Abstract
Dendrobium officinale is an important traditional Chinese medicinal plant and crop, which contains many kinds of medicinal components. The quality of medicinal plants is closely related to the ecological factors in a growing environment. The main components of D. officinale determined in this study were polysaccharides, total alkaloids and total flavonoids. In addition, this study dealt with the correlation of these components to 16 ecological factors under three different cultivation modes (Greenhouse, Bionic, Wild; Lu’an, Anhui Province, China). The relationship between ecological factors and quality factors was analyzed step by step using correlation analysis, principal component analysis and stepwise multiple linear regression. Eight ecological factors: maximum relative humidity, minimum relative humidity, maximum temperature, sunshine duration, soil pH, soil total nitrogen, soil total phosphorus and soil available phosphorus were considered as key factors that influenced the main medicinal qualities of cultivated D. officinale. This study provides an insight for exploring the complex relationship between ecological factors and D. officinale medicinal value in artificial cultivation.
1. Introduction
Dendrobium is a perennial medicinal plant belonging to Orchids family in plant taxonomy, and it is one the largest genera in Orchidaceae, comprising approximately 1500–2000 species distributed in the tropical and subtropical regions of Asia and North Australia [1,2]. In China, there are 74 species and two varieties of Dendrobium [3], mainly distributed in Anhui, Zhejiang, Fujian and other places in China, which contain polysaccharides, alkaloids, flavonoids, essential amino acids and some trace mineral elements [4,5]. It has been documented as a high-grade drug in Shennong’s Herbal, as the stems of Dendrobium have been used to make a tonic or a functional medicine to nourish the stomach, relieve throat inflammation, improve eyesight and promote body fluid production in Chinese medicine for thousands of years [6,7]. Among the medicinal species, Dendrobium officinale is officially listed in the Pharmacopoeia of the People’s Republic of China (2010 Edition, Chinese Pharmacopoeia Commission, 2010) [8], which is rich for polysaccharides, and its dried stems can be directly used or twisted like a spring named “Tiepi Fengdou” [7]. D. officinale metabolites including organic acids, amino acids and their derivatives, nucleotides and their derivatives and flavones [9]. Wild resources of D. officinale, known as “giant panda in the pharmaceutical field”, are very scarce. Meanwhile, with the further study and application of the medicinal value of D. officinale, its market demand is also gradually expanding; therefore, the scale of cultivation of D. officinale has expanded rapidly.
To protect wild D. officinale resources and meet market demand, in recent years, many provinces in China, including traditional and non-traditional D. officinale-producing areas, have been vigorously carrying out cultivation of D. officinale. This includes the development of the greenhouse cultivation mode, the bionic cultivation mode and the potted cultivation mode [10]. As D. officinale requires stringent environmental conditions during its growth and development, creating an artificial environment suitable for plant growth is the key to successful greenhouse cultivation. The greenhouse cultivation mode is a kind of facility cultivation mode. Facility cultivation refers to creating artificially controlled environmental conditions that allow plants to grow and develop normally, without adverse environmental effects on production, to ensure stable production and access to high-yield, high-quality agricultural products [11]. However, in the greenhouse cultivation, due to the increase in plant density, as well as the use of fertilizers, plant growth regulators and pesticides, the residues of toxic and hazardous substances are substantially exceeded. Therefore, the bionic cultivation mode has been developed as an alternative and will likely become an important development direction of D. officinale cultivation in the future. The bionic cultivation mode is based on medicinal plant growth and development habits, as well as on the requirements of the ecological environment, using simple sunshade greenhouse or forest resources, including appropriate shading trees for cultivation [12]. The question arises whether greenhouse cultivation mode is the best way to control the quality of D. officinale during the growth process, through control of the temperature, humidity, sunshine and other environmental factors.
Ecological factors are environmental factors that have direct or indirect effects on the growth, development, reproduction, behavior, and distribution of organisms, such as climate, soil, topography and other related organisms [13]. They can be roughly divided into climatic factors, geographical factors and soil factors. Plants have the capacity to overcome biological, physical, chemical and ecological constraints by regulating the improvement of secondary metabolites. As a result, investigations on the control of ecological factors on the build-up of secondary metabolites in medicinal plants has become a hot topic of major concern [14,15]. Different ecological factors cause differences in the quality of the genuine regional drug. For instance, in cultivated Polygala tenuifolia, annual mean temperature, annual sunshine duration and soil pH, Cl, Sr, Ca, S, B and exchangeable K concentration were considered as key factors that influenced the quality [16]; in Scutellaria baicalensis, most chemical constituents had negative correlation with latitude and positive correlation with temperature [17]. The ecological factors in the growing of D. officinale have not been reported, thus, it is necessary to study ecological factors influencing the quality of D. officinale.
In order to study the relationship between the main medicinal components and the ecological factors of D. officinale, we selected three different cultivation modes in Ta-pieh Mountains area. The objectives of this present study were to evaluate D. officinale quality and investigate the important ecological factors that affect it. These results can provide an effective reference basis for better cultivation of D. officinale, improve the content of main medicinal components of D. officinale through effective control of ecological and environmental factors, and provide a theoretical basis for high yield, stable yield, high quality and large-scale standardized cultivation of D. officinale.
2. Materials and Methods
2.1. Plant Material
Plants in the greenhouse and bionic cultivation modes were artificially cultivated and collected from Anhui Tongjisheng Biotechnology Company, Lu’an, China. Seeds germination and protocorm-like bodies growth were cultured on half-strength Murashige and Skoog (PhytoTechnology Laboratories, Shawnee Mission, KS) medium [18] adding 6-BA 0.1 mg·L−1, NAA 0.5 mg·L−1 and 1% additives (30 g·L−1 sucrose + 4 g·L−1 agar + 20% potato) (Sigma-Aldrich, St Louis, MO, USA). After 18 months, the plants were separately transplanted into pots to cultivate. Wild D. officinale plants were collected from the edge of cliff. The wild 2-year-old D. officinale were authenticated by Professor Jinchi Zhang and all the plants were deposited at the College of Forestry in Nanjing Forestry University, Nanjing, China. Due to the scarcity of wild D. officinale resources, it was difficult to collect samples; thus, we could only do three replicates for each plant. The wild D. officinale collection method is to fasten to the top of the cliff with a rope, then tie the other side to people and climb down to collect plants.
