Collembolan Assemblages Response to Wild Boars (Sus scrofa L.) Rooting in Pine Forest Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites and Sampling
2.2. Data Analysis
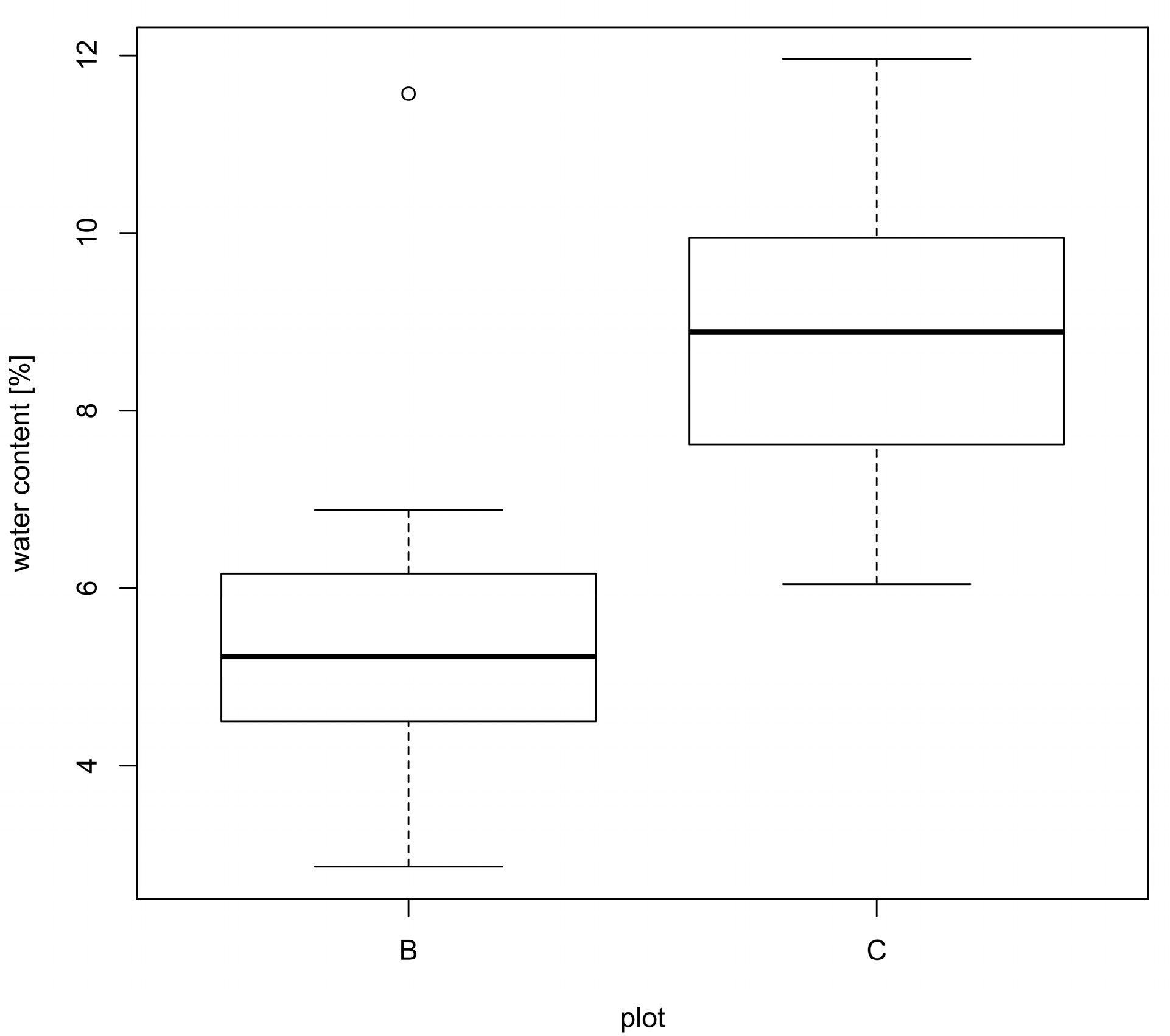
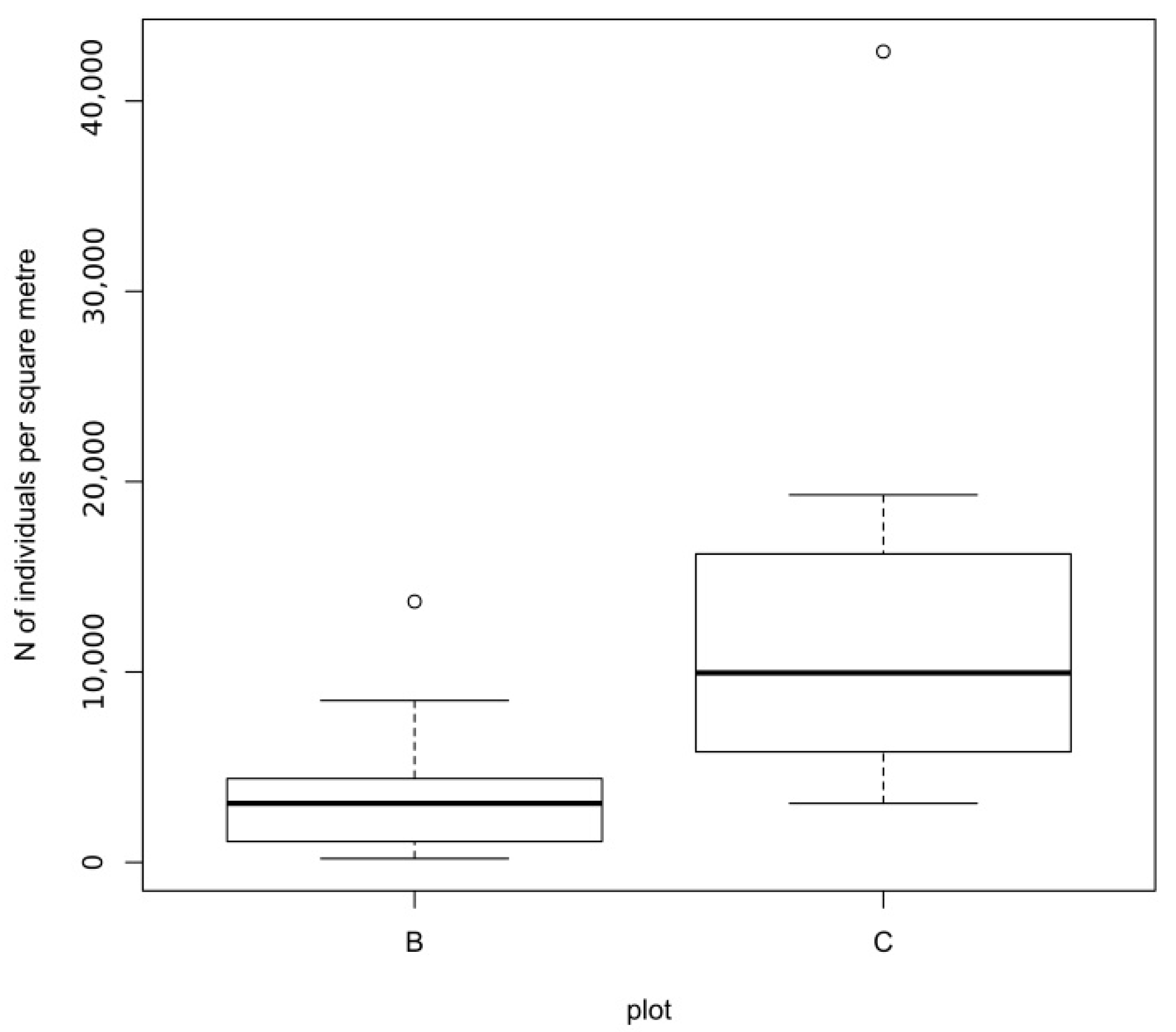
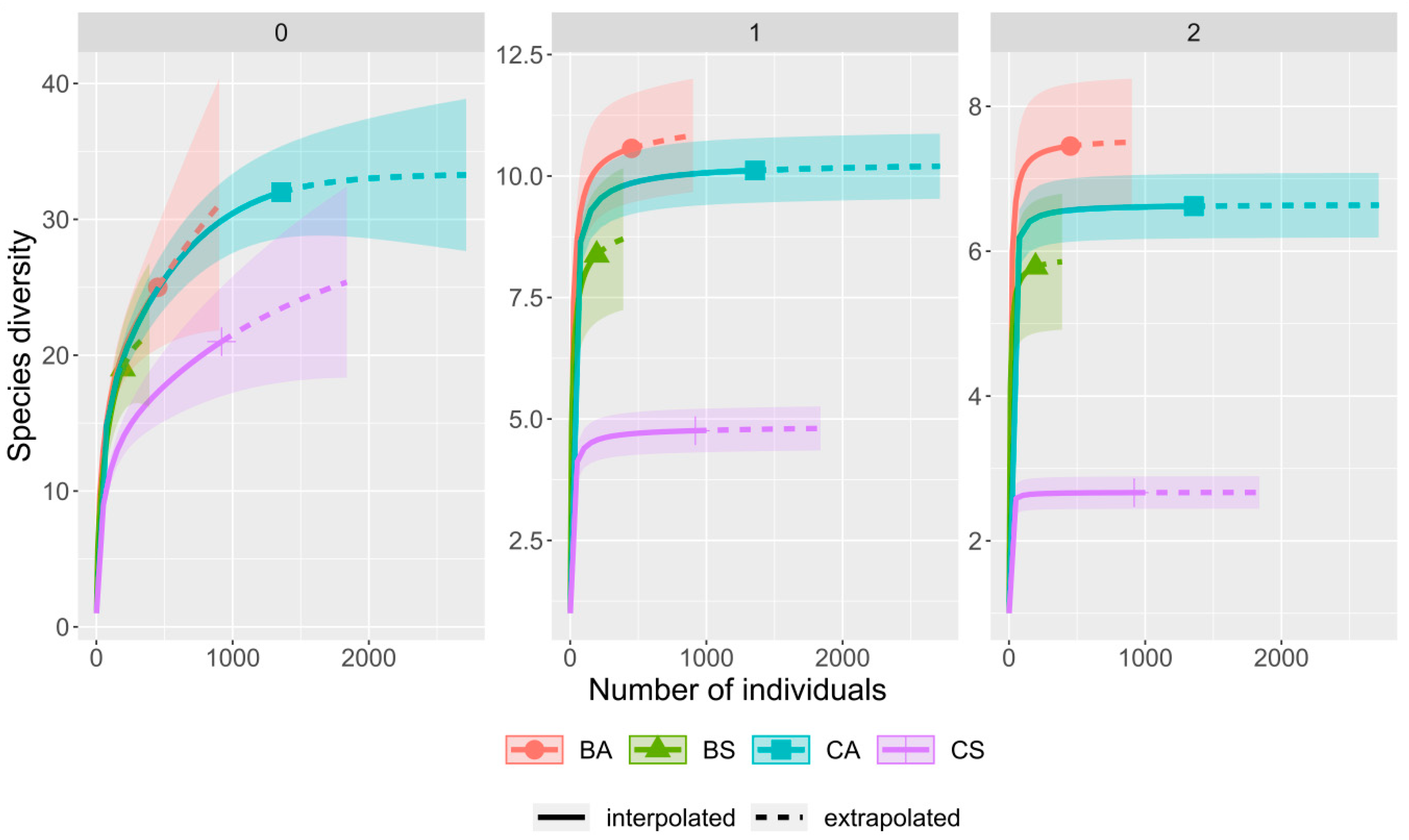
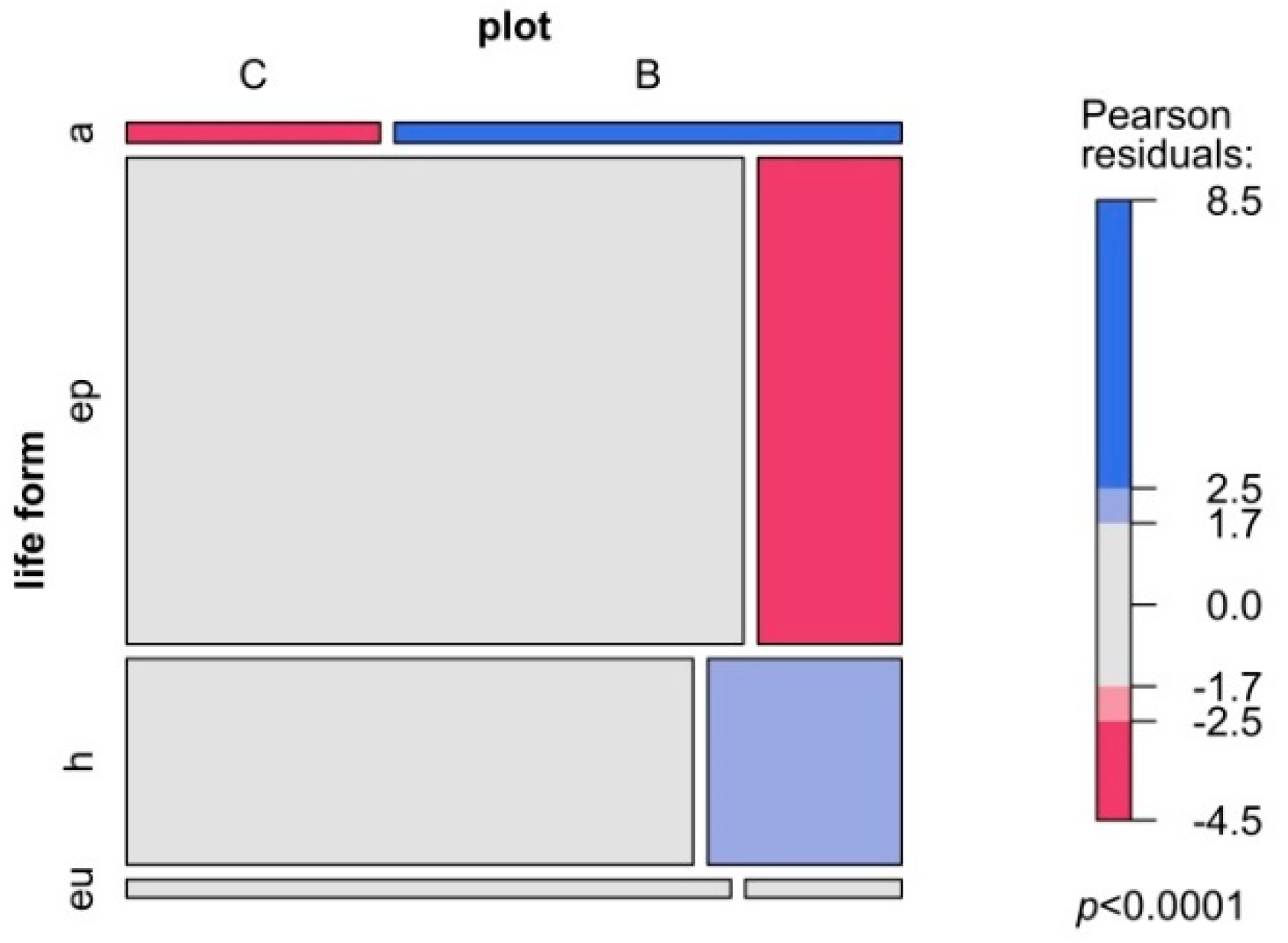
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Seastedt, T.R.; Crossley, D.A. Effects of microarthropods on the seasonal dynamics of nutrients in forest litter. Soil Biol. Biochem. 1980, 12, 337–342. [Google Scholar] [CrossRef]
- Rusek, J. Biodiversity of Collembola and their functional role in the ecosystem. Biodivers. Conserv. 1998, 7, 1207–1219. [Google Scholar] [CrossRef]
- Eisenhauer, N.; Sabais, A.C.W.; Scheu, S. Collembola species composition and diversity effects on ecosystem functioning vary with plant functional group identity. Soil Biol. Biochem. 2011, 43, 1697–1704. [Google Scholar] [CrossRef]
- Filser, J.; Faber, J.H.; Tiunov, A.V.; Brussaard, L.; Frouz, J.; De Deyn, G.; Uvarov, A.V.; Berg, M.P.; Lavelle, P.; Loreau, M.; et al. Soil fauna: Key to new carbon models. Soil 2016, 2, 565–582. [Google Scholar] [CrossRef] [Green Version]
- Petersen, H. Collembolan communities in shrublands along climatic gradients in Europe and the effect of experimental warming and drought on population density, biomass and diversity. Soil Org. 2011, 83, 463–488. [Google Scholar]
