Sparse Pinus Tabuliformis Stands Have Higher Canopy Transpiration Than Dense Stands Three Decades After Thinning
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Stand Selection
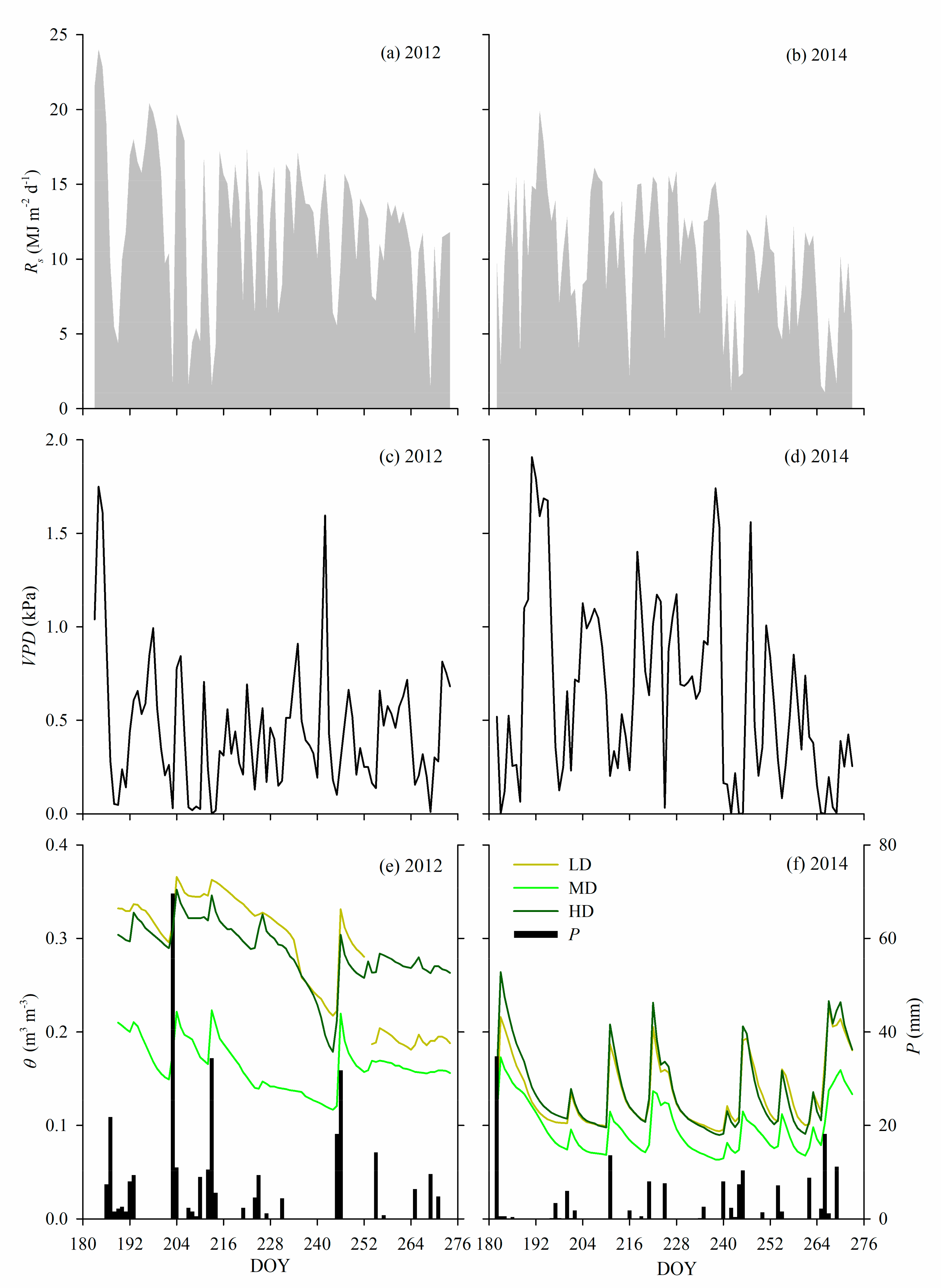
2.2. Meteorology and Soil Water Content
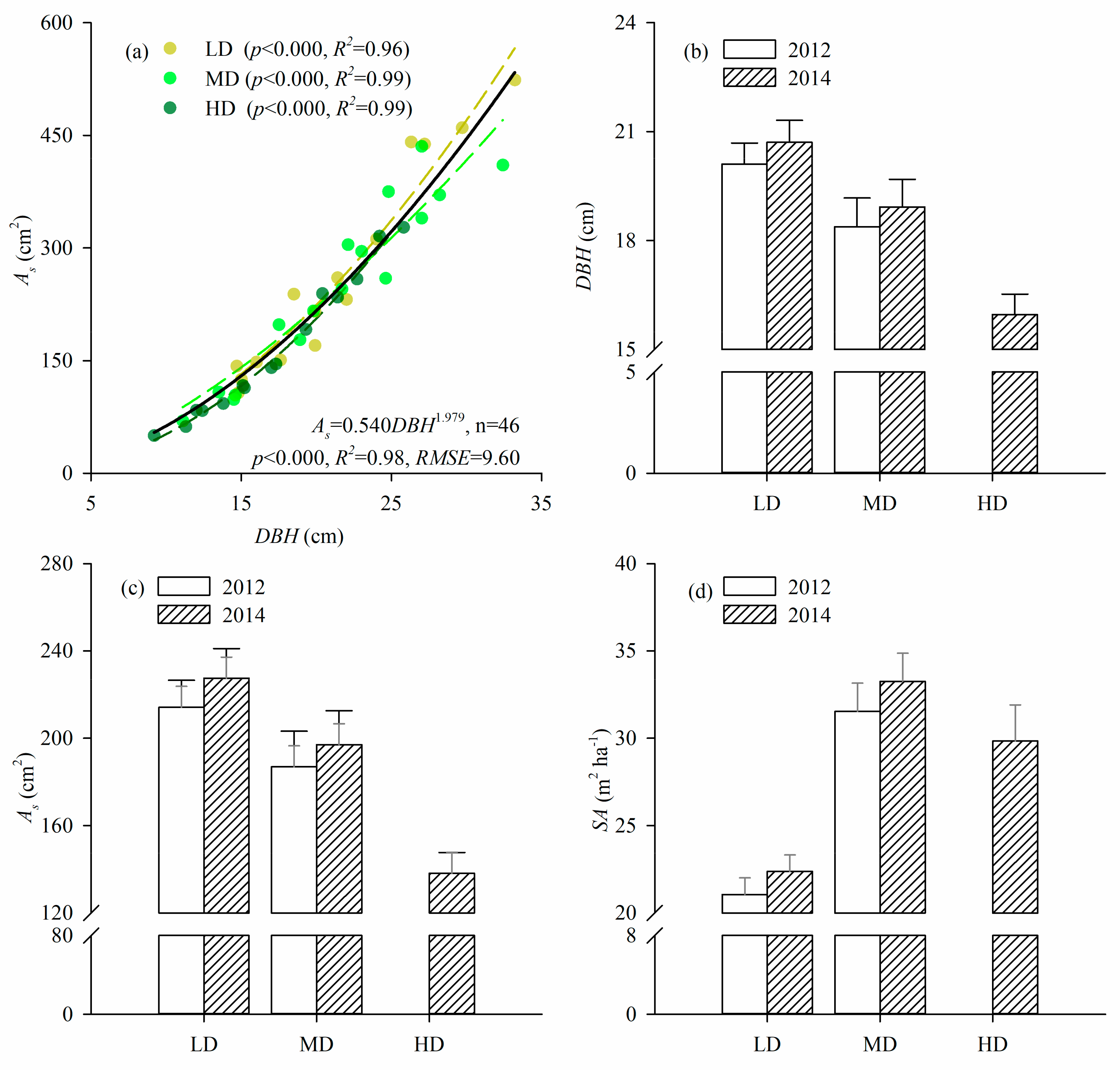
2.3. Sapwood Area Estimates
2.4. Sap Flow Measurements and Transpiration Calculation
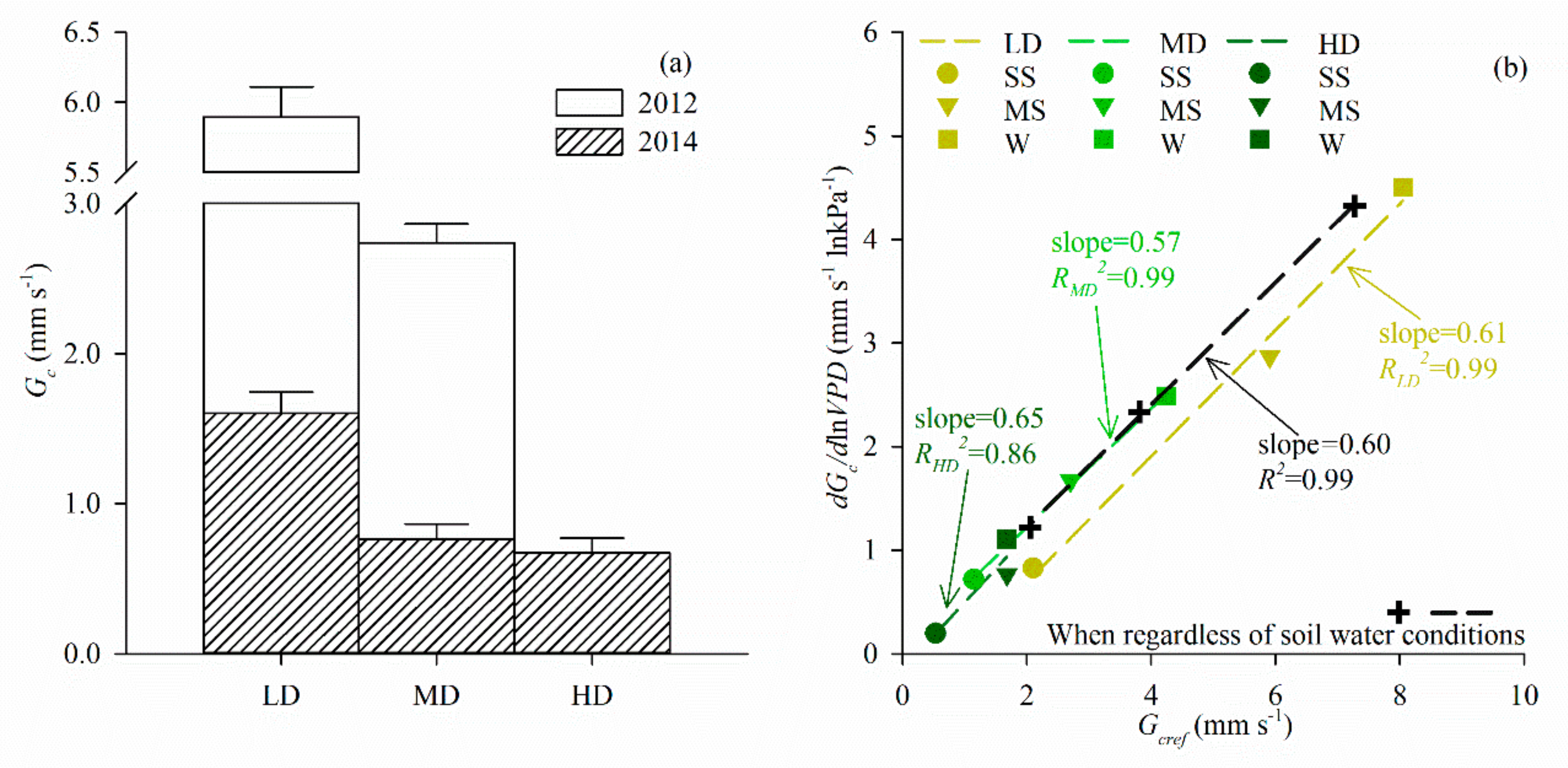
2.5. Canopy Conductance Estimates
2.6. Data Analysis
3. Results
3.1. Climatic and Soil Water Variations
3.2. DBH and Sapwood Area
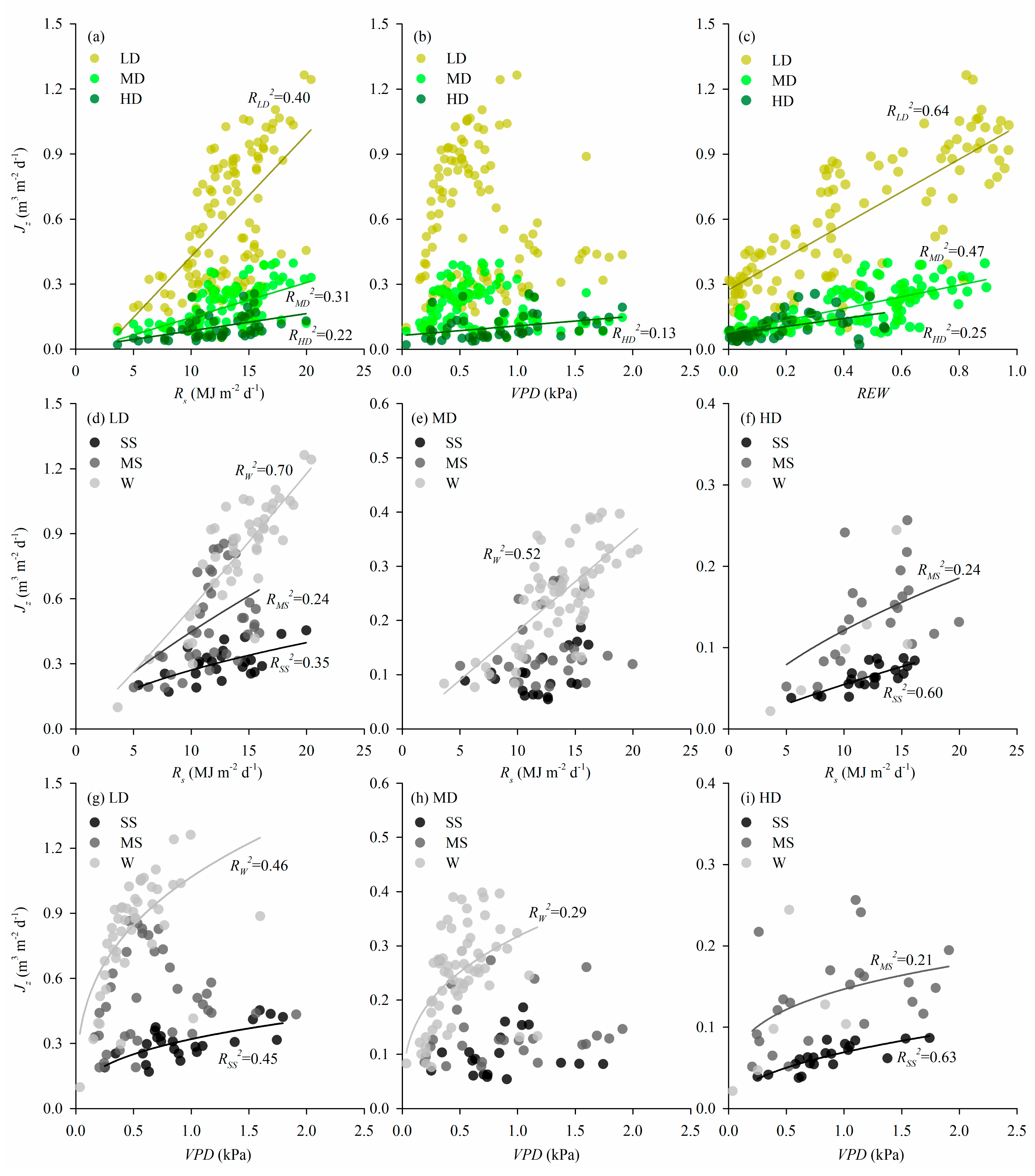
3.3. Biophysical Controls
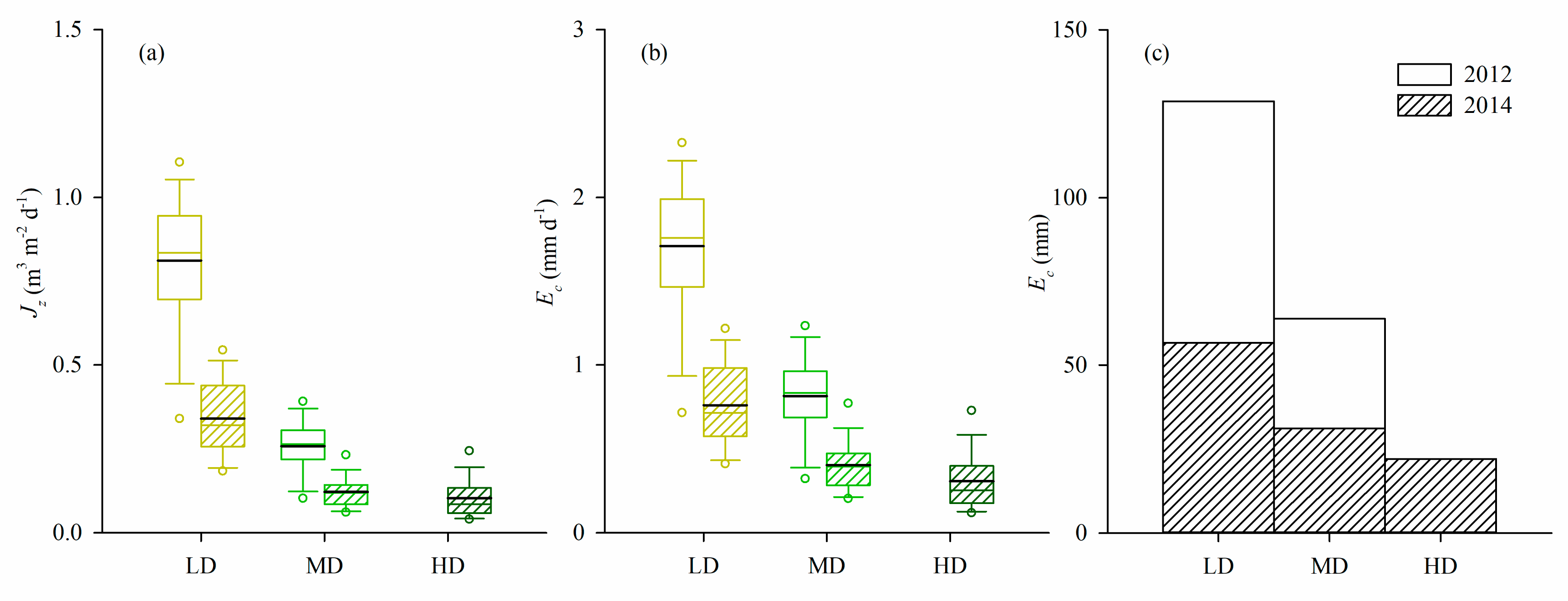
3.4. Sap Flux Density and Canopy Transpiration
4. Discussion
4.1. DBH and Sapwood Area
4.2. Biophysical Controls
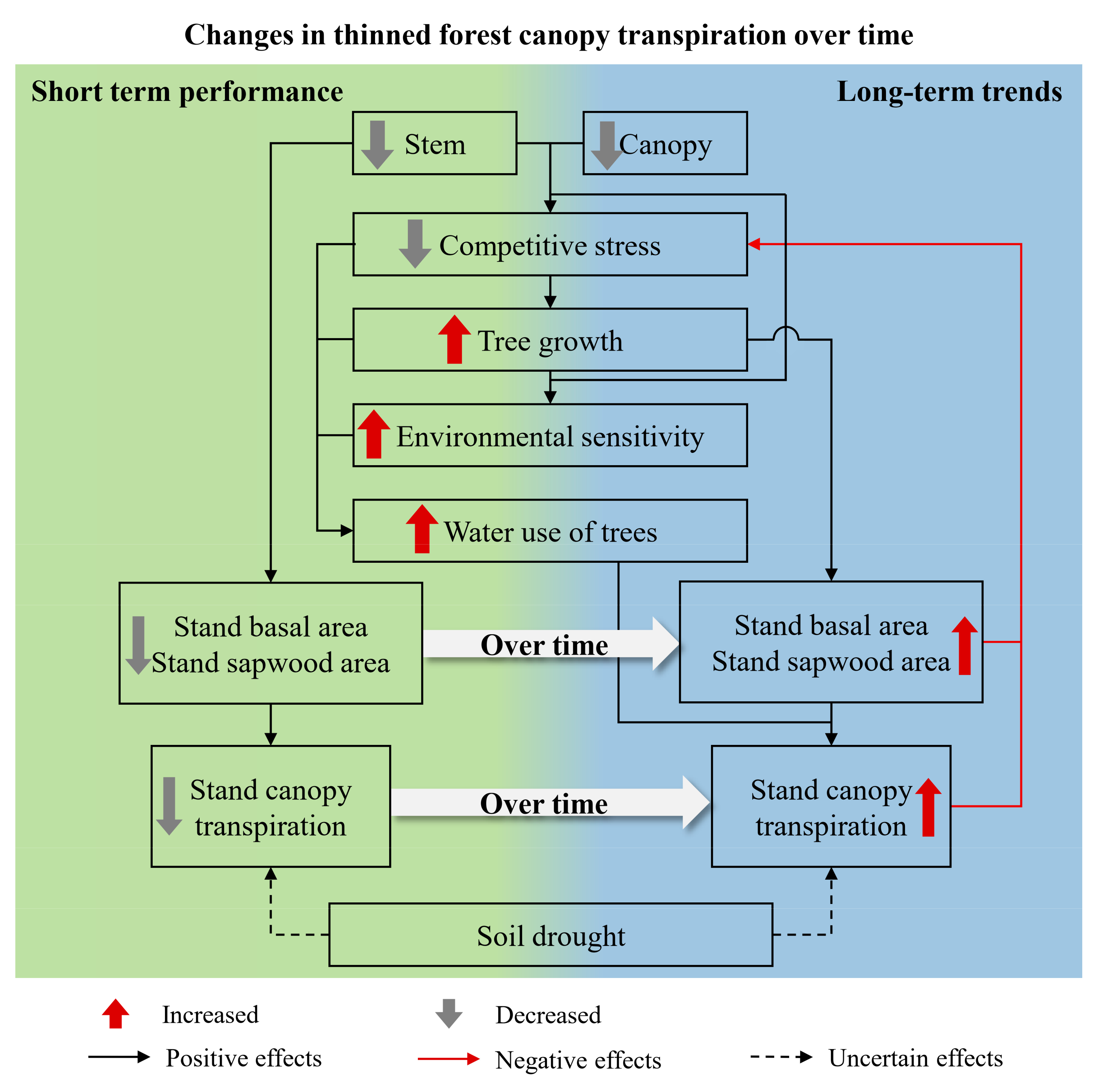
4.3. Influence of Stand Density and Soil Drought on Canopy Transpiration
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moreno, H.A.; Gupta, H.V.; White, D.D.; Sampson, D.A. Modeling the distributed effects of forest thinning on the long-term water balance and streamflow extremes for a semi-arid basin in the southwestern US. Hydrol. Earth Syst. Sci. 2016, 20, 1241–1267. [Google Scholar] [CrossRef]
- Dung, B.X.; Gomi, T.; Miyata, S.; Sidle, R.C.; Kosugi, K.; Onda, Y. Runoff responses to forest thinning at plot and catchment scales in a headwater catchment draining Japanese cypress forest. J. Hydrol. 2012, 444–445, 51–62. [Google Scholar] [CrossRef]
- Boggs, J.; Sun, G.; Domec, J.C.; McNulty, S.; Treasure, E. Clearcutting upland forest alters transpiration of residual trees in the riparian buffer zone. Hydrol. Process. 2015, 29, 4979–4992. [Google Scholar] [CrossRef]
- Tague, C.L.; Moritz, M.; Hanan, E. The changing water cycle: The eco-hydrologic impacts of forest density reduction in Mediterranean (seasonally dry) regions. Wiley Interdiscip. Rev. Water 2019, 6, e1350. [Google Scholar] [CrossRef]
- Elliott, K.J.; Caldwell, P.V.; Brantley, S.T.; Miniat, C.F.; Vose, J.M.; Swank, W.T. Water yield following forest-grass-forest transitions. Hydrol. Earth Syst. Sci. 2017, 21, 981–997. [Google Scholar] [CrossRef]
- Hawthorne, S.N.D.; Lane, P.N.J.; Bren, L.J.; Sims, N.C. The long term effects of thinning treatments on vegetation structure and water yield. For. Ecol. Manag. 2013, 310, 983–993. [Google Scholar] [CrossRef]
- Swank, W.T.; Knoepp, J.D.; Vose, J.M.; Laseter, S.N.; Webster, J.R. Response and Recovery of Water Yield and Timing, Stream Sediment, Abiotic Parameters, and Stream Chemistry Following Logging. In Long-Term Response of a Forest Watershed Ecosystem; Oxford University Press: Oxford, UK, 2014; pp. 36–56. ISBN 9780190267933. [Google Scholar]