After removal of roots and leaves, we obtained stem samples. Stems were collected from 2-year-old D. officinale plants. All stem samples were washed with pure water, placed in a 60 °C and oven dried to constant weight and then crushed and sieved for the extraction of polysaccharide, total alkaloid and total flavonoid in March 2018.
2.2. Experimental Design
We selected three different cultivation modes in the Ta-pieh Mountains area (Figure 1). The greenhouse cultivation mode involves the application of an intelligent control system to the greenhouse cultivation, using the most advanced bio-simulation technology to simulate the environment most suitable for plant growth in the greenhouse. Temperature, humidity, CO2 and illuminance sensors are used to sense various environmental indicators in the greenhouse, and the water curtain, fan and sun visor facilities in the shed are monitored by the microcomputer (Microsoft, Redmond, WA, USA) to change the biological growth environment inside the greenhouse (Figure 2A(a–c)). Bionic cultivation mode involves the building of a simple greenhouse around the natural environment where the wild D. officinale grows. Except for the simple sunshade and planting in a pot, the other environment conditions are similar to the wild D. officinale growth environment (Figure 2B(a–c)). Wild cultivation mode involves looking for natural growth of wild D. officinale in the Ta-pieh Mountains, which is often difficult to find and is collected along cliffs (Figure 2C(a–c)). We collected the two-year-old D. officinale in these three different cultivation modes, and determined the main medicinal components and collected the different ecological factors data in these three environments for analysis.
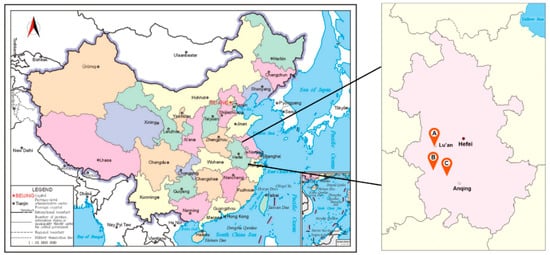
Figure 1.
Location map of different cultivation modes of Dendrobium officinale. Maps from Standard mapping service system of Ministry of Natural Resources of China (http://bzdt.ch.mnr.gov.cn/). (A) Greenhouse cultivation mode. (B) Bionic cultivation mode. (C) Wild cultivation mode.
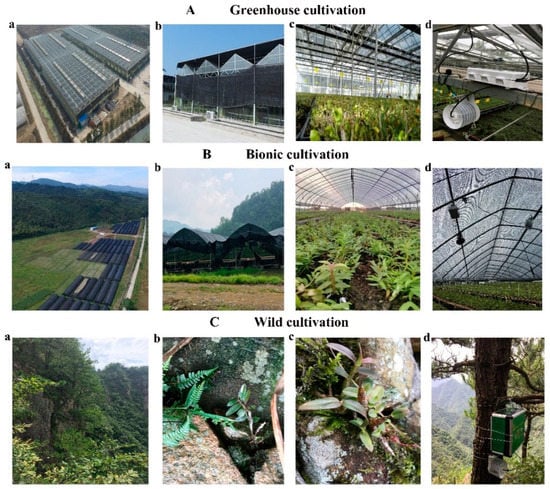
Figure 2.
Different cultivation modes of Dendrobium officinale. (A) Greenhouse cultivation mode. (a–c) The growth environment of Dendrobium officinale in the greenhouse cultivation mode. (d) The position where the EM50 instrument (Decagon Devices Inc., Pullman, WA, USA) is placed in the greenhouse. (B) Bionic cultivation mode. (a–c) The growth environment of Dendrobium officinale in the bionic cultivation mode. (d) The position where the EM50 instrument is placed in the bionic cultivation mode. (C) Wild cultivation mode. (a–c) The growth environment of Dendrobium officinale in wild cultivation mode. (d) The growth environment of Dendrobium officinale in a wild environment.
2.3. Ecological Factors
2.3.1. Climate Factors
Three automatic five-channel data loggers (EM-50, Decagon Devices Inc, Pullman, WA, USA) were installed, respectively, at the sites indicated in Figure 2(A(d),B(d),C(d)) and data were collected in half an hour interval. Net radiation was assessed with a PYR solar radiation sensor (Decagon Devices Inc., Pullman, WA, USA), air temperature and relative humidity were assessed with a VP-3 vapor pressure (EHT: relative humidity and temperature sensor, Decagon Devices Inc, Decagon Devices Inc., Pullman, WA, USA, accuracy ± 2% from 5 to 90% RH, ±3% from 90%–100% RH). These three sensors were placed in good contact with the microenvironment. The data record started from September 2017 to August 2019 for two years.
2.3.2. Soil Factors
For soil samples collected, the surface mulch was removed, and 500 g soil samples from 20 cm deep of topsoil were obtained from every root digging location, mixed fully, packed and labeled. The collected soil samples were removed from the obvious coarse impurities and placed in an ice box for temporary storage and brought back to the laboratory in time. The fresh sample was sieved through a 2 mm mesh to remove debris such as gravel and roots and then mixed well. A portion was stored in a refrigerator at 4 °C for pH, ammonium nitrogen and nitrate nitrogen content and determination of available phosphorus. Another part of the air-dried soil was sieved through a 0.1 mm mesh for the determination of soil organic carbon, total nitrogen and total phosphorus.
Soil pH was measured with a soil/deionized water (Sigma-Aldrich, St Louis, MO, USA) ratio of 1/2.5. Soil total nitrogen (TN) was determined using H2SO4 digestion and measured with the Kjeldahl method [19]. Soil total phosphorus (TP) was measured with H2SO4 and HClO4 (Sinopharm Chemical Reagent Co., Ltd, Shanghai, China) digestion followed by the colorimetric method using ascorbic acid (Sinopharm Chemical Reagent Co., Ltd, Shanghai, China). Soil ammonium nitrogen (AN) was extracted using 2 M KCl solution (Sinopharm Chemical Reagent Co., Ltd, Shanghai, China) followed by the method of detection using colorimetric-indophenol blue [20]. The nitrate nitrogen (NN) was determined using dual wavelength spectrophotometry [21]. Available phosphorus (AP) of soil samples was extracted with ammonium fluoride (NH4F, 0.03 M) and hydrochloric acid (HCl, 0.025 M) (Sinopharm Chemical Reagent Co., Ltd, Shanghai, China), and measured by UV–Vis spectrophotometer (Thermo Fisher Scientific, San Jose, CA, USA) [22]. Available potassium (AK) of soil samples was determined by the extraction with CH3COONH4 (1 M) (Sinopharm Chemical Reagent Co., Ltd, Shanghai, China), and measured by a flame photometer (Sherwood Scientific Ltd, Cambridge, UK) [23]. There were three replicates for each sample and each indicator.