- Xu, G.L.; Kuster, T.M.; Günthardt-Goerg, M.S.; Dobbertin, M.; Li, M.H. Seasonal exposure to drought and air warming affects soil collembola and mites. PLoS ONE 2012, 7, 23–27. [Google Scholar] [CrossRef] [Green Version]
- Russell, D.J.; Gergócs, V. Forest-management types similarly influence soil collembolan communities throughout regions in Germany–A data bank analysis. For. Ecol. Manag. 2019, 434, 49–62. [Google Scholar] [CrossRef]
- Hägvar, S. Effects of liming and artificial acid rain on collembola and Protura in coniferous forest. Pedobiologia (Jena) 1984, 27, 341–354. [Google Scholar]
- Loranger, G.; Bandyopadhyaya, I.; Razaka, B.; Ponge, J.F. Does soil acidity explain altitudinal sequences in collembolan communities? Soil Biol. Biochem. 2001, 33, 381–393. [Google Scholar] [CrossRef] [Green Version]
- Salamon, J.A.; Alphei, J. The Collembola community of a Central European forest: Influence of tree species composition. Eur. J. Soil Biol. 2009, 45, 199–206. [Google Scholar] [CrossRef]
- Lindo, Z.; Visser, S. Forest floor microarthropod abundance and oribatid mite (Acari: Oribatida) composition following partial and clear-cut harvesting in the mixed wood boreal forest. Can. J. For. Res. 2004, 34, 998–1006. [Google Scholar] [CrossRef] [Green Version]
