The Effect of Pine Forest Structure on Bird-Mobbing Behavior: From Individual Response to Community Composition
Abstract
1. Introduction
1.1. Forest Structure, Bird-Community Structure, and Bird Behavior
1.2. Vigilance in Forest Habitats
1.3. The Effect of Season on Mobbing Behavior
1.4. Inter and Intraspecific Responses to Mobbing
2. Materials and Methods
2.1. Study Plots
2.2. Bird Survey
2.3. Vegetation Structure in the Three Types of Forest Habitat
2.4. Estimated Response Level to Mobbing Calls
2.5. Statistical Analysis
3. Results
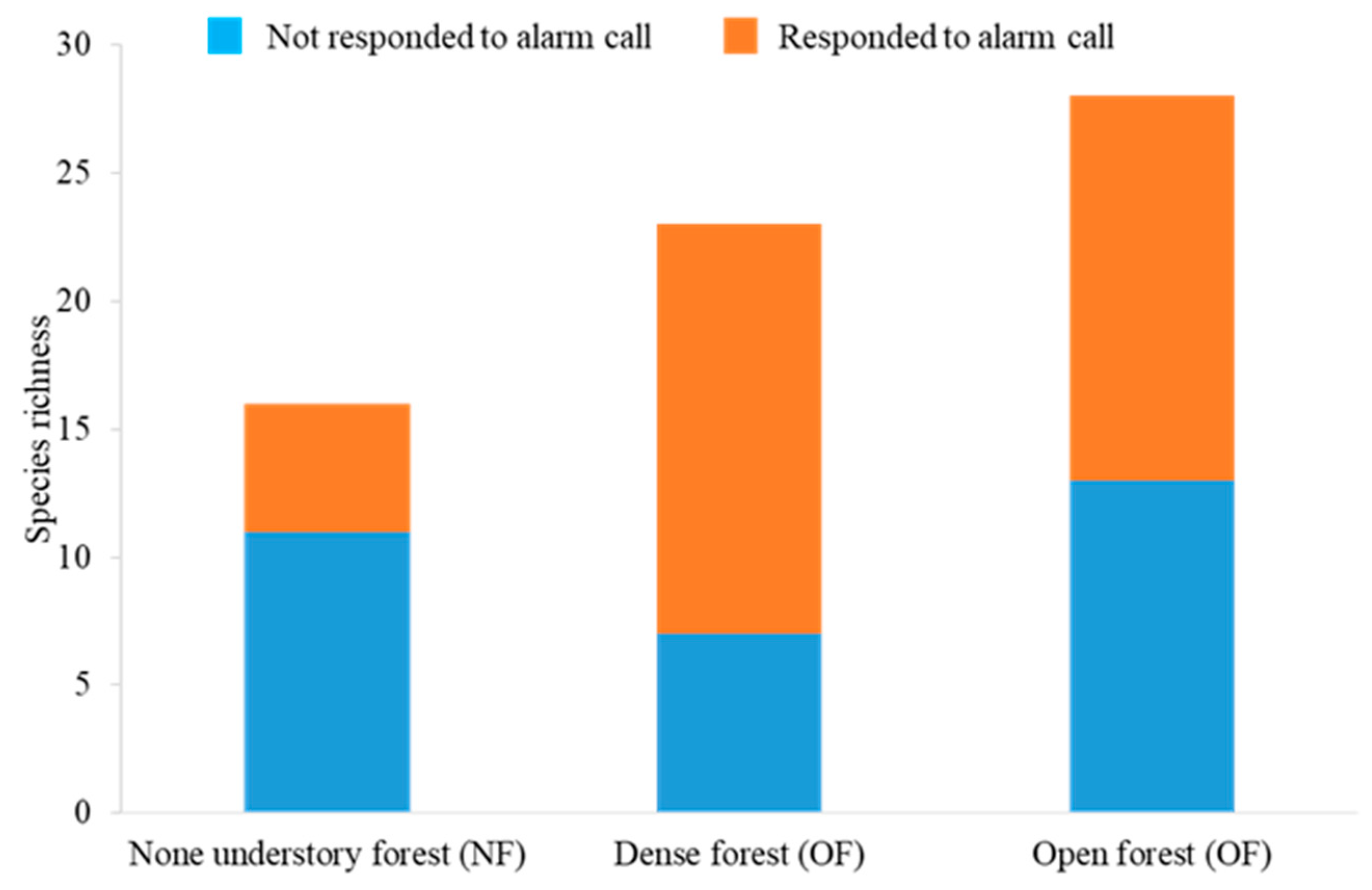
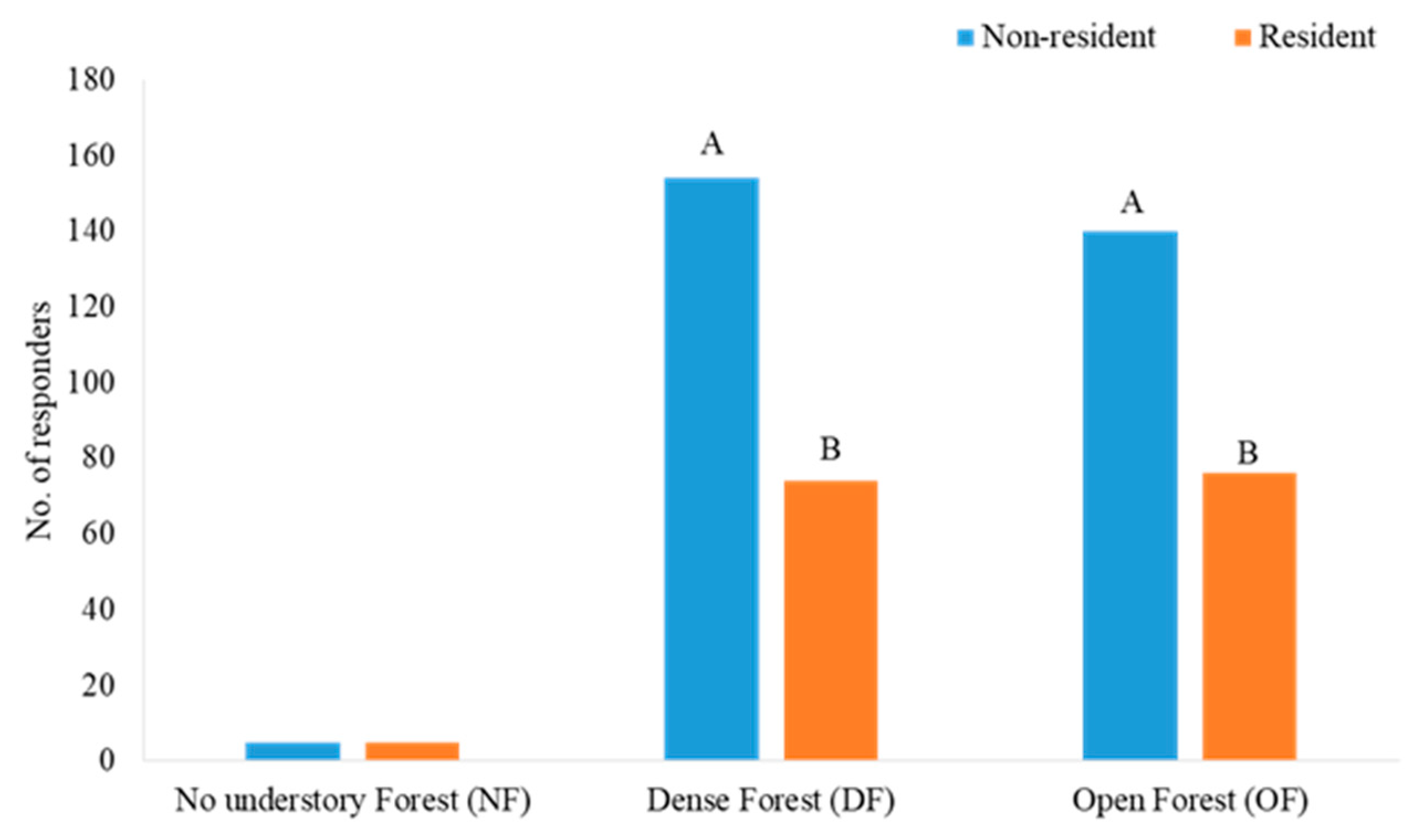
3.1. Responders to Mobbing Calls in Each Forest Type Habitat
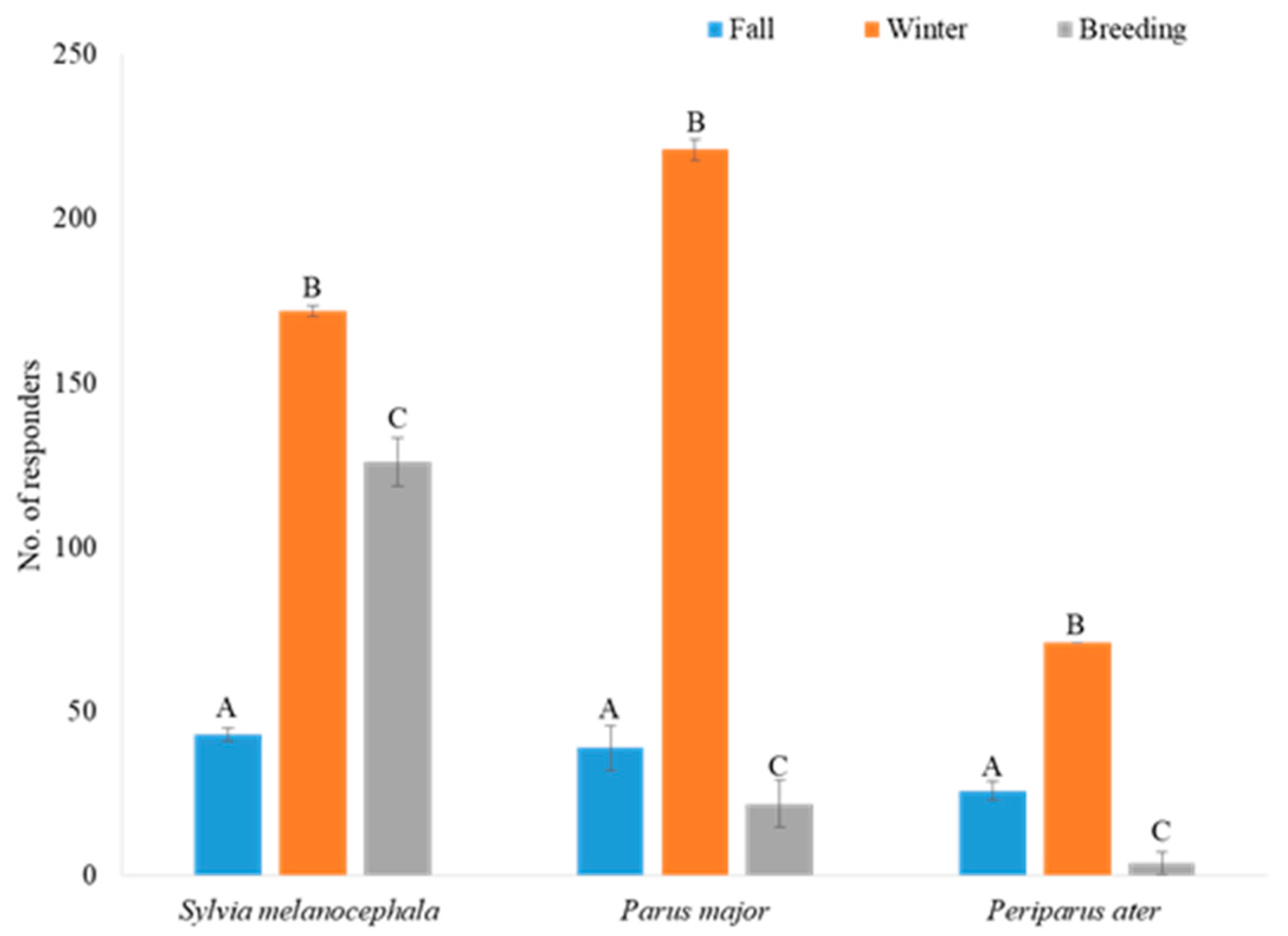
3.2. The Effect of Habitat, Season, and Caller Species on the Responding Bird Community
3.2.1. The Effect on Bird Abundance
3.2.2. The Effect on Species Richness
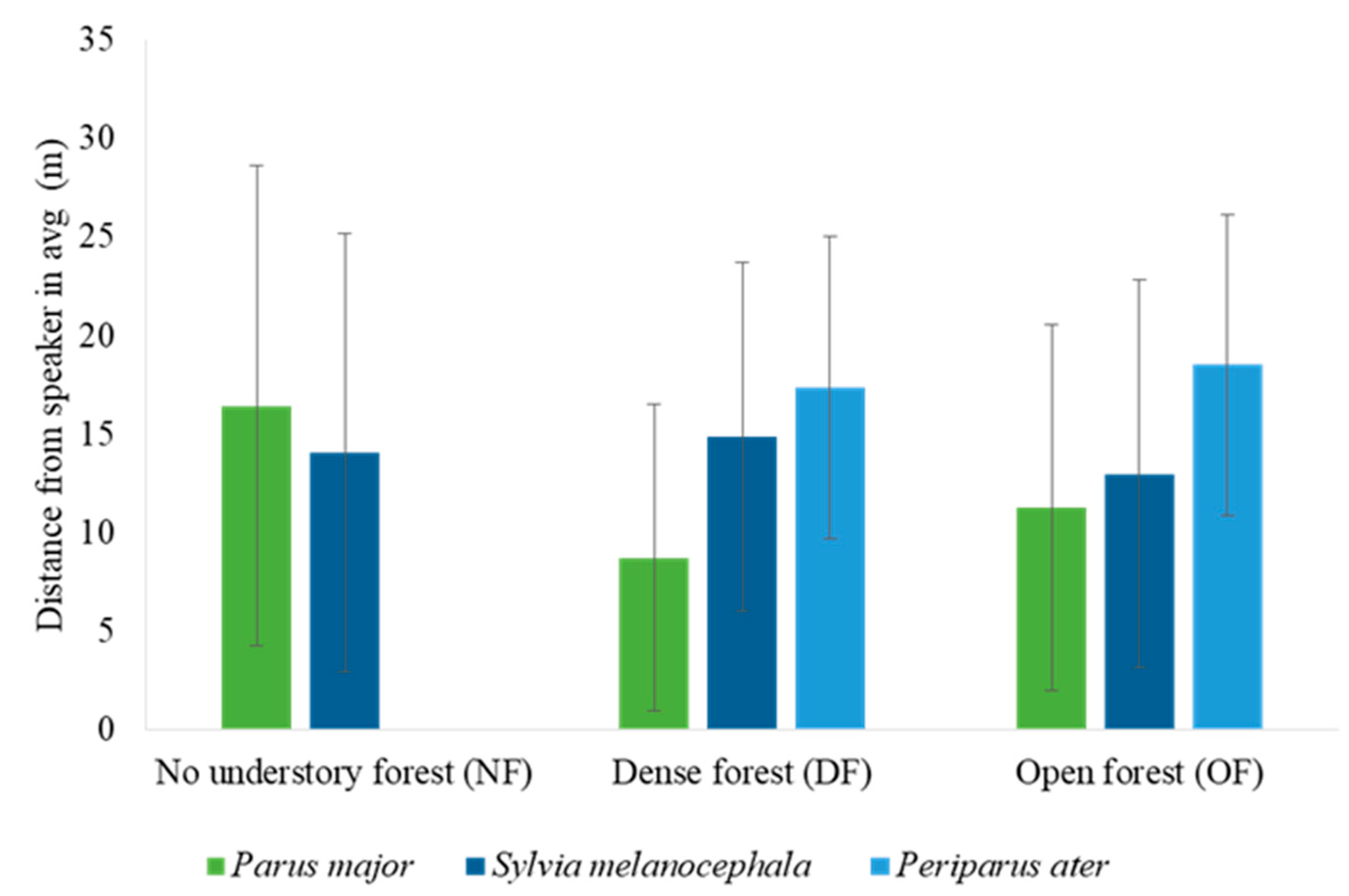
3.3. The Effect of Habitat, Season, and Caller Species on the Individual Level of Response
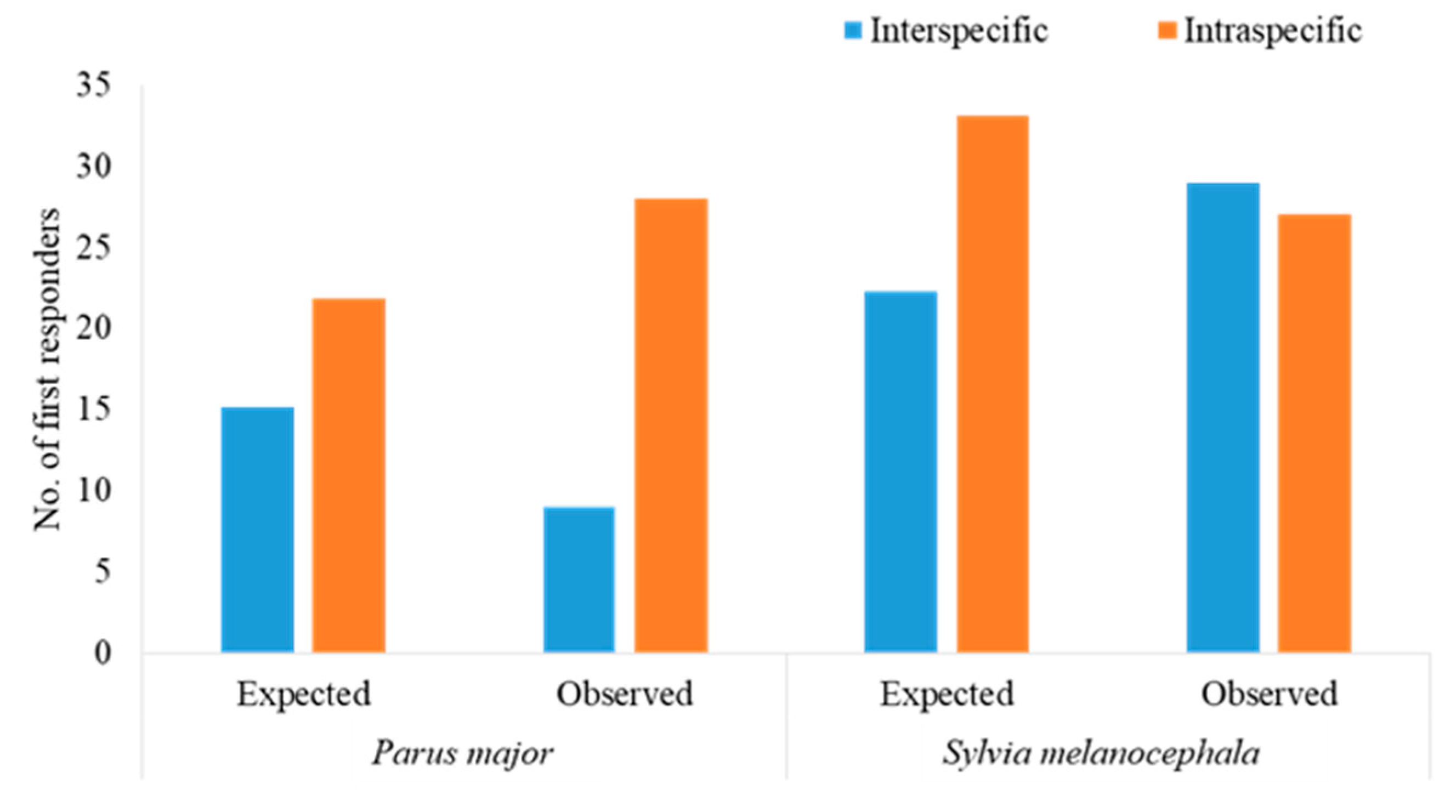
3.4. Intra- versus Interspecific Responses
3.5. The Responses of Wintering versus All-Year Residents
4. Discussion
4.1. The Effect of Forest Structure on Mobbing Behavior
4.2. The Effect of Season on Mobbing Behavior
4.3. Inter- versus Intraspecific Responses
4.4. Local versus Non-Resident Responses
4.5. Using Mobbing Calls in Forest Habitats
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dagan, U.; Izhaki, I. Understory vegetation in planted pine forests governs bird community composition and diversity in the eastern Mediterranean region. For. Ecosyst. 2019, 6, 29. [Google Scholar] [CrossRef]
- Villard, M.-A.; Trzcinski, M.K.; Merriam, G. Fragmentation effects on forest birds: Relative influence of woodland cover and configuration on landscape occupancy. Biology 1999, 13, 774–783. [Google Scholar] [CrossRef]
- Hannon, S. Effect of stand vs. landscape level forest structure on species abundance. Landscape 2005, 51. [Google Scholar] [CrossRef]
