Irrigation of Young Cork Oaks under Field Conditions—Testing the Best Water Volume
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
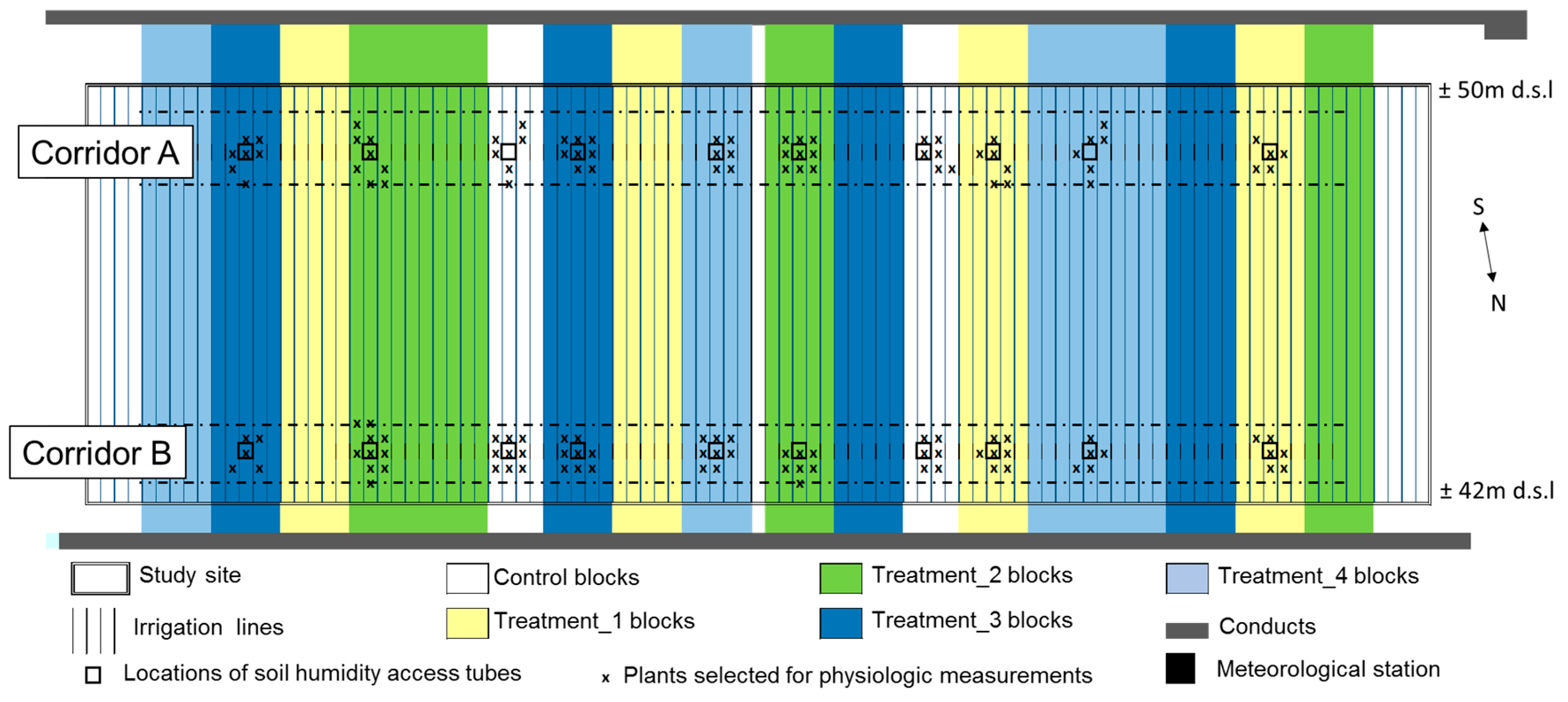
2.2. Plot Installation
2.3. Experimental Plot Design
2.4. Irrigation Treatments
2.5. Data Collection
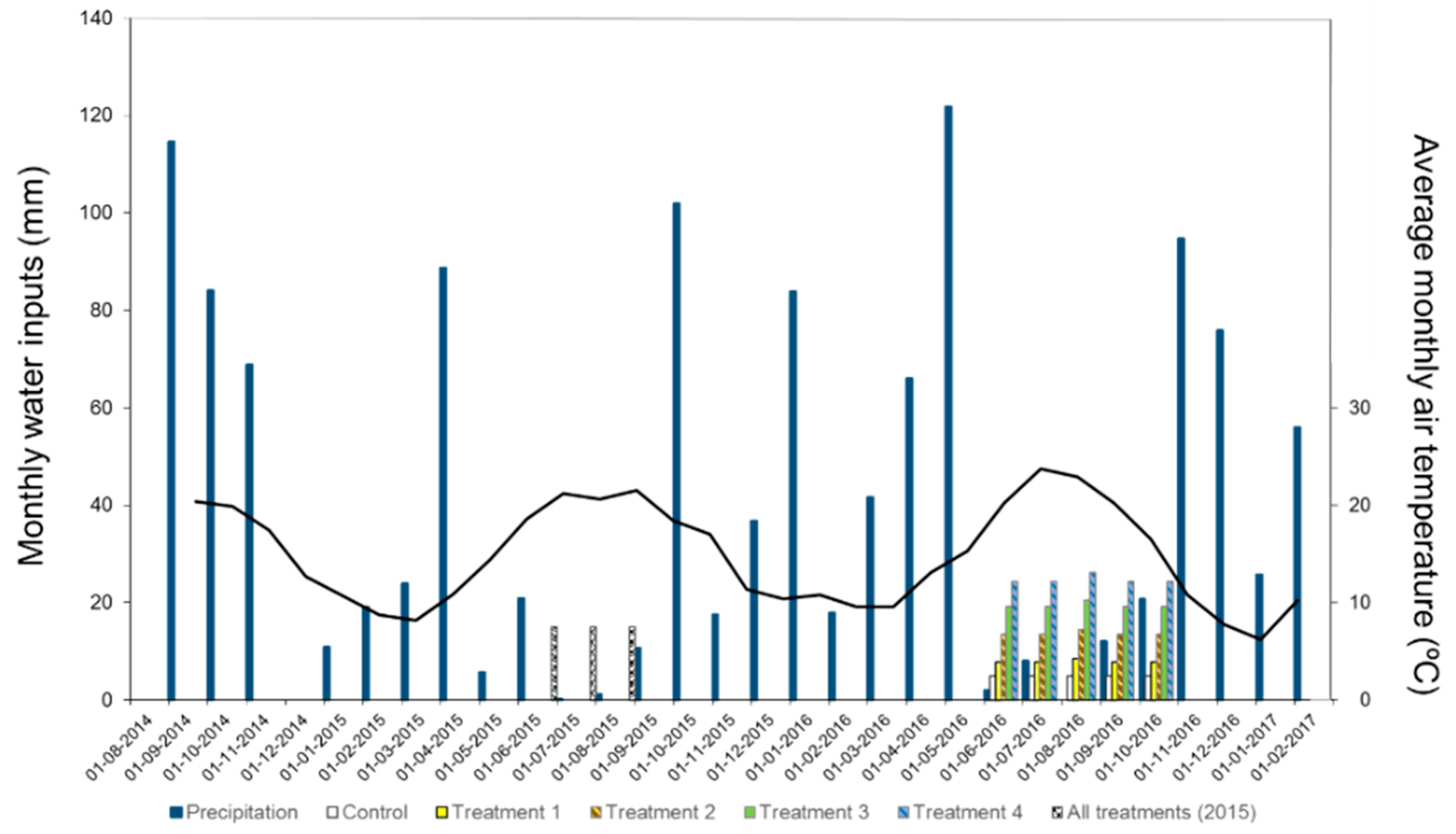
2.5.1. Meteorological Data
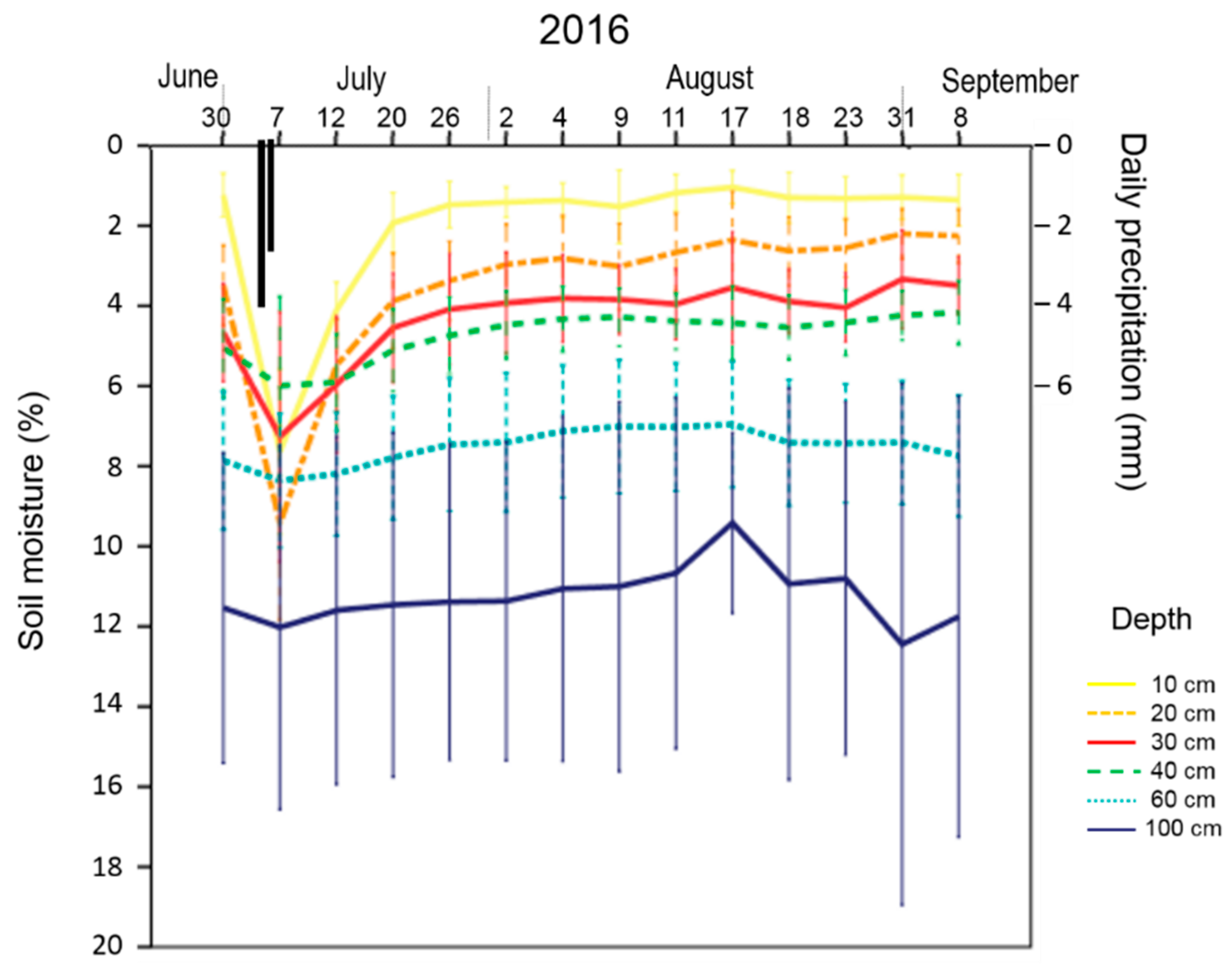
2.5.2. Soil Moisture
2.5.3. Dendrometric Measurements
2.5.4. Stomatal Conductance
2.5.5. Statistical Analysis
3. Results
3.1. Meteorology
3.2. Soil Moisture
3.3. Functional Parameters: Stomatal Conductance
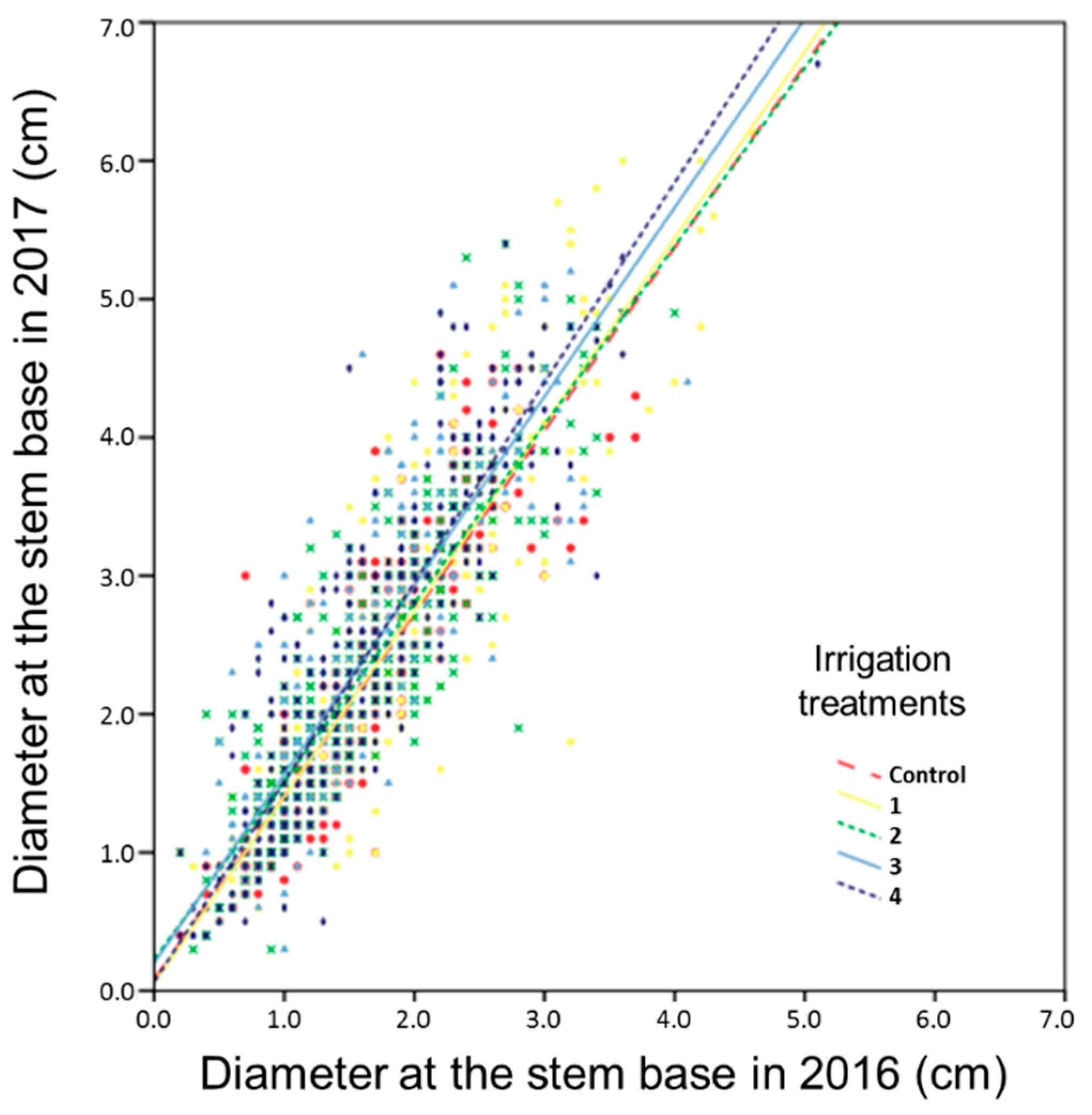
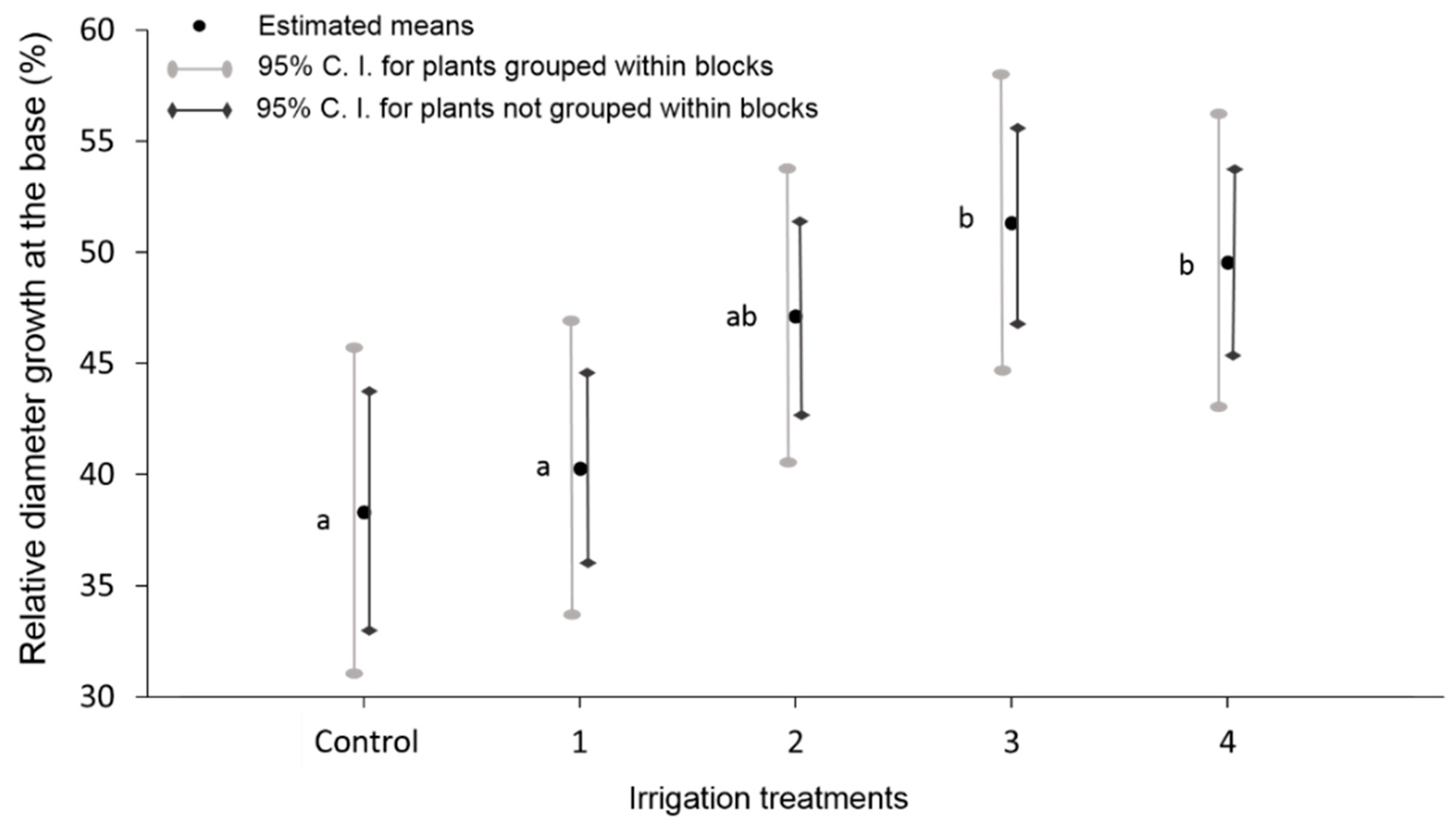
3.4. Structural Parameters: Dendrometry
4. Discussion
4.1. Water Volume for the Best Structural Functional Response
4.2. Soil Water Profile as an Indicator of Irrigation Requirements
4.3. Stomatal Conductance as an Indicator of Irrigation Requirements