2.4. Determination of Main Medicinal Components
2.4.1. Determination of Polysaccharide Content
The polysaccharide content was determined by the phenol-sulphuric acid method [24,25]. The method was described in our previous study [26]. The absorbance was measured at 490 nm using UV-visible spectrophotometer (Thermo Fisher Scientific, San Jose, CA, USA) with 1 mL of water as a blank, and the test was performed in parallel three times. The calibration curve was prepared from the glucose reference, and the equation of regression was: Y = 7.3717X − 0.0025, R2 = 0.9998, where Y is the absorbance and X is the concentration. Quantification of polysaccharides was performed on the basis of linear calibration plots of the absorbance versus the corresponding concentration.
2.4.2. Determination of Total Alkaloid Content
The total alkaloid content was determined using the method described as follows [27]. The extraction method is the same as the previous study [26]. 2 mL of the sample solution was precisely transferred to a 15 mL centrifuge tube, diluted to 10 mL with chloroform 5 mL of pH 4.5 buffer and 1 mL of 0.04% bromocresol green solution (Sinopharm Chemical Reagent Co., Ltd, Shanghai, China) were added, after which the mixture was left to stand for 30 min after strongly shaking for 3 min. Subsequently, 1 mL of 0.01 M NaOH (Sinopharm Chemical Reagent Co., Ltd, Shanghai, China) was added to 5 mL of the lower fractions for analysis. The alkaloid was determined by using a UV-visible spectrophotometer (Thermo Fisher Scientific, San Jose, CA, USA) at 620 nm with dendrobine (Solarbio Life Sciences, Beijing, China) as a reference standard. The calibration curve was prepared from the dendrobine (Solarbio Life Sciences, Beijing, China) reference, and the equation of regression was: Y = 9.5429X − 0.038, R2 = 0.9998, where Y is the absorbance and X is the concentration. Quantification of total alkaloids was performed on the basis of linear calibration plots of the absorbance versus the corresponding concentration.
2.4.3. Determination of Total Flavonoid Content
Powdered samples (0.1 g) were digested in 10 mL of 70% (v/v) ethanol (Sinopharm Chemical Reagent Co., Ltd, Shanghai, China) at 60 °C for 3 h and filtered through filter paper to determine flavonoid content. The filtrate was centrifuged at 5000 rpm for 10 min, and the supernatant was collected and adjusted to a final volume of 25 mL by adding 70% (v/v) ethanol (Sinopharm Chemical Reagent Co., Ltd, Shanghai, China). Total flavonoid content was determined by using the aluminum chloride colorimetric method with minor modifications [28]. Briefly, 0.5 mL of extracts, 1.5 mL of methanol, 0.1 mL of aluminum chloride (10%) (Sinopharm Chemical Reagent Co., Ltd, Shanghai, China), 0.1 mL of sodium acetate (1 M) (Sinopharm Chemical Reagent Co., Ltd, Shanghai, China) and 2.8 mL of distilled water (Sigma-Aldrich, St Louis, MO, USA) were mixed for 5 min by vortexing (MoBio Laboratories Inc., CA, USA). The reaction mixture was kept at room temperature for 30 min and the absorbance was measured at 415 nm [29]. The content of flavonoids was calculated using standard graph of quercetin (Sigma-Aldrich, St Louis, MO, USA) and the results were expressed as quercetin equivalent (mg·g−1).
2.5. Statistical Analysis
Differences in three cultivation modes of D. officinale main medicinal components were evaluated. Linear discriminant analysis (LDA), correlation analysis (CA), principal component analysis (PCA) and stepwise multiple linear regression (SMLR) analysis were applied by using the Statistical Product and Service Solutions (SPSS) version 21.0 for Windows (IBM, Chicago, IL, USA). LDA could give the probability distribution of factors belonging to each classification and the probability distribution of factors on each classification. CA was used to investigate whether ecological factors had a correlation with measured medicinal components and laid a foundation for subsequent data analysis. PCA could reduce data matrix dimension and determine the primary ecological factors in this study. Statistical analysis of other data (mean, annual mean, minimum and maximum values, standard deviation,) was done using Excel 2016 (Microsoft, Redmond, WA, USA).
3. Results
3.1. Main Medicinal Components Variation in Different Cultivation Modes
The important medicinal components of D. officinale are polysaccharide, total alkaloid and total flavonoid, and the content of three medicinal components was evaluated at different cultivation modes (Figure 3). This result showed that the highest contents of polysaccharide, total flavonoid and total alkaloid were in the wild cultivation mode and the lowest contents were in the greenhouse cultivation mode. Among them, the average content of polysaccharides in the wild cultivation mode was as high as 650.56 mg·g−1 dry weight and the total flavonoid content was up to 5.07 mg·g−1 dry weight.
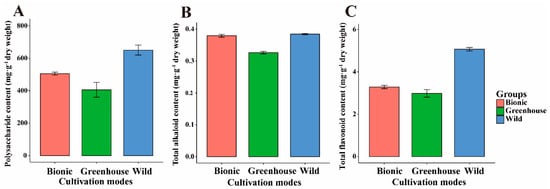
Figure 3.
Main medicinal components variation in different cultivation modes. (A) Polysaccharide content variation in different cultivation modes. (B) Total alkaloid content variation in different cultivation modes. (C) Total flavonoid content variation in different cultivation modes.
