Phytotoxic Effect of Macerates and Mulches from Cupressus leylandii Leaves on Clover and Cress: Role of Chemical Composition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Leaf Sampling
2.2. Macerate Preparations
2.3. Germination Tests with Macerates or Chemicals
2.4. Seedling Growth Tests with Mulches
2.5. Chemical Analysis
2.6. Germination Indices
2.7. Statistical Analysis
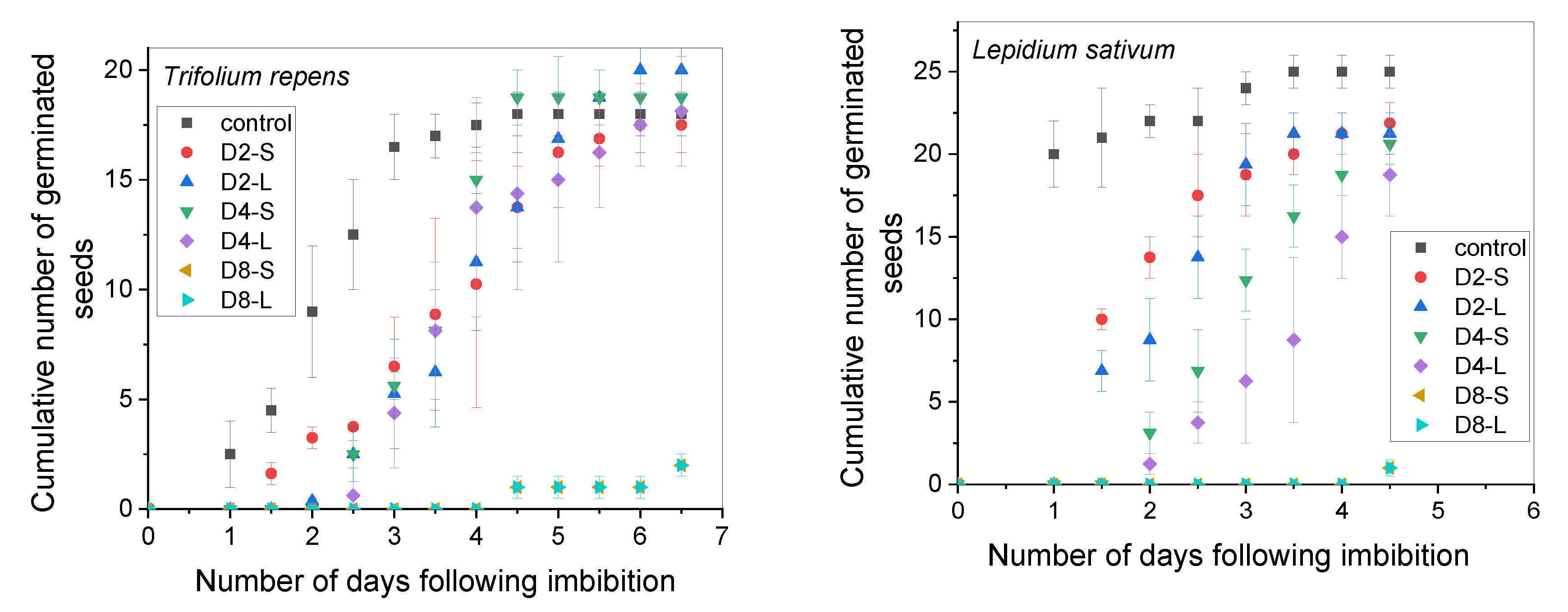
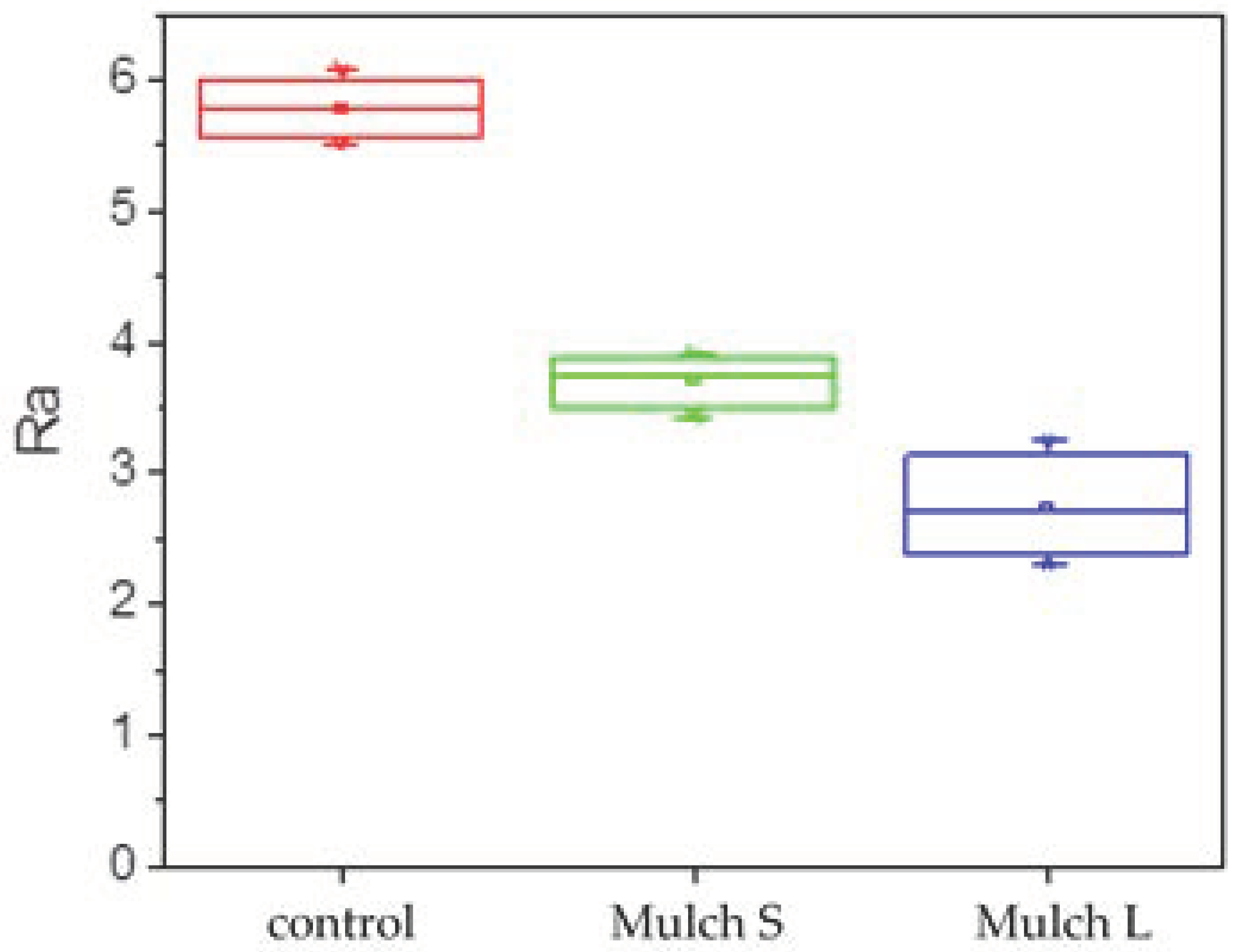
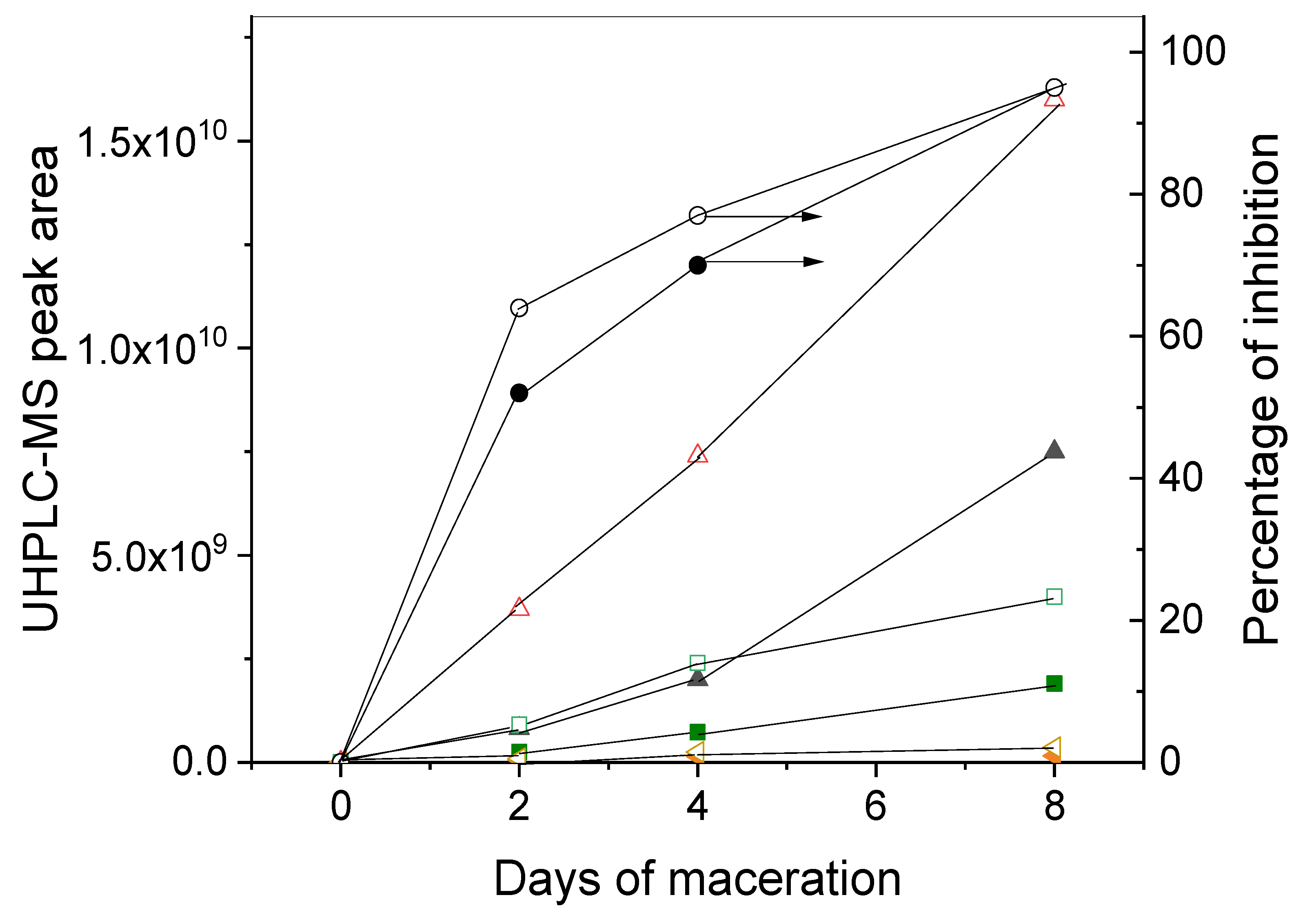
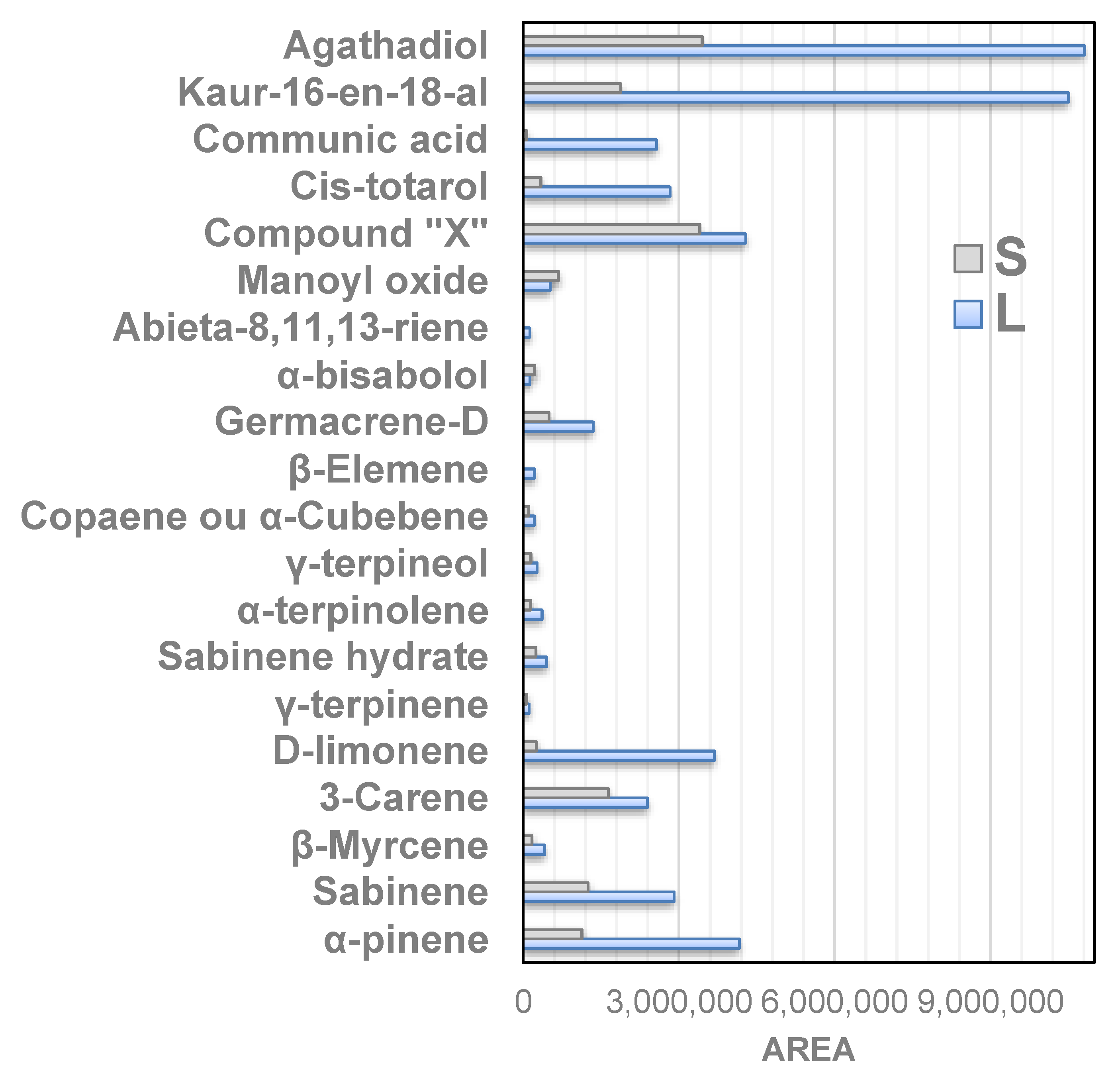
3. Results
3.1. Effect of Macerates on Seed Germination
3.2. Effect of Mulch on Seedling Growth
3.3. Chemical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Weston, L.A.; Alsaadawi, I.S.; Baerson, S.R. Sorghum Allelopathy—From Ecosystem to Molecule. J. Chem. Ecol. 2013, 39, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Inderjit; Weston, L.A.; Duke, S.O. Challenges, achievements and opportunities in allelopathy research. J. Plant Interact. 2005, 1, 69–81. [Google Scholar] [CrossRef]
- Macías, F.A.; Molinillo, J.M.G.; Varela, R.M.; Galindo, J.C.G. Allelopathy—A natural alternative for weed control. Pest Manag. Sci. 2007, 63, 327–348. [Google Scholar] [CrossRef] [PubMed]
- Inderjit, S.; Cheng, H.H.; Nishimura, H. Plant phenolics and terpenoids: Transformation, degradation, and potential for allelopathic interactions. In Principles and Practices in Plant Ecology: Allelochemical Interactions; Inderjit, S., Dakshini, K.M.M., Foy, C.L., Eds.; CRC Press: Boca Raton, FL, USA, 1999; pp. 255–266. [Google Scholar]