- Bren, L.; Lane, P.; Hepworth, G. Longer-term water use of native eucalyptus forest after logging and regeneration: The Coranderrk experiment. J. Hydrol. 2010, 384, 52–64. [Google Scholar] [CrossRef]
- Calev, A.; Zoref, C.; Tzukerman, M.; Moshe, Y.; Zangy, E.; Osem, Y. High-intensity thinning treatments in mature Pinus halepensis plantations experiencing prolonged drought. Eur. J. For. Res. 2016, 135, 551–563. [Google Scholar] [CrossRef]
- Liu, X.; Sun, G.; Mitra, B.; Noormets, A.; Gavazzi, M.J.; Domec, J.C.; Hallema, D.W.; Li, J.; Fang, Y.; King, J.S.; et al. Drought and thinning have limited impacts on evapotranspiration in a managed pine plantation on the southeastern United States coastal plain. Agric. For. Meteorol. 2018, 262, 14–23. [Google Scholar] [CrossRef]
- Huang, J.; Yu, H.; Guan, X.; Wang, G.; Guo, R. Accelerated dryland expansion under climate change. Nat. Clim. Chang. 2016, 6, 166–171. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Jasechko, S. Transpiration in the global water cycle. Agric. For. Meteorol. 2014, 189–190, 115–117. [Google Scholar] [CrossRef]
- Dierick, D.; Hölscher, D. Species-specific tree water use characteristics in reforestation stands in the Philippines. Agric. For. Meteorol. 2009, 149, 1317–1326. [Google Scholar] [CrossRef]
- Tfwala, C.M.; Van Rensburg, L.D.; Schall, R.; Zietsman, P.C.; Dlamini, P. Whole tree water use: Effects of tree morphology and environmental factors. Ecol. Ind. 2019, 102, 366–373. [Google Scholar] [CrossRef]
- Tsuruta, K.; Komatsu, H.; Kume, T.; Otsuki, K.; Kosugi, Y.; Kosugi, K. Relationship between stem diameter and transpiration for Japanese cypress trees: Implications for estimating canopy transpiration. Ecohydrology 2019, 12, e2097. [Google Scholar] [CrossRef]
- Sun, X.; Onda, Y.; Otsuki, K.; Kato, H.; Hirata, A.; Gomi, T. The effect of strip thinning on tree transpiration in a Japanese cypress (Chamaecyparis obtusa Endl.) plantation. Agric. For. Meteorol. 2014, 197, 123–135. [Google Scholar] [CrossRef]
- Tateishi, M.; Xiang, Y.; Saito, T.; Otsuki, K.; Kasahara, T. Changes in canopy transpiration of Japanese cypress and Japanese cedar plantations because of selective thinning. Hydrol. Process. 2015, 29, 5088–5097. [Google Scholar] [CrossRef]
- Gartner, B.L.; Meinzer, F.C. Structure-Function Relationships in Sapwood Water Transport and Storage. In Vascular Transport in Plants; Academic Press: San Diego, CA, USA, 2005; pp. 307–331. ISBN 978-0-12-088457-5. [Google Scholar]
- Komatsu, H.; Kume, T. Changes in the sapwood area of Japanese cedar and cypress plantations after thinning. J. For. Res. 2014, 20, 43–51. [Google Scholar] [CrossRef]