- Cassagne, N.; Gers, C.; Gauquelin, T. Relationships between Collembola, soil chemistry and humus types in forest stands (France). Biol. Fertil. Soils 2003, 37, 355–361. [Google Scholar] [CrossRef]
- Ponge, J.F. Plant-soil feedbacks mediated by humus forms: A review. Soil Biol. Biochem. 2013, 57, 1048–1060. [Google Scholar] [CrossRef] [Green Version]
- Bird, S.B.; Coulson, R.N.; Fisher, R.F. Changes in soil and litter arthropod abundance following tree harvesting and site preparation in a loblolly pine (Pinus taeda L.) plantation. For. Ecol. Manag. 2004, 202, 195–208. [Google Scholar] [CrossRef]
- Malmström, A.; Persson, T.; Ahlström, K.; Gongalsky, K.B.; Bengtsson, J. Dynamics of soil meso- and macrofauna during a 5-year period after clear-cut burning in a boreal forest. Appl. Soil Ecol. 2009, 43, 61–74. [Google Scholar] [CrossRef]
- Rousseau, L.; Handa, I.T.; Venier, L.; Fleming, R.; Hazlett, P.; Morris, D. Long-term effects of biomass removal on soil mesofaunal communities in northeastern Ontario (Canada) jack pine (Pinus banksiana) stands. For. Ecol. Manag. 2018, 421, 72–83. [Google Scholar] [CrossRef]
- Rousseau, L.; Venier, L.; Aubin, I.; Gendreau-Berthiaume, B.; Moretti, M.; Salmon, S.; Handa, I.T. Woody biomass removal in harvested boreal forest leads to a partial functional homogenization of soil mesofaunal communities relative to unharvested forest. Soil Biol. Biochem. 2019, 133, 129–136. [Google Scholar] [CrossRef]
