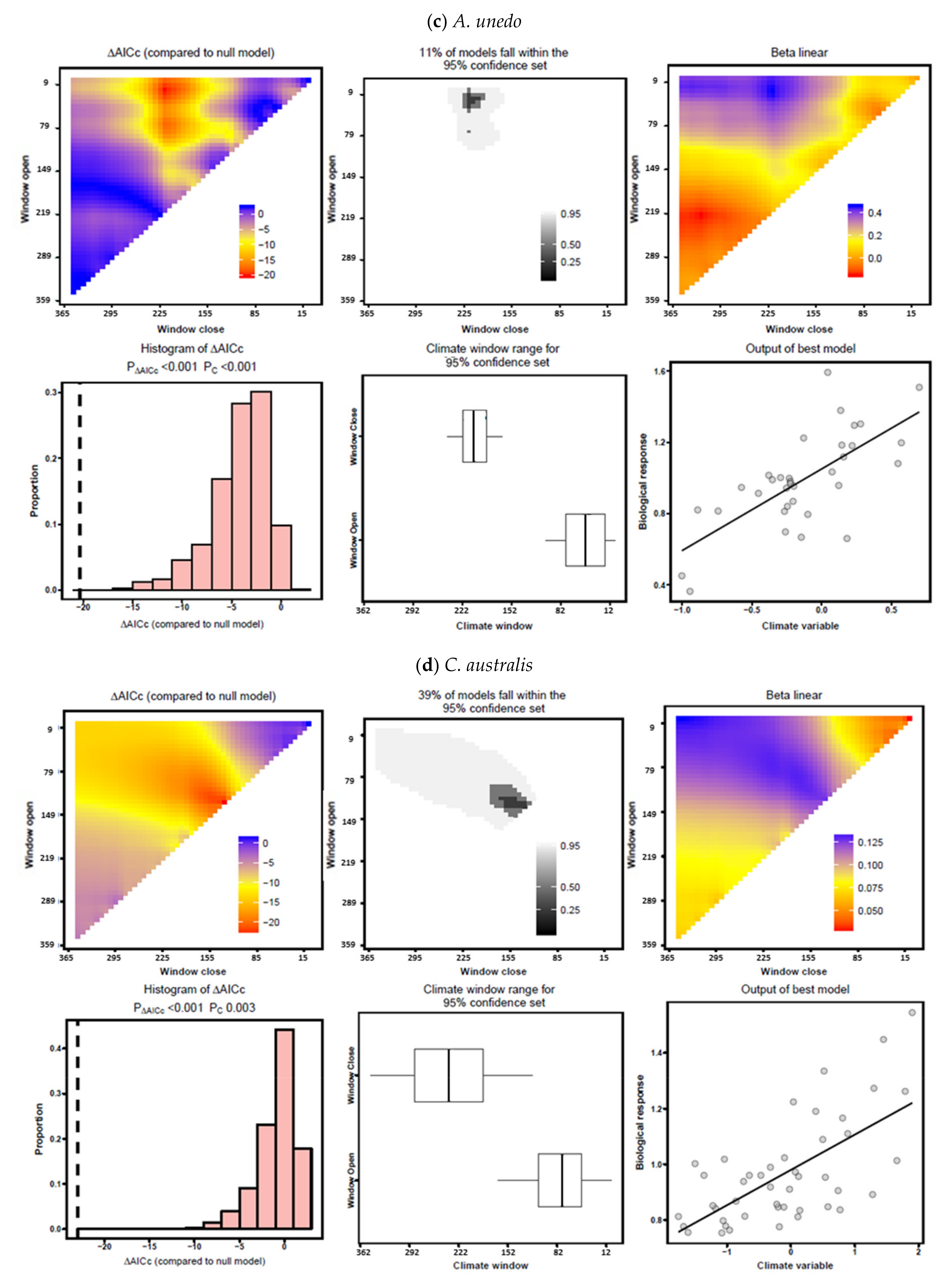
3.4. Drought–Growth Relationships According to “Climwin” Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Médail, F.; Quézel, P. Hot-spots analysis for conservation of plant biodiversity in the Mediterranean Basin. Ann. Mo. Bot. Gard. 1997, 84, 112–127. [Google Scholar] [CrossRef]
- Giorgi, F.; Lionello, P. Climate change projections for the Mediterranean region. Glob. Planet. Chang. 2008, 63, 90–104. [Google Scholar] [CrossRef]
- Cook, B.I.; Smerdon, J.E.; Seager, R.; Coats, S. Global warming and 21st century drying. Clim. Dyn. 2014, 43, 2607–2627. [Google Scholar] [CrossRef]
- Fritts, H.C. Tree-Rings and Climate; Academic Press: London, UK, 1976. [Google Scholar]
- Camarero, J.J. Linking functional traits and climate-growth relationships in Mediterranean species through wood density. IAWA J. 2018, 40, 215–240. [Google Scholar] [CrossRef]
- Gazol, A.; Sangüesa-Barreda, G.; Granda, E.; Camarero, J.J. Tracking the impact of drought on functionally different woody plants in a Mediterranean scrubland ecosystem. Plant Ecol. 2017, 218, 1009–1020. [Google Scholar] [CrossRef]
- Cherubini, P.; Gartner, B.L.; Tognetti, R.; Bräker, O.U.; Schoch, W.; Innes, J.L. Identification, measurement and interpretation of tree rings in woody species from mediterranean climates. Biol. Rev. 2003, 78, 119–148. [Google Scholar] [CrossRef]
- Camarero, J.J.; Sánchez-Salguero, R.; Ribas, M.; Touchan, R.; Andreu-Hayles, L.; Dorado-Liñá, I.; Meko, D.M.; Gutiérrez, E. Biogeographic, atmospheric and climatic factors influencing tree growth in Mediterranean Aleppo pine forests. Forests 2020, 11, 736. [Google Scholar] [CrossRef]
- Sánchez-Salguero, R.; Camarero, J.J.; Carrer, M.; Gutiérrez, E.; Alla, A.Q.; Andreu-Hayles, L.; Hevia, A.; Koutavas, A.; Martínez-Sancho, E.; Nola, P.; et al. Climate extremes and predicted warming threaten Mediterranean Holocene firs forests refugia. Proc. Natl. Acad. Sci. USA 2017, 114, E10142–E10150. [Google Scholar] [CrossRef]