- Olivar, J.; Bogino, S.; Rathgeber, C.; Bonnesoeur, V.; Bravo, F. Thinning has a positive effect on growth dynamics and growth-climate relationships in Aleppo pine (Pinus halepensis) trees of different crown classes. Ann. For. Sci. 2014, 71, 395–404. [Google Scholar] [CrossRef]
- Tsamir, M.; Gottlieb, S.; Preisler, Y.; Rotenberg, E.; Tatarinov, F.; Yakir, D.; Tague, C.; Klein, T. Stand density effects on carbon and water fluxes in a semi-arid forest, from leaf to stand-scale. For. Ecol. Manag. 2019, 453, 117573. [Google Scholar] [CrossRef]
- Del Campo, A.D.; Fernandes, T.J.G.; Molina, A.J. Hydrology-oriented (adaptive) silviculture in a semiarid pine plantation: How much can be modified the water cycle through forest management? Eur. J. For. Res. 2014, 133, 879–894. [Google Scholar] [CrossRef]
- Gebhardt, T.; Häberle, K.H.; Matyssek, R.; Schulz, C.; Ammer, C. The more, the better? Water relations of Norway spruce stands after progressive thinning. Agric. For. Meteorol. 2014, 197, 235–243. [Google Scholar] [CrossRef]
- Bréda, N.; Granier, A.; Aussenac, G. Effects of thinning on soil and tree water relations, transpiration and growth in an oak forest (Quercus petraea (Matt.) Liebl.). Tree Physiol. 1995, 15, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Medhurst, J.L.; Beadle, C.L. Crown structure and leaf area index development in thinned and unthinned Eucalyptus nitens plantations. Tree Physiol. 2001, 21, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Heithecker, T.D.; Halpern, C.B. Variation in microclimate associated with dispersed-retention harvests in coniferous forests of western Washington. For. Ecol. Manag. 2006, 226, 60–71. [Google Scholar] [CrossRef]
- Hale, S.E. The effect of thinning intensity on the below-canopy light environment in a Sitka spruce plantation. For. Ecol. Manag. 2003, 179, 341–349. [Google Scholar] [CrossRef]
- Anderson, P.D.; Larson, D.J.; Chan, S.S. Riparian buffer and density management influences on microclimate of young headwater forests of western Oregon. For. Sci. 2007, 53, 254–269. [Google Scholar]
- Rambo, T.R.; North, M.P. Canopy microclimate response to pattern and density of thinning in a Sierra Nevada forest. For. Ecol. Manag. 2009, 257, 435–442. [Google Scholar] [CrossRef]
- Ma, S.; Concilio, A.; Oakley, B.; North, M.; Chen, J. Spatial variability in microclimate in a mixed-conifer forest before and after thinning and burning treatments. For. Ecol. Manag. 2010, 259, 904–915. [Google Scholar] [CrossRef]
- Del Campo, A.D.; González-Sanchis, M.; Molina, A.J.; García-Prats, A.; Ceacero, C.J.; Bautista, I. Effectiveness of water-oriented thinning in two semiarid forests: The redistribution of increased net rainfall into soil water, drainage and runoff. For. Ecol. Manag. 2019, 438, 163–175. [Google Scholar] [CrossRef]