- Setälä, H.; Haimi, J.; Siira-Pietikäinen, A. Sensitivity of soil processes northern forest soils: Are management practices a threat? For. Ecol. Manag. 2000, 133, 5–11. [Google Scholar] [CrossRef]
- Čuchta, P.; Miklisová, D.; Kováč, L. The impact of disturbance and ensuing forestry practices on Collembola in monitored stands of windthrown forest in the Tatra National Park (Slovakia). Environ. Monit. Assess. 2013, 185, 5085–5098. [Google Scholar] [CrossRef]
- Čuchta, P.; Miklisová, D.; Kováč, Ľ. The succession of soil Collembola communities in spruce forests of the High Tatra Mountains five years after a windthrow and clear–cut logging. For. Ecol. Manag. 2019, 433, 504–513. [Google Scholar] [CrossRef]
- Mohr, D.; Cohnstaedt, L.W.; Topp, W. Wild boar and red deer affect soil nutrients and soil biota in steep oak stands of the Eifel. Soil Biol. Biochem. 2005, 37, 693–700. [Google Scholar] [CrossRef]
- Barrios-Garcia, M.N.; Ballari, S.A. Impact of wild boar (Sus scrofa) in its introduced and native range: A review. Biol. Invasions 2012, 14, 2283–2300. [Google Scholar] [CrossRef]
- Howe, T.; Singer, F.; Ackerman, B. Forage Relationships of European Wild Boar Invading Northern Hardwood Forest. J. Wildl. Manag. 1981, 45, 748–754. [Google Scholar] [CrossRef]