- Garfi, G. Climatic signal in tree-rings of Quercus pubescens s.l. and Celtis australis L. in South-Eastern Sicily. Dendrochronologia 2000, 18, 41–51. [Google Scholar]
- Camarero, J.J.; Sánchez-Salguero, R.; Sangüesa-Barreda, G.; Matías, L. Tree species from contrasting hydrological niches show divergent growth and water-use efficiency. Dendrochronologia 2018, 52, 87–95. [Google Scholar] [CrossRef]
- Altieri, S.; Mereu, S.; Cherubini, P.; Castaldi, S.; Sirignano, C.; Lubritto, C.; Battipaglia, G. Tree-ring carbon and oxygen isotopes indicate different water use strategies in three Mediterranean shrubs at Capo Caccia (Sardinia, Italy). Trees Struct. Funct. 2015, 29, 1593–1603. [Google Scholar] [CrossRef]
- Copenheaver, C.A.; Gärtner, H.; Schäfer, I.; Vaccari, F.P.; Cherubini, P. Drought-triggered false ring formation in a Mediterranean shrub. Botany 2010, 88, 545–555. [Google Scholar] [CrossRef]
- Nijland, W.; Jansma, E.; Addink, E.A.; Domínguez-Delmás, M.; De Jong, S.M. Relating ring width of Mediterranean evergreen species to seasonal and annual variations of precipitation and temperature. Biogeosci. Discuss. 2011, 8, 355–383. [Google Scholar] [CrossRef]
- Battipagli, G.; De Micco, V.; Brand, W.A.; Linke, P.; Aronne, G.; Saurer, M.; Cherubini, P. Variations of vessel diameter and δ13C in false rings of Arbutus unedo L. reflect different environmental conditions. New Phytol. 2010, 188, 1099–1112. [Google Scholar] [CrossRef]
- Suc, J.P. Origin and evolution of the Mediterranean vegetation and climate in Europe. Nature 1984, 307, 409–432. [Google Scholar] [CrossRef]
- Palamarev, E. Paleobotanical evidences of the Tertiary history and origin of the Mediterranean sclerophyll dendroflora. Plant Syst. Evol. 1989, 162, 93–107. [Google Scholar] [CrossRef]