- Whitehead, D.; Jarvis, P.G.; Waring, R.H. Stomatal conductance, transpiration, and resistance to water uptake in a Pinus sylvestris spacing experiment. Can. J. For. Res. 1984, 14, 692–700. [Google Scholar] [CrossRef]
- Alsheimer, M.; Köstner, B.; Falge, E.; Tenhunen, J.D. Temporal and spatial variation in transpiration of Norway spruce stands within a forested catchment of the Fichtelgebirge, Germany. Ann. Sci. For. 1998, 55, 103–123. [Google Scholar] [CrossRef]
- Lagergren, F.; Lankreijer, H.; Kučera, J.; Cienciala, E.; Mölder, M.; Lindroth, A. Thinning effects on pine-spruce forest transpiration in central Sweden. For. Ecol. Manag. 2008, 255, 2312–2323. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Campbell, G.S.; Norman, J.M. An Introduction to Environmental Biophysics; Springer Science & Business Media: New York, NY, USA, 1998; ISBN 978-0-387-94937-6. [Google Scholar]
- Chen, L.; Zhang, Z.; Zha, T.; Mo, K.; Zhang, Y.; Fang, X. Soil water affects transpiration response to rainfall and vapor pressure deficit in poplar plantation. New For. 2014, 45, 235–250. [Google Scholar] [CrossRef]
- Bréda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate forest trees and stands under severe drought: A review of ecophysiological responses, adaptation processes and long-term consequences. Ann. For. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef]
- Granier, A.; Loustau, D.; Bréda, N. A generic model of forest canopy conductance dependent on climate, soil water availability and leaf area index. Ann. For. Sci. 2000, 57, 755–765. [Google Scholar] [CrossRef]
- Wullschleger, S.D.; Hanson, P.; Todd, D. Transpiration from a multi-species deciduous forest as estimated by xylem sap flow techniques. For. Ecol. Manag. 2001, 143, 205–213. [Google Scholar] [CrossRef]
- Jonard, F.; André, F.; Ponette, Q.; Vincke, C.; Jonard, M. Sap flux density and stomatal conductance of European beech and common oak trees in pure and mixed stands during the summer drought of 2003. J. Hydrol. 2011, 409, 371–381. [Google Scholar] [CrossRef]
- Bai, Y.; Li, X.; Liu, S.; Wang, P. Modelling diurnal and seasonal hysteresis phenomena of canopy conductance in an oasis forest ecosystem. Agric. For. Meteorol. 2017, 246, 98–110. [Google Scholar] [CrossRef]
- Oren, R.; Sperry, J.S.; Ewers, B.E.; Pataki, D.E.; Phillips, N.; Megonigal, J.P. Sensitivity of mean canopy stomatal conductance to vapor pressure deficit in a flooded Taxodium distichum L. forest: Hydraulic and non-hydraulic effects. Oecologia 2001, 126, 21–29. [Google Scholar] [CrossRef]
- Spicer, R.; Gartner, B.L. The effects of cambial age and position within the stem on specific conductivity in Douglas-fir (Pseudotsuga menziesii) sapwood. Trees—Struct. Funct. 2001, 15, 222–229. [Google Scholar] [CrossRef]