- Duke, S.O. Plant terpenoids as pesticides. In Toxicology of Plant and Fungal Compounds; Keeler, R.F., Tu, A.T., Eds.; Marcel-Dekker: New York, NY, USA, 1991; Volume 6, pp. 269–296. [Google Scholar]
- Kato-Noguchi, H.; Kitajima, S. Momilactone Sensitive Proteins in Arabidopsis thaliana. Nat. Prod. Com. 2015, 10. [Google Scholar] [CrossRef] [Green Version]
- Kordali, S.; Cakir, A.; Sutay, S. Inhibitory Effects of Monoterpenes on Seed Germination and Seedling Growth. Z. Nat. C 2007, 62, 207–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Q.; Xie, H.; Xiao, H.; Lin, L.; Wei, X. Two new ent-kaurene diterpene glucosides from the roots of Mikania micrantha. Phytochem. Lett. 2013, 6, 425–428. [Google Scholar] [CrossRef]
- Eom, S.H.; Yang, H.S.; Weston, L.A. An Evaluation of the Allelopathic Potential of Selected Perennial Groundcovers: Foliar Volatiles of Catmint (Nepeta × faassenii) Inhibit Seedling Growth. J. Chem. Ecol. 2006, 32, 1835–1848. [Google Scholar] [CrossRef]
- Araniti, F.; Lupini, A.; Sunseri, F.; Abenavoli, M.R. Allelopatic Potential of Dittrichia viscosa (L.) W. Greuter Mediated by VOCs: A Physiological and Metabolomic Approach. PLoS ONE 2017, 12, e0170161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, A.