3.2. Ecological Factors Variation in Different Cultivation Modes
As for medicinal plants, the ecological differences in their growing locations may cause their active components to vary in concentrations, proportions and even types. Thus, we installed EM-50 microenvironment climate data loggers (Decagon Devices Inc., Pullman, WA, USA) in three different cultivation modes to monitor their microenvironment climate in real time. We have obtained a large amount of data, which is shown in the boxplot of Figure 4. In order to compare the microenvironment climate differences under the three different cultivation modes, we also use line charts to display these differences (Figure 5).
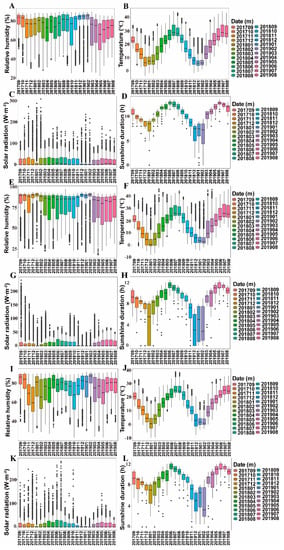
Figure 4.
Climate factors under different cultivation modes. (A–D): Greenhouse cultivation mode; (E–H): Bionic cultivation mode; (I–L): Wild cultivation mode. Each box plot represents 1440 replicates, the inner line shows the median, the notches represent the bootstrapped 95% confidence interval of the median, the ends of the box represent the first and third quartiles and the dots represent outliers.
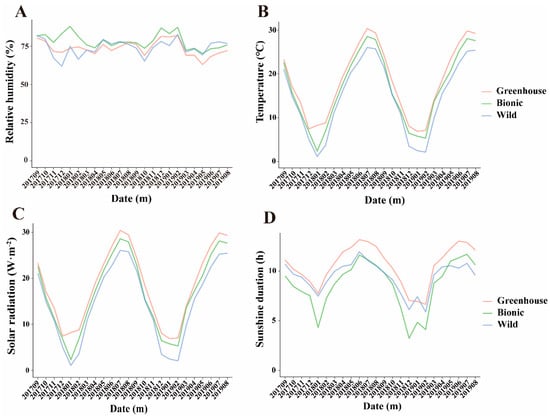
Figure 5.
Monthly average line chart of four major climatic factors. The dates go from September 2017 to August 2019. (A) Relative humidity. (B) Temperature. (C) Solar radiation. (D) Sunshine duration.
Appropriate soil is very important to improve the quality of Chinese medicine. We selected these indicators (pH, total nitrogen, ammonium nitrogen, nitrate nitrogen, total phosphorus, available phosphorus and available potassium) as a measure of soil factors under different cultivation modes. Since the substrates of the greenhouse cultivation and the bionic cultivation are consistent, we also used consistent data for subsequent analysis (Table 1).

Table 1.
Soil factors in different cultivation modes.
3.3. Ecological Factors Influencing the Main Medicinal Components
3.3.1. LDA
Linear discriminant analysis (LDA) is an unsupervised dimensionality reduction and classification technique based on sample category output that can maximize the variance between groups and minimize the variance within the same group. For achieving better classification and identification of different cultivation modes based on the ecological factors affecting the growth and quality of D. officinale, LDA was carried out on the basis of nine climate factors. The stepwise discriminant procedure was carried out to extract the best discriminant variable separating different climate factors affecting the growth and quality of D. officinale, which enters or removes variables by analyzing their effects on the classification of ecological factors based on the Wilks’ lambda criterion.
The discriminant function is used to separate the different groups in the discriminant space. As shown in Figure 6, function 1 explained the variance of 67.3% of the ecological factors influencing the growth of D. officinale, while function 2 explained the variance of 32.7%. Therefore, the total variance of the independent variable ecological factor explained by these two functions is 100%. Different cultivation modes were clumped in different regions of the space. The bionic cultivation mode was entirely separated from the other two cultivation modes. The greenhouse cultivation mode and wild cultivation mode were a little closer but there was no overlap among them. These results distinguished the microclimate of three different cultivation modes.
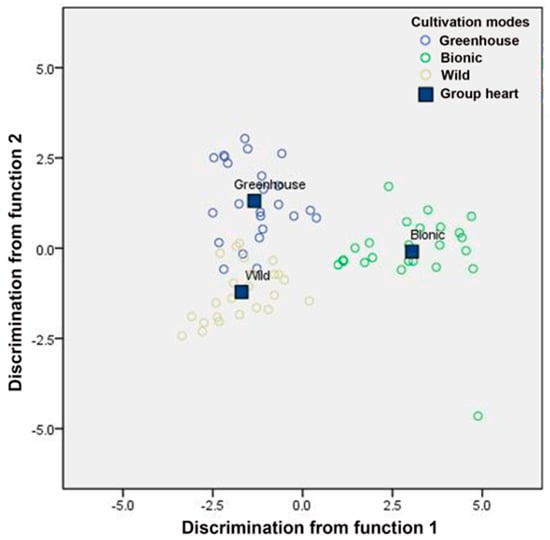
Figure 6.
Linear discriminant analysis plot for different cultivation modes based on the climate factors. Purple circle indicates greenhouse cultivation mode; green circle indicates bionic cultivation mode; yellow circle indicates wild cultivation mode.
3.3.2. CA (Correlation Analysis)
Correlation analysis between ecological factors and main medicinal components is shown in Figure 7. This analysis was performed to understand the relationships a between ecological factors and the main medicinal components. The correlation between ecological factors under different cultivation modes provides important information for artificial cultivation of D. officinale. As can be seen from Figure 7A–C, in the greenhouse cultivation mode, AN (Ammonium nitrogen) has a significant negative correlation with both polysaccharides and total alkaloid, while for total flavonoid, both MaxSR (Maximum monthly average solar radiation) and AK (Available potassium) have significant positive correlations, but NN (Nitrate nitrogen), TP (Total phosphorus) and AP (Total phosphorus) have significant negative correlations. In the bionic cultivation mode (Figure 7D–F), TN (Total nitrogen) and AK were significantly positively correlated with polysaccharide, AN and total flavonoid were also significantly positively correlated. In the wild cultivation mode (Figure 7G–I), pH value was significantly positively correlated with both polysaccharide and total alkaloid, while for total flavonoid, TN has significant positive correlations. When we did not consider any cultivation mode, we found that all soil factors were significantly positively related to polysaccharides and total flavonoids (Figure 7J–L). Except for RH (Relative humidity), SR (Solar radiation) and MaxSR, all other ecological factors are positively related to the three main medicinal components of Dendrobium.
