Baseline of Carbon Stocks in Pinus radiata and Eucalyptus spp. Plantations of Chile
Abstract
1. Introduction
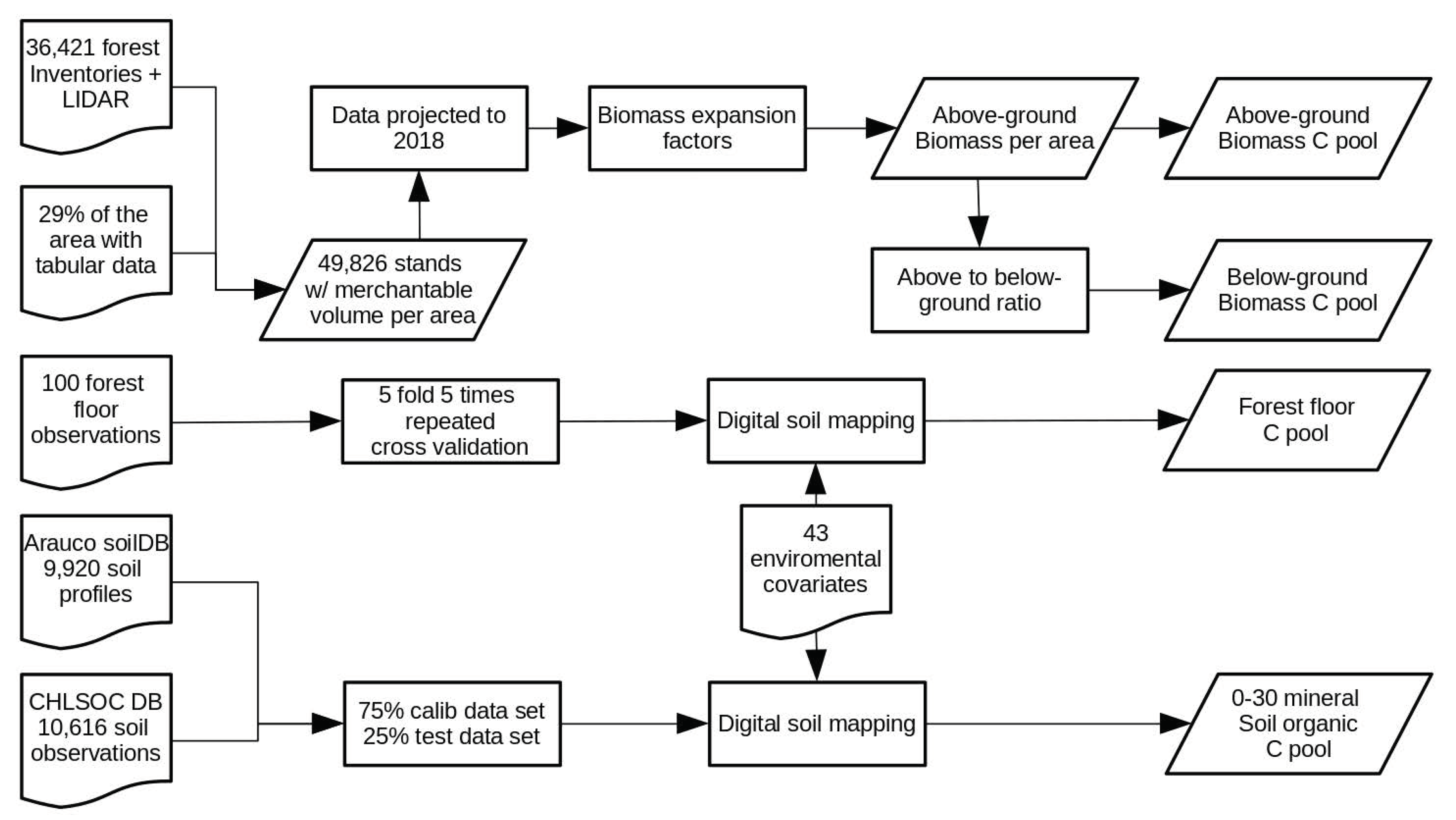
2. Materials and Methods
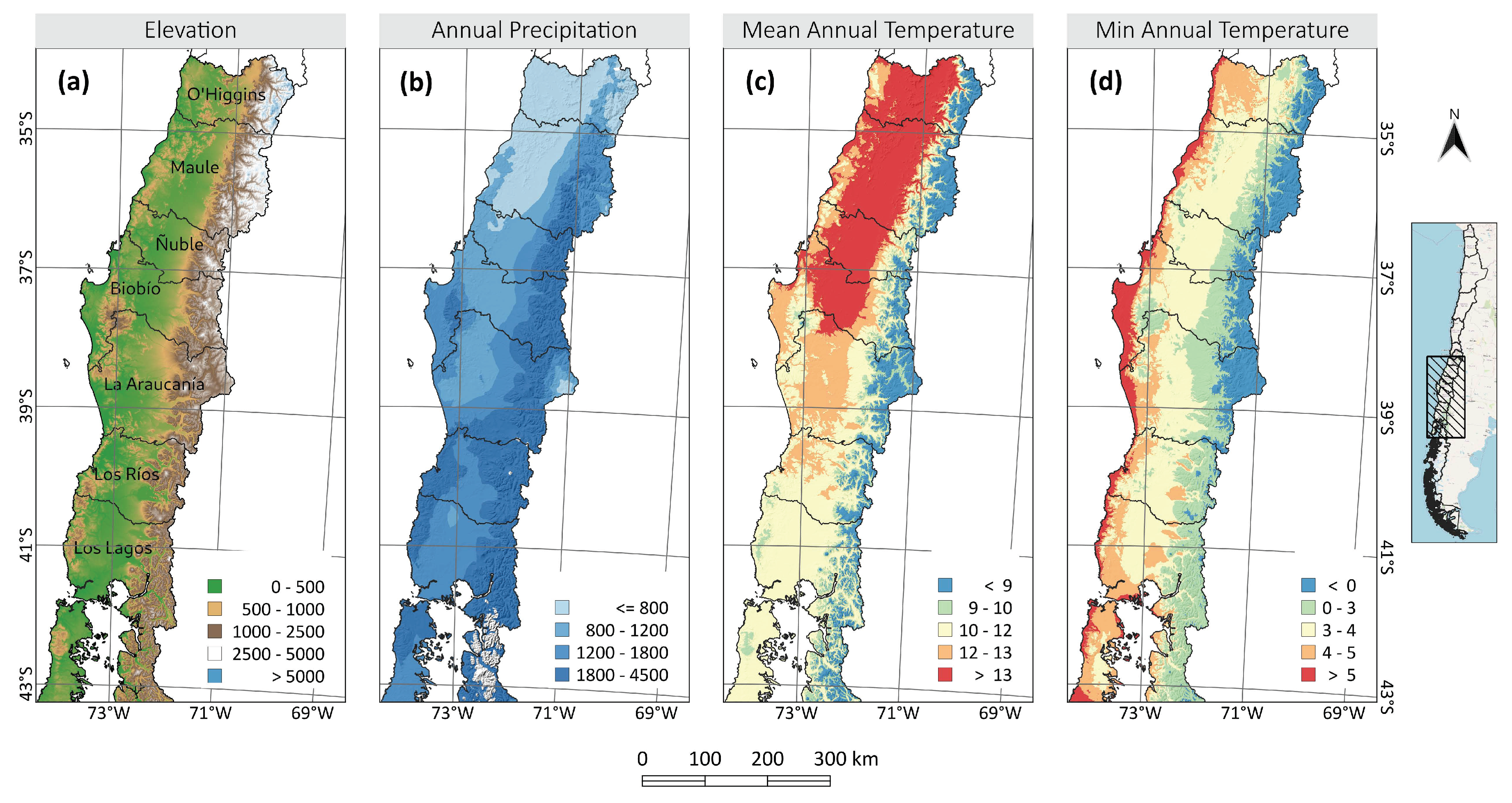
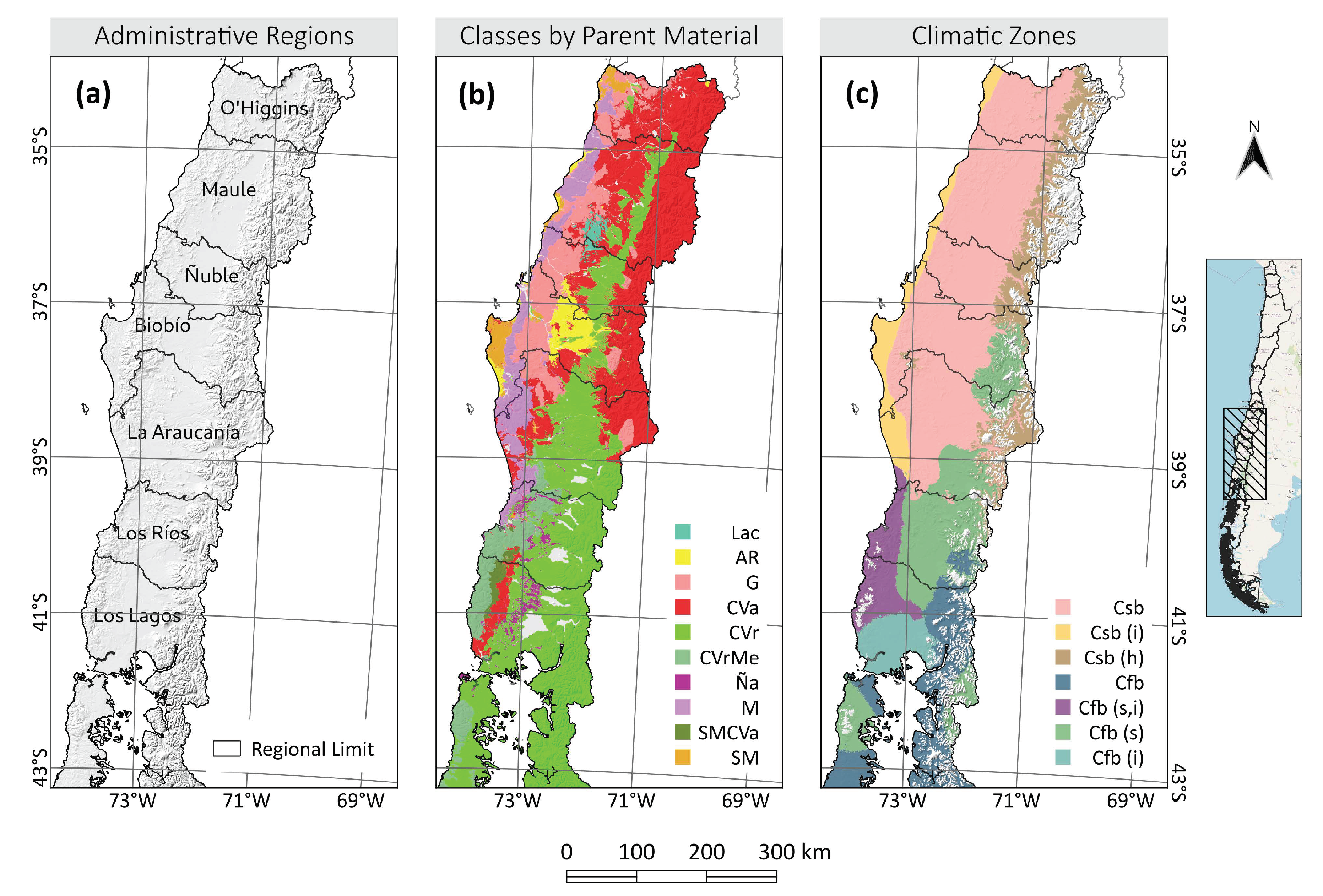
2.1. Area of Study
2.2. Above-Ground and Below-Ground Biomass Carbon Pool
2.3. Soil Organic Carbon Pool
2.4. Forest Floor Carbon Pool
2.5. Integration of the Results
2.6. Statistical Comparison of Mean Values
3. Results
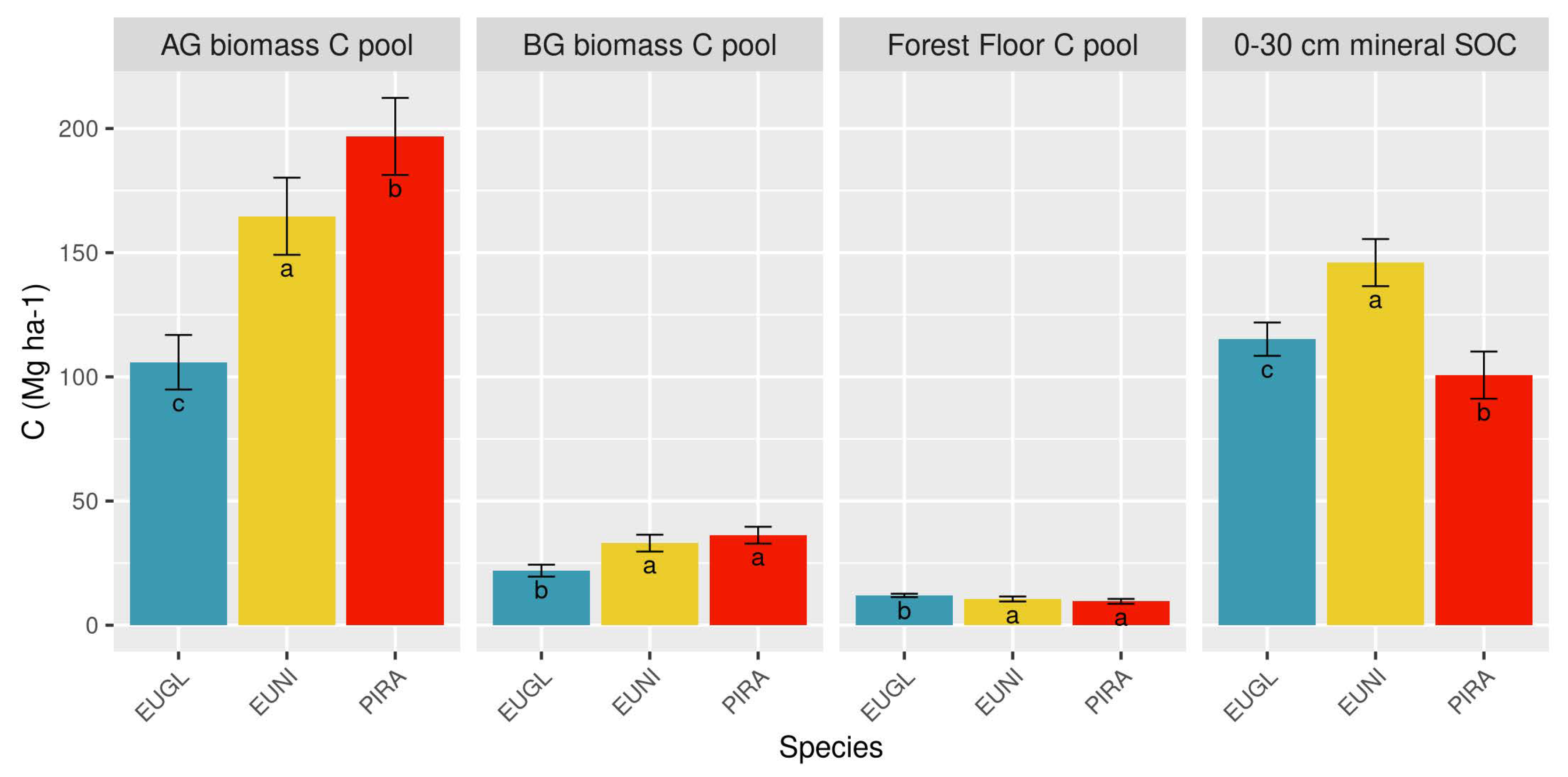
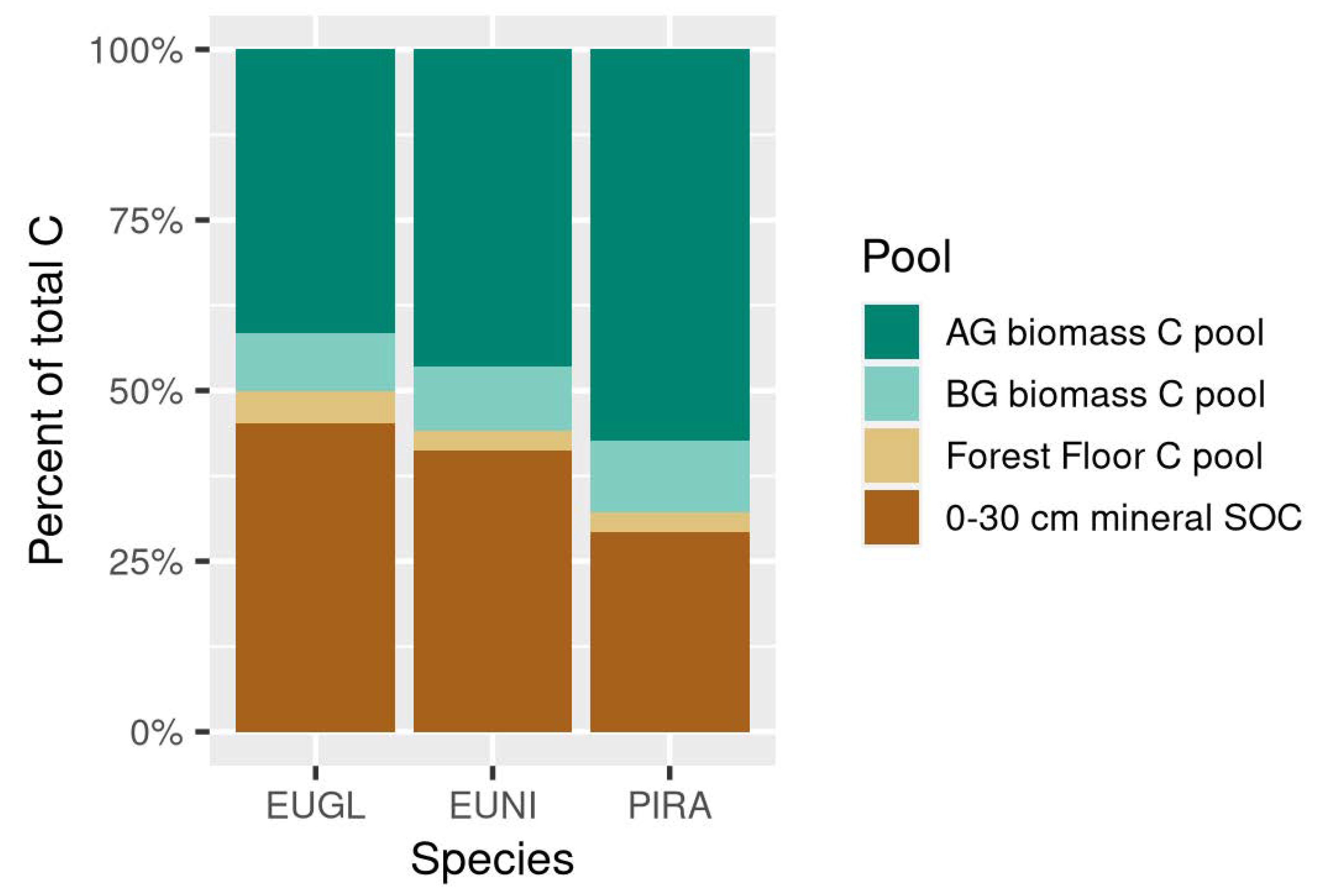
3.1. How Is the C Stock Distributed between the Different C Pools on a Forest Plantation?
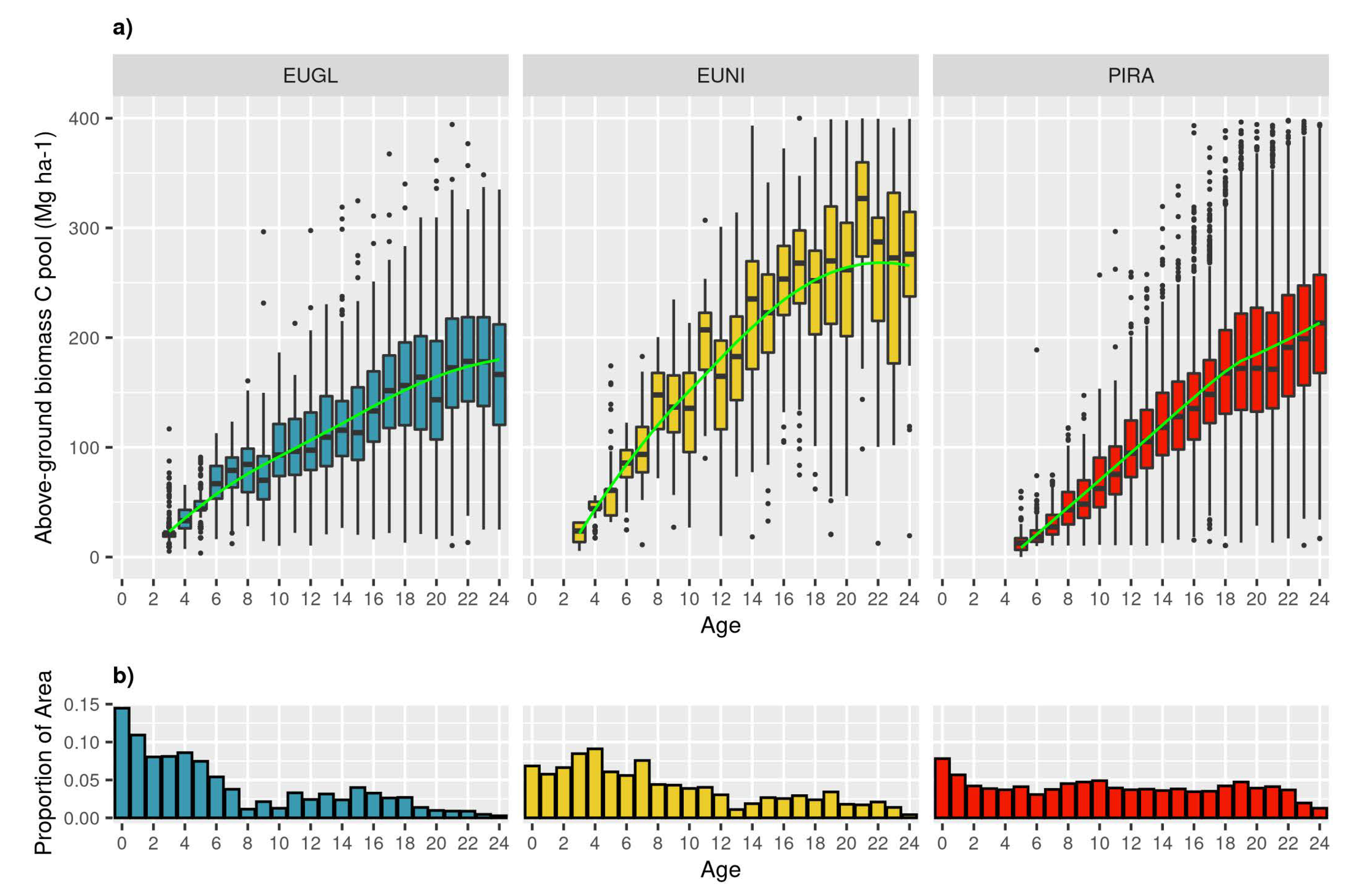
3.2. How Much C Is Present in Above-Ground Biomass at Different Ages for Different Species?
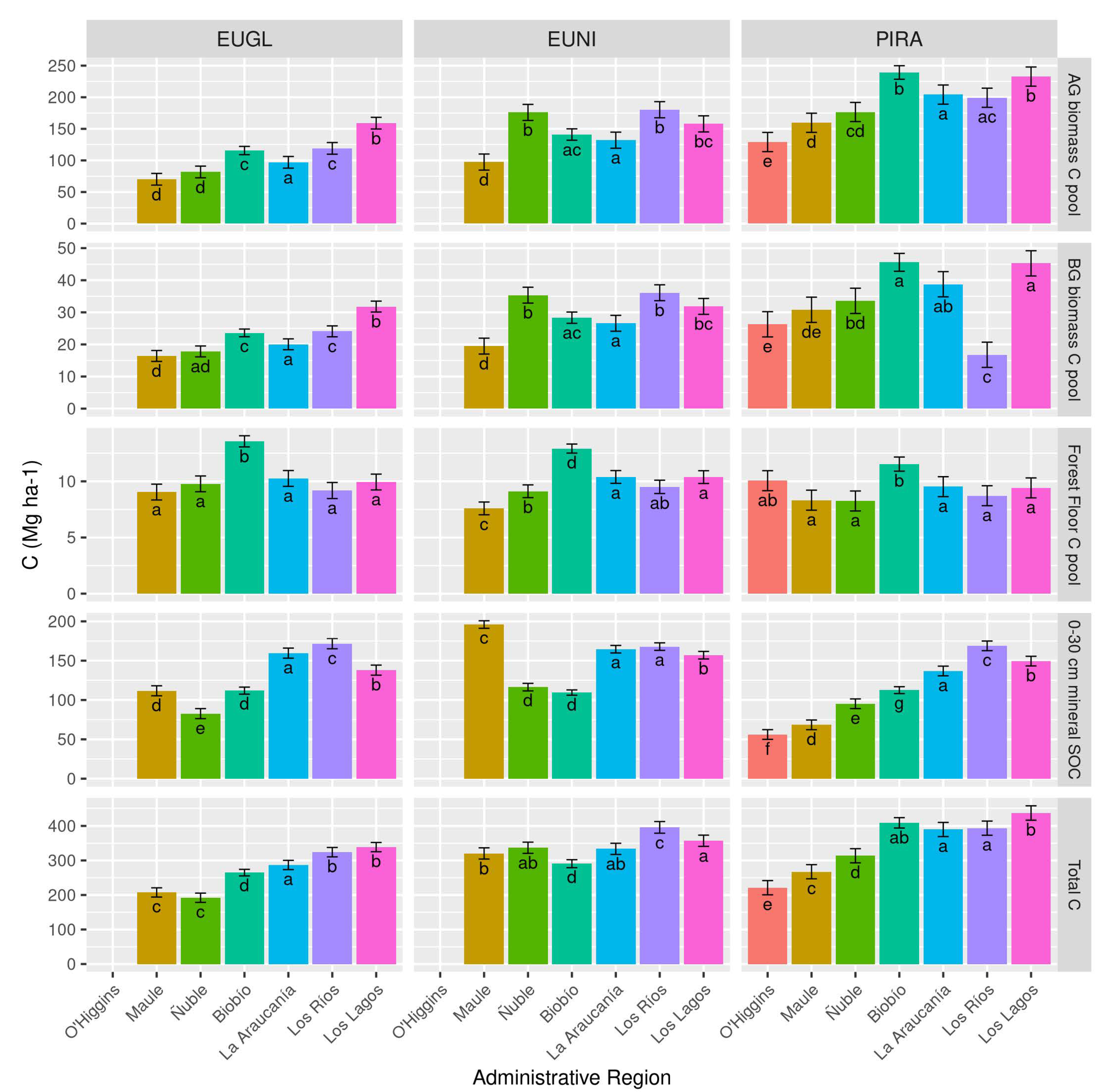
3.3. What Is the Capacity of Forest Plantations to Store C across the Different Forest Regions of Chile?
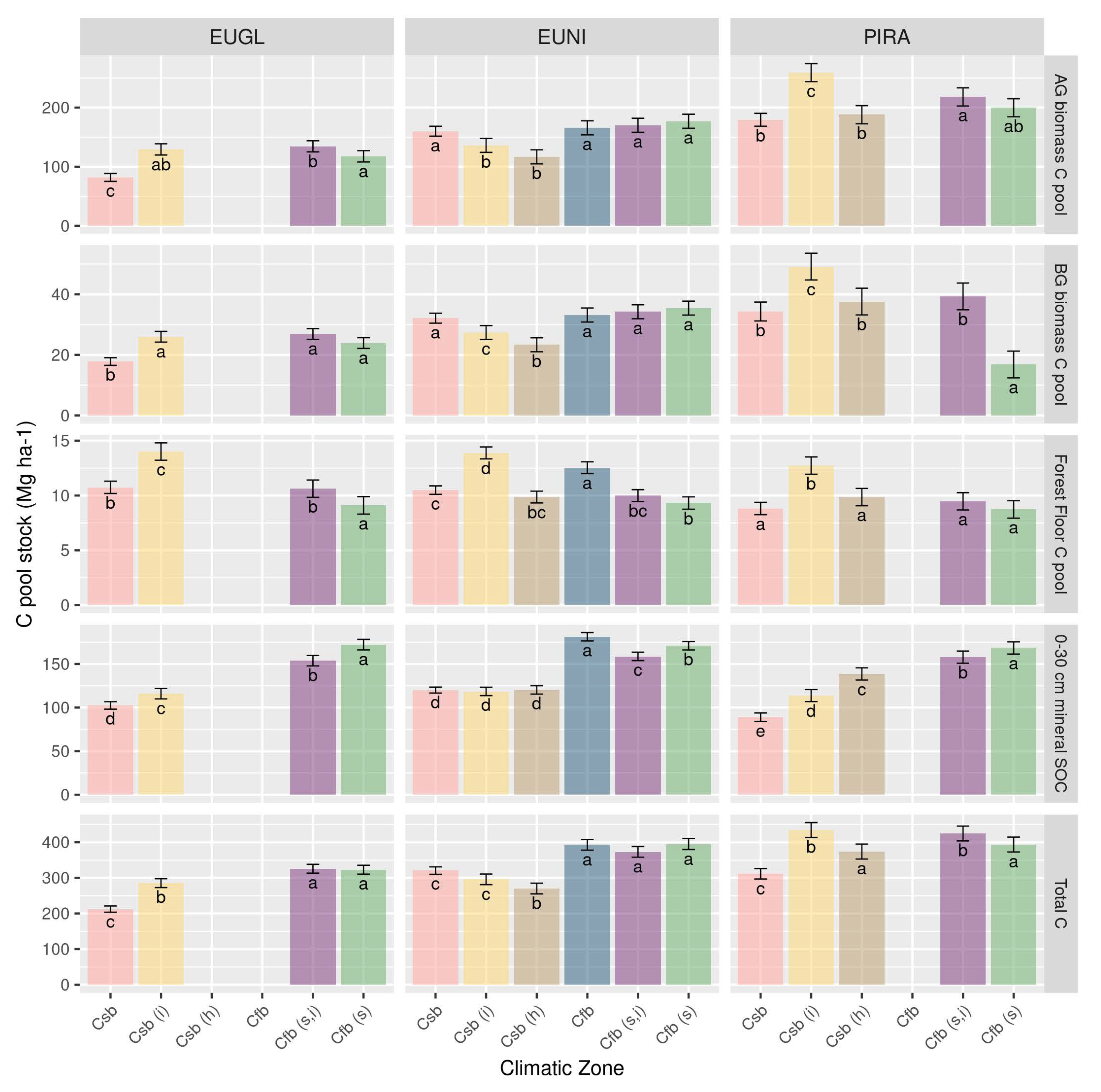
3.4. How Does This Change with Climate and Soil Types?
4. Discussion
4.1. The Capacity of Forests to Store Carbon by Species
4.2. The Capacity of Forests to Store Carbon in Different Regions
4.3. The Capacity of Forests to Store Carbon in Different Climatic Zones
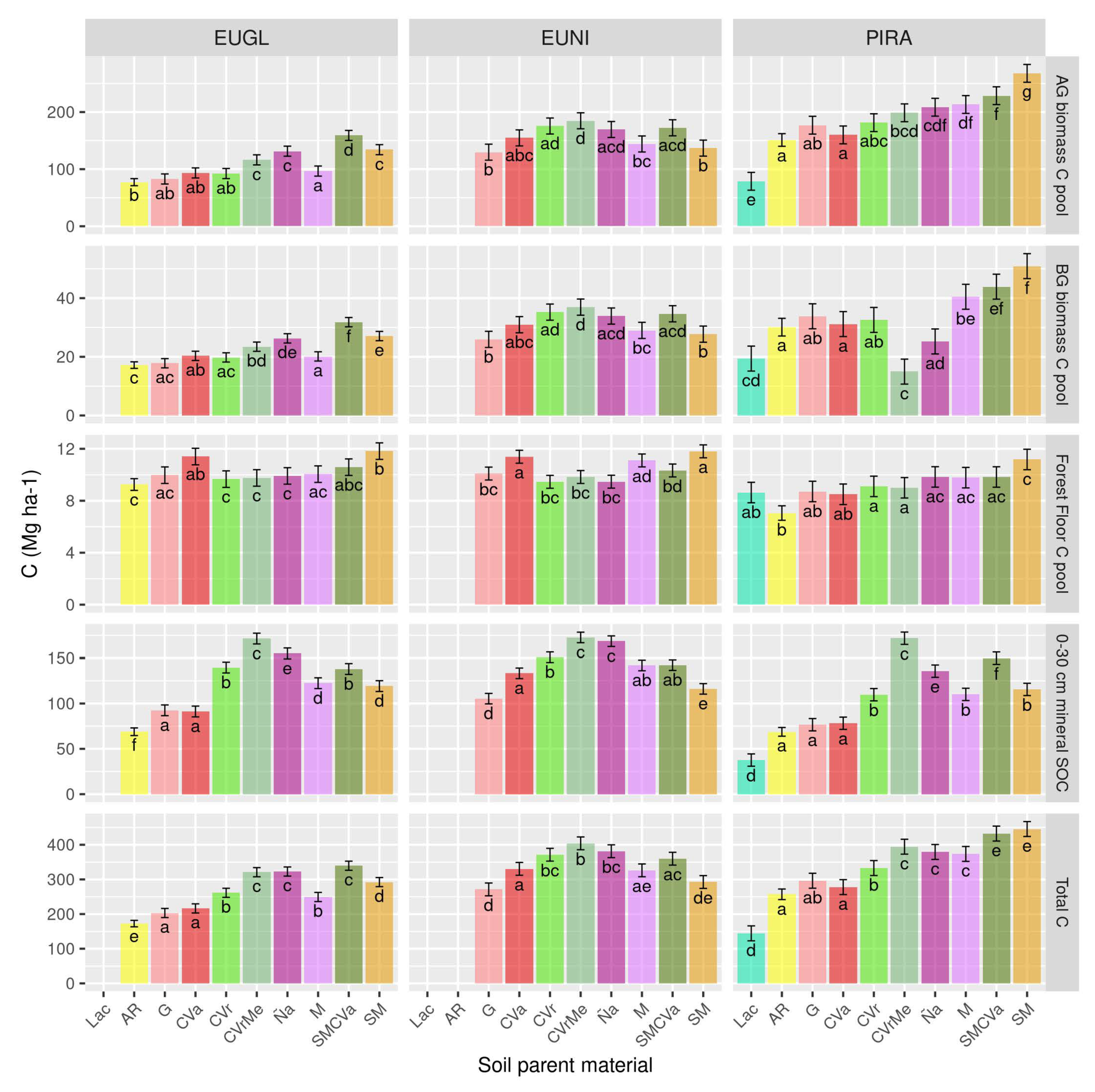
4.4. The Capacity of Forests to Store Carbon in Different Soils
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Environmental Covariates Used in Digital Soil Mapping
| Category | Source | Resolution | Variables |
|---|---|---|---|
| Topography | SRTM | 90 m | Elevation model, Aspect, Slope, Chanel Network Base Level, Channel Network Distance, Longitudinal Curvature, Convergence Index, Slope Length and Steepness Factor, Maximal Curvature, Minimal Curvature, Profile Curvature, Tangential Curvature, Terrain Surface Convexity, Terrain ruggedness index, Topographic Position Index, Topographic Wetness Index, Valley Depth |
| Climate | CR2-Uchile | 500 m | Mean annual temperature, Mean diurnal range (mean of max temp-min temp), Isothermality, Temperature seasonality, Max temperature of warmest month, Min temperature of coldest month, Temperature annual range, Mean temperature of the wettest quarter, Mean temperature of driest quarter, Mean temperature of warmest quarter, Mean temperature of coldest quarter, Total (annual) precipitation, Precipitation of wettest month, Precipitation of driest month, Precipitation seasonality, Precipitation of wettest quarter, Precipitation of driest quarter, Precipitation of warmest quarter, Precipitation of coldest quarter, average number of cold Day |
| Soil Morphology | CIREN | 90 m | Parent Material |
| Vegetation indices | MODIS | 250 m | Average Enhance vegetation index from last 20 years, Minimum Enhance vegetation index, Maximum Enhance vegetation index, Enhanced vegetation index range, Standard deviation for EVI from last 20 years |
Appendix B. INFOR Reference Values
| Growth Area | Site Class | Management Scheme | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| 1 | 1 | 207.1 | 188.5 | 180.6 | 179.9 | 171.4 | 162.8 | 171.4 | 60.5 | 92.2 |
| 2 | 164.2 | 149.2 | 141.4 | 145.7 | 51.4 | 128.5 | 137.1 | 64.5 | 78.6 | |
| 3 | 121.4 | 110.0 | 102.1 | 102.8 | 102.8 | 94.2 | 102.8 | 48.4 | 72.6 | |