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Chemical profiling, cytotoxicity and phytotoxicity of foliar volatiles of Hyptis suaveolens. Ecotoxicol. Environ. Saf. 2019, 171, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Puig, C.G.; Gonçalves, R.F.; Valentão, P.; Andrade, P.B.; Reigosa, M.J.; Pedrol, N. The Consistency between Phytotoxic Effects and the Dynamics of Allelochemicals Release from Eucalyptus globulus Leaves Used as Bioherbicide Green Manure. J. Chem. Ecol. 2018, 44, 658–670. [Google Scholar] [CrossRef]
- Cregg, B.M.; Schutzki, R. Weed Control and Organic Mulches Affect Physiology and Growth of Landscape Shrubs. HortScience 2009, 44, 1419–1424. [Google Scholar] [CrossRef]
- Saha, D.; Marble, S.C.; Pearson, B.J. Allelopthic effects of Common Landscape and Nursery Mulch Materials on Weed Control. Front. Plant Sci. 2018, 9, 733. [Google Scholar] [CrossRef]
- Billeaud, L.A.; Zajicek, J.M. Influence of Mulches on Weed Control, Soil pH, Soil Nitrogen Content, and Growth of Ligustrum japonicum. J. Environ. Hortic. 1989, 7, 155–157. [Google Scholar] [CrossRef]
- Broschat, T.K. Effects of Mulch Type and Fertilizer Placement on Weed Growth and Soil pH and Nutrient Content. HortTechnology 2007, 17, 174–177. [Google Scholar] [CrossRef] [Green Version]
- Duryea, M.L.; English, R.J.; Hermansen, L.A. A comparison of landscape mulches. J. Arboricult. 1999, 25, 88–97. [Google Scholar]
- Pauly, G.; Yani, A.; Piovetti, L.; Bernard-Dagan, C. Volatile constituents of the leaves of Cupressus dupreziana and Cupressus semperiverens. Phytochemistry 1983, 22, 957–959. [Google Scholar] [CrossRef]
