Dynamics of Abies nephrolepis Seedlings in Relation to Environmental Factors in Seorak Mountain, South Korea
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Survey and Data Collection
2.3. Analysis Methods
3. Results
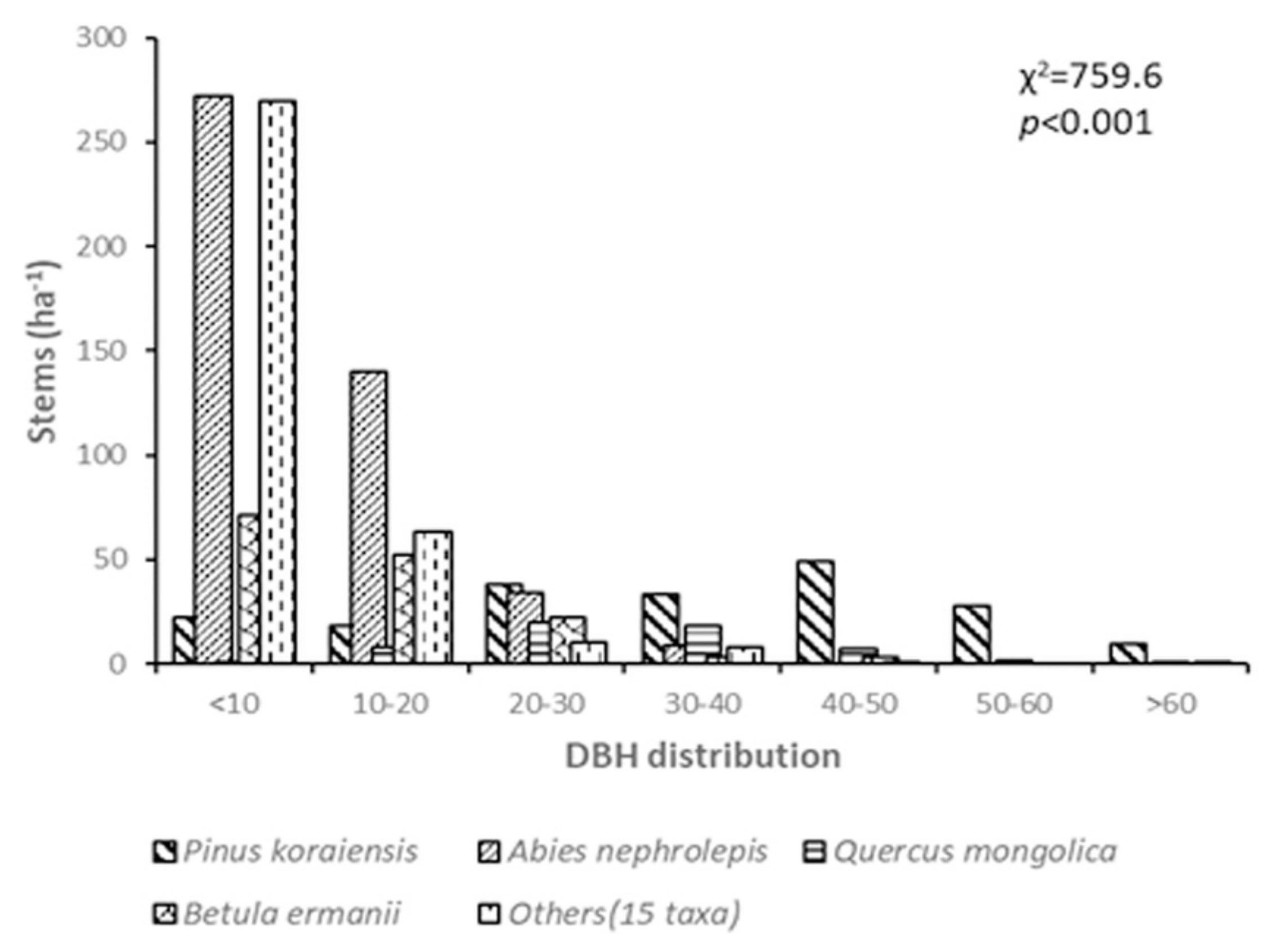
3.1. Short-Term Dynamics of Tree Density and DBH Distribution in Conifer Forests
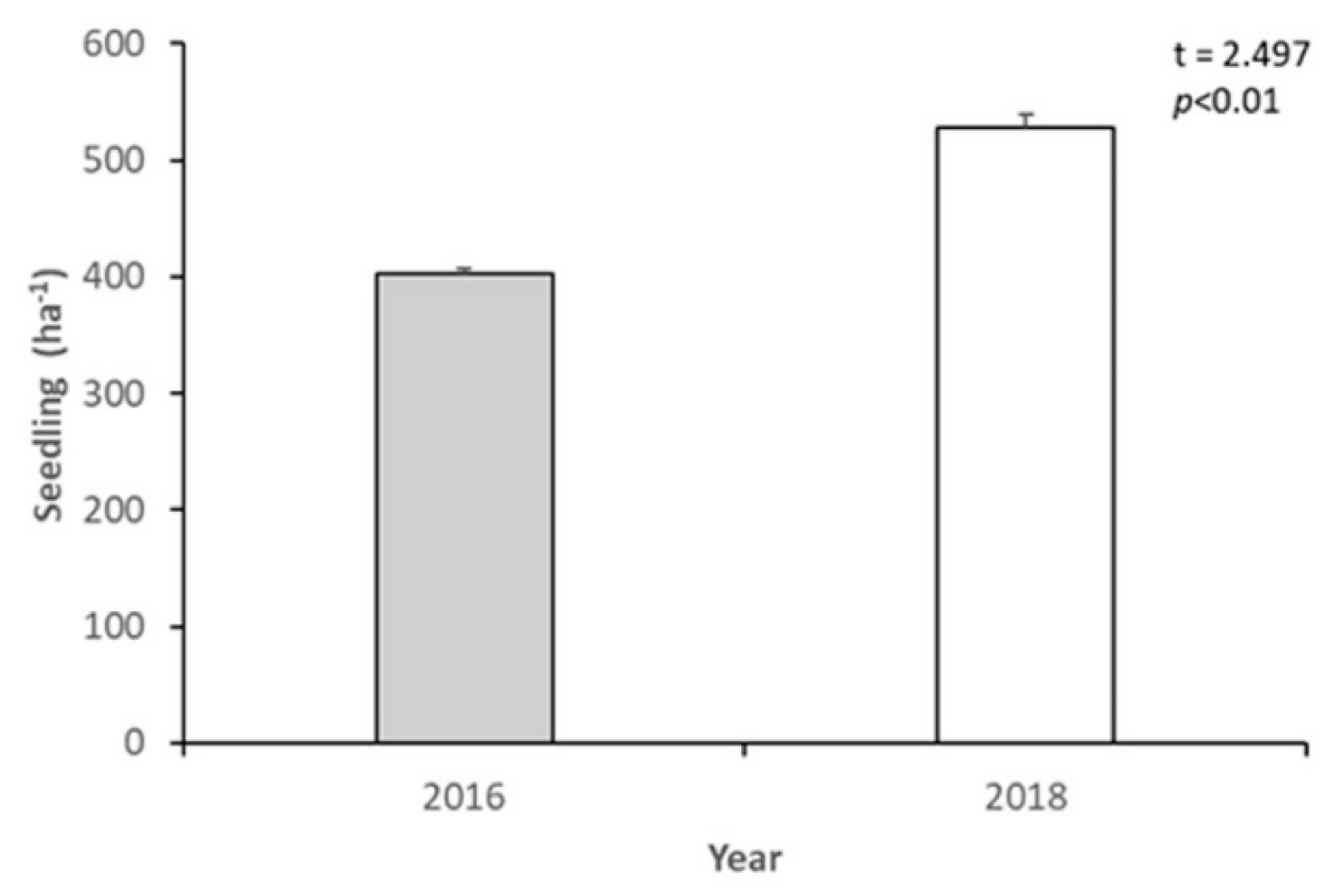
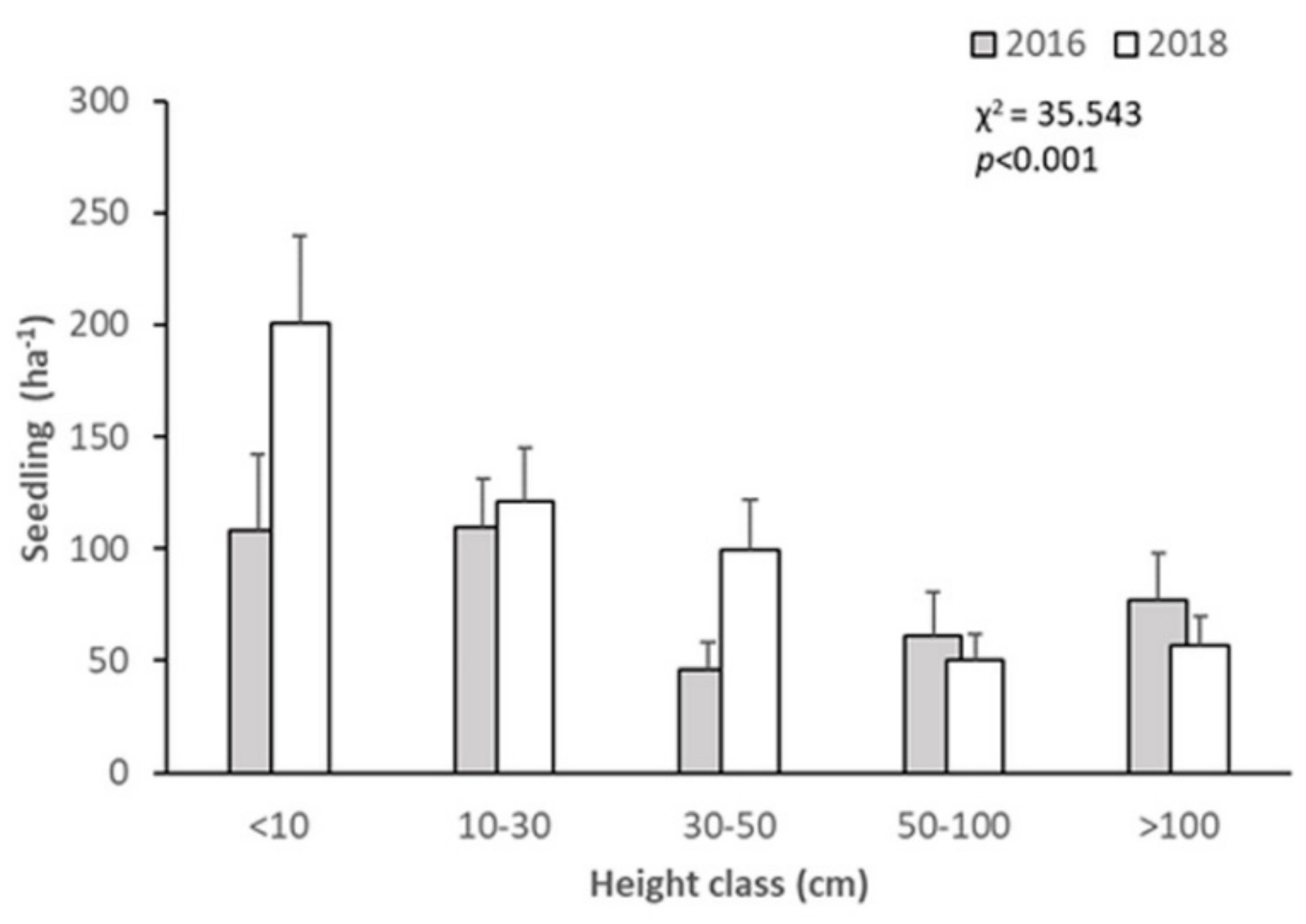
3.2. Short-Term Dynamics of Abies nephrolepis Seedlings
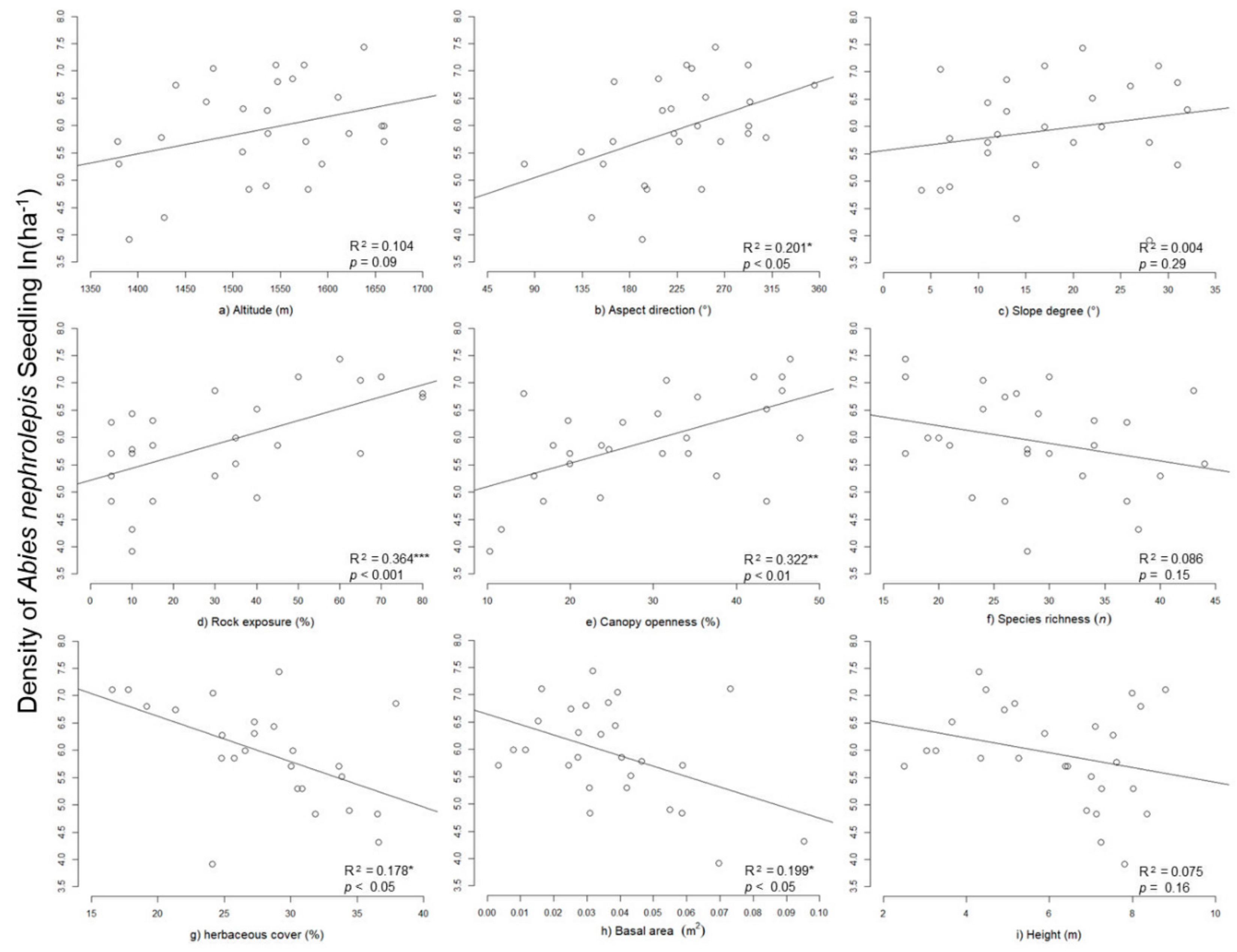
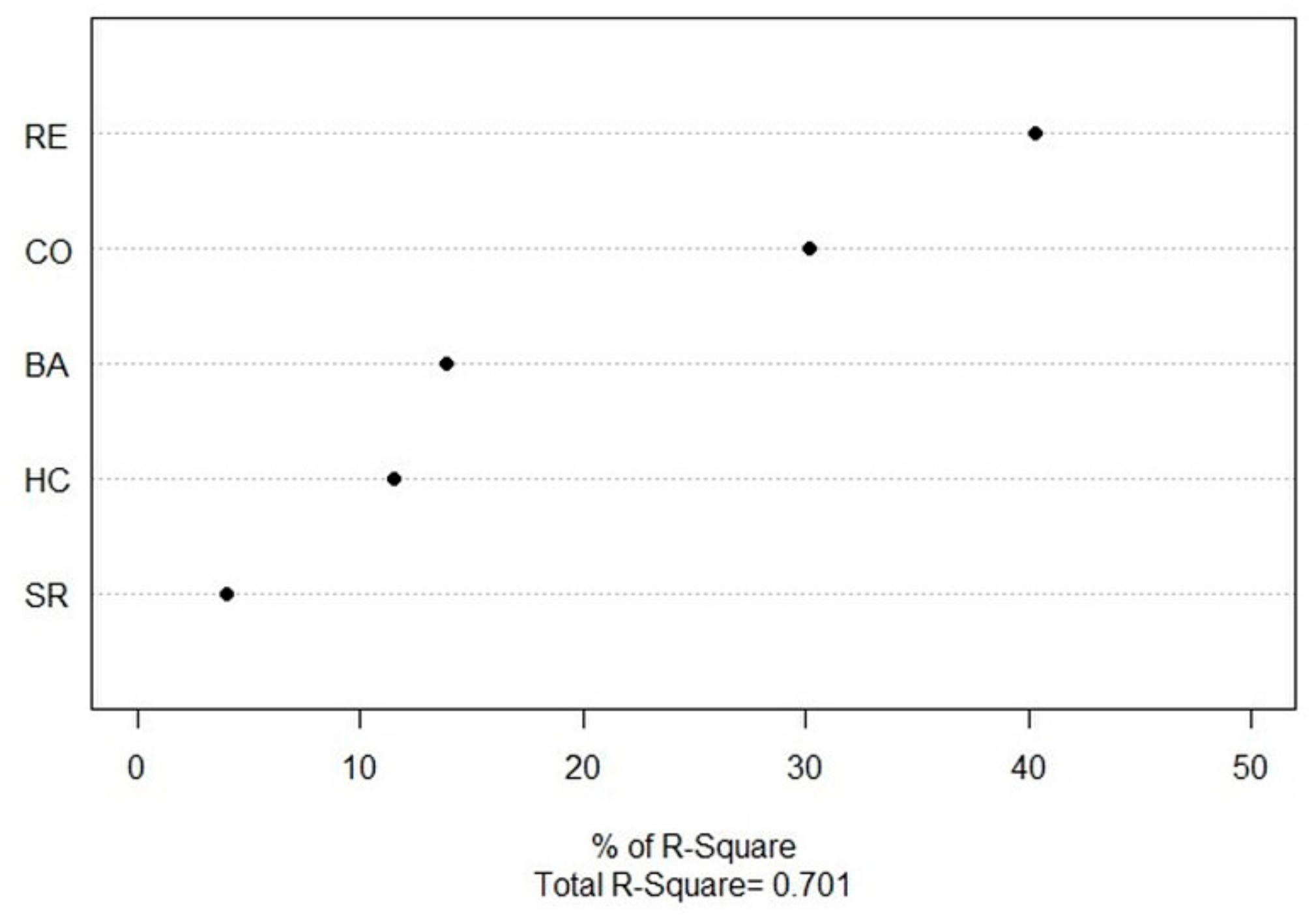
3.3. Abies nephrolepis Seedlings and Their Relationships with Environmental Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kimmins, J.P. Forest Ecology: A Foundation for Sustainable Forest Management and Environmental Ethics in Forestry, 3rd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2004; pp. 349–364. ISBN 978130662583. [Google Scholar]
- Tsuyama, I.; Higa, M.; Nakao, K.; Matsui, T.; Horikawa, M.; Tanaka, N. How Will Subalpine Conifer Distributions be Affected by Climate Change? Impact Assessment for Spatial Conservation Planning. Reg. Environ. Chang. 2015, 15, 393–404. [Google Scholar] [CrossRef]
- Mathys, A.S.; Coops, N.C.; Waring, R.H. An Ecoregion Assessment of Projected Tree Species Vulnerabilities in Western North America through the 21st Century. Glob. Chang. Biol. 2017, 23, 920–932. [Google Scholar] [CrossRef]
- Thompson, L.G.; Mosley-Thompson, E.; Davis, M.E.; Brecher, H.H. Tropical glaciers, recorders and indicators of climate change, are disappearing globally. Ann. Glaciol. 2011, 52, 23–34. [Google Scholar] [CrossRef]
- Cullen, N.J.; Sirguey, P.; Mölg, T.; Kaser, G.; Winkler, M.; Fitzsimons, S.J. A century of ice retreat on Kilimanjaro: The mapping reloaded. Cryosphere 2013, 7, 419–431. [Google Scholar] [CrossRef]
- Juvik, J.; Kueffer, C.; Juvik, S.; Nagata, S. Introduction—Losing the High Ground: Rapid Transformation of Tropical Island Alpine and Subalpine Environments. Arct. Antarct. Alp. Res. 2014, 46, 705–708. [Google Scholar] [CrossRef][Green Version]
- Gobiet, A.; Kotlarski, S.; Beniston, M.; Heinrich, G.; Rajczak, J.; Stoffel, M. 21st century climate change in the European Alps—A review. Sci. Total Environ. 2014, 493, 1138–1151. [Google Scholar] [CrossRef]
- Kohler, T.; Wehrli, A.; Jurek, M. Mountains and Climate Change: A Global Concern; Geographica Bernensia: Bern, Switzerland, 2014; pp. 1–140. ISBN 9783905835380. [Google Scholar]
- Rangwala, I.; Miller, J.R. Climate change in mountains: A review of elevation-dependent warming and its possible causes. Clim. Chang. 2012, 114, 527–547. [Google Scholar] [CrossRef]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolström, M.; et al. Climate Change Impacts, Adaptive Capacity, and Vulnerability of European Forest Ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Gitzen, R.A.; Millspaugh, J.J.; Cooper, A.B.; Licht, D.S. Design and Analysis of Long-Term Ecological Monitoring Studies; Cambridge University Press: Cambridge, UK, 2012; pp. 1–584. ISBN 9780521191548. [Google Scholar]