- Schieck, J. Biased detection of bird vocalizations affects comparisons of bird abundance among forested habitats. Condor 1997, 99, 179–190. [Google Scholar] [CrossRef]
- Whelan, C.J.; Maina, G.G. Effects of season, understorey vegetation density, habitat edge and tree diameter on patch-use by bark-foraging birds. Funct. Ecol. 2005, 19, 529–536. [Google Scholar] [CrossRef]
- Izhaki, I. Passerine bird communities in Mediterranean pine forests. In Ecology, Biogeography and Management of Pinus hiaepensis and P. brutia Forest Ecosystems in the Mediterranean Basin; Ne’eman, G., Trabaud, L., Eds.; Backhuys Publisher: Leiden, The Netherlands, 2000; pp. 237–250. [Google Scholar]
- Gil-Tena, A.; Saura, S.; Brotons, L. Effects of forest composition and structure on bird species richness in a Mediterranean context: Implications for forest ecosystem management. For. Ecol. Manag. 2007, 242, 470–476. [Google Scholar] [CrossRef]
- Smith, K.M.; Keeton, W.S.; Donovan, T.M.; Mitchell, B. Stand-level forest structure and avian habitat: Scale dependencies in predicting occurrence in a heterogeneous forest. For. Sci. 2008, 54, 36–46. [Google Scholar]
- Batary, P.; Scherber, C.; Fronczek, S.; Normann, C.; Tscharntke, T. How do edge effect and tree species diversity change bird diversity and avian nest survival in Germany’s largest deciduous forest? For. Ecol. Manag. 2014, 319, 44–50. [Google Scholar] [CrossRef]
- Díaz, L. Influences of forest type and forest structure on bird communities in oak and pine woodlands in Spain. For. Ecol. Manag. 2006, 223, 54–65. [Google Scholar] [CrossRef]
- Moning, C.; Müller, J. Critical forest age thresholds for the diversity of lichens, molluscs and birds in beech (Fagus sylvatica L.) dominated forests. Ecol. Indic. 2009, 9, 922–932. [Google Scholar] [CrossRef]
- Diaz, M.; Carbonell, R.; Santos, T.; Telleria, J. Breeding bird communities in pine plantations of the Spanish plateaux: Biogeography, landscape and vegetation effects. J. Appl. Ecol. 1998, 35, 562–574. [Google Scholar] [CrossRef]