- Ghimire, C.P.; Lubczynski, M.W.; Bruijnzeel, L.A.; Chavarro-Rincón, D. Transpiration and canopy conductance of two contrasting forest types in the Lesser Himalaya of Central Nepal. Agric. For. Meteorol. 2014, 197, 76–90. [Google Scholar] [CrossRef]
- Reyes-Acosta, J.L.; Lubczynski, M.W. Optimization of dry-season sap flow measurements in an oak semi-arid open woodland in Spain. Ecohydrology 2014, 7, 258–277. [Google Scholar] [CrossRef]
- Wang, H.; Ouyang, Z.; Zheng, H.; Wang, X.; Ni, Y.; Yu-Fen, R.E.N. Characteristics of spatial variations in xylem sap flow in urban greening tree species Pinus tabulaeformis, Cedrus deodara and Robinia pseudoacacia in Beijing, China. Chin. J. Plant Ecol. 2010, 34, 924–937. [Google Scholar]
- Moon, M.; Kim, T.; Park, J.; Cho, S.; Ryu, D.; Kim, H.S. Variation in sap flux density and its effect on stand transpiration estimates of Korean pine stands. J. For. Res. 2014, 20, 85–93. [Google Scholar] [CrossRef]
- Wang, R. Research on Water Consumption and Irrigation Regime of Main Landscape Tree Species in Beijing City; Beijing Forestry University: Beijing, China, 2006. [Google Scholar]
- Granier, A. Evaluation of transpiration in a Douglas-fir stand by means of sap flow measurements. Tree Physiol. 1987, 3, 309–320. [Google Scholar] [CrossRef]
- Rabbel, I.; Diekkrüger, B.; Voigt, H.; Neuwirth, B. Comparing ΔTmax determination approaches for Granier-based sapflow estimations. Sensors 2016, 16, 2042. [Google Scholar] [CrossRef]
- Oishi, A.C.; Hawthorne, D.A.; Oren, R. Baseliner: An open-source, interactive tool for processing sap flux data from thermal dissipation probes. SoftwareX 2016, 5, 139–143. [Google Scholar] [CrossRef]
- Pataki, D.E.; McCarthy, H.R.; Litvak, E.; Pincetl, S. Transpiration of urban forests in the Los Angeles metropolitan area. Ecol. Appl. 2011, 21, 661–677. [Google Scholar] [CrossRef]
- Litvak, E.; McCarthy, H.R.; Pataki, D.E. Transpiration sensitivity of urban trees in a semi-arid climate is constrained by xylem vulnerability to cavitation. Tree Physiol. 2012, 32, 373–388. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, W.; Wang, X.; Gao, F.; Zheng, H.; Tong, L.; Ouyang, Z. Ozone uptake by adult urban trees based on sap flow measurement. Environ. Pollut. 2012, 162, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Ma, L.; Wang, X.; Zhai, M. Temporal and spacial variation of sap flow of Chinese pine (Pinus tabulaeformis). J. Beijing For. Univ. 2010, 22, 1–6. [Google Scholar]
- Morikawa, Y.; Hattori, S.; Kiyono, Y. Transpiration of a 31-year-old Chamaecyparis obtusa Endl. stand before and after thinning. Tree Physiol. 1986, 2, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, H. Values of the decoupling factor observed on forest canopies. J. Jpn. Soc. Hydrol. Water Resour. 2003, 16, 423–438. [Google Scholar] [CrossRef]