- Sergeant, C.J.; Moynahan, B.J.; Johnson, W.F. Practical advice for implementing long-term ecosystem monitoring. J. Appl. Ecol. 2012, 49, 969–973. [Google Scholar] [CrossRef]
- Elzinga, C.L.; Salzer, D.W.; Willoughby, J.W. Measuring and Monitoring Plant Populations; Bureau of Land Management: Denver, CO, USA, 1998; pp. 1–7. [Google Scholar]
- Pereira, H.M.; Leadley, P.W.; Proença, V.; Alkemade, R.; Scharlemann, J.P.W.; Fernandez-Manjarrés, J.F.; Araújo, M.B.; Balvanera, P.; Biggs, R.; Cheung, W.W.L.; et al. Scenarios for Global Biodiversity in the 21st Century. Science 2010, 330, 1496–1501. [Google Scholar] [CrossRef] [PubMed]
- Snyman, H. Dynamics and sustainable utilization of rangeland ecosystems in arid and semi-arid climates of southern Africa. J. Arid. Environ. 1998, 39, 645–666. [Google Scholar] [CrossRef]
- Ludwig, J.A.; Wilcox, B.P.; Breshears, D.D.; Tongway, D.J.; Imeson, A.C. Vegetation patches and runoff–erosion as interacting ecohydrological processes in semiarid landscapes. Ecology 2005, 86, 288–297. [Google Scholar] [CrossRef]
- Lim, J.H.; Kim, G.T.; Yun, C.W.; Shin, J.H.; Bae, S.W. Stand Structure and Dynamics of the Pinus parviflora Forest in Ulleungdo Island. J. Korean For. Soc. 2004, 93, 194–201. [Google Scholar]
- Kim, G.S.; Kim, G.D.; Min, B.M.; Park, D.S.; Son, Y.H.; You, Y.H.; Lee, K.E.; Lee, K.S.; Lee, W.S.; Lee, E.J.; et al. Ecological Research in Terrestrial Ecosystem; National Institute of Ecology: Seocheon, Korea, 2014; pp. 7–18. ISBN 9791186197325. [Google Scholar]
- Chun, J.H.; Lim, J.H.; Kim, S.H.; Park, C.R.; Kwon, T.S.; Yang, H.M.; Cho, J.H.; Choi, H.T.; Lee, L.K.; Kim, C.S.; et al. Long-Term Ecological Research (LTER) on Forest Ecosystem Responses to Global Environmental Change; Korea Forest Research Institute: Seoul, Korea, 2014; pp. 27–55. ISBN 9788981762636. [Google Scholar]
- Kim, N.-S.; Lee, H.-C. A Study on Changes and Distributions of Korean Fir in Sub-Alpine Zone. J. Korea Soc. Environ. Restor. Technol. 2013, 16, 49–57. [Google Scholar] [CrossRef]
- Kim, J.D.; Park, G.E.; Lim, J.H.; Yun, C.W. Phytosociological Community Type Classification and Flora of Vascular Plants for the Forest Vegetation of Daecheongbong Area in Mt. Seorak. J. Korean For. Soc. 2017, 106, 130–149. [Google Scholar]
- Mencuccini, M.; Piussi, P.; Sulli, A.Z. Thirty years of seed production in a subalpine Norway spruce forest: Patterns of temporal and spatial variation. For. Ecol. Manag. 1995, 76, 109–125. [Google Scholar] [CrossRef]
- Marr, J.W. The Development and Movement of Tree Islands near the Upper Limit of Yree Growth in the Southern Rocky Mountains. Ecology 1977, 58, 1159–1164. [Google Scholar] [CrossRef]