- Ross, B.D.; Morrison, M.L.; Hoffman, W.; Fredericksen, T.S.; Sawicki, R.J.; Ross, E.; Lester, M.B.; Beyer, J.; Johnson, B.N. Bird relationships to habitat characteristics created by timber harvesting in Pennsylvania. J. Pa. Acad. Sci. 2001, 74, 71–84. [Google Scholar]
- Rey-Benayas, J.M.; Galván, I.; Carrascal, L.M. Differential effects of vegetation restoration in Mediterranean abandoned cropland by secondary succession and pine plantations on bird assemblages. For. Ecol. Manag. 2010, 260, 87–95. [Google Scholar] [CrossRef]
- Robbins, C.S. Effect of forest fragmentation on breeding bird populations in the Piedmont of the mid-Atlantic region. In Proceedings of the 1979 Mid-Atlantic Natural History Symposium: Bird Populations—A Litmus Test of the Environment; Lynch, J.F., Ed.; Patuxent Wildlife Research Center: Laurel, MD, USA, 1980; pp. 31–36. Available online: https://pubs.er.usgs.gov/publication/5210240 (accessed on 30 August 2019).
- Lynch, J.F.; Whigham, D.F. Effects of forest fragmentation on breeding bird communities in Maryland, USA. Biol. Conserv. 1984, 28, 287–324. [Google Scholar] [CrossRef]
- Patterson, I.J.; Ollason, J.G.; Doyle, P. Bird populations in upland spruce plantations in northern Britain. For. Ecol. Manag. 1995, 79, 107–131. [Google Scholar] [CrossRef]
- Sweeney, O.; Wilson, M.; Irwin, S. woodlands and plantation for breeding birds of native nests in Ireland. Irish Birds 2011, 9, 181–196. [Google Scholar]
- Fuller, R.J. Bird Life of Woodland and Forest; Cambridge University Press: Cambridge, UK, 2003; ISBN 0521543479. [Google Scholar]
- Robinson, S.K.; Holmes, R.T. Effects of plant species and foliage structure on the foraging behavior of forest birds. Auk 1984, 101, 672–684. [Google Scholar] [CrossRef]
- Curio, E.; Ernst, U.; Vieth, W. The adaptive significance of avian mobbing. Etology 1978, 48, 184–202. [Google Scholar] [CrossRef]
- Frankenberg, E. The Adaptive significance of avian mobbing. Etology 1981, 55, 97–118. [Google Scholar] [CrossRef]