- Monteith, J.; Unsworth, M. Principles of Environmental Physics: Plants, Animals, and the Atmosphere; Academic Press: London, UK, 2013; ISBN 9780123869104. [Google Scholar]
- Kumagai, T.; Tateishi, M.; Shimizu, T.; Otsuki, K. Transpiration and canopy conductance at two slope positions in a Japanese cedar forest watershed. Agric. For. Meteorol. 2008, 148, 1444–1455. [Google Scholar] [CrossRef]
- Ewers, B.E.; Oren, R. Analysis of assumptions and errors in the calculation of stomatal conductance from sap-flow measurements. Tree Physiol. 2000, 20, 579–589. [Google Scholar] [CrossRef]
- Schäfer, K.V.R.; Oren, R.; Tenhunen, J.D. The effect of tree height on crown level stomatal conductance. Plant Cell Environ. 2000, 23, 365–375. [Google Scholar] [CrossRef]
- Granier, A.; Biron, P.; Kiistner, B.; Gay, L.W.; Najjar, G. Comparisons of Xylem Sap Flow and Water Vapour Flux at the Stand Level and Derivation of Canopy Conductance for Scots Pine. Theor. Appl. Clim. 1996, 53, 115–122. [Google Scholar] [CrossRef]
- Domec, J.C.; King, J.S.; Ward, E.; Christopher Oishi, A.; Palmroth, S.; Radecki, A.; Bell, D.M.; Miao, G.; Gavazzi, M.; Johnson, D.M.; et al. Conversion of natural forests to managed forest plantations decreases tree resistance to prolonged droughts. For. Ecol. Manag. 2015, 355, 58–71. [Google Scholar] [CrossRef]
- Ewers, B.E.; Oren, R.; Johnsen, K.H.; Landsberg, J.J. Estimating maximum mean canopy stomatal conductance for use in models. Can. J. For. Res. 2001, 31, 198–207. [Google Scholar] [CrossRef]
- Oren, R.; Sperry, J.; Katul, G.; Pataki, D.; Ewers, B.; Phillips, N.; Schäfer, K. Survey and synthesis of intra-and interspecific variation in stomatal sensitivity to vapour pressure deficit. Plant Cell Environ. 1999, 22, 1515–1526. [Google Scholar] [CrossRef]
- Cabon, A.; Mouillot, F.; Lempereur, M.; Ourcival, J.M.; Simioni, G.; Limousin, J.M. Thinning increases tree growth by delaying drought-induced growth cessation in a Mediterranean evergreen oak coppice. For. Ecol. Manag. 2018, 409, 333–342. [Google Scholar] [CrossRef]
- Chang, J.; Li, X.; Liu, S.; Lv, J.; Ren, Q. Variations in amount and ring number of sapwood and heartwood of Pinus tabulaeformis. Sci. Silvae Sin. 2009, 45, 76–82. [Google Scholar]
- Giuggiola, A.; Bugmann, H.; Zingg, A.; Dobbertin, M.; Rigling, A. Reduction of stand density increases drought resistance in xeric Scots pine forests. For. Ecol. Manag. 2013, 310, 827–835. [Google Scholar] [CrossRef]
- Elkin, C.; Giuggiola, A.; Rigling, A.; Bugmann, H. Short- and long-term efficacy of forest thinning to mitigate drought impacts in mountain forests in the European Alps. Ecol. Appl. 2015, 25, 1083–1098. [Google Scholar] [CrossRef]