- Körner, C.; Paulsen, J. A world-wide study of high altitude treeline temperatures. J. Biogeogr. 2004, 31, 713–732. [Google Scholar] [CrossRef]
- Holtmeier, F.K.; Broll, G.; Müterthies, A.; Anschlag, K. Regeneration of trees in the treeline ecotone: Northern Finnish Lapland. Fenn. Int. J. Geogr. 2003, 181, 103–128. [Google Scholar]
- Maher, E.L.; Germino, M.J.; Hasselquist, N.J. Interactive effects of tree and herb cover on survivorship, physiology, and microclimate of conifer seedlings at the alpine tree-line ecotone. Can. J. For. Res. 2005, 35, 567–574. [Google Scholar] [CrossRef]
- Bonan, G.B.; Shugart, H. Environmental Factors and Ecological Processes in Boreal Forests. Annu. Rev. Ecol. Syst. 1989, 20, 1–28. [Google Scholar] [CrossRef]
- Horton, J.L.; Culatta, K.E. Physiological Characteristics of Southern Appalachian High-Elevation Rock Outcrop Herbs on Clear and Cloudy Days. Castanea 2016, 81, 270–279. [Google Scholar] [CrossRef]
- Frei, E.R.; Bianchi, E.; Bernareggi, G.; Bebi, P.; Dawes, M.A.; Brown, C.D.; Trant, A.J.; Mamet, S.D.; Rixen, C. Biotic and abiotic drivers of tree seedling recruitment across an alpine treeline ecotone. Sci. Rep. 2018, 8, 10894. [Google Scholar] [CrossRef] [PubMed]
- Hättenschwiler, S.; Smith, W.K. Seedling occurrence in alpine treeline conifers: A case study from the central Rocky Mountains, USA. Acta Oecol. 1999, 20, 219–224. [Google Scholar] [CrossRef]
- Pansing, E.R.; Tomback, D.F.; Wunder, M.B.; French, J.P.; Wagner, A.C. Microsite and elevation zone effects on seed pilferage, germination, and seedling survival during early whitebark pine recruitment. Ecol. Evol. 2017, 7, 9027–9040. [Google Scholar] [CrossRef] [PubMed]
- Resler, L.M. Geomorphic Controls of Spatial Pattern and Process at Alpine Treeline. Prof. Geogr. 2006, 58, 124–138. [Google Scholar] [CrossRef]
- Wagner, A.; Tomback, D.; Resler, L.; Pansing, E. Whitebark Pine Prevalence and Ecological Function in Treeline Communities of the Greater Yellowstone Ecosystem, USA: Potential Disruption by White Pine Blister Rust. Forests 2018, 9, 635. [Google Scholar] [CrossRef]
- Brang, P.; Moran, J.; Puttonen, P.; Vyse, A. Regeneration of Picea engelmannii and Abies lasiocarpa in high-elevation forests of south-central British Columbia depends on nurse logs. For. Chron. 2003, 79, 273–279. [Google Scholar] [CrossRef]
- Claveau, Y.; Comeau, P.G.; Coates, K.D.; Messier, C. Growth and crown morphological responses of boreal conifer seedlings and saplings with contrasting shade tolerance to a gradient of light and height. Can. J. For. Res. 2002, 32, 458–468. [Google Scholar] [CrossRef]
- Takahashi, K.; Obata, Y. Growth, Allometry and Shade Tolerance of Understory Saplings of Four Subalpine Conifers in Central Japan. J. Plant Res. 2014, 127, 329–338. [Google Scholar] [CrossRef]