| 4 | 85.7 | 78.5 | 70.7 | 68.5 | 68.5 | 68.5 | 77.1 | 40.3 | 66.5 | |
| 2 | 1 | 214.2 | 212.1 | 196.3 | 197.1 | 188.5 | 179.9 | 197.1 | 96.8 | 87.6 |
| 2 | 178.5 | 164.9 | 157.1 | 154.2 | 154.2 | 145.7 | 154.2 | 66.5 | 82.9 | |
| 3 | 142.8 | 141.4 | 125.7 | 128.5 | 128.5 | 120.0 | 128.5 | 64.5 | 96.8 | |
| 4 | 107.1 | 94.2 | 86.4 | 85.7 | 85.7 | 77.1 | 94.2 | 64.5 | 96.8 | |
| 4 | 1 | 228.5 | 204.2 | 196.3 | 179.9 | 179.9 | 154.2 | 188.5 | 96.8 | 170.5 |
| 2 | 178.5 | 157.1 | 141.4 | 128.5 | 137.1 | 128.5 | 137.1 | 101.4 | 147.5 | |
| 3 | 149.9 | 133.5 | 125.7 | 120.0 | 111.4 | 102.8 | 102.8 | 82.9 | 119.8 | |
| 4 | 121.4 | 102.1 | 102.1 | 85.7 | 77.1 | 85.7 | 94.2 | 96.8 | 92.2 | |
| 5 | 1 | 100.0 | 86.4 | 78.5 | 77.1 | 77.1 | 68.5 | 77.1 | 87.6 | 96.8 |
| 2 | 78.5 | 70.7 | 62.8 | 60.0 | 60.0 | 60.0 | 60.0 | 96.8 | 82.9 | |
| 3 | 64.3 | 55.0 | 47.1 | 51.4 | 51.4 | 42.8 | 51.4 | 66.5 | 84.7 | |
| 4 | 50.0 | 39.3 | 39.3 | 36.7 | 36.7 | 42.8 | 42.8 | 56.4 | 66.5 | |
| 6 | 1 | 221.3 | 204.2 | 188.5 | 188.5 | 179.9 | 154.2 | 188.5 | 142.8 | 198.1 |
| 2 | 171.4 | 157.1 | 141.4 | 137.1 | 137.1 | 128.5 | 145.7 | 129.0 | 165.9 | |
| 3 | 142.8 | 125.7 | 117.8 | 145.7 | 120.0 | 102.8 | 120.0 | 115.2 | 129.0 | |
| 4 | 107.1 | 94.2 | 86.4 | 85.7 | 85.7 | 77.1 | 85.7 | 82.9 | 96.8 | |
| 7 | 1 | 207.1 | 196.3 | 188.5 | 179.9 | 179.9 | 171.4 | 179.9 | 101.4 | 165.9 |
| 2 | 178.5 | 164.9 | 157.1 | 154.2 | 154.2 | 137.1 | 154.2 | 82.9 | 147.5 | |
| 3 | 149.9 | 141.4 | 133.5 | 128.5 | 128.5 | 120.0 | 128.5 | 96.8 | 115.2 | |
| 4 | 114.2 | 102.1 | 102.1 | 94.2 | 94.2 | 85.7 | 94.2 | 66.5 | 78.3 | |
| 9 | 1 | 214.2 | 188.5 | 172.8 | 171.4 | 162.8 | 154.2 | 171.4 | 110.6 | 198.1 |
| 2 | 164.2 | 141.4 | 133.5 | 120.0 | 120.0 | 120.0 | 128.5 | 96.8 | 175.1 | |
| 3 | 121.4 | 110.0 | 102.1 | 94.2 | 94.2 | 85.7 | 94.2 | 78.3 | 152.1 | |
| 4 | 92.8 | 78.5 | 70.7 | 60.0 | 60.0 | 60.0 | 60.0 | 84.7 | 115.2 | |
Appendix C. Average C Stock Values of the Different Pool Per Specie, Region, Climatic Zones and Parent Material
| Specie | Age | Above-Ground Biomass C | Below-Ground Biomass C | Forest Floor C | Soil Organic C (0–30 cm) | Total Ecosystem C |
|---|---|---|---|---|---|---|
| EUGL | 10–14 | 105.9 ± 5.6 | 22.0 ± 1.2 | 12.0 ± 0.4 | 115.2 ± 3.4 | 254.6 ± 8.5 |
| EUNI | 10–14 | 164.7 ± 8.0 | 33.0 ± 1.7 | 10.5 ± 0.5 | 146.0 ± 4.9 | 351.9 ± 12.3 |
| PIRA | 20–24 | 196.8 ± 8.0 | 36.2 ± 1.7 | 9.6 ± 0.5 | 100.7 ± 4.9 | 343.4 ± 12.1 |
| Specie | Administrative Region | Above-Ground Biomass C | Below-Ground Biomass C | Forest Floor C | Soil Organic C (0–30 cm) | Total Ecosystem C |
|---|---|---|---|---|---|---|
| EUGL | Maule | 70.2 ± 4.7 | 16.4 ± 0.9 | 9.0 ± 0.4 | 111.7 ± 3.3 | 207.4 ± 6.9 |
| Ñuble | 81.7 ± 4.7 | 17.8 ± 0.9 | 9.8 ± 0.4 | 82.7 ± 3.3 | 192.0 ± 6.9 | |
| Biobío | 115.7 ± 3.4 | 23.6 ± 0.6 | 13.6 ± 0.3 | 111.9 ± 2.3 | 264.7 ± 4.9 | |
| La Araucanía | 97.0 ± 4.7 | 20.0 ± 0.9 | 10.3 ± 0.4 | 159.6 ± 3.3 | 286.9 ± 6.9 | |
| Los Ríos | 119.1 ± 4.7 | 24.1 ± 0.9 | 9.2 ± 0.4 | 171.5 ± 3.3 | 324.0 ± 7.0 | |
| Los Lagos | 159.0 ± 4.7 | 31.8 ± 0.9 | 9.9 ± 0.4 | 138.0 ± 3.3 | 338.7 ± 6.9 | |
| EUNI | Maule | 97.4 ± 6.5 | 19.5 ± 1.3 | 7.6 ± 0.3 | 195.9 ± 2.5 | 320.4 ± 8.3 |
| Ñuble | 176.0 ± 6.5 | 35.3 ± 1.3 | 9.1 ± 0.3 | 116.3 ± 2.5 | 336.8 ± 8.3 | |