- Lorenz, K. On Aggression; Routledge Publishing: London, UK, 1963; ISBN 9780415283205. [Google Scholar]
- Sullivan, K.A. Information Exploitation by Downy Woodpeckers in mixed-species flocks. Behaviour 1984, 91, 294–311. [Google Scholar] [CrossRef]
- Newman, J.A.; Recer, G.M.; Zwicker, S.M.; Caraco, T. Effects of predation hazard on foraging constraints: Patch-use strategies in grey squirrels [Sciurus cardinensis]. Oikos 1988, 93–97. [Google Scholar] [CrossRef]
- Brown, J.S. Vigilance, patch use and habitat selection: Foraging under predation risk. Evol. Ecol. Res. 1999, 1, 49–71. [Google Scholar]
- Rae, L.F.; Whitaker, D.M.; Warkentin, I.G. Variable effect of playback of chickadee mobbing calls on detection probability of boreal forest birds. J. Field Ornithol. 2015, 86, 51–64. [Google Scholar] [CrossRef]
- Wheatcroft, D.; Price, T.D. Learning and signal copying facilitate communication among bird species. Proc. Biol. Sci. 2013, 280, 20123070. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez-A’Lamo, J.D.; Magrath, R.D.; Oteyza, J.C.; Chalfoun, A.D.; Haff, T.M.; Schmidt, K.A.; Thomson, R.L.; Martin, T.E. Nest predation research: Recent findings and future perspectives. J. Ornithol. 2015, 156, 247–262. [Google Scholar] [CrossRef]
- Hurd, C.R. Interspecific attraction to the mobbing calls of black-capped chickadees (Parus atricapillus). Behav. Ecol. Sociobiol. 1996, 38, 287–292. [Google Scholar] [CrossRef]
- Sieving, K.E.; Contreras, T.A.; Maute, K.L. Heterospecific facilitation of forest-boundary crossing by mobbing understory birds in North-Central Florida. Auk 2004, 121, 738–751. [Google Scholar] [CrossRef]
- Dutour, M.; Lena, J.P.; Lengagne, T. Mobbing behaviour in a passerine community increases with prevalence in predator diet. Ibis 2017, 159, 324–330. [Google Scholar] [CrossRef]
- Lind, J.; Jöngren, F.; Nilsson, J.; Schönberg Alm, D.; Strandmark Lind, A. Information, predation risk and foraging decisions during mobbing in Great Tits Parus major. Ornis Fenn. 2005, 82, 89–96. [Google Scholar]