- Barrón, E.; Rivas-Carballo, R.; Postigo-Mijarra, J.; Alcalde-Olivares, C.; Vieira, M.; Castro, L.; Pais, J.; Valle-Hernández, M. The Cenozoic vegetation of the Iberian Peninsula: A synthesis. Rev. Palaeobot. Palynol. 2010, 162, 382–402. [Google Scholar] [CrossRef]
- Bailey, L.D.; van de Pol, M. Climwin: An R toolbox for climate window analysis. PLoS ONE 2016, 11, e0167980. [Google Scholar] [CrossRef]
- Van de Pol, M.; Bailey, L.D.; McLean, N.; Rijsdijk, L.; Lawson, C.R.; Brouwer, L. Identifying the best climatic predictors in ecology and evolution. Methods Ecol. Evol. 2016, 7, 1246–1257. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. 2019. Available online: https://www.R-project.org/ (accessed on 1 October 2020).
- Mitrakos, K. A theory for Mediterranean plant life. Acta Oecol. (Oecol. Plant.) 1980, 1, 245–252. [Google Scholar]
- Camarero, J.J.; Olano, J.M.; Parras, A. Plastic bimodal xylogenesis in conifers from continental Mediterranean climates. New Phytol. 2010, 185, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Montserrat-Martí, G.; Camarero, J.J.; Palacio, S.; Pérez-Rontomé, C.; Milla, R.; Albuixech, J.; Maestro, M. Summer-drought constrains the phenology and growth of two co-existing Mediterranean oaks with contrasting leaf habit: Implications for their persistence and reproduction. Trees Struct. Funct. 2009, 23, 787–799. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Tomas-Burguera, M.; Beguería, S.; Reig, F.; Latorre, B.; Peña-Gallardo, M.; Luna, M.Y.; Morata, A.; González-Hidalgo, J.C. A high-resolution dataset of drought indices for Spain. Data 2017, 2, 22. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Beguería, S.; López-Moreno, J.I. A multiscalar drought index sensitive to global warming: The standardized precipitation evapotranspiration index. J. Clim. 2010, 23, 1696–1718. [Google Scholar] [CrossRef]