4.4. Plants Structural-Functional Variability
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Parameters | F Value | df1 | df2 | p Value |
|---|---|---|---|---|
| Corrected model | 5.14 | 6 | 214 | <0.001 |
| Treatments | 2.33 | 4 | 214 | 0.057 |
| Elevation (m a.s.l.) | 9.52 | 1 | 214 | 0.002 |
| Time (days) | 12.30 | 1 | 214 | <0.001 |
| Parameter | Estimate | S.E. | Wald Z | 95% Confidence Interval | |||
|---|---|---|---|---|---|---|---|
| p Value | Lower | Upper | |||||
| Repeated Measures | AR diagonal | 4043.20 | 243.78 | 16.58 | <0.001 | 3592.55 | 4550.38 |
| AR rho | 0.34 | 0.05 | 7.47 | <0.001 | 0.25 | 0.43 | |
| Intercept (location) | Variance | 346.26 | 205.55 | 1.68 | 0.092 | 108.17 | 1108.42 |
| Parameter | Estimate | S.E. | t Value | 95% Confidence Interval | |||
|---|---|---|---|---|---|---|---|
| p Value | Lower | Upper | |||||
| Intercept | 183.76 | 17.25 | 10.65 | <0.001 | 149.79 | 217.72 | |
| Time (day of measurement) | −1.96 | 0.18 | −10.62 | <0.001 | −2.32 | −1.60 | |
| Deep water storage (mm) | 1.12 | 0.28 | 4.00 | <0.001 | 0.57 | 1.67 | |
| Initial diameter_base (cm) | 1.40 | 0.40 | −10.62 | 0.001 | 0.61 | 2.20 | |
| Irrigation water (mm week−1) | ≈1 * | −33.26 | 8.54 | −3.90 | <0.001 | −50.08 | −16.44 |
| 1.88 | −24.17 | 8.90 | −2.72 | 0.007 | −41.71 | −6.64 | |
| 3.12 | −8.33 | 8.60 | −0.97 | 0.333 | −25.28 | 8.61 | |
| 4.38 | −4.44 | 9.33 | −0.48 | 0.635 | −22.83 | 13.95 | |
| 5.62 | 0 a | ||||||
| Water (Treatment) Repeated Contrast | Dependent Variable: Rg.Db2016 (%) | ||
|---|---|---|---|
| control vs. Level 1 | Contrast Estimate | −2.36 | |
| Std. Error | 3.51 | ||
| p value. | 0.501 | ||
| 95% Confidence Interval for Difference | Lower Bound | −9.25 | |
| Upper Bound | 4.52 | ||
| Level 1 vs. Level 2 | Contrast Estimate | −6.29 | |
| Std. Error | 3.11 | ||
| p value. | 0.044 | ||
| 95% Confidence Interval for Difference | Lower Bound | −12.40 | |