- Piovetti, L.; Gonzalez, E.; Diara, A. Diterpene composition of Cupressus dupreziana and Cupressus semperiverens. Phytochemistry 1980, 19, 2772–2773. [Google Scholar] [CrossRef]
- Khan, M.F.; Ahamad, T.; Rawat, P. Biomedicial and chemical profile of Cupressus sempervirens: A mini review. Insight Biomed. 2017, 2, 1–5. [Google Scholar]
- M’Barek, K. Chemical Composition and Phytotoxicity ofCupressus sempervirensLeaves Against Crops. J. Essent. Oil Bear. Plants 2016, 19, 1582–1599. [Google Scholar] [CrossRef]
- Olate, V.R.; Soto, A.E.; Schmeda-Hirschmann, G. Seasonal Variation and Resin Composition in the Andean Tree Austrocedrus chilensis. Molecules 2014, 19, 6489–6503. [Google Scholar] [CrossRef] [Green Version]
- Chiapusio, G.; Sanchez, A.M.; Reigosa, M.J.; González, L.; Pellissier, F. Do Germination Indices Adequately Reflect Allelochemical Effects on the Germination Process? J. Chem. Ecol. 1997, 23, 2445–2453. [Google Scholar] [CrossRef]
- Comino, C.; Lanteri, S.; Portis, E.; Acquadro, A.; Romani, A.; Hehn, A.; Larbat, R.; Bourgaud, F. Isolation and functional characterization of a cDNA coding a hydroxycinnamoyltransferase involved in phenylpropanoid biosynthesis in Cynara cardunculus L. BMC Plant Biol. 2007, 7, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hennequin, J.R.; Juste, C.; Lasserre, M. Presence of free phenolic acids in the soil. Investigation of their effects on germination and growth of plants. Ann. Agron. 1967, 18, 545–569. [Google Scholar]