- Hilaire, L.R.S.; Leopold, D.J. Conifer seedling distribution in relation to microsite conditions in a central New York forested minerotrophic peatland. Can. J. For. Res. 1995, 25, 261–269. [Google Scholar] [CrossRef]
- Bergeron, Y.; Sirois, L.; Simard, M.-J.; Simard, M. Conifer seedling recruitment in a southeastern Canadian boreal forest: The importance of substrate. J. Veg. Sci. 1998, 9, 575–582. [Google Scholar]
- Germino, M.J.; Smith, W.K.; Resor, A.C. Conifer seedling distribution and survival in an alpine-treeline ecotone. Plant Ecol. 2002, 162, 157–168. [Google Scholar] [CrossRef]
- Hunziker, U.; Brang, P. Microsite patterns of conifer seedling establishment and growth in a mixed stand in the southern Alps. For. Ecol. Manag. 2005, 210, 67–79. [Google Scholar] [CrossRef]
- Kelly, D. The evolutionary ecology of mast seeding. Trends Ecol. Evol. 1994, 9, 465–470. [Google Scholar] [CrossRef]
- Monks, A.; Tanentzap, A.J. Resource limitation underlying multiple masting models makes mast seeding sensitive to future climate change. New Phytol. 2016, 210, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Bogdziewicz, M.; Steele, M.A.; Marino, S.; Crone, E.E. Correlated seed failure as an environmental veto to synchronize reproduction of masting plants. New Phytol. 2018, 219, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Bogdziewicz, M.; Żywiec, M.; Espelta, J.M.; Fernández-Martinez, M.; Calama, R.; Ledwoń, M.; McIntire, E.; Crone, E.E.; Fernandez-Martinez, M.; Martinez, M.F. Environmental Veto Synchronizes Mast Seeding in Four Contrasting Tree Species. Am. Nat. 2019, 194, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.S. Geoecology on the Subalpine Vegetation and Landscape of Mt. Seorak. J. Korean Geogr. Soc. 2000, 35, 177–187. [Google Scholar]
- Kong, W.S.; Lim, J.H. Disjunctive Distribution of Vaccinium Vitis-Idaea and Thermal Condition. J. Korean Geogr. Soc. 2008, 43, 495–510. [Google Scholar]
- Lee, J.H.; Shin, H.S.; Cho, H.J.; Yun, C.W. Subalpine Conifer Forest Communities; National Institute of Ecology: Seoul, Korea, 2014; pp. 1–136. ISBN 9791186197189. [Google Scholar]
- Sala, O.E.; Vuuren, D.; Pereira, H.M. Chapter 10: Biodiversity across Scenarios. In Ecosystems and Human Well-Being: Scenarios; Carpenter, S.R., Pingali, P.L., Bennett, E.M., Zurek, M.B., Eds.; Island Press: New York, NY, USA, 2005; Volume 2, pp. 375–408. ISBN 9781559633918. [Google Scholar]
- Frankham, R.; Ballou, J.D.; Briscoe, D.A. A Primer of Conservation Genetics; Cambridge University Press: Cambridge, UK, 2004; pp. 1–215. ISBN 9780521538275. [Google Scholar]
- Yim, Y.J.; Baek, S.D. Vegetation of Daecheong-Bong. Mt. Seolag. Korean J. Ecol. 1983, 6, 1–13. [Google Scholar]
- Chun, Y.M.; Kwon, J.H.; Hong, M.P.; Lee, J.S.; Choung, H.L.; Lee, S.H. Habitat Environment and Succession of Abies nephrolepis Forest in Mt. Seorak. Geogr. J. Korea 2010, 44, 93–102. [Google Scholar]