| Biobío | 141.0 ± 4.6 | 28.3 ± 0.9 | 12.9 ± 0.2 | 109.4 ± 1.7 | 290.6 ± 5.9 | |
| La Araucanía | 132.0 ± 6.5 | 26.6 ± 1.3 | 10.4 ± 0.3 | 164.6 ± 2.5 | 333.6 ± 8.3 | |
| Los Ríos | 180.2 ± 6.5 | 36.1 ± 1.3 | 9.5 ± 0.3 | 167.9 ± 2.5 | 395.8 ± 8.7 | |
| Los Lagos | 157.8 ± 6.5 | 31.9 ± 1.3 | 10.4 ± 0.3 | 157.0 ± 2.5 | 357.0 ± 8.3 | |
| PIRA | O’Higgins | 129.2 ± 7.8 | 26.3 ± 2.0 | 10.1 ± 0.5 | 56.1 ± 3.2 | 221.1 ± 10.5 |
| Maule | 159.6 ± 7.8 | 30.8 ± 2.0 | 8.3 ± 0.5 | 68.4 ± 3.2 | 267.3 ± 10.5 | |
| Ñuble | 176.6 ± 7.8 | 33.6 ± 2.0 | 8.3 ± 0.5 | 95.2 ± 3.2 | 313.6 ± 10.5 | |
| Biobío | 239.2 ± 5.5 | 45.6 ± 1.4 | 11.5 ± 0.3 | 112.5 ± 2.2 | 408.5 ± 7.4 | |
| La Araucanía | 204.2 ± 7.8 | 38.7 ± 2.0 | 9.5 ± 0.5 | 136.9 ± 3.2 | 389.6 ± 10.5 | |
| Los Ríos | 199.2 ± 7.8 | 16.8 ± 2.0 | 8.7 ± 0.5 | 168.8 ± 3.2 | 393.5 ± 10.5 | |
| Los Lagos | 232.7 ± 7.8 | 45.3 ± 2.0 | 9.4 ± 0.5 | 149.4 ± 3.2 | 436.8 ± 10.5 |
| Specie | Climatic Zone | Above-Ground Biomass C | Below-Ground Biomass C | Forest Floor C | Soil Organic C (0–30 cm) | Total Ecosystem C |
|---|---|---|---|---|---|---|
| EUGL | Csb | 81.8 ± 3.4 | 17.8 ± 0.6 | 10.7 ± 0.3 | 102.4 ± 2.2 | 212.3 ± 4.5 |
| Csb (i) | 129.2 ± 4.9 | 26.0 ± 0.9 | 14.0 ± 0.4 | 115.9 ± 3.1 | 285.2 ± 6.4 | |
| Cfb (s,i) | 134.4 ± 4.9 | 26.9 ± 0.9 | 10.6 ± 0.4 | 153.8 ± 3.1 | 325.8 ± 6.4 | |
| Cfb (s) | 117.5 ± 4.9 | 23.9 ± 0.9 | 9.1 ± 0.4 | 172.2 ± 3.1 | 323.0 ± 6.4 | |
| EUNI | Csb | 160.1 ± 4.3 | 32.1 ± 0.8 | 10.5 ± 0.2 | 120.1 ± 1.8 | 320.5 ± 5.4 |
| Csb (i) | 136.1 ± 6.1 | 27.4 ± 1.2 | 13.9 ± 0.3 | 118.5 ± 2.5 | 295.9 ± 7.6 | |
| Csb (h) | 116.7 ± 6.1 | 23.3 ± 1.2 | 9.9 ± 0.3 | 120.3 ± 2.5 | 270.2 ± 7.6 | |
| Cfb | 165.8 ± 6.1 | 33.2 ± 1.2 | 12.5 ± 0.3 | 181.2 ± 2.5 | 392.7 ± 7.6 | |
| Cfb (s,i) | 170.1 ± 6.1 | 34.2 ± 1.2 | 10.0 ± 0.3 | 158.7 ± 2.5 | 373.1 ± 7.6 | |
| Cfb (s) | 176.9 ± 6.1 | 35.4 ± 1.2 | 9.3 ± 0.3 | 170.9 ± 2.5 | 395.1 ± 8.0 | |
| PIRA | Csb | 179.4 ± 5.6 | 34.3 ± 1.6 | 8.8 ± 0.3 | 88.9 ± 2.5 | 311.6 ± 7.6 |
| Csb (i) | 259.1 ± 7.9 | 49.1 ± 2.3 | 12.7 ± 0.4 | 113.8 ± 3.6 | 434.5 ± 10.7 | |
| Csb (h) | 188.0 ± 7.9 | 37.6 ± 2.3 | 9.9 ± 0.4 | 138.6 ± 3.6 | 374.0 ± 10.7 | |
| Cfb (s,i) | 218.1 ± 7.9 | 39.3 ± 2.3 | 9.5 ± 0.4 | 157.8 ± 3.6 | 424.7 ± 10.7 | |
| Cfb (s) | 199.7 ± 7.9 | 16.8 ± 2.3 | 8.7 ± 0.4 | 168.4 ± 3.6 | 393.6 ± 10.7 |
| Specie | Soil Parent Material | Above-Ground Biomass C | Below-Ground Biomass C | Forest Floor C | Soil Organic C (0–30 cm) | Total Ecosystem C |
|---|---|---|---|---|---|---|
| EUGL | AR | 77.2 ± 3.2 | 17.2 ± 0.6 | 9.3 ± 0.2 | 68.9 ± 2.2 | 172.5 ± 4.8 |
| G | 82.8 ± 4.5 | 17.8 ± 0.8 | 10.0 ± 0.3 | 92.5 ± 3.0 | 203.1 ± 6.8 | |
| CVa | 93.4 ± 4.5 | 20.3 ± 0.8 | 11.4 ± 0.3 | 91.2 ± 3.0 | 216.3 ± 6.8 | |
| CVr | 92.3 ± 4.5 | 19.8 ± 0.8 | 9.7 ± 0.3 | 139.6 ± 3.0 | 261.4 ± 6.8 | |
| CVrMe | 116.3 ± 4.5 | 23.5 ± 0.8 | 9.8 ± 0.3 | 171.3 ± 3.0 | 320.8 ± 6.8 | |
| Ña | 131.5 ± 4.5 | 26.3 ± 0.8 | 9.9 ± 0.3 | 155.1 ± 3.0 | 322.7 ± 6.8 | |
| M | 96.7 ± 4.5 | 20.1 ± 0.8 | 10.0 ± 0.3 | 122.4 ± 3.0 | 249.3 ± 6.8 | |
| SMCVa | 159.1 ± 4.5 | 31.8 ± 0.8 | 10.6 ± 0.3 | 138.0 ± 3.0 | 339.5 ± 6.8 | |
| SM | 134.1 ± 4.5 | 27.1 ± 0.8 | 11.8 ± 0.3 | 119.2 ± 3.0 | 292.3 ± 6.8 | |
| EUNI | G | 129.8 ± 7.2 | 26.0 ± 1.4 | 10.1 ± 0.3 | 105.4 ± 3.0 | 271.2 ± 9.5 |