- Moreno, B.P.; Mantovanelli, G.C.; Ricardo, L.L.; Silva, A.A.; De Oliveira, R.S.; Ishii-Iwamoto, E.L.; Sarragiotto, M.H.; Baldoqui, D.C. Chemical Characterization and Phytotoxic Effects of the Aerial Parts of Ruzigrass (Urochloa ruziziensis). Chem. Biodivers. 2020, 17, e1900694. [Google Scholar] [CrossRef]
- Peters, R.J. Two rings in them all: The labdane-related diterpenoids. Nat. Prod. Rep. 2010, 27, 1521–1530. [Google Scholar] [CrossRef] [Green Version]
- Ding, L.; Qi, L.; Jing, H.; Li, J.; Wang, W.; Wang, T. Phytotoxic Effects of Leukamenin E (anent-kaurene diterpenoid) on Root Growth and Root Hair Development in Lactuca sativa L. seedlings. J. Chem. Ecol. 2008, 34, 1492–1500. [Google Scholar] [CrossRef]
- Das, K.R.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. A kaurene-type novel phytotoxic substance in Wedelia chinensis. Tetrahedron Lett. 2020, 61, 151600. [Google Scholar] [CrossRef]
- Yang, W.H.; Zheng, L.P.; Yuan, H.Y.; Wang, J.W. Glaucocalyxin A and B Regulate Growth and Induce Oxidative Stress in Lettuce (Lactuca sativa L.) Roots. J. Plant Growth Regul. 2013, 33, 384–396. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, D.; Oliveros-Bastidas, A.; Alonso-Amelot, M.E.; Calcagno-Pissarelli, M.P. Two new labdane diterpenoids from the foliar exudates of Blakiella bartsiifolia. Phytochem. Lett. 2017, 20, 269–273. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, S.-H.; Hua, J.; Liu, Y.; Jing, S.-X.; Li, X.-N.; Li, S.-H. Capitate Glandular Trichomes of Paragutzlaffia henryi Harbor New Phytotoxic Labdane Diterpenoids. J. Agric. Food Chem. 2015, 63, 10004–10012. [Google Scholar] [CrossRef]