- Kim, G.T.; Choo, G.C.; Um, T.W. Studies on the Structure of Forest Community at Taech’ongbong-Soch’ongbong Area in Soraksan national Park. Korean J. Environ. Ecol. 1997, 10, 240–250. [Google Scholar]
- Kim, H.S.; Bae, S.W.; Jang, S.C.; Jeong, J.M. Stand Structure and Growth Characteristics at Different Elevations of the Korean Pine (Pinus Koraiensis) Natural Forest on Mt. Seorak. J. For. Environ. Sci. 2011, 27, 157–167. [Google Scholar]
- Kwon, H.J.; Gwon, J.H.; Han, K.S.; Kim, M.Y.; Song, H.K. Subalpine Forest Vegetation of Daecheongbong Area, Mt. Seoraksan. Korean J. Environ. Ecol. 2010, 24, 194–201. [Google Scholar]
- Park, I.H.; Ryu, S.B.; Choi, Y.C. Forest structure in relation to slope aspect and altitude in Osaek-Taech’ongbong-Shinhungsa area at Soraksan National Park. Korean J. Environ. Ecol. 1998, 11, 486–492. [Google Scholar]
- Song, Y.H.; Yun, C.W. Community Type and Stand Structure of the Korean Pine (Pinus Koraiensis) Natural Forest in Seoraksan National Park. Korean J. Environ. Ecol. 2006, 20, 29–40. [Google Scholar]
- Hong, M.P.; Lee, H.J.; Chun, Y.M.; Hong, B.R. Flora of Mt. Seorak, Gangwon-do. Korean J. Environ. Ecol. 2010, 24, 436–486. [Google Scholar]
- Yun, J.H.; Kim, J.H.; Kim, S.Y.; Park, C.H.; Lee, B.Y. Vertical Distribution of Vascular Plants in Osaek Valley, Seoraksan National Park by Temperature Gradient. Korean J. Environ. Ecol. 2012, 26, 156–185. [Google Scholar]
- Lee, C.B.; Kim, H.H. Elevational Patterns of Plant Species Richness and Relative Importance of Climatic and Topographic Factors on the Mt. Seorak, South Korea. J. Agric. Life Sci. 2018, 52, 1–11. [Google Scholar] [CrossRef]
- Park, H.-C.; Lee, J.-H.; Lee, G.-G. Predicting the suitable habitat of the Pinus pumila under climate change. J. Environ. Impact Assess. 2014, 23, 379–392. [Google Scholar] [CrossRef]
- Hong, S.G.; Kim, J.J.; Cho, H.K. Studies on Natural Regeneration of Abies koreana. Natl. Acad. Sci. 2008, 47, 71–84. [Google Scholar]
- Song, K.-M.; Kang, Y.-J.; Hyeon, H.-J. Vegetation Structure at the Slope Direction and Characteristic of Seedlings of Abies koreana in Hallasan Mountain. J. Environ. Sci. Int. 2014, 23, 39–46. [Google Scholar] [CrossRef]
- Kim, J.D.; Park, G.E.; Lim, J.-H.; Yun, C.W. The Change of Seedling Emergence of Abies koreana and Altitudinal Species Composition in the Subalpine Area of Mt. Jiri over Short-Term (2015–2017). Korean J. Environ. Ecol. 2018, 32, 313–322. [Google Scholar] [CrossRef]
- Jang, W.; Park, P.S. Stand Structure and Maintenance of Picea jezoensis in a Northern Temperate Forest, South Korea. J. Plant Boil. 2010, 53, 180–189. [Google Scholar] [CrossRef]
- Ko, S.Y.; Han, S.H.; Yun, C.W. Population Structure and Dynamics of the Picea jezoensis Stand in Mt. Gyebangsan. J. Korean Soc. For. Sci. 2013, 102, 355–364. [Google Scholar]