| CVa | 154.8 ± 7.2 | 31.0 ± 1.4 | 11.4 ± 0.3 | 133.3 ± 3.0 | 330.4 ± 9.5 | |
| CVr | 175.6 ± 7.2 | 35.2 ± 1.4 | 9.5 ± 0.3 | 150.9 ± 3.0 | 371.2 ± 9.5 | |
| CVrMe | 184.7 ± 7.2 | 36.9 ± 1.4 | 9.8 ± 0.3 | 172.6 ± 3.0 | 404.0 ± 9.5 | |
| Ña | 169.5 ± 7.2 | 33.9 ± 1.4 | 9.5 ± 0.3 | 168.5 ± 3.0 | 381.3 ± 9.5 | |
| M | 144.1 ± 7.2 | 29.0 ± 1.4 | 11.1 ± 0.3 | 141.9 ± 3.0 | 326.1 ± 9.5 | |
| SMCVa | 172.5 ± 7.2 | 34.7 ± 1.4 | 10.3 ± 0.3 | 142.3 ± 3.0 | 359.7 ± 9.5 | |
| SM | 136.8 ± 7.2 | 27.7 ± 1.4 | 11.8 ± 0.3 | 116.1 ± 3.0 | 292.5 ± 9.5 | |
| PIRA | Lac | 78.8 ± 8.0 | 19.4 ± 2.2 | 8.6 ± 0.4 | 37.6 ± 3.5 | 144.4 ± 11.0 |
| AR | 151.1 ± 5.7 | 30.1 ± 1.5 | 7.1 ± 0.3 | 68.7 ± 2.5 | 257.0 ± 7.8 | |
| G | 177.0 ± 8.0 | 33.8 ± 2.2 | 8.7 ± 0.4 | 76.6 ± 3.5 | 296.1 ± 11.0 | |
| CVa | 160.0 ± 8.0 | 31.2 ± 2.2 | 8.5 ± 0.4 | 78.2 ± 3.5 | 277.7 ± 11.0 | |
| CVr | 181.4 ± 8.0 | 32.6 ± 2.2 | 9.1 ± 0.4 | 109.7 ± 3.5 | 332.8 ± 11.0 | |
| CVrMe | 198.7 ± 8.0 | 14.9 ± 2.2 | 9.0 ± 0.4 | 171.7 ± 3.5 | 394.4 ± 11.0 | |
| Ña | 208.5 ± 8.0 | 25.2 ± 2.2 | 9.8 ± 0.4 | 135.7 ± 3.5 | 379.2 ± 11.0 | |
| M | 213.3 ± 8.0 | 40.5 ± 2.2 | 9.8 ± 0.4 | 110.1 ± 3.5 | 373.6 ± 11.0 | |
| SMCVa | 228.7 ± 8.0 | 43.9 ± 2.2 | 9.8 ± 0.4 | 149.9 ± 3.5 | 432.3 ± 11.0 | |
| SM | 267.8 ± 8.0 | 50.9 ± 2.2 | 11.2 ± 0.4 | 115.5 ± 3.5 | 445.4 ± 11.0 |
References
- Oreskes, N. The Scientific Consensus on Climate Change. Science 2005, 306, 2004–2005. [Google Scholar] [CrossRef] [PubMed]
- Stocker, T.F.; Qin, D.; Plattner, G.K.; Tignor, M.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M.; et al. Climate change 2013: The physical science basis. In Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; Volume 1535. [Google Scholar]
- Rosenzweig, C.; Karoly, D.; Vicarelli, M.; Neofotis, P.; Wu, Q.; Casassa, G.; Menzel, A.; Root, T.L.; Estrella, N.; Seguin, B.; et al. Attributing physical and biological impacts to anthropogenic climate change. Nature 2008, 453, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Meinshausen, M.; Vogel, E.; Nauels, A.; Lorbacher, K.; Meinshausen, N.; Etheridge, D.M.; Fraser, P.J.; Montzka, S.A.; Rayner, P.J.; Trudinger, C.M.; et al. Historical greenhouse gas concentrations for climate modelling (CMIP6). Geosci. Model Dev. 2017, 10, 2057–2116. [Google Scholar] [CrossRef]
- Keller, D.P.; Lenton, A.; Littleton, E.W.; Oschlies, A.; Scott, V.; Vaughan, N.E. The effects of carbon dioxide removal on the carbon cycle. Curr. Clim. Chang. Rep. 2018, 4, 250–265. [Google Scholar] [CrossRef]
- Smith, P.; Bustamante, M.; Ahammad, H.; Clark, H.; Dong, H.; Elsiddig, E.; Haberl, H.; Harper, R.; House, J.; Jafari, M.; et al. Agriculture, forestry and other land use (AFOLU). Climate change 2014: Mitigation of climate change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Chapter 2014, 11, 811–922. [Google Scholar]
- McKinley, D.C.; Ryan, M.G.; Birdsey, R.A.; Giardina, C.P.; Harmon, M.E.; Heath, L.S.; Houghton, R.A.; Jackson, R.B.; Morrison, J.F.; Murray, B.C.; et al. A synthesis of current knowledge on forests and carbon storage in the United States. Ecol. Appl. 2011, 21, 1902–1924. [Google Scholar] [CrossRef]