- Pasho, E.; Camarero, J.J.; de Luis, M.; Vicente‒Serrano, S.M. Spatial variability in large‒scale and regional atmospheric drivers of Pinus halepensis growth in eastern Spain. Agric. For. Meteorol. 2011, 151, 1106–1119. [Google Scholar] [CrossRef]
- Pasho, E.; Camarero, J.J.; Vicente-Serrano, S.M. Climatic impacts and drought control of radial growth and seasonal wood formation in Pinus halepensis. Trees Struct. Funct. 2012, 26, 1875–1886. [Google Scholar] [CrossRef]
- Holmes, R.K. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull. 1983, 43, 69–78. [Google Scholar]
- Cook, E.R.; Krusic, P. A Tree-Ring Standardization Program Based on Detrending and Autoregressive Time Series Modeling, with Interactive Graphics; Tree-Ring Laboratory, Lamont Doherty Earth Observatory, Columbia University: New York, NY, USA, 2005. [Google Scholar]
- Briffa, K.R.; Jones, P.D. Basic chronology statistics and assessment. In Methods of Dendrochronology: Applications in the Environmental Sciences; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1990; pp. 137–152. [Google Scholar]
- Wigley, T.M.; Briffa, K.R.; Jones, P.D. On the average value of correlated time series, with applications in dendroclimatology and hydrometeorology. J. Clim. Appl. Meteorol. 1984, 23, 201–213. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration: Guidelines for Computing Crop Requirements; Irrigation and Drainage Paper No. 56; FAO: Rome, Italy, 1998. [Google Scholar]
- Gutiérrez, E.; Campelo, F.; Camarero, J.J.; Ribas, M.; Muntán, E.; Nabais, C.; Freitas, H. Climate controls act at different scales on the seasonal pattern of Quercus ilex L. stem radial increments in NE Spain. Trees Struct. Funct. 2011, 25, 637–646. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach; Springer: New York, NY, USA, 2002. [Google Scholar]