- Groot Bruinderink, G.W.T.A.; Hazebroek, E. Wild boar (Sus scrofa L.) rooting and forest regeneration on podzolic soils in the Netherlands. For. Ecol. Manag. 1996, 88, 71–80. [Google Scholar] [CrossRef]
- Risch, A.C.; Wirthner, S.; Busse, M.D.; Page-Dumroese, D.S.; Schütz, M. Grubbing by wild boars (Sus scrofa L.) and its impact on hardwood forest soil carbon dioxide emissions in Switzerland. Oecologia 2010, 164, 773–784. [Google Scholar] [CrossRef] [Green Version]
- Vtorov, I. Feral Pig Removal: Effects on Soil Microarthropods in a Hawaiian Rain Forest. J. Wildl. Manag. 1993, 57, 875–880. [Google Scholar] [CrossRef]
- Taylor, D.L.; Leung, L.K.-P.; Gordon, I.J. The impact of feral pigs (Sus scrofa) on an Australian lowland tropical rainforest. Wildl. Res. 2011, 38, 445. [Google Scholar] [CrossRef]
- Fagiani, S.; Fipaldini, D.; Santarelli, L.; Burrascano, S.; DelVico, E.; Giarrizzo, E.; Mei, M.; Taglianti, A.; Boitani, L.; Mortellti, A. Monitoring protocols for the evaluation of the impact of wild boar (Sus scrofa) rooting on plants and animals in forest ecosystems. Ital. J. Mammal. 2014, 25, 31–38. [Google Scholar]
- IUSS Working Group WRB. World reference base for soil resources 2014, update 2015: International soil classification system for naming soils and creating legends for soil maps. World Soil Resour. 2015, 192.