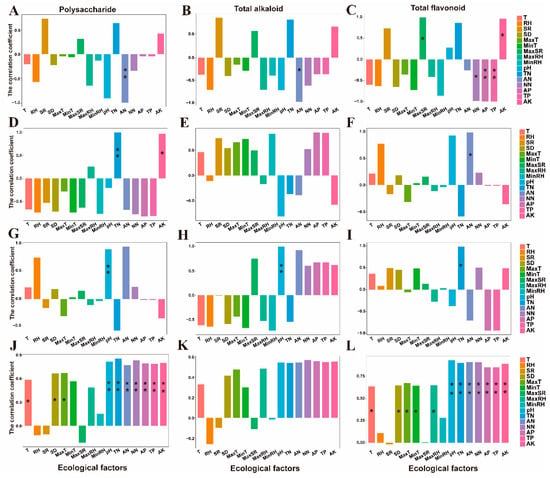
Figure 7.
Correlation analysis between ecological factors and main medicinal components. (A–C): Greenhouse cultivation mode; (D–F): Bionic cultivation mode; (G–I): Wild cultivation mode; (J–L): All the ecological factors and main medicinal components under regardless of cultivation modes. T: Temperature; RH: Relative humidity; SR: Solar radiation; SD: Sunshine duration; MaxT: Maximum monthly average temperature; MinT: Minimum monthly average temperature; MaxSR: Maximum monthly average solar radiation; MaxRH: Maximum monthly average relative humidity; MinRH: Minimum monthly average relative humidity; TN: Total nitrogen; AN: Ammonium nitrogen; NN: Nitrate nitrogen; AP: Available phosphorus; TP: Total phosphorus; AK: Available potassium. “*” indicates p < 0.05, “**” indicates p < 0.01.
3.3.3. PCA of Ecological Factors
The Principal Components Analysis (PCA) model was applied to all ecological factors and three medicinal components to determine the most important variables that explain the relationships between ecological factors and the main medicinal components of D. officinale. In addition, PCA was carried out all ecological factors regardless of the cultivation mode of D. officinale.
The cumulative contribution rate of the first four eigenvalues sums to 92.287%, and the individual eigenvalues were greater than 1 (Table 2). Therefore, these components could be analyzed to get the load level of each ecological factor and the component matrix (Table 3). The important ecological factors that influenced the first principal component (PC1) were pH (0.995), TN (0.989), AN (0.994), NN (0.996), AP (0.993), TP (0.993) and AK (0.992). For the second principal component (PC2), Temperature (0.978) and MinT (0.946) were the important ecological factors. The third principal component (PC3) had a proportion in SR (0.85), MaxSR (0.885) and MaxRH (−0.733). Meanwhile, the fourth principal component (PC4) mainly described the information about RH (0.807) and MinRH (0.754). Since most of the ecological factors are in these four principal components, we carried out stepwise multiple linear regression analysis using all ecological factors.

Table 2.
Eigenvalues and cumulative contribution rates of principal components.

Table 3.
Component analysis matrix of the principal components.
3.3.4. Stepwise Multiple Linear Regression (SMLR) Analysis
SMLR has been applied to identify the best prediction model in many studies. The standardized regression coefficient reveals the significance of an individual descriptor in the regression model. A high absolute value of this coefficient corresponds to a high weight of variables in the model [30,31]. We used SMLR analysis to establish three prediction models of three main medicinal components (Table 4).

Table 4.
Stepwise multiple linear regression (SMLR) analysis of ecological factors influencing the main medicinal components in D. offficinale.
The model I showed a positive linear correlation with the TN, MaxRH and MaxT, but a negative linear correlation with MinRH. For polysaccharide, the main ecological factors affecting it were as follows: TN > MinRH > MaxRH > MaxT in sequence. The Model II showed a positive linear correlation with the MaxRH, TP and MaxT, but a negative linear correlation with MinRH and SD. For total alkaloid, the main ecological factors affecting it were as follows: TP > MaxRH > MaxT > SD > Min RH in sequence. The Model III showed a positive linear correlation with the pH, but a negative linear correlation with MaxRH and AP. For total flavonoid, the main ecological factors affecting it were as follows: pH > AP > MaxRH in sequence. These findings demonstrated that extreme climatic conditions, such as excessive high temperature and excessive sunshine, could cause the main medicinal components to increase or decrease.
4. Discussion
Although the production of active ingredients in medicinal plants is guided by genetic processes, it is also strongly influenced by environmental factors. Most plants regulate the sorts and amounts of secondary metabolites according to the growing environment [32]. Therefore, environmental factors cause changes in the growth of medicinal plants, as well as the quantity and quality of their active ingredients, such as alkaloids, glycosides and steroids [33]. In our study, three different cultivation modes of D. officinale were selected, and we collected the ecological data and determined the main medicinal components, so as to obtain the most important ecological factors affecting the three main medicinal components in D. officinale. Due to the lack of replication, other factors not measured in the study (soils, disturbance, presence of insects or disease etc.) may have impacted the variables that we measured, in addition to the measured weather variables.
We determined polysaccharide, total alkaloid and total flavonoid of D. officinale under different cultivation modes. We found that in the three cultivation modes, the contents of the three main medicinal components are arranged according to amount as follows: Wild > Bionic > Greenhouse. The most obvious difference is the polysaccharide and total flavonoid in the wild cultivation mode, which are significantly higher than the other two. Other studies also show that these two contents are higher, indicating that the quality of wild D. officinale grown under natural conditions is much better than artificial cultivation [34,35]. However, due to our large market demand and scarce wild resources, we cannot obtain these wild D. officinale in large quantities. Hence, we can try to control their growing environment in greenhouse cultivation, in order to get the maximum medicinal value.