- Shimazaki, A.; Yamaura, Y.; Senzaki, M.; Yabuhara, Y.; Nakamura, F. Mobbing call experiment suggests the enhancement of forest bird movement by tree cover in urban landscapes across seasons. Avian Conserv. Ecol. 2017, 12, 16. [Google Scholar] [CrossRef]
- Desrochers, A.; Bélisle, M.; Bourque, J. Do mobbing calls affect the perception of predation risk by forest birds? Anim. Behav. 2002, 64, 709–714. [Google Scholar] [CrossRef]
- Griesser, M.; Suzuki, T.N. Naive Juveniles Are More Likely to Become Breeders after Witnessing Predator Mobbing. Am. Nat. 2017, 189, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Barash, D.P. Evolutionary aspects of parental behavior: Distraction behavior of the alpine accentor. Wilson Bull. 1975, 367–373. [Google Scholar]
- Thys, B.; Lambreghts, Y.; Pinxten, R.; Eens, M. Nest defence behavioural reaction norms: Testing life-history and parental investment theory predictions. R. Soc. Open Sci. 2019, 6, 182180. [Google Scholar] [CrossRef] [PubMed]
- Montgomerie, R.D.; Weatherhead, P.J. Risks and rewards of nest defence by parent birds. Q. Rev. Biol. 1988, 63, 167–187. [Google Scholar] [CrossRef]
- Lima, S.L. Predators and the breeding bird: Behavioral and reproductive flexibility under the risk of predation. Biol. Rev. 2009, 84, 485–513. [Google Scholar] [CrossRef] [PubMed]
- Pettifor, R.A. The effects of avian mobbing on a potential predator, the European kestrel, Falco tinnunculus. Anim. Behav. 1990, 39, 821–827. [Google Scholar] [CrossRef]
- Shedd, D.H. Seasonal variation in mobbing intensity in the Black-capped Chickadee. Wilson Bull. 1983, 95, 343–348. [Google Scholar]
- Shedd, D.H. Seasonal variation and function of mobbing and related antipredator behaviors of the American Robin (Turdus migratorius). Auk 1982, 99, 342–346. [Google Scholar]
- Forsman, J.T.; Monkkonen, M.; Inkeroinen, J.; Reunanen, P. Aggregate dispersion of birds after encountering a predator: Experimental evidence. J. Avian Biol. 1998, 29, 44. [Google Scholar] [CrossRef]