- Medhurst, J.L.; Battaglia, M.; Beadle, C.L. Measured and predicted changes in tree and stand water use following high-intensity thinning of an 8-year-old Eucalyptus nitens plantation. Tree Physiol. 2002, 22, 775–784. [Google Scholar] [CrossRef]
- Climent, J.; Chambel, M.R.; Pérez, E.; Gil, L.; Pardos, J. Relationship between heartwood radius and early radial growth, tree age, and climate in Pinus canariensis. Can. J. For. Res. 2002, 32, 103–111. [Google Scholar] [CrossRef]
- Benyon, R.G.; Lane, P.N.J.; Jaskierniak, D.; Kuczera, G.; Haydon, S.R. Use of a forest sapwood area index to explain long-term variability in mean annual evapotranspiration and streamflow in moist eucalypt forests. Water Resour. Res. 2015, 51, 5318–5331. [Google Scholar] [CrossRef]
- Granier, A.; Bréda, N. Modelling canopy conductance and stand transpiration of an oak forest from sap flow measurements. Ann. Sci. For. 1996, 53, 537–546. [Google Scholar] [CrossRef]
- Forrester, D.I.; Bertram, C.A.; Murphy, S. Impact of competition from coppicing stumps on the growth of retained trees differs in thinned Eucalyptus globulus and Eucalyptus tricarpa plantations in southeastern Australia. Can. J. For. Res. 2012, 42, 841–848. [Google Scholar] [CrossRef]
- Gebauer, R.; Volaík, D.; Urban, J.; Børja, I.; Nagy, N.E.; Eldhuset, T.D.; Krokene, P. Effect of thinning on anatomical adaptations of Norway spruce needles. Tree Physiol. 2011, 31, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.C.; McDowell, N.G.; Allen, C.D.; Anderson-Teixeira, K.J. Larger trees suffer most during drought in forests worldwide. Nat. Plants 2015, 1, 15139. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, H.; Isomäki, A. Thinning intensity and long-term changes in increment and stem form of Scots pine trees. For. Ecol. Manag. 2004, 203, 21–34. [Google Scholar] [CrossRef]
- Zimmermann, R.; Schulze, E.D.; Wirth, C.; Schulze, E.E.; Mcdonald, K.C.; Vygodskaya, N.N.; Ziegler, W. Canopy transpiration in a chronosequence of Central Siberian pine forests. Glob. Chang. Biol. 2000, 6, 25–37. [Google Scholar] [CrossRef]
- Sohn, J.A.; Saha, S.; Bauhus, J. Potential of forest thinning to mitigate drought stress: A meta-analysis. For. Ecol. Manag. 2016, 380, 261–273. [Google Scholar] [CrossRef]
| Stand Name | LD | MD | HD |
|---|---|---|---|
| Elevation (m) | c. 650 | c. 650 | c. 650 |
| Aspect | NE | NE | NE |
| Slope (°) | 23 | 24 | 21 |
| Planting year | 1968 | 1968 | 1968 |
| Planting density (stems ha−1) | 5000 | 5000 | 5000 |
| Thinning year | 1981 | 1981 | 1981 |
| Thinning intensity (% of stems removed) | 80 | 65 | 55 |
| Current density (stems ha−1) 1 | 983 | 1688 | 2160 |