- Chun, Y.M.; Ahn, J.K.; Hong, M.P.; Shin, J.T.; Won, H.J.; Lee, S.H. Structure and Dynamics of Abies nephrolepis Community in Odaesan National Park. Geogr. J. Korea 2011, 45, 559–570. [Google Scholar]
- Narukawa, Y.; Yamamoto, S. Effects of dwarf bamboo (Sasa sp.) and forest floor microsites on conifer seedling recruitment in a subalpine forest, Japan. For. Ecol. Manag. 2002, 163, 61–70. [Google Scholar] [CrossRef]
- Fukasawa, Y.; Ando, Y. Species Effects of Bryophyte Colonies on Tree Seeding Regeneration on Coarse Woody Debris. Ecol. Res. 2018, 33, 191–197. [Google Scholar] [CrossRef]
- Bossard, C.C.; Cao, Y.; Wang, J.; Rose, A.; Tang, Y. New patterns of establishment and growth of Picea, Abies and Betula tree species in subalpine forest gaps of Jiuzhaigou National Nature Reserve, Sichuan, southwestern China in a changing environment. For. Ecol. Manag. 2015, 356, 84–92. [Google Scholar] [CrossRef]
- Chhetri, P.K.; Bista, R.; Cairns, D.M. Population structure and dynamics of Abies spectabilis at treeline ecotone of Barun Valley, Makalu Barun National Park, Nepal. Acta Ecol. Sin. 2016, 36, 269–274. [Google Scholar] [CrossRef]
- Wei, X.; Wu, H.; Meng, H.; Pang, C.; Jiang, M. Regeneration dynamics of Euptelea pleiospermum along latitudinal and altitudinal gradients: Trade-offs between seedling and sprout. For. Ecol. Manag. 2015, 353, 232–239. [Google Scholar] [CrossRef]
- Lim, J.H.; Kim, E.S.; Park, G.E.; Kim, Y.S.; Jang, G.C.; Han, J.G.; Jung, S.C.; Lim, H.I.; Lee, B.R.; Song, U.K.; et al. Present Status and Conservation Measure of High Mountain Coniferous Forest in the Endangered; Korea Forest Research Institute: Seoul, Korea, 2019; pp. 87–95. [Google Scholar]
- Walter, H.; Harnickell, E.; Mueller-Dombois, D. Climate-Diagram Maps of the Individual Continents and the Ecological Climatic Regions of the Earth; Springer: Berlin, Germany, 1975; pp. 445–446. [Google Scholar]
- Braun-Blanquet, J. Pflanzensoziologie: Grundzüge Der Vegetationskunde; Springer: Wien, Austria, 1964; pp. 17–205. ISBN 9783709181119. [Google Scholar]
- Frazer, G.W.; Canham, C.; Lertzman, K. Gap Light Analyzer (GLA), Version 2.0: Imaging Software to Extract Canopy Structure and Gap Light Transmission Indices from True-Colour Fisheye Photographs, Users Manual and Program Documentation; Millbrook: New York, NY, USA, 1999; pp. 1–40. [Google Scholar]
- Derksen, S.; Keselman, H.J. Backward, forward and stepwise automated subset selection algorithms: Frequency of obtaining authentic and noise variables. Br. J. Math. Stat. Psychol. 1992, 45, 265–282. [Google Scholar] [CrossRef]
- Ratner, B. Variable selection methods in regression: Ignorable problem, outing notable solution. J. Target. Meas. Anal. Mark. 2010, 18, 65–75. [Google Scholar] [CrossRef]
- Johnson, J.W. A Heuristic Method for Estimating the Relative Weight of Predictor Variables in Multiple Regression. Multivar. Behav. Res. 2000, 35, 1–19. [Google Scholar] [CrossRef]