- Coninck, H.; Revi, A.; Babiker, M.; Bertoldi, P.; Buckeridge, M.; Cartwright, A.; Dong, W.; Ford, J.; Fuss, S.; Hourcade, J.C.; et al. Chapter 4—Strengthening and implementing the global response. In Global Warming of 1.5 °C; IPCC: Geneva, Switzerland, 2018; pp. 313–443. [Google Scholar]
- Bellassen, V.; Luyssaert, S. Carbon sequestration: Managing forests in uncertain times. Nature 2014, 506, 153–155. [Google Scholar] [CrossRef]
- Beer, C.; Reichstein, M.; Tomelleri, E.; Ciais, P.; Jung, M.; Carvalhais, N.; Rödenbeck, C.; Arain, M.A.; Baldocchi, D.; Bonan, G.B.; et al. Terrestrial gross carbon dioxide uptake: Global distribution and covariation with climate. Science 2010, 329, 834–838. [Google Scholar] [CrossRef]
- Pregitzer, K.S.; Euskirchen, E.S. Carbon cycling and storage in world forests: Biome patterns related to forest age. Glob. Chang. Biol. 2004, 10, 2052–2077. [Google Scholar] [CrossRef]
- Ravindranath, N.H.; Murthy, I.K.; Samantaray, R. Enhancing Carbon Stocks and Reducing CO2 Emissions in Agriculture and Natural Resource Management Projects: Toolkit; Technical Report; The World Bank: Washington, DC, USA, 2012. [Google Scholar]
- Köhl, M.; Neupane, P.R.; Mundhenk, P. REDD+ measurement, reporting and verification—A cost trap? Implications for financing REDD+ MRV costs by result-based payments. Ecol. Econ. 2020, 168, 106513. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Temesgen, H.; Affleck, D.; Poudel, K.; Gray, A.; Sessions, J. A review of the challenges and opportunities in estimating above ground forest biomass using tree-level models. Scand. J. For. Res. 2015, 30, 326–335. [Google Scholar] [CrossRef]
- Rodriguez-Veiga, P.; Wheeler, J.; Louis, V.; Tansey, K.; Balzter, H. Quantifying forest biomass carbon stocks from space. Curr. For. Rep. 2017, 3, 1–18. [Google Scholar] [CrossRef]
- Aalde, H.; Gonzalez, P.; Gytarsky, M.; Krug, T.; Kurz, W.A.; Ogle, S.; Raison, J.; Schoene, D.; Ravindranath, N.H.; Elhassan, N.G.; et al. Forest land. IPCC Guidel. Natl. Greenh. Gas Invent. 2006, 4, 1–83. [Google Scholar]
- Wutzler, T.; Profft, I.; Mund, M. Quantifying tree biomass carbon stocks, their changes and uncertainties using routine stand taxation inventory data. Silva Fenn. 2011, 45, 359–377. [Google Scholar] [CrossRef][Green Version]
- Salas, C.; Donoso, P.J.; Vargas, R.; Arriagada, C.A.; Pedraza, R.; Soto, D.P. The forest sector in Chile: An overview and current challenges. J. For. 2016, 114, 562–571. [Google Scholar] [CrossRef]
- Scharlemann, J.P.; Tanner, E.V.; Hiederer, R.; Kapos, V. Global soil carbon: Understanding and managing the largest terrestrial carbon pool. Carbon Manag. 2014, 5, 81–91. [Google Scholar] [CrossRef]
- Pfeiffer, M.; Padarian, J.; Osorio, R.; Bustamante, N.; Olmedo, G.F.; Guevara, M.; Aburto, F.; Albornoz, F.; Antilén, M.; Araya, E.; et al. CHLSOC: The Chilean Soil Organic Carbon database, a multi-institutional collaborative effort. Earth Syst. Sci. Data 2020, 12, 457–468. [Google Scholar] [CrossRef]
- Silver, W.L.; Ostertag, R.; Lugo, A.E. The potential for carbon sequestration through reforestation of abandoned tropical agricultural and pasture lands. Restor. Ecol. 2000, 8, 394–407. [Google Scholar] [CrossRef]
- Lal, R. Forest soils and carbon sequestration. For. Ecol. Manag. 2005, 220, 242–258. [Google Scholar] [CrossRef]