| F | p | Paired Comparison 1 | F | p | Paired Comparison 1 | ||
|---|---|---|---|---|---|---|---|
| 2012 | 2014 | ||||||
| DBH | Density | 3.258 | 0.074 | LD a, MD a | 16.806 | <0.001 | LD a, MD a, HD b |
| With interaction | Without interaction | ||||||
| As2 | Density | 0.227 | 0.798 | LD a, MD a, HD a | 1.341 | 0.272 | LD a, MD a, HD a |
| DBH | 859.663 | <0.001 | 913.341 | <0.001 | |||
| Density*DBH | 0.206 | 0.815 | |||||
| Rs | VPD | REW | |||||
|---|---|---|---|---|---|---|---|
| Environmental controls | r | p | r | p | r | p | |
| Jz | LD | 0.647 | <0.001 | / | 0.570 | 0.793 | <0.001 |
| MD | 0.564 | <0.001 | / | 0.267 | 0.680 | <0.001 | |
| HD | 0.469 | 0.001 | 0.355 | 0.012 | 0.495 | <0.001 | |
| Climatic controls 2 | r | p | r | p | |||
| Jz | LD | 0.629 | <0.001 | 0.497 | <0.001 | ||
| MD | 0.525 | <0.001 | 0.229 | 0.018 | |||
| HD | 0.581 | <0.001 | 0.556 | <0.001 | |||
| Density differences in environmental responses 3 | F | p | F | p | |||
| Jz | Density | 1.328 | 0.267 | 28.564 | <0.001 | ||
| Environmental variable | 75.862 | <0.001 | 86.545 | <0.001 | |||
| Density*Environmental variable | 26.731 | <0.001 | 35.111 | <0.001 | |||
| Density differences in physiological controls 4 | F | p | |||||
| dGc/dlnVPD | Density | 4.779 | 0.069 | ||||
| Gcref | 329.812 | <0.001 | |||||
| 2012 | 2014 | |||||
|---|---|---|---|---|---|---|
| F | p | Paired Comparison 2 | F | p | Paired Comparison 2 | |
| Sap flux density 3 | 3.899 | 0.074 | LD a, MD a | 9.508 | 0.003 | LD a, MD b, HD b |
| Canopy transpiration 4 | 1.894 | 0.199 | LD a, MD a | 16.893 | <0.001 | LD a, MD b, HD b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Zhang, Z.; Chen, L.; Cai, Y.; Zhang, H.; Lou, J.; Xu, Z.; Xu, H.; Song, C. Sparse Pinus Tabuliformis Stands Have Higher Canopy Transpiration Than Dense Stands Three Decades After Thinning. Forests 2020, 11, 70. https://doi.org/10.3390/f11010070
Chen Z, Zhang Z, Chen L, Cai Y, Zhang H, Lou J, Xu Z, Xu H, Song C. Sparse Pinus Tabuliformis Stands Have Higher Canopy Transpiration Than Dense Stands Three Decades After Thinning. Forests. 2020; 11(1):70. https://doi.org/10.3390/f11010070
Chicago/Turabian StyleChen, Zuosinan, Zhiqiang Zhang, Lixin Chen, Yongmao Cai, Haiquan Zhang, Junpeng Lou, Zhou Xu, Hang Xu, and Conghe Song. 2020. "Sparse Pinus Tabuliformis Stands Have Higher Canopy Transpiration Than Dense Stands Three Decades After Thinning" Forests 11, no. 1: 70. https://doi.org/10.3390/f11010070
APA StyleChen, Z., Zhang, Z., Chen, L., Cai, Y., Zhang, H., Lou, J., Xu, Z., Xu, H., & Song, C. (2020). Sparse Pinus Tabuliformis Stands Have Higher Canopy Transpiration Than Dense Stands Three Decades After Thinning. Forests, 11(1), 70. https://doi.org/10.3390/f11010070