- Tonidandel, S.; Lebreton, J.M. Relative Importance Analysis: A Useful Supplement to Regression Analysis. J. Bus. Psychol. 2011, 26, 1–9. [Google Scholar] [CrossRef]
- Houle, G. Mast seeding in Abies balsamea, Acer saccharum and Betula alleghaniensis in an old growth, cold temperate forest of north-eastern North America. J. Ecol. 1999, 87, 413–422. [Google Scholar] [CrossRef]
- Manabe, T.; Nishimura, N.; Miura, M.; Yamamoto, S. Population structure and spatial patterns for trees in a temperate old-growth evergreen broad-leaved forest in Japan. Plant Ecol. 2000, 151, 181–197. [Google Scholar] [CrossRef]
- Peñuelas, J.; Ogaya, R.; Boada, M.; Jump, A. Migration, invasion and decline: Changes in recruitment and forest structure in a warming-linked shift of European beech forest in Catalonia (NE Spain). Ecography 2007, 30, 829–837. [Google Scholar] [CrossRef]
- Schwartz, J.W.; Nagel, L.M.; Webster, C.R. Effects of uneven-aged management on diameter distribution and species composition of northern hardwoods in Upper Michigan. For. Ecol. Manag. 2005, 211, 356–370. [Google Scholar] [CrossRef]
- Gaire, N.; Dhakal, Y.; Lekhak, H.; Bhuju, D.; Shah, S. Vegetation Dynamics in Treeline Ecotone of Langtang National Park, Central Nepal. Nepal J. Sci. Technol. 2010, 11, 107–114. [Google Scholar] [CrossRef]
- Dang, H.; Zhang, Y.; Zhang, K.; Jiang, M.; Zhang, Q. Age structure and regeneration of subalpine fir (Abies fargesii) forests across an altitudinal range in the Qinling Mountains, China. For. Ecol. Manag. 2010, 259, 547–554. [Google Scholar] [CrossRef]
- Kuuluvainen, T.; Penttinen, A.; Leinonen, K.; Nygren, M. Statistical opportunities for comparing stand structural heterogeneity in managed and primeval forests: An example from boreal spruce forest in southern Finland. Silva Fenn. 1996, 30, 315–328. [Google Scholar] [CrossRef]
- Motta, R.; Nola, P.; Piussi, P. Structure and Stand Development in Three Subalpine Norway Spruce (Picea abies (L.) Karst.) Stands in Paneveggio (Trento, Italy). Glob. Ecol. Biogeogr. 1999, 8, 455–471. [Google Scholar] [CrossRef]
- Svensson, J.S.; Jeglum, J.K. Structure and dynamics of an undisturbed old-growth Norway spruce forest on the rising Bothnian coastline. For. Ecol. Manag. 2001, 151, 67–79. [Google Scholar] [CrossRef]
- LaMedica, S.; Lingua, E.; Popa, I.; Motta, R.; Carrer, M. Spatial structure in four Norway spruce stands with different management history in the Alps and Carpathians. Silva Fenn. 2011, 45, 865–873. [Google Scholar] [CrossRef]
- Omelko, A.; Ukhvatkina, O.; Zhmerenetsky, A. Disturbance history and natural regeneration of an old-growth Korean pine-broadleaved forest in the Sikhote-Alin mountain range, Southeastern Russia. For. Ecol. Manag. 2016, 360, 221–234. [Google Scholar] [CrossRef]