| Upper Bound | −0.18 | ||
| Level 2 vs. Level 3 | Contrast Estimate | −4.76 | |
| Std. Error | 3.20 | ||
| p value. | 0.138 | ||
| 95% Confidence Interval for Difference | Lower Bound | −11.04 | |
| Upper Bound | 1.52 | ||
| Level 3 vs. Level 4 | Contrast Estimate | 1.66 | |
| Std. Error | 3.14 | ||
| p value. | 0.598 | ||
| 95% Confidence Interval for Difference | Lower Bound | −4.50 | |
| Upper Bound | 7.81 | ||
| Parameter | Estimate | S.E. | t Value | 95% Confidence Interval | |||
|---|---|---|---|---|---|---|---|
| p Value. | Lower | Upper | |||||
| Intercept | 77.85 | 8.01 | 9.72 | <0.001 | 62.14 | 93.56 | |
| Initial diameter_base (cm) | −0.69 | 0.15 | −4.77 | <0.001 | −0.98 | −0.41 | |
| Distance to the stream (m) | −0.08 | 0.03 | −2.47 | 0.014 | −0.14 | −0.02 | |
| Irrigation water (mm week−1) | ≈1 * | −11.75 | 3.50 | −3.35 | <0.001 | −18.63 | −4.88 |
| 1.88 | −9.39 | 3.06 | −3.07 | 0.002 | −15.38 | −3.39 | |