- Marler, P. Specific distinctiveness in the communication signals of birds. Behaviour 1957, 11, 13–39. [Google Scholar] [CrossRef]
- Turcotte, Y.; Desrochers, A. Playbacks of mobbing calls of Black-capped Chickadees help estimate the abundance of forest birds in winter. J. Field Ornithol. 2002, 73, 303–307. [Google Scholar] [CrossRef]
- Forsman, J.T.; Mönkkönen, M. Responses by breeding birds to heterospecific song and mobbing call playbacks under varying predation risk. Anim. Behav. 2001, 62, 1067–1073. [Google Scholar] [CrossRef]
- Johnson, F.R.; Mcnaughton, E.J.; Shelley, C.D.; Blumstein, D.T. Mechanisms of heterospecific recognition in avian mobbing calls. Aust. J. Zool. 2003, 51, 577–585. [Google Scholar] [CrossRef]
- Nolen, M.T.; Lucas, J.R. Asymmetries in mobbing behaviour and correlated intensity during predator mobbing by nuthatches, chickadees and titmice. Anim. Behav. 2009, 77, 1137–1146. [Google Scholar] [CrossRef]
- Suzuki, T.N. Referential calls coordinate multi-species mobbing in a forest bird community. J. Ethol. 2016, 34, 79–84. [Google Scholar] [CrossRef]
- Morris, D.W.; Davidson, D.L. Optimally foraging mice match patch use with habitat differences in fitness. Ecology 2000, 81, 2061–2066. [Google Scholar] [CrossRef]
- Hua, F.; Sieving, K.E. Understory avifauna exhibits altered mobbing behavior in tropical forest degraded by selective logging. Oecologia 2016, 182, 743–754. [Google Scholar] [CrossRef]
- Goldreich, Y. The spatial distribution of annual rainfall in Israel—A review. Theor. Appl. Climatol. 1994, 50, 45–59. [Google Scholar] [CrossRef]
- CBS—Statistical Abstract of Israel Forest, Planted Forest Area. Available online: http://www.cbs.gov.il/reader/shnaton/templ_shnaton.html?num_tab=st19_07&CYear=2014 (accessed on 26 August 2015).
- JNF. JNF Forests Data to 2014. Available online: http://www.kkl.org.il/afforestation-and-environment/afforestation/forest-data-2014/ (accessed on 26 August 2015).