- Malmström, A. Life-history traits predict recovery patterns in Collembola species after fire: A 10 year study. Appl. Soil Ecol. 2012, 56, 35–42. [Google Scholar] [CrossRef]
- Martins da Silva, P.; Carvalho, F.; Dirilgen, T.; Stone, D.; Creamer, R.; Bolger, T.; Sousa, J.P. Traits of collembolan life-form indicate land use types and soil properties across an European transect. Appl. Soil Ecol. 2016, 97, 69–77. [Google Scholar] [CrossRef]
- GISIN, H. Okologie und Levensgemenischaften der Collembolen im schweizerischen Exkursionsgebiet Basels. Rev. Suisse Zool. 1943, 50, 131–224. [Google Scholar]
- Potapov, A.A.; Semenina, E.E.; Korotkevich, A.Y.; Kuznetsova, N.A.; Tiunov, A.V. Connecting taxonomy and ecology: Trophic niches of collembolans as related to taxonomic identity and life forms. Soil Biol. Biochem. 2016, 101, 20–31. [Google Scholar] [CrossRef]
- Lorenc, H. Climate atlas of Poland (In Polish); Instytut Meteorologii i Gospodarki Wodnej: Warszawa, Poland, 2005. [Google Scholar]
- Potapov, A.M.; Korotkevich, A.Y.; Tiunov, A.V. Non-vascular plants as a food source for litter-dwelling Collembola: Field evidence. Pedobiologia (Jena) 2018, 66, 11–17. [Google Scholar] [CrossRef]
- Babenko, A.B.; Chernova, N.M.; Potapov, M.B.; Stebaeva, S.K. Collembola of Russia and Adjacent Countries: Family Hypogastruridae; Nauka: Moscow, Russia, 1994. [Google Scholar]
- Fjellberg, A. The Collembola of Fennoscandia and Denmark, Part I: Poduromorpha; Brill: Leiden, The Netherlands, 1998; Volume 35. [Google Scholar]
- Fjellberg, A. The Collembola of Fennoscandia and Denmark, Part II: Entomobryomorpha and Symphypleona; Brill: Leiden, The Netherlands, 2007; Volume 42. [Google Scholar]
- Pomorski, R.J. Onychiuridae of Poland (Collembola: Onychiuridae); BS: Wrocław, Poland, 1998. [Google Scholar]
- Bretfeld, G. Synopses on Palaearctic Collembola: Symphypleona. Abh. Ber. Naturkundemus. Gorlitz 1999, 71, 1–318. [Google Scholar]
- Potapov, M. Synopses on Palaearctic Collembola. Isotomidae. Abh. Ber. Naturkundemus. Gorlitz 2001, 73, 1–603. [Google Scholar]
- Thibaud, J.; Schulz, H.; da Gama Assalino, M. Synopses on Palaearctic Collembola. Hypogastruridae. Abh. Ber. Naturkundemus. Gorlitz 2004, 75, 1–287. [Google Scholar]
- Babenko, A.; Kuznetsova, N.; Potapov, M.; Stebaeva, C.; Chanislamova, G.; Chernova, H. Identification keys of Collembola of the USSR; Nauka: Moscow, Russia, 1988. [Google Scholar]
- Rusek, J. A new classification of Collembola and Protura life forms. In: Contribution to soil zoology in central Europe II. ISB BC ASCR. Ces. Budejovice 2007, 109–115. [Google Scholar]
- Chao, A.; Jost, L. Coverage-based rarefaction and extrapolation: Standardizing samples by completeness rather than size. Ecology 2012, 93, 2533–2547. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Lawrence Erlbaum Assos: Hillsdale, MI, USA, 1988. [Google Scholar]
- Anderson, M.J.; Walsh, D.C.I. PERMANOVA, ANOSIM, and the Mantel test in the face of heterogeneous dispersions: What null hypothesis are you testing? Ecol. Monogr. 2013, 83, 557–574. [Google Scholar] [CrossRef]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: http://www.R-project.org/ (accessed on 21 October 2020).