| 3.12 | −3.10 | 3.11 | −1.00 | 0.319 | −9.20 | 2.99 | |
| 4.38 | 1.66 | 3.14 | 0.53 | 0.598 | −4.50 | 7.81 | |
| 5.62 | 0 a | ||||||
| Parameter | Estimate | S.E | Wald Z | p Value | 95% Confidence Interval | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Residual | 1683.86 | 59.69 | 28.21 | <0.001 | 1570.84 | 1805.02 |
| Variance Intercept (subject = block) | 17.24 | 15.00 | 1.15 | 0.250 | 3.13 | 94.84 |
References
- Serrasolses, I.; Pérez-Devesa, M.; Vilagrosa, A.; Pausas, J.G.; Sauras, T.; Cortina, J.; Vallejo, R. Soil properties constraining cork oak distribution. In Cork Oak Woodlands on the Edge: Ecology, Adaptive Management, and Restoration; Aronson, J., Pereira, J.S., Pausas, J.G., Eds.; Island Press: Washington, WA, USA, 2012; pp. 89–102. [Google Scholar]
- APCOR. Associação Portuguesa de Cortiça. Cork Yearbook 18/19. Available online: http://www.apcor.pt/wp-content/uploads/2018/12/Anuario_APCOR_2018.pdf (accessed on 3 November 2018).