- Burnham, K.P.; Anderson, D.R.; Huyvaert, K.P. AIC model selection and multimodel inference in behavioral ecology: Some background, observations, and comparisons. Behav. Ecol. Sociobiol. 2011, 65, 23–35. [Google Scholar] [CrossRef]
- Campelo, F.; Gutiérrez, E.; Ribas, M.; Sánchez-Salguero, R.; Nabais, C.; Camarero, J.J. The facultative bimodal growth pattern in Quercus ilex—A simple model to predict sub-seasonal and inter-annual growth. Dendrochronologia 2018, 49, 77–88. [Google Scholar] [CrossRef]
- Lamas, S.; Rozas, V. Crecimiento radial de las principales especies arbóreas de la isla de Cortegada (Parque Nacional de las Islas Atlánticas) en relación con la historia y el clima. Investig. Agrar. Sist. Recur. For. 2007, 16, 3–14. [Google Scholar] [CrossRef]
- Gimeno, T.E.; Camarero, J.J.; Granda, E.; Pías, B.; Valladares, F. Enhanced growth of Juniperus thurifera under a warmer climate is explained by a positive carbon gain under cold and drought. Tree Physiol. 2012, 32, 326–336. [Google Scholar] [CrossRef]
- Trifilò, P.; Barbera, P.M.; Raimondo, F.; Nardini, A.; Lo Gullo, M.A. Coping with drought-induced xylem cavitation: Coordination of embolism repair and ionic effects in three Mediterranean evergreens. Tree Physiol. 2014, 34, 109–122. [Google Scholar] [CrossRef]
- Liphschitz, N.; Lev-Yadun, S. Cambial activity of evergreen and seasonal dimorphics around the Mediterranean. IAWA Bull. 1986, 7, 145–153. [Google Scholar] [CrossRef]
- Larcher, W. Temperature stress and survival ability of Mediterranean sclerophyllous plants. Plant Biosyst. Int. J. Deal. Asp. Plant. Biol. 2000, 134, 279–295. [Google Scholar] [CrossRef]
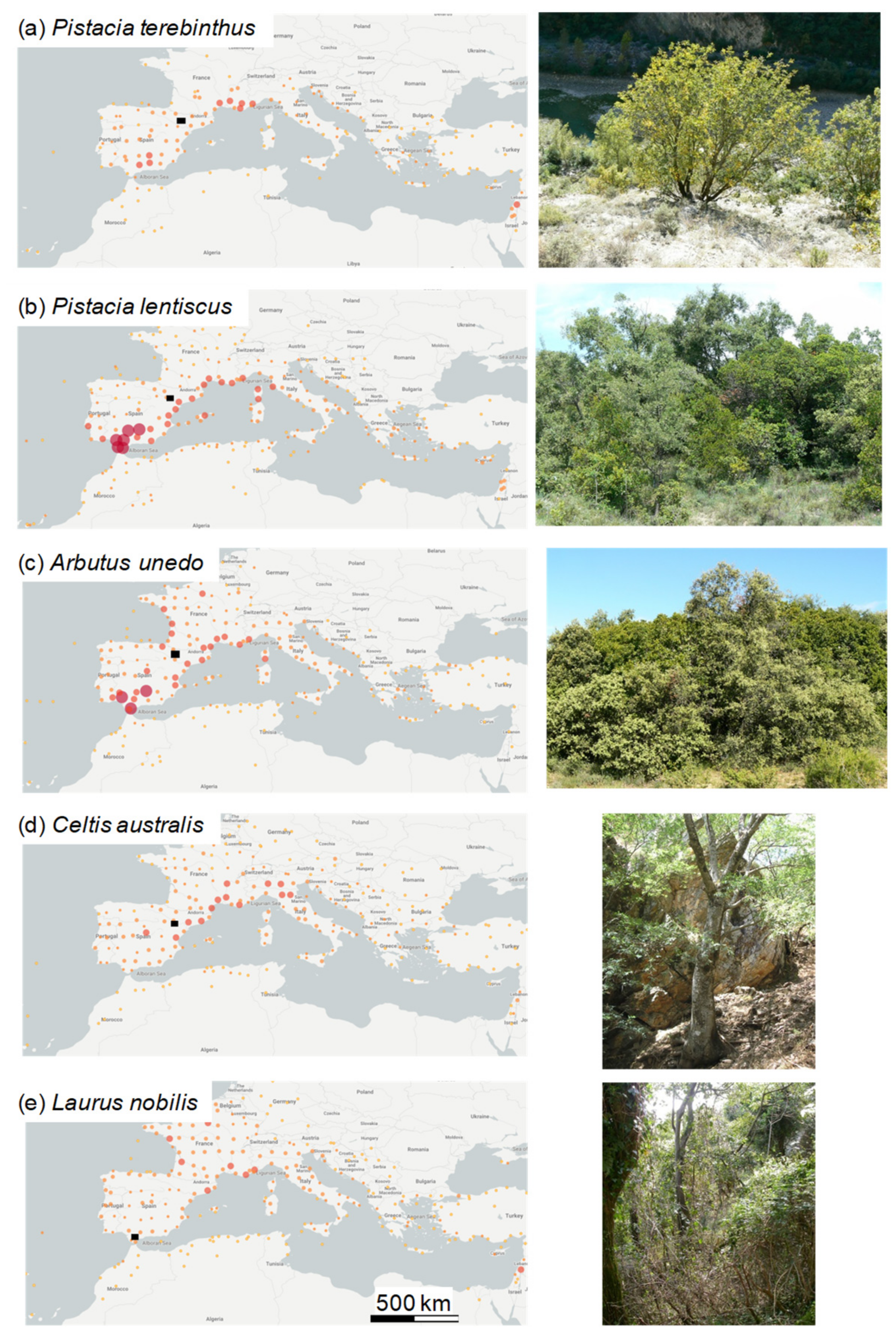
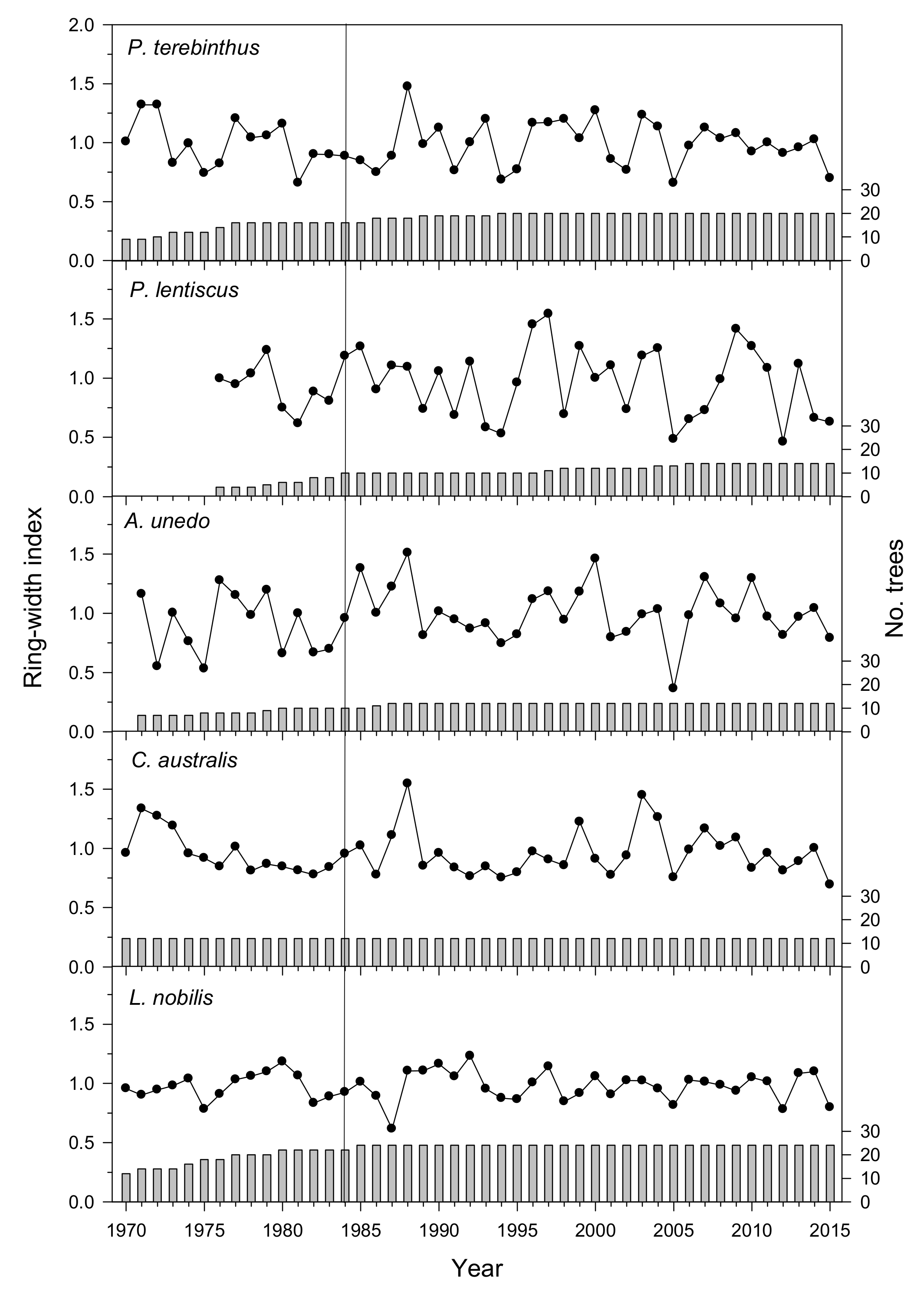

| Species | Diameter (cm) | No. Individuals | No. Radii | Best Replicated Period | Mean Ring Width (mm) | AC1 | MS | Rbar | Related Reference |