- Espinosa, M.; Acuña, E.; Cancino, J.; Muñoz, F.; Perry, D.A. Carbon sink potential of radiata pine plantations in Chile. Forestry 2005, 78, 11–19. [Google Scholar] [CrossRef]
- Bernier, P.; Schoene, D. Adapting forests and their management to climate change: An overview. Unasylva 2009, 60, 5–11. [Google Scholar]
- Maier, C.; Johnsen, K. Quantifying carbon sequestration in forest plantations by modeling the dynamics of above and below ground carbon pools. In Proceedings of the 14th Biennial Southern Silvicultural Research Conference; U.S. Department of Agriculture, Forest Service, Southern Research Station: Asheville, NC, USA, 2010; Volume 121, pp. 3–8. [Google Scholar]
- Sanquetta, C.; Dalla Corte, A.; Benedet Maas, G. The role of forests in climate change. Quebracho (Santiago Del Estero) 2011, 19, 84–96. [Google Scholar]
- Aba, S.C.; Ndukwe, O.; Amu, C.J.; Baiyeri, K.P. The role of trees and plantation agriculture in mitigating global climate change. Afr. J. Food Agric. Nutr. Dev. 2017, 17, 12691–12707. [Google Scholar] [CrossRef]
- Bastin, J.F.; Finegold, Y.; Garcia, C.; Mollicone, D.; Rezende, M.; Routh, D.; Zohner, C.M.; Crowther, T.W. The global tree restoration potential. Science 2019, 364, 76–79. [Google Scholar] [CrossRef]
- Friedlingstein, P.; Allen, M.; Canadell, J.G.; Peters, G.P.; Seneviratne, S.I. Comment on “The global tree restoration potential”. Science 2019, 366. [Google Scholar] [CrossRef]
- Veldman, J.W.; Aleman, J.C.; Alvarado, S.T.; Anderson, T.M.; Archibald, S.; Bond, W.J.; Boutton, T.W.; Buchmann, N.; Buisson, E.; Canadell, J.G.; et al. Comment on “The global tree restoration potential”. Science 2019, 366. [Google Scholar] [CrossRef]
- Lewis, S.L.; Mitchard, E.T.A.; Prentice, C.; Maslin, M.; Poulter, B. Comment on “The global tree restoration potential”. Science 2019, 366. [Google Scholar] [CrossRef]
- Reed, W.P.; Kaye, M.W. Bedrock type drives forest carbon storage and uptake across the mid-Atlantic Appalachian Ridge and Valley, U.S.A. For. Ecol. Manag. 2020, 460, 117881. [Google Scholar] [CrossRef]
- Heilmayr, R.; Echeverría, C.; Lambin, E.F. Impacts of Chilean forest subsidies on forest cover, carbon and biodiversity. Nat. Sustain. 2020. [Google Scholar] [CrossRef]
- Soto, L.; Galleguillos, M.; Seguel, O.; Sotomayor, B.; Lara, A. Assessment of soil physical properties’ statuses under different land covers within a landscape dominated by exotic industrial tree plantations in south-central Chile. J. Soil Water Conserv. 2019, 74, 12–23. [Google Scholar] [CrossRef]
- Casanova, M.; Salazar, O.; Seguel, O.; Luzio, W. The soils of Chile; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Buchner, C.; Martin, M.; Sagardia, R.; Avila, A.; Molina, E.; Rojas, Y.; Muñoz, J.; Barros, S.; Rose, J.; Barrientos, M.; et al. Disponibilidad de Madera de Plantaciones de Pino Radiata y Eucalipto (2017–2047) [Wood Availability from Radiata Pine and Eucalyptus Plantations (2017–2047)]; Instituto Forestal [Forestry Institute]: Santiago, Chile, 2018. [Google Scholar]
- Peters-Nario, R.J. Simulador de árbol Individual de Pino Radiata (Pinus Radiata D. Don): Arquitectura de Copa y Calidad de Madera; Agencia Nacional de Investigación y Desarrollo (ANID); FONDEF: Santiago, Chile, 2001; Available online: http://repositorio.conicyt.cl/handle/10533/109507 (accessed on 30 September 2020).