The metabolism and accumulation of active ingredients is the reflection of integrated influences of multiple ecological factors on the medicinal plant during their developmental and growth periods. In particular, certain metabolites are only synthesized under specific environments, or their contents significantly increase under specific environments [36]. Both temperature and sunshine illumination could affect the plant growth and the concentrations of active ingredients in medicinal plants [37,38,39]. Previous study reported the influence of environmental factors on the contents of secondary metabolites in the leaves of Eucommia ulmoides Oliv. and found that annual sunshine duration was significantly and positively correlated to the contents of geniposidic acid [40]. In Salvia miltiorrhiza, temperature is the primary climatic factor affecting the accumulation of tanshinones [34,41]. Sunshine duration was paramount ecological factor and had positive correlation with notopterol in Notopterygii incisum Ting ex H. T. Chang, while temperature was negatively correlated with isoimperatorin in N. incisum [42]. From our correlation analysis, we can see that temperature, relative humidity and sunshine duration have significant correlations with the medicinal components of D. officinale in different varying degrees. Furthermore, we performed a regression analysis and found that the MaxRH had an effect on all three medicinal components, followed by temperature and SD. As shown in our climate data records of the three cultivation modes, we can see that D. officinale is suitable for growing in a place with relatively high humidity. The wild D. officinale has high medicinal components, but its temperature has always been the lowest trend among the three cultivation modes (Figure 5). Moreover, D. officinale is suitable for growing in shade, and its growth temperature cannot be too high, which is also detrimental to the accumulation of medicinal ingredients. Many studies also point out that low temperature is beneficial to the accumulation of active ingredients, which are found in Panax ginseng and Astragalus membranaceus [43,44].
When it comes to soil condition, physico-chemical properties together with inorganic elements have an important effect on the growth and secondary metabolites in medicinal plants [45]. For instance, Liu et al. [46] found that soil pH is an important limiting factor that has the strongest direct effect on the concentrations of active ingredients. Shah et al. [47] highlighted that soil pH has positive effects on paeonol and paeoniflorin concentrations. Through regression analysis, we found that the main soil factor affecting flavonoids is pH, which is consistent with previous research conclusions. The soil pH had positive and direct effects on the concentrations of total flavonoid. Since the content of total flavonoids in wild cultivation is high, and since the pH value of soil factors in the wild cultivation that we detected is higher than that of the other two cultivation modes, we can pay attention to the regulation of pH value in the future cultivation. The type of soil, pH value, fertility and trace elements will all have a certain effect on the secondary metabolites of the plants [48]. Appropriate soil type is very important to improve the quality of traditional Chinese medicine.
Proper nutrient supply is a sufficient condition for the normal functioning of the plant’s secondary metabolic pathways. It is generally believed that lack of nitrogen-nutrient will lead to the accumulation of nitrogen-free secondary metabolites such as phenols. On the contrary, it will promote the synthesis of nitrogen-containing secondary metabolites such as alkaloids and cyanogenic glycosides, reflecting the nitrogen-nutrient balance requirements of plants. Previous studies found that nitrogen and phosphorus can increase the content of Fritillaria alkaloids, while potassium fertilizer reduces its content [49]. Studies have also found that soil nitrogen can affect the content of tripterine in Tripterygium wilfordii [50]. K coupled with Fe, Mn, B, Ba and Zn had a great influence on polysaccharides, total flavonoids and total saponins in Ophiopogon japonicus root [51]. In our study, after analysis, soil TN, AP and TP all affected the medicinal ingredients of D. officinale to varying degrees. These results are consistent with previous studies. Importantly, comprehensive assessment revealed medicinal components in D. officinale under different cultivation modes and found out the most important ecological factors that affect the medicinal components, which provides a good reference for the future artificial cultivation of D. officinale.
5. Conclusions
In our study, we determined three main components of D. officinale under three different cultivation modes in China. And we obtained the ecological factor data of these three different cultivation modes for analysis. Among the three cultivation modes, the best medicinal value of D. officinale is the wild cultivation mode. After a series of statistical analysis, the most important ecological factors affecting polysaccharide are soil total nitrogen, maximum relative humidity, maximum temperature and minimum relative humidity; the most important ecological factors affecting total alkaloid are maximum relative humidity, maximum temperature, minimum relative humidity, soil total phosphorus and sunshine duration; the most important ecological factors affecting total flavonoid are maximum relative humidity, soil pH and soil available phosphorus. Hence, this study provides a good reference for artificial cultivation D. officinale and investigating medicinal value and its ecological influence of other medicinal plants.
Author Contributions
Conception and design of the research: Y.Y. and X.T.; acquisition of data: Z.J.; analysis and interpretation of data: C.L.; statistical analysis: X.T.; drafting the manuscript: Y.Y. and J.Z.; revision of manuscript for important intellectual content: J.M. All authors read and approved the final manuscript.