- Macara, A.M. Estimativa em 1975 dos prejuízos causados pelas principais doenças do sobreiro num montado da região ribatejana. Inst. Prod. Florestais Cortica 1975, 205–212. [Google Scholar]
- Cabral, M.T.; Ferreira, M.C.; Moreira, T.; Carvalho, E.C.; Diniz, A.C. Diagnostico das causas da anormal mortalidade dos sobreiros a sul do Tejo. Sci. Gerund. 1992, 18, 205–214. [Google Scholar]
- Brasier, C.M.; Robredo, F.; Ferraz, J.F.P. Evidence for Phytophthora cinnamomi involvement in Iberian oak decline. Plant Pathol. 1993, 42, 140–145. [Google Scholar] [CrossRef]
- Moreira, A.C.; Martins, J.M.S. Influence of site factors on the impact of Phytophthora cinnamomi in cork oak stands in Portugal. For. Pathol. 2005, 35, 145–162. [Google Scholar] [CrossRef]
- Sousa, E.; Santos, M.N.; Varela, M.C.; Henriques, J. Perda de Vigor dos Montados de Sobro e Azinho: Analise da Situacao e Perspectivas (Documento Sıntese); DGRF, INRB: Lisboa, Portugal, 2007. [Google Scholar]
- Camilo-Alves, C.; Clara, M.I.E.; Almeida Ribeiro, N.M.C. Decline of Mediterranean oak trees and its association with Phytophthora cinnamomi: A review. Eur. J. For. Res. 2013, 132, 411–432. [Google Scholar] [CrossRef]
- Camilo-Alves, C.S.P.; Vaz, M.M.; Clara, M.I.E.; Almeida Ribeiro, N.M.C. Chronic cork oak decline and water status: New insights. New For. 2017, 48, 753–772. [Google Scholar] [CrossRef]
- Lloret, F.; Siscart, D. Los efectos demográficos de la sequía en población de encina. Cuad. Soc. Española Cienc. For. 1995, 2, 77–81. [Google Scholar]
- Peñuelas, J.; Lloret, F.; Montoya, R. Severe drought effects on Mediterranean woody flora in Spain. For. Sci. 2001, 47, 214–218. [Google Scholar]
- Vessella, F.; Parlante, A.; Schirone, A.; Sandoletti, G.; Bellarosa, R.; Piovesan, G.; Schirone, B. Irrigation regime as a key factor to improve growth performance of Quercus suber L. Scand. J. For. Res. 2010, 25, 68–74. [Google Scholar] [CrossRef]
- Otieno, D.O.; Schmidt, M.W.T.; Vale-do-Lobo, R.; Pereira, J.S.; Tenhunen, J.D. Regulation of transpirational water loss in Quercus suber trees in a Mediterranean-type ecosystem. Tree Physiol. 2007, 27, 1179–1187. [Google Scholar] [CrossRef]
- Grant, O.M.; Tronina, Ł.; Ramalho, J.C.; Kurz Besson, C.; Lobo-do-Vale, R.; Santos Pereira, J.; Chaves, M.M. The impact of drought on leaf physiology of Quercus suber L. trees: Comparison of an extreme drought event with chronic rainfall reduction. J. Exp. Bot. 2010, 61, 4361–4371. [Google Scholar] [CrossRef] [PubMed]
- Vaz, M.; Pereira, J.S.; Gazarini, L.C.; David, T.S.; David, J.S.; Rodrigues, A.; Chaves, M.M. Drought-induced photosynthetic inhibition and autumn recovery in two Mediterranean oak species (Quercus ilex and Quercus suber). Tree Physiol. 2010, 30, 946–956. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C.A.; David, J.S.; Cochard, H.; Caldeira, M.C.; Henriques, M.O.; Quilhó, T.; David, T.S. Drought-induced embolism in current-year shoots of two Mediterranean evergreen oaks. For. Ecol. Manag. 2012, 285, 1–10. [Google Scholar] [CrossRef]
- ICNF. 5° Inventário Florestal Nacional; Instituto da Conservação da Natureza e das Florestas: Lisboa, Portugal, 2010. Available online: http://www2.icnf.pt/portal/florestas/ifn/ifn5/rel-fin (accessed on 9 October 2018).