|---|---|---|---|---|---|---|---|---|---|
| Pistacia terebinthus | 15.7 ± 1.1 | 20 | 40 | 1973–2015 | 1.06 ± 0.51 | 0.49 | 0.26 | 0.54 | - |
| Pistacia lentiscus | 12.0 ± 0.9 | 14 | 28 | 1984–2015 | 0.65 ± 0.35 | 0.28 | 0.39 | 0.59 | [7,13] |
| Arbutus unedo | 20.2 ± 1.8 | 12 | 24 | 1980–2015 | 0.93 ± 0.63 | 0.45 | 0.41 | 0.46 | [8,14,15,16] |
| Celtis australis | 27.2 ± 3.8 | 12 | 24 | 1961–2015 | 0.85 ± 0.21 | 0.62 | 0.27 | 0.55 | [11,12] |
| Laurus nobilis | 22.0 ± 2.1 | 24 | 48 | 1971–2015 | 2.37 ± 1.04 | 0.46 | 0.21 | 0.40 | - |
| Arbutus unedo | Celtis australis | Pistacia terebinthus | Pistacia lentiscus | Laurus nobilis | |
|---|---|---|---|---|---|
| Arbutus unedo | 0.001 | 0.006 | 0.001 | 0.164 | |
| Celtis australis | 0.526 *** | 0.001 | 0.020 | 0.625 | |
| Pistacia terebinthus | 0.484 ** | 0.580 *** | 0.022 | 0.021 | |
| Pistacia lentiscus | 0.518 *** | 0.372 * | 0.411 * | 0.204 | |
| Laurus nobilis | 0.234 | 0.081 | 0.414 * | 0.208 |
| Species | Climate Variable | Linear Model | Linear Model Using K-Fold Cross-Validation and Randomization Method | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Climate Window | ΔAICc | R2 | p | Climate Window | ΔAICc | R2 | p of the Randomization | ||
| P. terebinthus | Temperature | June–July | −4.57 | 0.136 | 0.0108 | July | −2.40 | 0.136 | 0.4468 |
| Precipitation | April–June | −21.15 | 0.393 | 0.0001 | April–July | −16.73 | 0.362 | 0.0008 | |
| P. lentiscus | Temperature | June–July | 0.47 | 0.082 | 0.1664 | May–July | −2.29 | 0.0685 | 0.4976 |
| Precipitation | January–August | −15.14 | 0.508 | 0.0001 | January–August | −2.75 | 0.508 | 0.4378 | |
| A. unedo | Temperature | June–July | −3.64 | 0.163 | 0.0178 | June | −0.84 | 0.013 | 0.7376 |
| Precipitation | February–July | −17.13 | 0.437 | 0.0001 | March–July | −8.36 | 0.427 | 0.0510 | |