Funding
This project was funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), National Foundation of Forestry Science and Technology Popularization (Grant No. [2015]17) and the Major Fund for Natural Science of Jiangsu Higher Education Institutions (Grant No. 15KJA220004). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Acknowledgments
We would thank Donald L. DeAngelis, from the Wetland and Aquatic Research Center, U.S. Geological Survey, for his valuable comments and suggestions that have greatly improved the quality of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hou, B.; Tian, M.; Luo, J.; Ji, Y.; Xue, Q. Genetic diversity assessment and ex situ conservation strategy of the endangered Dendrobium officinale (orchidaceae) using new trinucleotide microsatellite markers. Plant Syst. Evolut. 2012, 298, 1483–1491. [Google Scholar] [CrossRef]
- Hou, B.; Luo, J.; Zhang, Y.; Niu, Z.; Xue, Q.; Ding, X. Iteration expansion and regional evolution: Phylogeography of Dendrobium officinale and four related taxa in southern china. Sci. Rep. 2017, 7, 43525. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, Z.; Ding, X.; Zhou, K.; Xu, L. Differentiation of dendrobium species used as ”huangcao shihu” by rdna its sequence analysis. Planta Med. 2006, 72, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ding, X.; Chu, B.; Zhou, Q.; Ding, G.; Gu, S. Genetic diversity analysis and conservation of the endangered chinese endemic herb Dendrobium officinale kimura et migo (orchidaceae) based on aflp. Genetica 2008, 133, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.Y.; Feng, Z.Y.; Zhang, X.X.; Xu, W.; Hou, B.W.; Ding, X.Y. Genetic diversity and population structure of an endangered orchid (Dendrobium loddigesii rolfe) from china revealed by srap markers. Sci. Hortic. 2011, 129, 877–881. [Google Scholar] [CrossRef]
- Chang, C.-C.; Ku, A.F.; Tseng, Y.-Y.; Yang, W.-B.; Fang, J.-M.; Wong, C.-H. 6,8-di-c-glycosyl flavonoids from Dendrobium huoshanense. J. Nat. Prod. 2010, 73, 229–232. [Google Scholar] [CrossRef]
- Xu, J.; Han, Q.-B.; Li, S.-L.; Chen, X.-J.; Wang, X.-N. Chemistry, bioactivity and quality control of Dendrobium, a commonly used tonic herb in traditional chinese medicine. Phytochem. Rev. 2013, 12, 341–367. [Google Scholar] [CrossRef]
- National Pharmacopoeia Committee. Pharmacopoeia of the People’s Republic of China. 1 (2010); China Medical Science Press: Beijing, China, 2010. [Google Scholar]
- Cao, H.; Ji, Y.; Li, S.; Lu, L.; Tian, M.; Yang, W.; Li, H. Extensive metabolic profiles of leaves and stems from the medicinal plant Dendrobium officinale kimura et migo. Metabolites 2019, 9, 215. [Google Scholar] [CrossRef]
- Jin-Ping, S.I.; Qiao-Xian, Y.U.; Song, X.S.; Shao, W.J. Artificial cultivation modes for Dendrobium officinale. China J. Chin. Mater. Med. 2013, 38, 481–484. [Google Scholar] [CrossRef]
- Wang, H.; You, D.; Cui, X.; Guo, Y. Effect of plastic greenhouse cultivation on inoculation and growth of Cistanche tubulosa. Chin. Agric. Sci. Bull. 2012, 28, 279–283. [Google Scholar]
- Shao, Q.S.; Ye, S.Y.; Zhou, A.C.; Wang, H.Z.; Xu, J.W. Current researches and prospects of seedling propagation and cultivation modes of jinxianlian. China J. Chin. Mater. Med. 2016, 41, 160–166. [Google Scholar]
- Kebaili, Z.; Hameurlaine, S.; Fellah, O.; Djermane, M.; Gherraf, N.; Zellagui, A.; Abidi, A.; Derouiche, K. Assessment of alkaloid content and antibacterial activity of hyoscyamus albus and hyoscyamus muticus collected in two different climatic regions in Algeria. J. Biochem. Technol. 2019, 10, 1–6. [Google Scholar]
- Hanif, A.; Juahir, H.; Lananan, F.; Kamarudin, M.; Adiana, G.; Azemin, A.; Yusra, A.I. Spatial variation of melaleuca cajuputi powell essential oils. J. Fundam. Appl. Sci. 2018, 10, 139–155. [Google Scholar]
- Fellah, O.; Hameurlaine, S.; Gherraf, N.; Zellagui, A.; Ali, T.; Abidi, A.; Altun, M.; Demirtas, I.; SahinYaglioglu, A. Anti-proliferative activity of ethyl acetate extracts of tamarix gallica l. Grown at different climatic conditions in Algeria. Acta Sci. Nat. 2018, 5, 23–31. [Google Scholar] [CrossRef]
- Pu, Y.; Wang, D.; Yan, Y.; Tian, H.; Peng, B.; Qin, X.; Ma, C.; Du, C.; Hu, B.; Zhang, F. Analysis of influencing factors of secondary metabolites contents in cultivated Polygala tenuifolia. China J. Chin. Mater. Med. 2017, 42, 3167–3177. [Google Scholar]
- Guo, L.P.; Wang, S.; Zhang, J.; Yang, G.; Zhao, M.X. Effects of ecological factors on secondary metabolites and inorganic elements ofscutellaria baicalensisand analysis of geoherblism. Sci. China Life Sci. 2013, 56, 1047–1056. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Thomas, R.; Sheard, R.; Moyer, J. Comparison of conventional and automated procedures for nitrogen, phosphorus, and potassium analysis of plant material using a single digestion 1. Agron. J. 1967, 59, 240–243. [Google Scholar] [CrossRef]
- Lu, R. Analysis methods of soil agricultural chemistry. China Agric. Sci. Technol. 2000, 107, 147–150. [Google Scholar]
- Huang, Y.; Ye, Y.; Yang, S. Feasibility of no3-n determination by dual wavelength spectrophotometric method. Chin. Agric. Sci. Bull. 2009, 25, 43–45. [Google Scholar]
- Yan, J.; Li, K.; Peng, X.; Huang, Z.; Liu, S.; Zhang, Q. The mechanism for exclusion of pinus massoniana during the succession in subtropical forest ecosystems: Light competition or stoichiometric homoeostasis? Sci. Rep. 2015, 5, 10994. [Google Scholar] [CrossRef] [PubMed]
- Abliz, A.; Halik, Ü.; Welp, M.; Zhang, L.-X. Effects of shelterbelt afforestation on soil properties in kökyar, nw china. Int. J. Appl. Environ. Sci. 2015, 10, 2017–2036. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Yang, F.; Wei, N.-N.; Gao, R.; Piao, X.-C.; Lian, M.-L. Effect of several medium factors on polysaccharide and alkaloid accumulation in protocorm-like bodies ofdendrobium candidumduring bioreactor culture. Acta Physiol. Plant. 2015, 37, 94. [Google Scholar] [CrossRef]