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: An R package for rarefaction and extrapolation of species diversity (H ill numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; Mcglinn, D.; Minchin, P.R.; O’hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R package version 2.5-5. 2019. URL. Available online: https://cran.r-project.org/web/packages/vegan (accessed on 21 October 2020).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression; SAGE: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Meyer, D.; Zeileis, A.; Hornik, K. The strucplot framework: Visualizing multi-way contingency tables with vcd. J. Stat. Softw. 2006, 17, 1–48. [Google Scholar] [CrossRef]
- Berch, S.M.; Battigelli, J.P.; Hope, G.D. Responses of soil mesofauna communities and oribatid mite species to site preparation treatments in high-elevation cutblocks in southern British Columbia. Pedobiologia (Jena) 2007, 51, 23–32. [Google Scholar] [CrossRef]
- Verhoef, H.A.; van Selm, A.J. Distribution and population dynamics of Collembola in relation to soil moisture. Ecography (Cop.) 1983, 6, 387–388. [Google Scholar] [CrossRef]
- Pflug, A.; Wolters, V. Influence of drought and litter age on Collembola communities. Eur. J. Soil Biol. 2001, 37, 305–308. [Google Scholar] [CrossRef]
- Lindberg, N.; Engtsson, J.B.; Persson, T. Effects of experimental irrigation and drought on the composition and diversity of soil fauna in a coniferous stand. J. Appl. Ecol. 2002, 39, 924–936. [Google Scholar] [CrossRef]
- Tsiafouli, M.A.; Kallimanis, A.S.; Katana, E.; Stamou, G.P.; Sgardelis, S.P. Responses of soil microarthropods to experimental short-term manipulations of soil moisture. Appl. Soil Ecol. 2005, 29, 17–26. [Google Scholar] [CrossRef]
- Lindberg, N.; Bengtsson, J. Recovery of forest soil fauna diversity and composition after repeated summer droughts. Oikos 2006, 114, 494–506. [Google Scholar] [CrossRef]
- Flórián, N.; Ladányi, M.; Ittzés, A.; Kröel-Dulay, G.; Ónodi, G.; Mucsi, M.; Szili-Kovács, T.; Gergócs, V.; Dányi, L.; Dombos, M. Effects of single and repeated drought on soil microarthropods in a semi-arid ecosystem depend more on timing and duration than drought severity. PLoS ONE 2019, 14, e0219975. [Google Scholar] [CrossRef] [Green Version]
- Blok, D.; Heijmans, M.M.P.D.; Schaepman-Strub, G.; van Ruijven, J.; Parmentier, F.J.W.; Maximov, T.C.; Berendse, F. The Cooling Capacity of Mosses: Controls on Water and Energy Fluxes in a Siberian Tundra Site. Ecosystems 2011, 14, 1055–1065. [Google Scholar] [CrossRef] [Green Version]
- Bokhorst, S.; Wardle, D.A.; Nilsson, M.C.; Gundale, M.J. Impact of understory mosses and dwarf shrubs on soil micro-arthropods in a boreal forest chronosequence. Plant Soil 2014, 379, 121–133. [Google Scholar] [CrossRef]
- Krull, C.R.; Choquenot, D.; Burns, B.R.; Stanley, M.C. Feral pigs in a temperate rainforest ecosystem: Disturbance and ecological impacts. Biol. Invasions 2013, 15, 2193–2204. [Google Scholar] [CrossRef]


| Taxa | Life Form | C1 | C2 | C3 | B1 | B2 | B3 |
|---|---|---|---|---|---|---|---|
| Xenylla maritima Tullberg, 1869 | ep | 450 | 400 | 50 | 267 | 233 | 300 |
| Xenylla sp. juv. | ep | 67 | - | - | - | - | - |
| Willemia anopthalma Börner, 1901 | eu | 33 | 17 | 33 | - | - | - |