| C. australis | Temperature | February–March | −6.41 | 0.124 | 0.0040 | February–March | −2.72 | 0.124 | 0.3502 |
| Precipitation | January–May | −19.87 | 0.288 | 0.0001 | January–December | −4.10 | 0.197 | 0.2730 | |
| L. nobilis | Temperature | February–May | −3.68 | 0.140 | 0.0174 | October | −2.36 | 0.004 | 0.4038 |
| Precipitation | June–July | −6.86 | 0.206 | 0.0033 | April–October | −2.49 | 0.018 | 0.5224 | |
| Species | SPEI Period (Months) | Climate Window (DOY) | ∆AICc | R2 | p | p of the Randomization |
|---|---|---|---|---|---|---|
| P. terebinthus | 1 | 86–204 | −23.37 | 0.501 | 9.51 × 10−7 | <0.001 |
| 3 | 100–239 | −22.40 | 0.488 | 1.52 × 10−8 | <0.001 | |
| 6 | 2–330 | −24.21 | 0.513 | 6.31 × 10−7 | <0.001 | |
| 9 | 156–169 | −26.95 | 0.547 | 1.68 × 10−7 | <0.001 | |
| 12 | 121–330 | −26.76 | 0.545 | 1.84 × 10−7 | <0.001 | |
| 24 | 324–330 | −16.24 | 0.395 | 3.08 × 10−8 | <0.001 | |
| 36 | 233–267 | −6.14 | 0.206 | 4.82 × 10−3 | 0.012 | |
| P. lentiscus | 1 | 2–232 | −10.65 | 0.411 | 5.51 × 10−4 | 0.042 |
| 3 | 2–239 | −13.19 | 0.468 | 1.62 × 10−4 | 0.005 | |
| 6 | 2–232 | −17.62 | 0.555 | 1.96 × 10−9 | 0.001 | |
| 9 | 149–155 | −18.80 | 0.575 | 1.12 × 10−9 | <0.001 | |
| 12 | 212–218 | −17.49 | 0.552 | 2.09 × 10−8 | <0.001 | |
| 24 | 240–246 | −1.96 | 0.166 | 4.29 × 10−2 | 0.175 | |
| 36 | 233–239 | −0.04 | 0.100 | 1.23 × 10−1 | 0.327 | |
| A. unedo | 1 | 16–218 | −20.33 | 0.488 | 4.37 × 10−7 | <0.001 |
| 3 | 65–239 | −17.24 | 0.439 | 1.96 × 10−9 | <0.001 | |
| 6 | 163–211 | −19.09 | 0.469 | 7.99 × 10−8 | 0.001 | |
| 9 | 198–204 | −13.98 | 0.383 | 9.68 × 10−7 | 0.002 | |
| 12 | 191–218 | −10.49 | 0.316 | 5.44 × 10−4 | 0.007 | |
| 24 | 331–337 | −2.43 | 0.133 | 3.41 × 10−2 | 0.135 | |
| 36 | 205–211 | 0.66 | 0.050 | 2.03 × 10−1 | 0.467 | |
| C.australis | 1 | 86–155 | −7.66 | 0.199 | 2.15 × 10−3 | 0.11 |