- Knapp, N.; Fischer, R.; Huth, A. Linking lidar and forest modeling to assess biomass estimation across scales and disturbance states. Remote. Sens. Environ. 2018, 205, 199–209. [Google Scholar] [CrossRef]
- Burkhart, H.E.; Tomé, M. Modeling Forest Trees and Stands; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 9789048131, pp. 1–457. [Google Scholar] [CrossRef]
- Kilkki, P.; Saramäki, M.; Varmola, M. A simultaneous equation model to determine taper curve. Silva Fenn. 1978, 12, 120–125. [Google Scholar] [CrossRef][Green Version]
- McBratney, A.B.; Mendonça Santos, M.L.; Minasny, B. On digital soil mapping. Geoderma 2003, 117, 3–52. [Google Scholar] [CrossRef]
- Schoeneberger, P.J.; Wysocki, D.A.; Benham, E.C. Field Book for Describing and Sampling Soils; Government Printing Office: Washington, DC, USA, 2012.
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Reyes Rojas, L.A.; Adhikari, K.; Ventura, S.J. Projecting Soil Organic Carbon Distribution in Central Chile under Future Climate Scenarios. J. Environ. Qual. 2018, 47, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Guevara, M.; Olmedo, G.; Stell, E.; Yigini, Y.; Aguilar Duarte, Y.; Arellano Hernández, C.; Arévalo, G.; Eduardo Arroyo-Cruz, C.; Bolivar, A.; Bunning, S.; et al. No silver bullet for digital soil mapping: Country-specific soil organic carbon estimates across Latin America. SOIL 2018, 4. [Google Scholar] [CrossRef]
- Díaz, J.S.G.; Delgado, N.O.; Gamboa, A.B.; Bunning, S.; Guevara, M.; Medina, E.; Olivera, C.; Olmedo, G.F.; Rodríguez, L.M.; Sevilla, V.; et al. Estimación del carbono orgánico en los suelos de ecosistema de páramo en Colombia. Rev. Ecosistemas 2020, 29, 1855. [Google Scholar]
- Heuvelink, G.B.M.; Angelini, M.E.; Poggio, L.; Bai, Z.; Batjes, N.H.; van den Bosch, R.; Bossio, D.; Estella, S.; Lehmann, J.; Olmedo, G.F.; et al. Machine learning in space and time for modelling soil organic carbon change. Eur. J. Soil Sci. 2020. [Google Scholar] [CrossRef]
- Hengl, T.; MacMillan, R.A. Predictive Soil Mapping with R; Lulu.com: Morrisville, NC, USA, 2019. [Google Scholar]
- Baritz, R.; Guevara, M.A.; Mulder, T.V.L.; Olmedo, G.F.; Omuto, C.T.; Vargas, R.R.; Yigini, Y. Mapping methods. In Soil Organic Carbon Mapping Cookbook, 2nd ed.; Yigini, Y., Olmedo, G.F., Reiter, S., Baritz, R., Viatkin, K., Vargas, R.R., Eds.; FAO: Rome, Italy, 2018; Chapter 6; pp. 51–109. [Google Scholar]
- Meinshausen, N. Quantile regression forests. J. Mach. Learn. Res. 2006, 7, 983–999. [Google Scholar]
- Achat, D.L.; Fortin, M.; Landmann, G.; Ringeval, B.; Augusto, L. Forest soil carbon is threatened by intensive biomass harvesting. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Minasny, B.; McBratney, A.B. A conditioned Latin hypercube method for sampling in the presence of ancillary information. Comput. Geosci. 2006, 32, 1378–1388. [Google Scholar] [CrossRef]
- Sarricolea, P.; Herrera-Ossandon, M.; Meseguer-Ruiz, Ó. Climatic regionalisation of continental Chile. J. Maps 2017, 13, 66–73. [Google Scholar] [CrossRef]
- Centro de Información de Recursos Naturales (Chile). Estudio Agrológico VI Región. Descripciones de Suelos. Materiales y Símbolos. Actualización 1996; CIREN: Santiago, Chile, 1996; Volume N° 114.
- Centro de Información de Recursos Naturales (Chile). Estudio Agrológico Región Metropolitana. Descripciones de Suelos. Materiales y Símbolos. Actualización 1996; CIREN: Santiago, Chile, 1996; Volume N° 115.
- Centro de Información de Recursos Naturales (Chile). Estudio Agrológico V Región. Descripciones de Suelos. Materiales y Símbolos. Actualización 1997; CIREN: Santiago, Chile, 1997; Volume N° 116.
- Centro de Información de Recursos Naturales (Chile). Estudio Agrológico VII Región. Descripciones de Suelos. Materiales y Símbolos. Actualización 1997; CIREN: Santiago, Chile, 1997; Volume N° 117.
- Centro de Información de Recursos Naturales (Chile). Estudio Agrológico VIII Región. Descripciones de Suelos. Materiales y Símbolos. Actualización 1997; CIREN: Santiago, Chile, 1999; Volume N° 121.
- Centro de Información de Recursos Naturales (Chile). Estudio Agrológico IX Región. Descripciones de Suelos. Materiales y Símbolos. Actualización 2002; CIREN: Santiago, Chile, 2002.
- Centro de Información de Recursos Naturales (Chile). Estudio Agrológico X Región. Descripciones de Suelos. Materiales y Símbolos. Actualización 2003; CIREN: Santiago, Chile, 2003; Volume N° 123.
- Centro de Información de Recursos Naturales (Chile). Estudio Agrológico IV Región. Descripciones de Suelos. Materiales y Símbolos. Actualización 2005; CIREN: Santiago, Chile, 2005; Volume N° 129.
- Centro de Información de Recursos Naturales (Chile). Estudio Agrológico XI Región. Descripciones de Suelos. Materiales y Símbolos. Actualización 2005; CIREN: Santiago, Chile, 2005; Volume N° 130.
- Centro de Información de Recursos Naturales (Chile). Estudio Agrológico III Región. Descripciones de Suelos. Materiales y Símbolos. Actualización 2007; CIREN: Santiago, Chile, 2007; Volume N° 135.