- Dinis, C.; Camilo-Alves, C.; Vaz, M.; Almeida Ribeiro, N. 2018. Ripping Plantation Lines Improves Deep Root Development of Container-Grown Cork-Oak Seedlings; World Congress SilvoPastoral Systems: Evora, Portugal, 2016. [Google Scholar]
- Tetens, O. Uber einige meteorologische Begriffe. Z. Geophys. 1930, 6, 297–309. [Google Scholar]
- Campbell, G.S.; Norman, J.M. An Introduction to Environmental Biophysics, 2nd ed.; Springer Science & Business Media: New York, NY, USA, 2012; pp. 129–144. [Google Scholar]
- Hao, A.; Marui, A.; Haraguchi, T. Estimation of Wet-bulb Formation in Various Soil during Drip Irrigation. J. Fac. Agric. Kyushu Univ. 2007, 52, 187–193. [Google Scholar]
- Saxton, K.E.; Rawls, W.J. Soil water characteristic estimates by texture and organic matter for hydrologic solutions. Soil Sci. Soc. Am. J. 2006, 70, 1569–1578. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Sharkey, T.D. Stomatal conductance and photosynthesis. Annu. Rev. Plant Physiol. 1982, 33, 317–345. [Google Scholar] [CrossRef]
- Jones, H.G. Plant water relations and implications for irrigation scheduling. Acta Hortic. 1990, 278, 67–76. [Google Scholar] [CrossRef]
- Dolman, A.J.; Van Den Burg, G.J. Stomatal behaviour in an oak canopy. Agric. For. Meteorol. 1988, 43, 99–108. [Google Scholar] [CrossRef]
- Matsumoto, K.; Ohta, T.; Tanaka, T. Dependence of stomatal conductance on leaf chlorophyll concentration and meteorological variables. Agric. For. Meteorol. 2005, 132, 44–57. [Google Scholar] [CrossRef]
- Vialet-Chabrand, S.; Dreyer, E.; Brendel, O. Performance of a new dynamic model for predicting diurnal time courses of stomatal conductance at the leaf level. Plant Cell Environ. 2013, 36, 1529–1546. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.; Ingwers, M.W.; McGuire, M.A.; Teskey, R.O. Increase in leaf temperature opens stomata and decouples net photosynthesis from stomatal conductance in Pinus taeda and Populus deltoides x nigra. J. Exp. Bot. 2017, 68, 1757–1767. [Google Scholar] [CrossRef] [PubMed]
- Dinis, C. Cork Oak (Quercus suber L.) Root System: A Structural-Functional 3D Approach. Ph.D. Thesis, Evora University, Evora, Portugal, 2014. [Google Scholar]
- Puértolas, J.; Pardos, M.; Jiménez, M.D.; Aranda, I.; Pardos, J.A. Interactive responses of Quercus suber L. seedlings to light and mild water stress: Effects on morphology and gas exchange traits. Ann. For. Sci. 2008, 65, 611. [Google Scholar] [CrossRef]
- Schmidt, M.W.T.; Schreiber, D.; Correia, A.; Ribeiro, N.; Surový, P.; Otieno, D.; Tenhunen, J.; Pereira, J.S. Sap flow in cork oak trees at two contrasting sites in portugal. Acta Hortic. 2009, 846, 345–352. [Google Scholar] [CrossRef]
- Rzigui, T.; Jazzar, L.; Baaziz Khaoula, B.; Fkiri, S.; Nasr, Z. Drought tolerance in cork oak is associated with low leaf stomatal and hydraulic conductances. iForest Biogeosci. For. 2018, 11, 728. [Google Scholar] [CrossRef]
- Aranda, I.; Castro, L.; Pardos, M.; Gil, L.; Pardos, J.A. Effects of the interaction between drought and shade on water relations, gas exchange and morphological traits in cork oak (Quercus suber L.) seedlings. For. Ecol. Manage. 2005, 210, 117–129. [Google Scholar] [CrossRef]
- Merouani, H.; Branco, C.; Almeida, M.H.; Pereira, J.S. Effects of acorn storage duration and parental tree on emergence and physiological status of Cork oak (Quercus suber L) seedlings. Ann. For. Sci. 2001, 58, 543–554. [Google Scholar] [CrossRef]
- Branco, M.; Branco, C.; Merouani, H.; Almeida, M.H. Germination success, survival and seedling vigour of Quercus suber acorns in relation to insect damage. For. Ecol. Manag. 2002, 166, 159–164. [Google Scholar] [CrossRef]