| 3 | 2–204 | −9.48 | 0.230 | 8.50 × 10−4 | 0.023 | |
| 6 | 2–246 | −14.85 | 0.317 | 5.68 × 10−5 | 0.001 | |
| 9 | 128–134 | −22.98 | 0.430 | 1.02 × 10−6 | <0.001 | |
| 12 | 205–211 | −17.39 | 0.354 | 1.60 × 10−5 | <0.001 | |
| 24 | 338–344 | −5.64 | 0.162 | 6.18 × 10−3 | 0.026 | |
| 36 | 275–281 | −1.72 | 0.085 | 5.13 × 10−2 | 0.135 | |
| L. nobilis | 1 | 282–288 | −2.95 | 0.142 | 2.59 × 10−2 | 0.609 |
| 3 | 338–351 | −0.88 | 0.089 | 8.11 × 10−2 | 0.726 | |
| 6 | 317–330 | −1.66 | 0.109 | 5.22 × 10−2 | 0.446 | |
| 9 | 338–351 | −0.20 | 0.072 | 1.20 × 10−1 | 0.568 | |
| 12 | 2–8 | −1.05 | 0.094 | 7.34 × 10−2 | 0.253 | |
| 24 | 338–351 | 0.74 | 0.046 | 2.14 × 10−1 | 0.478 | |
| 36 | 282–288 | 1.41 | 0.028 | 3.37 × 10−1 | 0.629 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camarero, J.J.; Rubio-Cuadrado, Á. Relating Climate, Drought and Radial Growth in Broadleaf Mediterranean Tree and Shrub Species: A New Approach to Quantify Climate-Growth Relationships. Forests 2020, 11, 1250. https://doi.org/10.3390/f11121250
Camarero JJ, Rubio-Cuadrado Á. Relating Climate, Drought and Radial Growth in Broadleaf Mediterranean Tree and Shrub Species: A New Approach to Quantify Climate-Growth Relationships. Forests. 2020; 11(12):1250. https://doi.org/10.3390/f11121250
Chicago/Turabian StyleCamarero, J. Julio, and Álvaro Rubio-Cuadrado. 2020. "Relating Climate, Drought and Radial Growth in Broadleaf Mediterranean Tree and Shrub Species: A New Approach to Quantify Climate-Growth Relationships" Forests 11, no. 12: 1250. https://doi.org/10.3390/f11121250
APA StyleCamarero, J. J., & Rubio-Cuadrado, Á. (2020). Relating Climate, Drought and Radial Growth in Broadleaf Mediterranean Tree and Shrub Species: A New Approach to Quantify Climate-Growth Relationships. Forests, 11(12), 1250. https://doi.org/10.3390/f11121250