- Ne’eman, G.; Izhaki, I. The effect of stand age and microhabitat on soil seed banks in Mediterranean Aleppo pine forests after fire. Plant Ecol. 1999, 144, 115–125. [Google Scholar] [CrossRef]
- Schiller, G.; Ne’eman, G.; Korol, L. Post-fire vegetation dynamics in a native Pinus halepensis Mill. forest on Mt. Carmel. Isr. J. Plant Sci. 1997, 45, 297–308. [Google Scholar] [CrossRef]
- Bibby, C.J. Bird Census Techniques; Elsevier: Amsterdam, The Netherlands, 2000; ISBN 0120958317. [Google Scholar]
- Shriner, S. Distribution of Breeding Birds in Great Smoky Mountains National Park; North Carolina State University: Raleigh, NC, USA, 2001. [Google Scholar]
- Brown, D.J.; Ferrato, J.R.; White, C.J.; Mali, I.; Forstner, M.R.J.; Simpson, T.R. Short-term changes in summer and winter resident bird communities following a high severity wildfire in a southern USA mixed pine/hardwood forest. For. Ecol. Manag. 2015, 350, 13–21. [Google Scholar] [CrossRef]
- Reynolds, R.T.; Scott, J.M.; Nussbaum, R.A. A Variable circular-plot method for estimating bird numbers. Condor 1980, 82, 309–313. [Google Scholar] [CrossRef]
- Marsden, S.J. Changes in Bird abundance following selective logging on Seram, Indonesia. Conserv. Biol. 1998, 12, 605–611. [Google Scholar] [CrossRef]
- Reynaud, P.; Thioulouse, J. Identification of birds as biological markers along a neotropical urban–rural gradient (Cayenne, French Guiana), using co-inertia analysis. J. Environ. Manag. 2000, 59, 121–140. [Google Scholar] [CrossRef]
- Ellis, T.M.; Betts, M.G. Bird abundance and diversity across a hardwood gradient within early seral plantation forest. For. Ecol. Manag. 2011, 261, 1372–1381. [Google Scholar] [CrossRef]
- Czeszczewik, D.; Zub, K.; Stanski, T.; Sahel, M.; Kapusta, A.; Walankiewicz, W. Effects of forest management on bird assemblages in the Bialowieza Forest, Poland. iForest-Biogeosci. For. 2015, 8, 377–385. [Google Scholar] [CrossRef]
- Gunn, J.S.; Desrochers, A.; Marc-André, V.; Bourque, J.; Ibarzabal, J. Playbacks of Mobbing Calls of Black-Capped Chickadees as a Method to Estimate Reproductive Activity of Forest Birds. J. Field Ornithol. 2000, 71, 472–483. [Google Scholar] [CrossRef]
- Roché, J.C. All the Bird Songs of Britain and Europe (Disc 4); Sittelle: Mens, France, 1993; Available online: https://www.discogs.com/Jean-C-Roch%C3%A9-All-The-Bird-Songs-Of-Britain-And Europe/release/777634 (accessed on 16 April 2015).
- Summers, R.W.; Jardine, D.C.; Marquiss, M.; Rae, R. The distribution and habitats of crossbills Loxia spp. in Britain, with special reference to the Scottish Crossbill Loxia scotica. Ibis 2002, 144, 393–410. [Google Scholar] [CrossRef]
- Kasprzykowski, Z.; Goławski, A. Does the use of playback affect the estimates of numbers of Grey Partridge Perdix perdix? Wildl. Biol. 2009, 15, 123–129. [Google Scholar] [CrossRef][Green Version]
- Peterson, R.T.; Mountfort, G.; Hollom, P.A.D. Birds of Britain and Europe; Collins: New York, NY, USA, 2004; ISBN 0007192347. [Google Scholar]
- Chang, Y.M. The Influence of avian aerial predator location on perceived predation risk and foraging location in five passerine species. J. Sci. Innov. 2013, 3, 97–111. [Google Scholar]
- Turney, S.; Godin, J.J. To forage or hide? Threat-sensitive foraging behaviour in wild, non-reproductive passerine birds. Curr. Zool. 2014, 60, 719–728. [Google Scholar] [CrossRef]
- Graw, B.; Manser, M.B. The function of mobbing in cooperative meerkats. Anim. Behav. 2007, 74, 507–517. [Google Scholar] [CrossRef]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kreisel, K.; Stein, S. Bird use of burned and unburned coniferous forests during winter. Wilson Bull. 1999, 111, 243–250. [Google Scholar]
- SPSS IBM SPSS Statistics. Available online: http://www-01.ibm.com/software/analytics/spss/products/statistics/ (accessed on 27 October 2015).