- Yuan, Y.; Yu, M.; Zhang, B.; Liu, X.; Zhang, J. Comparative nutritional characteristics of the three major chinese dendrobium species with different growth years. PLoS ONE 2019, 14, e0222666. [Google Scholar] [CrossRef]
- Bush, L.P.; Wilkinson, H.H.; Schardl, C.L. Bioprotective alkaloids of grass-fungal endophyte symbioses. Plant Physiol. 1997, 114, 1–7. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Hossain, M.A.; Rahman, S.M. Total phenolics, flavonoids and antioxidant activity of tropical fruit pineapple. Food Res. Int. 2011, 44, 672–676. [Google Scholar] [CrossRef]
- Latt, Z.Z.; Wittenberg, H. Improving flood forecasting in a developing country: A comparative study of stepwise multiple linear regression and artificial neural network. Water Resour. Manag. 2014, 28, 2109–2128. [Google Scholar] [CrossRef]
- Rahimmalek, M.; Heidari, E.F.; Ehtemam, M.H.; Mohammadi, S. Essential oil variation in iranian ajowan (trachyspermum ammi (l.) sprague) populations collected from different geographical regions in relation to climatic factors. Ind. Crops Prod. 2017, 95, 591–598. [Google Scholar] [CrossRef]
- Valls, J.; Richard, T.; Trotin, F.; Monti, J.-P.; Mérillon, J.-M.; Vitrac, X. Carbon-14 biolabeling of flavanols and chlorogenic acids in crataegus monogyna cell suspension cultures. Food Chem. 2007, 105, 879–882. [Google Scholar] [CrossRef]
- Gairola, S.; Shariff, N.M.; Bhatt, A. Influence of climate change on production of secondary chemicals in high altitude medicinal plants: Issues needs immediate attention. J. Med. Plants Res. 2010, 4, 1825–1829. [Google Scholar]
- Yuan, Y.; Zhang, J.; Liu, X.; Meng, M.; Wang, J.; Lin, J. Tissue-specific transcriptome for Dendrobium officinale reveals genes involved in flavonoid biosynthesis. Genomics 2019, 19, 30700–30701. [Google Scholar] [CrossRef]
- Shen, C.; Guo, H.; Chen, H.; Shi, Y.; Meng, Y.; Lu, J.; Feng, S.; Wang, H. Identification and analysis of genes associated with the synthesis of bioactive constituents in Dendrobium officinale using rna-seq. Sci. Rep. 2017, 7, 187. [Google Scholar] [CrossRef] [PubMed]
- Peñuelas, J.; Llusià, J. Effects of carbon dioxide, water supply, and seasonality on terpene content and emission by rosmarinus officinalis. J. Chem. Ecol. 1997, 23, 979–993. [Google Scholar] [CrossRef]
- Zhong, G.; Zhang, L.; Zhang, L.; Yang, R.; Ding, C. A study on photosynthetic characteristics of different Salvia miltiorrhiza varieties. Acta Pratac. Sin. 2011, 20, 116–122. [Google Scholar]
- Peng, X.; Zhang, S. Competition for light and crop productivity in an agro-forestry system in the hilly region, Shangluo, China. Acta Ecol. Sin. 2012, 32, 2692–2698. [Google Scholar] [CrossRef]
- Liu, J.; Shu, Z.; Liang, Z.; Shi, X.; Zhang, Y. Uv-b radiation effects on phenolic changes and antioxidant activity in Salvia miltiorrhiza bunge leaf. J. Food Agric. Environ. 2013, 11, 2788–2791. [Google Scholar]
- Dong, J.E.; Ma, X.H.; Wei, Q.; Peng, S.B.; Zhang, S.C. Effects of growing location on the contents of secondary metabolites in the leaves of four selected superior clones of eucommia ulmoides. Ind. Crops Prod. 2011, 34, 1607–1614. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, D.; Liang, Z.; Liu, J.; Yan, K.; Zhu, Y.; Yang, S. Climatic factors control the geospatial distribution of active ingredients in Salvia miltiorrhiza bunge in china. Sci. Rep. 2019, 9, 904. [Google Scholar] [CrossRef]
- Huang, L.; Wentao, L.I.; Wang, Z.; Juan, F.U.; Chen, S. Correlative study between chemical constituents and ecological factors of notopterygii rhizoma et radix of endangered plateau plant. Acta Ecol. Sin. 2013, 33, 7667–7678. [Google Scholar]
- Pan, H.; Fang, C.; Zhou, T.; Wang, Q.; Chen, J. Accumulation of calycosin and its 7-o-β-d-glucoside and related gene expression in seedlings of astragalus membranaceus bge. Var. Mongholicus (bge.) hsiao induced by low temperature stress. Plant Cell Rep. 2007, 26, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Nam, M.H.; Heo, E.J.; Kim, J.Y.; Kim, S.I.; Kwon, K.H.; Seo, J.B.; Kwon, O.; Yoo, J.S.; Park, Y.M. Proteome analysis of the responses of panax ginseng ca meyer leaves to high light: Use of electrospray ionization quadrupole-time of flight mass spectrometry and expressed sequence tag data. Proteomics 2003, 3, 2351–2367. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.p.; Sui, X.x.; Sun, Q. Biological functions of secondary metabolism of medicinal plants and influences of ecological environment. Nat. Prod. Res. Dev. 2006, 18, 1027. [Google Scholar]
- Liu, W.; Liu, J.; Yin, D.; Zhao, X. Influence of ecological factors on the production of active substances in the anti-cancer plant sinopodophyllum hexandrum (royle) t.S. Ying. PLoS ONE 2015, 10, e0122981. [Google Scholar] [CrossRef]
- Shah, F.A.; Ren, Y.; Yuan, Y.J.; Fu, S.; Wang, Y.; Chen, H. Effect of plant age and geographical location on active paeonol and paeoniflorin accumulation in the roots of paeonia ostii. Pak. J. Bot. 2018, 50, 1785–1790. [Google Scholar]
- Antoniadis, V.; Levizou, E.; Shaheen, S.M.; Ok, Y.S.; Sebastian, A.; Baum, C.; Prasad, M.N.; Wenzel, W.W.; Rinklebe, J. Trace elements in the soil-plant interface: Phytoavailability, translocation, and phytoremediation–A review. Earth Sci. Rev. 2017, 171, 621–645. [Google Scholar] [CrossRef]
- Yan, L.I.; Zhou, X.; Lou, Z.; Xiao, X. Review of plant secondary metabolites and the factors that influence its accumulation. Jiangxi For. Sci. Technol. 2012, 54–60. [Google Scholar]
- Du, W.; Huang, H.; Garden, W.B. Correlation analysis of secondary metabolites and environmental factors in tripterygium wilfordii. Chin. Bull. Bot. 2008, 25, 707–713. [Google Scholar]
- Zhang, L.; Ye, Z.; Guo, Q. Effects of soil factor on active components of radix ophiopogonis. China J. Chin. Mater. Med. 2010, 35, 1372–1377. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).