- Pons, J.; Pausas, J.G. Oak regeneration in heterogeneous landscapes: The case of fragmented Quercus suber forests in the eastern Iberian Peninsula. For. Ecol. Manag. 2006, 231, 196–204. [Google Scholar] [CrossRef]
- Trubat, R.; Cortina, J.; Vilagrosa, A. Nursery fertilization affects seedling traits but not field performance in Quercus suber L. J. Arid Environ. 2010, 74, 491–497. [Google Scholar] [CrossRef]
- Ribeiro, N.A.; Surový, P. Growth modeling in complex forest systems: CORKFITS a tree spatial growth model for cork oak woodlands. Formath 2011, 10, 263–278. [Google Scholar] [CrossRef]
- Tenhunen, J.; Geyer, R.; Carreiras, J.M.B.; Ribeiro, N.A.; Dinh, N.Q.; Otieno, D.; Pereira, J.S. Simulating function and vulnerability of cork oak woodland ecosystems. In Cork Oak Woodlands on the Edge: Ecology, Adaptive Management, and Restoration; Pereira, J.S., Pausas, J.G., Aronson, J., Eds.; Society for Ecological Restoration International; Island Press: Washington, DC, USA, 2008; pp. 227–234. [Google Scholar]
| a: Physical Properties | |||||||||
| Density (g cm−3) | Gross Sand (%) | Fine Sand (%) | Silt (%) | Clay (%) | |||||
| 1.60 ± 0.11 | 77.55 ± 3.48 | 11.47 ± 1.78 | 4.48 ± 0.97 | 6.50 ± 0.97 | |||||
| b: Chemical Properties | |||||||||
| O.M. (%) | pH (H2O) | P2O5 (ppm) | N (%) | K2O (ppm) | Mg (ppm) | Fe (ppm) | Mn (ppm) | Zn (ppm) | Cu (ppm) |
| 0.37 ± 0.30 | 5.39 ± 0.53 | 72.25 ± 33.03 | traces | 95.17 ± 76.57 | 27.33 ± 12.66 | 13.64 ± 7.75 | 17.20 ± 15.99 | 0.77 ± 0.65 | 0.07 ± 0.06 |
| Period | Irrigation Parameters | Treatments | ||||
|---|---|---|---|---|---|---|
| Control | 1 | 2 | 3 | 4 | ||
| Weekly | Total volume (mm) * | ** | 1.88 | 3.12 | 4.38 | 5.62 |
| Frequency (x) | ** | 3 | 3 | 3 | 3 | |
| Drip fertilization (min.) | ** | 30 | 30 | 30 | 30 | |
| Total season | Volume (mm) | 21 | 39.48 | 65.52 | 91.98 | 118.02 |
| Parameter | Estimate | S.E. | t Value | 95% Confidence Interval | |||
|---|---|---|---|---|---|---|---|
| p Value | Lower | Upper | |||||
| Intercept | 116.46 | 25.04 | 4.65 | <0.001 | 66.30 | 166.63 | |
| Time (day of measurement) | −1.29 | 0.18 | −6.95 | <0.001 | −1.65 | −0.92 | |
| Initial diameter_base (cm) | 1.86 | 0.51 | 3.65 | <0.001 | 0.85 | 2.87 | |
| Deep water storage (mm) | 1.41 | 0.38 | 3.67 | 0.001 | 0.62 | 2.18 | |
| Treatments | Control + 1 | −26.23 | 8.76 | −2.99 | 0.009 | −44.88 | −7.57 |
| 2 + 3 + 4 | 0 a | ||||||
| Parameter | Estimate | S.E | t Value | 95% Confidence Interval | |||
|---|---|---|---|---|---|---|---|
| p Value | Lower | Upper | |||||
| Intercept | 78.70 | 7.85 | 10.02 | <0.001 | 63.29 | 94.10 | |
| Initial diameter_base (cm) | −0.72 | 0.15 | −4.94 | <0.001 | −1.10 | −0.44 | |
| Treatments | Control + 1 | −9.95 | 2.87 | −3.47 | 0.003 | −16.03 | −3.87 |
| 2 + 3 + 4 | 0 a | 0.00 | |||||
| Distance to the stream (m) | −0.08 | 0.03 | −2.59 | 0.010 | −0.14 | −0.02 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camilo-Alves, C.; Dinis, C.; Vaz, M.; Barroso, J.M.; Ribeiro, N.A. Irrigation of Young Cork Oaks under Field Conditions—Testing the Best Water Volume. Forests 2020, 11, 88. https://doi.org/10.3390/f11010088
Camilo-Alves C, Dinis C, Vaz M, Barroso JM, Ribeiro NA. Irrigation of Young Cork Oaks under Field Conditions—Testing the Best Water Volume. Forests. 2020; 11(1):88. https://doi.org/10.3390/f11010088
Chicago/Turabian StyleCamilo-Alves, Constança, Cati Dinis, Margarida Vaz, João M. Barroso, and Nuno Almeida Ribeiro. 2020. "Irrigation of Young Cork Oaks under Field Conditions—Testing the Best Water Volume" Forests 11, no. 1: 88. https://doi.org/10.3390/f11010088
APA StyleCamilo-Alves, C., Dinis, C., Vaz, M., Barroso, J. M., & Ribeiro, N. A. (2020). Irrigation of Young Cork Oaks under Field Conditions—Testing the Best Water Volume. Forests, 11(1), 88. https://doi.org/10.3390/f11010088