| Friesea claviseta Axelson, 1900 | ep | 533 | 50 | 1717 | - | 133 | - |
| F. truncata Cassagnau, 1958 | ep | 950 | 50 | - | 317 | - | 67 |
| Friesea sp. juv. | ep | 17 | 17 | - | 17 | 17 | - |
| Pseudachorutes dubius Krausbauer, 1898 | ep | - | 33 | 17 | - | - | 17 |
| Pseudachorutes corticicolus (Schäffer, 1896) | ep | 17 | - | - | - | - | - |
| Pseudachorutes sp. juv. | ep | 33 | 17 | 17 | 33 | - | 17 |
| Micranurida pygmea Börner, 1901 | h | 50 | - | 17 | - | 17 | - |
| Neanura muscorum (Templeton, 1835) | h | 133 | 283 | 117 | 567 | 233 | 433 |
| Neanuridae juv. | h | 200 | 483 | 133 | 17 | 133 | 50 |
| Micraphorura absoloni (Börner, 1901) | eu | - | 33 | 17 | - | 50 | 33 |
| Mesaphorura yosii Rusek, 1967 | eu | - | - | 33 | - | - | - |
| Anurophorus atlanticus Fjellberg, 1974 | ep | - | 17 | 500 | 17 | - | 17 |
| A. laricis Nicolet, 1842 | ep | - | 33 | - | 17 | 100 | 17 |
| A. septentrionalis (Pallisa, 1966) | ep | 3483 | - | 683 | 83 | - | 1083 |
| Anurophorus sp. juv. | ep | 300 | 17 | 417 | 33 | - | 550 |
| Folsomia quadrioculata (Tullberg, 1871) | h | 17 | - | 800 | - | - | 33 |
| Proisotoma minima (Tullberg, 1871) | h | - | - | - | - | 17 | - |
| Isotomiella minor (Schäffer, 1896) | eu | 67 | 100 | 600 | 33 | 33 | 100 |
| Parisotoma notabilis (Schäffer, 1896) | h | 1767 | 1467 | 4383 | 850 | 350 | 783 |
| Desoria tolya Fjellberg, 2007 | ep | 633 | 17 | 467 | 583 | 233 | 233 |
| Desoria trispinata (Mac Gillivray, 1896) | ep | - | - | 3850 | 350 | - | 183 |
| Desoria sp. juv. | ep | 2817 | 3367 | 3233 | 17 | 717 | 267 |
| Tomoceridae juv. | ep | - | - | - | - | 17 | - |
| Orchesella bifasciata Nicolet, 1841 | a | - | 83 | 133 | 50 | 17 | 183 |
| O. flavescens (Bourlet, 1839) | a | - | 17 | - | - | - | - |
| O. multifasciata (Stscherbakow, 1898) | a | - | - | - | 83 | - | - |
| Orchesella sp. juv. | a | - | 17 | 33 | - | - | - |
| Entomobrya corticalis (Nicolet, 1841) | a | - | - | - | 33 | 33 | 267 |
| E. multifasciata (Tullberg, 1871) | a | 67 | - | - | 67 | - | - |
| Willowsia buski (Lubbock, 1869) | a | - | - | - | - | 17 | - |
| Lepidocyrtus lignorum (Fabricius, 1775) | ep | 83 | 467 | 1467 | 83 | 100 | - |
| Pseudosinella zygophora (Schille, 1908) | h | 100 | 267 | - | - | - | - |
| Entomobyidae juv. | a | 33 | 67 | - | 83 | 50 | 17 |
| Megalothorax minimus (Willem, 1900) | eu | - | - | 33 | - | - | - |
| Arrhopalites sp. juv. | h | - | 17 | - | - | 17 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sławski, M.; Sławska, M. Collembolan Assemblages Response to Wild Boars (Sus scrofa L.) Rooting in Pine Forest Soil. Forests 2020, 11, 1123. https://doi.org/10.3390/f11111123
Sławski M, Sławska M. Collembolan Assemblages Response to Wild Boars (Sus scrofa L.) Rooting in Pine Forest Soil. Forests. 2020; 11(11):1123. https://doi.org/10.3390/f11111123
Chicago/Turabian StyleSławski, Marek, and Małgorzata Sławska. 2020. "Collembolan Assemblages Response to Wild Boars (Sus scrofa L.) Rooting in Pine Forest Soil" Forests 11, no. 11: 1123. https://doi.org/10.3390/f11111123
APA StyleSławski, M., & Sławska, M. (2020). Collembolan Assemblages Response to Wild Boars (Sus scrofa L.) Rooting in Pine Forest Soil. Forests, 11(11), 1123. https://doi.org/10.3390/f11111123