- Venier, L.A.; Fahrig, L. Habitat availability causes the species abundance-distribution relationship. Oikos 1996, 76, 564–570. [Google Scholar] [CrossRef]
- Nana, D.E.; Sedlacek, O.; Dolezal, J.; Dancak, M.; Altman, J.; Svoboda, M.; Majesky, U.; Horak, D. Relationship between survival rate of avian artificial nests and forest vegetation structure along a tropical altitudinal gradient on Mount Cameroon. Biotropica 2015, 74, 758–764. [Google Scholar] [CrossRef]
- Paz, U. Plants and Animals of the Land of Israel-Volume 6-Birds; Alon, A., Ed.; Ministry of Defence-Publishing: Tel Aviv, Israel; Society for the Protection of Nature in Israel: Tel Aviv, Israel, 1986. [Google Scholar]
- Nijman, V. Seasonal variation in naturally occurring mobbing behaviour of drongos (Dicruridae) towards two avian predators. Artic. Ethol. Ecol. Evol. 2004, 16, 25–32. [Google Scholar] [CrossRef]
- Bērziņš, A.; Krama, T.; Krams, I.; Freeberg, T.M.; Kivleniece, I.; Kullberg, C.; Rantala, M.J. Mobbing as a trade-off between safety and reproduction in a songbird. Behav. Ecol. 2010, 21, 1054–1060. [Google Scholar] [CrossRef]
- Krams, I.; Krama, T. Interspecific reciprocity explains mobbing behaviour of the breeding chaffinches, Fringilla coelebs. Proc. R. Soc. B 2002, 269, 2345–2350. [Google Scholar] [CrossRef]
- Hinde, R.A. The behaviour of the Great Tit (Parus Major) and some other related species. Behav. Suppl. 1952, 2, 1–201. [Google Scholar]
- Da Cunha, F.C.R.; Fontenelle, J.C.R.; Griessera, M. Predation risk drives the expression of mobbing across bird species. Behav. Ecol. 2017, 28, 1517–1523. [Google Scholar] [CrossRef]
- Gottfried, B.M. Anti-predator aggression in birds nesting in old field habitats: An experimental analysis. Condor 1979, 81, 251–257. [Google Scholar] [CrossRef]
- Griesser, M.; Ekman, J. Nepotistic mobbing behaviour in the Siberian jay, Perisoreus infaustus. Anim. Behav. 2005, 69, 345–352. [Google Scholar] [CrossRef]
- Curio, E.; Klump, G.; Regelmann, K. An anti-predator response in the great tit (Parus major): Is it tuned to predator risk? Oecologia 1983, 60, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Gehlbach, F.R.; Leverett, J.S. Mobbing of Eastern Screech-Owls: Predatory cues, risk to mobbers and degree of threat. Condor 1995, 97, 831–834. [Google Scholar] [CrossRef]
- Lynch, J.F.; Morton, E.S.; Voort, M.E. Van der. Habitat segregation between the sexes of wintering Hooded Warblers (Wilsonia citrina). Auk 1985, 102, 714–721. [Google Scholar]
- Kelset, M.G. A comparison of the song and territorial behaviour of a long-distance migrant, the Marsh Warbler Acrocephalus palustris, in summer and winter. Ibis 1989, 131, 403–414. [Google Scholar] [CrossRef]
- Taku, A.I.; Evaristus, T.A.; Whytock, R.C.; Guilain, T.; Mallord, J. Habitat characteristics of wintering Wood Warbler Phylloscopus sibilatrix in the centre region of Cameroon: Conservation implications. Ostrich 2018, 89, 19–24. [Google Scholar]
- Schwabl, H.; Kriner, E. Territorial aggression and song of male European Robins (Erithacus rubecula) in autumn and spring: Effects of antiandrogen treatment. Horm. Behav. 1991, 25, 180–194. [Google Scholar] [CrossRef]
- Nocera, J.J.; Taylor, P.D.; Ratcliffe, L.M. Inspection of mob-calls as sources of predator information: Response of migrant and resident birds in the Neotropics. Behav. Ecol. Sociobiol. 2008, 62, 1769–1777. [Google Scholar] [CrossRef]
- Randler, C.; Vollmer, C. Asymmetries in commitment in an avian communication network. Naturwissenschaften 2013, 100, 199–203. [Google Scholar] [CrossRef] [PubMed]
- MacDicken, K.G.; Sola, P.; Hall, J.E.; Sabogal, C.; Tadoum, M.; de Wasseige, C. Global progress toward sustainable forest management. For. Ecol. Manag. 2015, 352, 47–56. [Google Scholar] [CrossRef]
- Mitchell, M.S.; Lancia, R.A.; Gerwin, J.A. Using landscape-level data to predict the distribution of birds on a managed forest: Effects of scale. Ecol. Appl. 2001, 11, 1692–1708. [Google Scholar] [CrossRef]
- Clawges, R.; Vierling, K.; Vierling, L.; Rowell, E. The use of airborne lidar to assess avian species diversity, density, and occurrence in a pine/aspen forest. Remote Sens. Environ. 2008, 112, 2064–2073. [Google Scholar] [CrossRef]



| F18,92 = 2.56 | R2 = 0.33 | p < 0.001 | ||
|---|---|---|---|---|
| Estimate | Std. Error | t Value | Pr (>|t|) | |
| (Intercept) | −54.1 | 37.9 | −1.426 | 0.16 |
| Shrub cover (m) | 0.0017 | 0.0005 | 3.184 | <0.001 |
| Trees per ha | 0.0528 | 0.0334 | 1.581 | 0.12 |
| Ground cover (m) | 0.027 | 0.040 | 0.675 | 0.50 |
| Tree height (m) | 2.51 | 2.04 | 1.228 | 0.22 |
| Low tree cover (m) | 0.44 | 0.24 | 1.864 | 0.07 |
| P. major mobbing | 4.75 | 1.37 | 3.468 | <0.001 |
| S. melanocephala mobbing | 3.35 | 1.27 | 2.625 | 0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dagan, U.; Izhaki, I. The Effect of Pine Forest Structure on Bird-Mobbing Behavior: From Individual Response to Community Composition. Forests 2019, 10, 762. https://doi.org/10.3390/f10090762
Dagan U, Izhaki I. The Effect of Pine Forest Structure on Bird-Mobbing Behavior: From Individual Response to Community Composition. Forests. 2019; 10(9):762. https://doi.org/10.3390/f10090762
Chicago/Turabian StyleDagan, Uzi, and Ido Izhaki. 2019. "The Effect of Pine Forest Structure on Bird-Mobbing Behavior: From Individual Response to Community Composition" Forests 10, no. 9: 762. https://doi.org/10.3390/f10090762
APA StyleDagan, U., & Izhaki, I. (2019). The Effect of Pine Forest Structure on Bird-Mobbing Behavior: From Individual Response to Community Composition. Forests, 10(9), 762. https://doi.org/10.3390/f10090762