- Ali, M.; Abbasi, B.H. Light-induced fluctuations in biomass accumulation, secondary metabolites production and antioxidant activity in cell suspension cultures of Artemisia absinthium L. J. Photochem. Photobiol. B Biol. 2014, 140, 223–227. [Google Scholar] [CrossRef]
- Abbasi, B.H.; Tian, C.-L.; Murch, S.J.; Saxena, P.K.; Liu, C.-Z. Light-enhanced caffeic acid derivatives biosynthesis in hairy root cultures of Echinacea purpurea. Plant Cell Rep. 2007, 26, 1367–1372. [Google Scholar] [CrossRef] [PubMed]
- Kreuzaler, F.; Hahlbrock, K. Flavonoid glycosides from illuminated cell suspension cultures of Petroselinum hortense. Phytochemistry 1973, 12, 1149–1152. [Google Scholar] [CrossRef]


| Treatment | Germinated Seeds | |
|---|---|---|
| Trifolium Repens | Lepidium Sativum | |
| Control | 71 ± 8 | 98 ± 2 |
| March, macerate S-D12 | 6 ± 2 | 4 ± 1 |
| March, macerate L-D12 | 1 ± 1 | 1 ± 1 |
| May, macerate S-D8 | 2 ± 1 | 1 ± 1 |
| May, macerate S-D8 neutralized | 1 ± 1 | |
| May, macerate L-D8 | 2 ± 1 | 2 ± 1 |
| May, macerate L-D8 neutralized | 1 ± 1 | |
| Macerate | Organic Acids | Phenols | Sugars |
|---|---|---|---|
| L-D2 | 6.2 × 109 | 2.0 × 109 | 5.2 × 108 |
| L-D4 | 14 × 109 | 2.3 × 109 | 1.9 × 109 |
| L-D8 | 28 × 109 | 10 × 109 | 3.0 × 109 |
| S-D2 | 3.0 × 109 | 4.1 × 109 | 3.8 × 108 |
| S-D4 | 5.5 × 109 | 3.2 × 109 | 8.7 × 108 |
| S-D8 | 15 × 109 | 5.3 × 109 | 1.3 × 109 |