- Sernageomin, S. Mapa Geológi co de Chile: Versión digital. In Servicio Nacional de Geología y Minería, Publicación Geológica Digital, No. 4 CD-Room, Versión 1.0, Base geológica Escala; CIREN: Santiago, Chile, 2003; Volume 1. [Google Scholar]
- Alcayaga, S. Características generales de los suelos ñadis. Mesa Redonda Suelos Volcánicos. Soc. Agron. Chile. Publ. Espec. 1964, 1, 1–14. [Google Scholar]
- Ryan, M.; Binkley, D.; Fownes, J.H. Age-related decline in forest productivity: Pattern and process. In Advances in Ecological Research; Elsevier: Amsterdam, The Netherlands, 1997; Volume 27, pp. 213–262. [Google Scholar]
- Oliver, G.R.; Beets, P.N.; Pearce, S.H.; Graham, J.D.; Garrett, L.G. Carbon accumulation in two Pinus radiata stands in the North Island of New Zealand. N. Z. J. For. Sci. 2011, 41, 71–86. [Google Scholar]
- Guo, L.B.; Cowie, A.L.; Montagu, K.D.; Gifford, R.M. Carbon and nitrogen stocks in a native pasture and an adjacent 16-year-old Pinus radiata D. Don. plantation in Australia. Agric. Ecosyst. Environ. 2008, 124, 205–218. [Google Scholar] [CrossRef]
- Guedes, B.S.; Olsson, B.A.; Egnell, G.; Sitoe, A.A.; Karltun, E. Plantations of Pinus and Eucalyptus replacing degraded mountain miombo woodlands in Mozambique significantly increase carbon sequestration. Glob. Ecol. Conserv. 2018, 14, e00401. [Google Scholar] [CrossRef]
- Ángel Balboa-Murias, M.; Rodríguez-Soalleiro, R.; Merino, A.; Álvarez González, J.G. Temporal variations and distribution of carbon stocks in aboveground biomass of radiata pine and maritime pine pure stands under different silvicultural alternatives. For. Ecol. Manag. 2006, 237, 29–38. [Google Scholar] [CrossRef]
- Fernández-Núñez, E.; Rigueiro-Rodríguez, A.; Mosquera-Losada, M. Carbon allocation dynamics one decade after afforestation with Pinus radiata D. Don and Betula alba L. under two stand densities in NW Spain. Ecol. Eng. 2010, 36, 876–890. [Google Scholar] [CrossRef]
- Soares, P.; Tomé, M. Biomass expansion factors for Eucalyptus globulus stands in Portugal. For. Syst. 2012, 21, 141–152. [Google Scholar] [CrossRef]
- Demessie, A.; Singh, B.R.; Lal, R. Soil Carbon and Nitrogen Stocks Under Plantations in Gambo District, Southern Ethiopia. J. Sustain. For. 2011, 30, 496–517. [Google Scholar] [CrossRef]
- Acuña, E.; Cancino, J.; Rubilar, R.; Sandoval, S. Aboveground biomass growth and yield of first rotation cutting cycle of Acacia and Eucalyptus short rotation dendreoenergy crops. Rev. Àrvore 2017, 41. [Google Scholar] [CrossRef]
- Bioenercel. Aprovechamiento Sustentable de Residuos de Cosecha Forestal e Industrial Para Producción de Biocombustibles de Segunda Generación; Bioenercel: Concepción, Chile, 2013; Number 6. [Google Scholar]
- Weiskittel, A.R.; Hann, D.W.; Kershaw, J.A.; Vanclay, J.K. Forest Growth and Yield Modeling; John Wiley & Sons: Hoboken, NJ, USA, 2011; p. 430. [Google Scholar]
- Houghton, R. Aboveground forest biomass and the global carbon balance. Glob. Chang. Biol. 2005, 11, 945–958. [Google Scholar] [CrossRef]
- Jackson, R.B.; Lajtha, K.; Crow, S.E.; Hugelius, G.; Kramer, M.G.; Piñeiro, G. The ecology of soil carbon: Pools, vulnerabilities, and biotic and abiotic controls. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 419–445. [Google Scholar] [CrossRef]
- Tifafi, M.; Guenet, B.; Hatté, C. Large differences in global and regional total soil carbon stock estimates based on SoilGrids, HWSD, and NCSCD: Intercomparison and evaluation based on field data from USA, England, Wales, and France. Glob. Biogeochem. Cycles 2018, 32, 42–56. [Google Scholar] [CrossRef]
- Crowther, T.W.; Todd-Brown, K.E.; Rowe, C.W.; Wieder, W.R.; Carey, J.C.; Machmuller, M.B.; Snoek, B.; Fang, S.; Zhou, G.; Allison, S.D.; et al. Quantifying global soil carbon losses in response to warming. Nature 2016, 540, 104–108. [Google Scholar] [CrossRef]
- James, J.; Devine, W.; Harrison, R.; Terry, T. Deep soil carbon: Quantification and modeling in subsurface layers. Soil Sci. Soc. Am. J. 2014, 78, S1–S10. [Google Scholar] [CrossRef]
- Dietzen, C.A.; Marques, E.R.; James, J.N.; Bernardi, R.H.; Holub, S.M.; Harrison, R.B. Response of deep soil carbon pools to forest management in a highly productive Andisol. Soil Sci. Soc. Am. J. 2017, 81, 970–978. [Google Scholar] [CrossRef]
- Heilmayr, R. Replication Data for “Impacts of Chilean Forest Subsidies on Forest Cover, Carbon and Biodiversity”. 2019. Available online: https://dataverse.harvard.edu/dataset.xhtml?persistentId=doi:10.7910/DVN/6RDDQH (accessed on 30 September 2020). [CrossRef]
- Gysling Caselli, A.J.; Alvarez González, V.; Soto Aguirre, D.A.; Pardo Velásquez, E.; Poblete, P. Anuario Forestal 2018; INFOR: Santiago, Chile, 2018. [Google Scholar]
- Flores, F.J.; Allen, H.L.E.E. Efectos del clima y capacidad de almacenamiento de agua del suelo en la productividad de rodales de pino radiata en Chile: Un análisis utilizando el modelo 3-PG. Bosque (Valdivia) 2004, 25, 11–24. [Google Scholar] [CrossRef][Green Version]
- Battaglia, M.; Beadle, C.; Loughhead, S. Photosynthetic temperature responses of Eucalyptus globulus and Eucalyptus nitens. Tree Physiol. 1996, 16, 81–89. [Google Scholar] [CrossRef]
- Fox, T.R. Sustained productivity in intensively managed forest plantations. For. Ecol. Manag. 2000, 138, 187–202. [Google Scholar] [CrossRef]
- Rubilar, R.A.; Allen, H.L.; Fox, T.R.; Cook, R.L.; Albaugh, T.J.; Campoe, O.C. Advances in Silviculture of Intensively Managed Plantations. Curr. For. Rep. 2018, 4, 23–34. [Google Scholar] [CrossRef]
- Bonilla, C.A.; Johnson, O.I. Soil erodibility mapping and its correlation with soil properties in Central Chile. Geoderma 2012, 189–190, 116–123. [Google Scholar] [CrossRef]
- Toro, J.; Gessel, S.P. Radiata pine plantations in Chile. New For. 1999, 18, 393–404. [Google Scholar] [CrossRef]
- Battaglia, M.; Cherry, M.L.; Beadle, C.L.; Sands, P.J.; Hingston, A. Prediction of leaf area index in eucalypt plantations: Effects of water stress and temperature. Tree Physiol. 2000, 18, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, R.; Real, P.; Espinosa, M.; Perry, D.A. A process-based model to evaluate site quality for Eucalyptus nitens in the Bio-Bio Region of Chile. Forestry 2009, 82, 149–162. [Google Scholar] [CrossRef]
- Matus, F.; Rumpel, C.; Neculman, R.; Panichini, M.; Mora, M. Soil carbon storage and stabilisation in andic soils: A review. CATENA 2014, 120, 102–110. [Google Scholar] [CrossRef]
- Valle, S.R.; Dörner, J.; Zúñiga, F.; Dec, D. Seasonal dynamics of the physical quality of volcanic ash soils under different land uses in southern Chile. Soil Tillage Res. 2018, 182, 25–34. [Google Scholar] [CrossRef]
- Ellies, A. Soil erosion and its control in Chile—An overview. Acta Geol. Hisp. 2000, 35, 279–284. [Google Scholar]
- Ramírez, P.B.; Calderón, F.J.; Fonte, S.J.; Bonilla, C.A. Environmental controls and long-term changes on carbon stocks under agricultural lands. Soil Tillage Res. 2019, 186, 310–321. [Google Scholar] [CrossRef]
- White, D.; Beadle, C.; Worledge, D.; Honeysett, J.; Cherry, M. The influence of drought on the relationship between leaf and conducting sapwood area in Eucalyptus globulus and Eucalyptus nitens. Trees 1998, 12, 406–414. [Google Scholar] [CrossRef]
- Close, D.C.; Beadle, C.L.; Brown, P.H.; Holz, G.K. Cold-induced photoinhibition affects establishment of Eucalyptus nitens (Deane and Maiden) Maiden and Eucalyptus globulus Labill. Trees 2000, 15, 32–41. [Google Scholar] [CrossRef]
- Davidson, N.; Battaglia, M.; Close, D. Photosynthetic responses to overnight frost in Eucalyptus nitens and E. globulus. Trees 2004, 18, 245–252. [Google Scholar] [CrossRef]
- Zúñiga, F.; Dec, D.; Valle, S.R.; Thiers, O.; Paulino, L.; Martínez, O.; Seguel, O.; Casanova, M.; Pino, M.; Horn, R.; et al. The waterlogged volcanic ash soils of southern Chile. A review of the “Ñadi” soils. CATENA 2019, 173, 99–113. [Google Scholar] [CrossRef]
- Friedlingstein, P.; Jones, M.W.; O’Sullivan, M.; Andrew, R.M.; Hauck, J.; Peters, G.P.; Peters, W.; Pongratz, J.; Sitch, S.; Le Quéré, C.; et al. Global Carbon Budget 2019. Earth Syst. Sci. Data 2019, 11, 1783–1838. [Google Scholar] [CrossRef]
| Growing Stock Level (m3) | |||||
|---|---|---|---|---|---|
| <20 | 21–40 | 41–100 | 100–200 | >200 | |
| Pinus radiata | 1.8 | 1.0 | 0.75 | 0.7 | 0.7 |
| Eucalyptus sp. | 3.0 | 1.7 | 1.4 | 1.05 | 0.8 |
| Above-Ground Biomass (tonnes · ha−1) | |||
|---|---|---|---|
| <50 | 50–150 | >150 | |
| Pinus radiata | 0.40 | 0.29 | 0.20 |
| Eucalyptus sp. | 0.44 | 0.28 | 0.20 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olmedo, G.F.; Guevara, M.; Gilabert, H.; Montes, C.R.; Arellano, E.C.; Barría-Knopf, B.; Gárate, F.; Mena-Quijada, P.; Acuña, E.; Bown, H.E.; et al. Baseline of Carbon Stocks in Pinus radiata and Eucalyptus spp. Plantations of Chile. Forests 2020, 11, 1063. https://doi.org/10.3390/f11101063
Olmedo GF, Guevara M, Gilabert H, Montes CR, Arellano EC, Barría-Knopf B, Gárate F, Mena-Quijada P, Acuña E, Bown HE, et al. Baseline of Carbon Stocks in Pinus radiata and Eucalyptus spp. Plantations of Chile. Forests. 2020; 11(10):1063. https://doi.org/10.3390/f11101063
Chicago/Turabian StyleOlmedo, Guillermo F., Mario Guevara, Horacio Gilabert, Cristián R. Montes, Eduardo C. Arellano, Beatriz Barría-Knopf, Francisco Gárate, Pablo Mena-Quijada, Eduardo Acuña, Horacio E. Bown, and et al. 2020. "Baseline of Carbon Stocks in Pinus radiata and Eucalyptus spp. Plantations of Chile" Forests 11, no. 10: 1063. https://doi.org/10.3390/f11101063
APA StyleOlmedo, G. F., Guevara, M., Gilabert, H., Montes, C. R., Arellano, E. C., Barría-Knopf, B., Gárate, F., Mena-Quijada, P., Acuña, E., Bown, H. E., & Ryan, M. G. (2020). Baseline of Carbon Stocks in Pinus radiata and Eucalyptus spp. Plantations of Chile. Forests, 11(10), 1063. https://doi.org/10.3390/f11101063