| AreaL-D4/AreaS-D4 | 2.5 | 0.71 | 2.2 |
| Analysis Technique | Sample | Organic Acids | Phenols | Terpenes | Terpenoids | Diterpenoids |
|---|---|---|---|---|---|---|
| UHPLC | L | 2.4 × 109 | 1.1 × 108 | 2.9 × 109 | ||
| S | 1.2 × 109 | 2.9 × 108 | 2.8 × 108 | |||
| AreaL/AreaS | 2.0 | 0.37 | 9.3 | |||
| GC | L | 1.4 × 107 | 2.7 × 105 | 2.1 × 107 | ||
| S | 4.5 × 106 | 1.5 × 105 | 3.4 × 106 | |||
| AreaL/AreaS | 3.0 | 1.8 | 6.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khaled, A.; Sleiman, M.; Goupil, P.; Richard, C. Phytotoxic Effect of Macerates and Mulches from Cupressus leylandii Leaves on Clover and Cress: Role of Chemical Composition. Forests 2020, 11, 1177. https://doi.org/10.3390/f11111177
Khaled A, Sleiman M, Goupil P, Richard C. Phytotoxic Effect of Macerates and Mulches from Cupressus leylandii Leaves on Clover and Cress: Role of Chemical Composition. Forests. 2020; 11(11):1177. https://doi.org/10.3390/f11111177
Chicago/Turabian StyleKhaled, Amina, Mohamad Sleiman, Pascale Goupil, and Claire Richard. 2020. "Phytotoxic Effect of Macerates and Mulches from Cupressus leylandii Leaves on Clover and Cress: Role of Chemical Composition" Forests 11, no. 11: 1177. https://doi.org/10.3390/f11111177
APA StyleKhaled, A., Sleiman, M., Goupil, P., & Richard, C. (2020). Phytotoxic Effect of Macerates and Mulches from Cupressus leylandii Leaves on Clover and Cress: Role of Chemical Composition. Forests, 11(11), 1177. https://doi.org/10.3390/f11111177