- Kohyama, T. Regeneration and coexistence of two Abies species dominating subalpine forests in central Japan. Oecologia 1984, 62, 156–161. [Google Scholar] [CrossRef]
- Hasegawa, S.F.; Mori, A. Structural characteristics of Abies mariesii saplings in a snowy subalpine parkland in central Japan. Tree Physiol. 2007, 27, 141–148. [Google Scholar]
- Oliver, W.W.; Dolph, K.L. Mixed-conifer seedling growth varies in response to overstory release. For. Ecol. Manag. 1992, 48, 179–183. [Google Scholar] [CrossRef]
- Mori, A.; Mizumachi, E.; Osono, T.; Doi, Y. Substrate-associated seedling recruitment and establishment of major conifer species in an old-growth subalpine forest in central Japan. For. Ecol. Manag. 2004, 196, 287–297. [Google Scholar] [CrossRef]
- Koski, V.; Selkäinaho, J. Experiments on the Joint Effect of Heat Sum and Photoperiod on Seedlings of Betula Pendula; The Finnish Forest Research Institute: Helsinki, Finland, 1982; pp. 1–44. ISBN 9514005740. [Google Scholar]
- Bonan, G.B. Environmental factors and ecological processes controlling vegetation patterns in boreal forests. Landsc. Ecol. 1989, 3, 111–130. [Google Scholar] [CrossRef]
- Tamm, C.O. Growth, Yield and Nutrition in Carpets of a Forest Moss (Hylocomium Splendens); Reports of the Forest Research Institute of Sweden: Stockholm, Sweden, 1953; pp. 38–46. [Google Scholar]
- Van Cleve, K.; Chapin, F., III; Flanagan, P.; Viereck, L.; Dyrness, C. Forest Ecosystems in the Alaskan Taiga: A Synthesis of Structure and Function; Oechel, W.C., Van Cleve, K., Eds.; Springer: Berlin, Germany, 1986; pp. 121–137. ISBN 9781461293538. [Google Scholar]
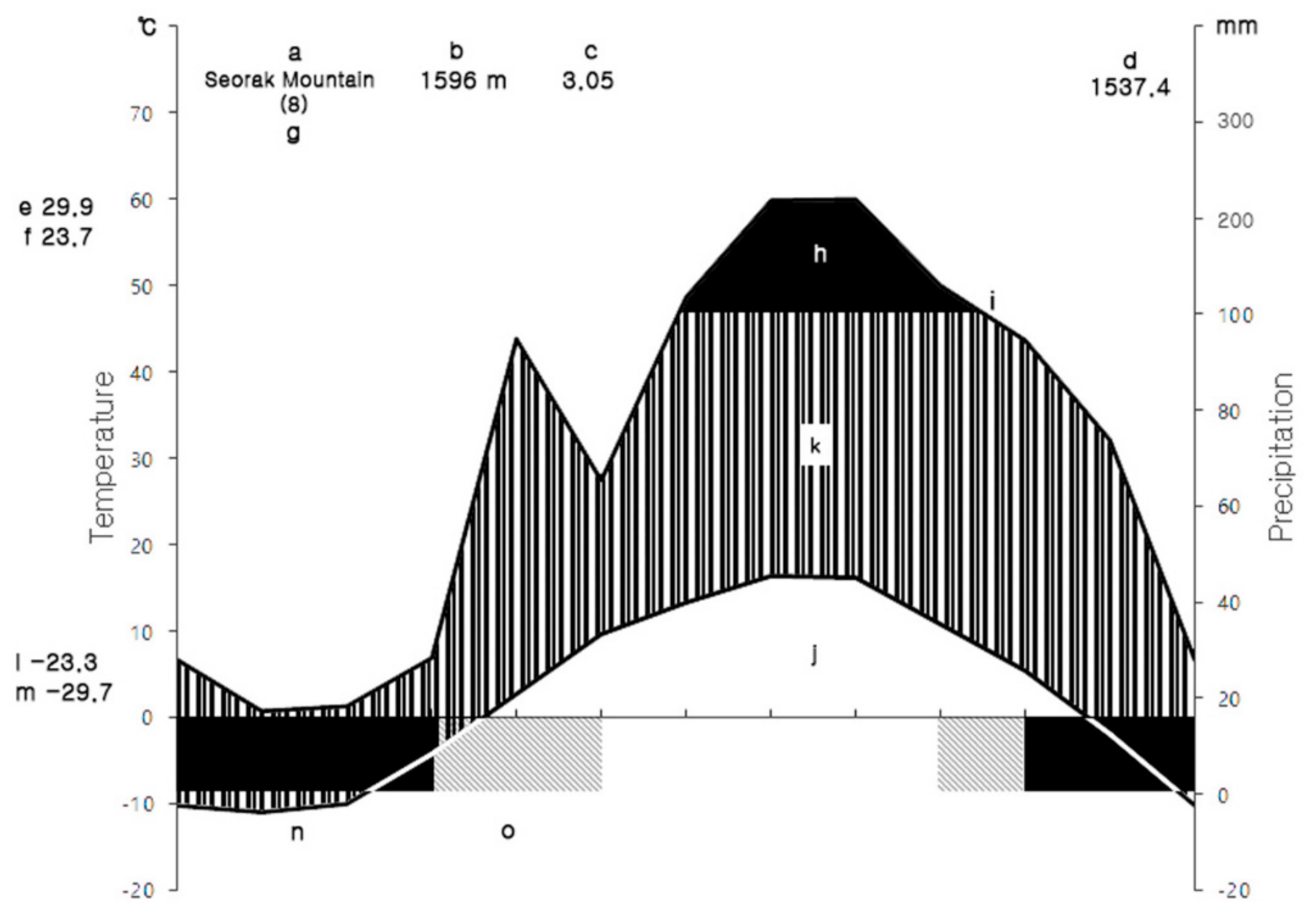
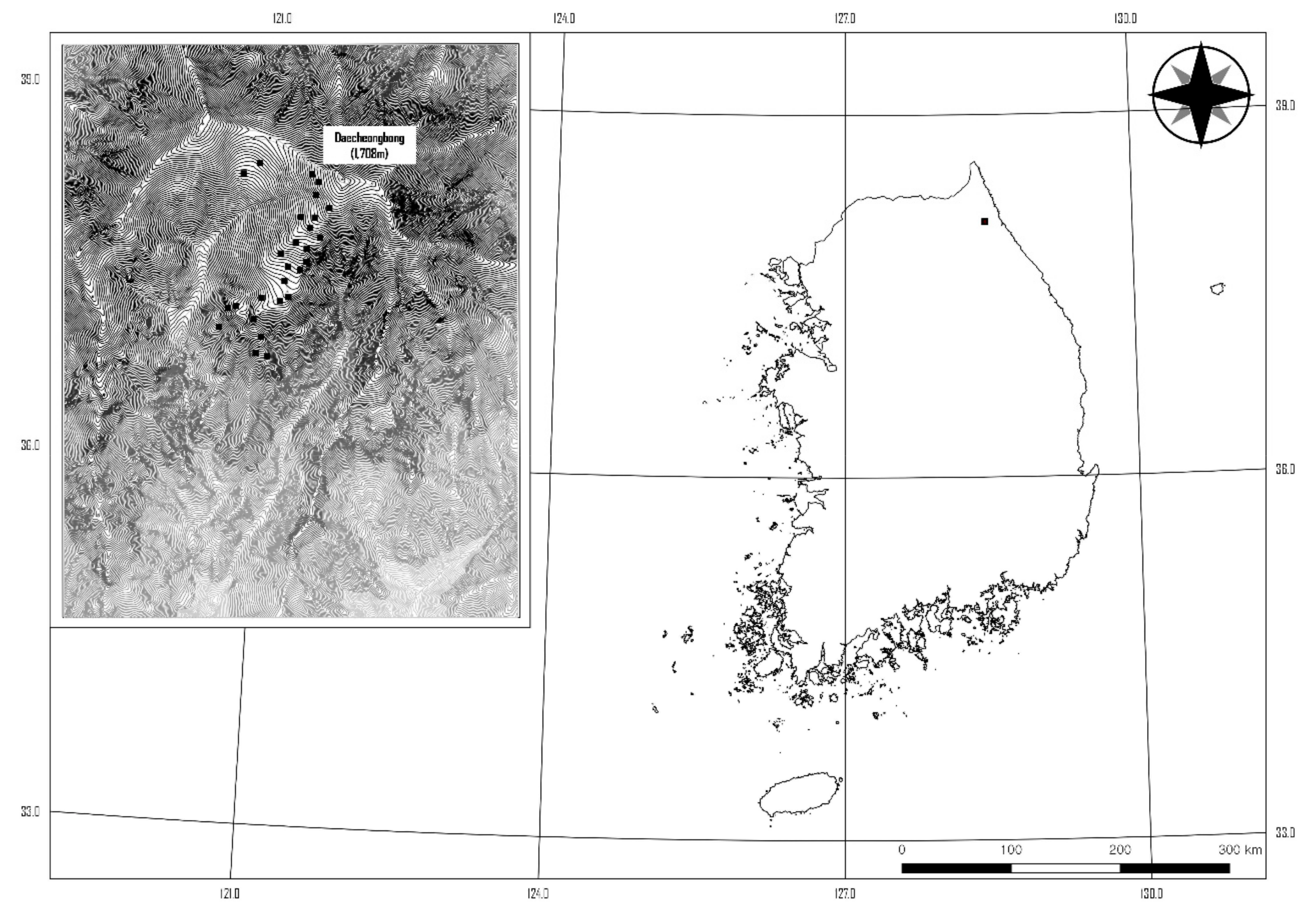
| Variable | Character | Type | Mean ± SE (n = 27) |
|---|---|---|---|
| Altitude (m) | A | N | 1532.1 ± 16.4 |
| Aspect direction (°) | A | N | 225.5 ± 11.9 |
| Slope degree (°) | A | N | 15.6 ± 1.6 |
| Rock exposure (%) | A | N | 32.4 ± 4.8 |
| Canopy openness (%) | A | N | 29 ± 3.3 |
| Species richness (n) | B | N | 28.7 ± 1.5 |
| Herbaceous cover (%) | B | N | 26.7 ± 1.5 |
| Basal area (m2) | B | N | 0.034 ± 0.1 |
| Height (m) | B | N | 6 ± 0.7 |
| Species Population | Alive | Dead | ND | Recruited Stems | ||
|---|---|---|---|---|---|---|
| 16′ | 18′ | 16′ | 18′ | |||
| Pinus koraiensis | 201 | 198 | 23 | 26 | 3 | 0 |
| Abies nephrolepis | 437 | 425 | 32 | 47 | 15 | 3 |
| Quercus mongolica | 60 | 59 | 3 | 4 | 1 | 0 |
| Betula ermanii | 136 | 133 | 5 | 8 | 3 | 0 |
| Dead tree (unknown) | - | - | 23 | 23 | - | 0 |
| Others (15 taxa) | 339 | 336 | 0 | 5 | 5 | 2 |
| Total | 1173 | 1151 | 86 | 113 | 27 | 5 |
| Variable | Estimate | SE | t-Value | p-Value |
|---|---|---|---|---|
| (Intercept) | 4.3783 | 0.7162 | 6.113 | 0 *** |
| Rock exposure (%) | 0.0202 | 0.0048 | 4.221 | 0.0004 *** |
| Canopy openness (%) | 0.0345 | 0.0109 | 3.167 | 0.0046 ** |
| Species richness (n) | 0.0442 | 0.0184 | 2.395 | 0.0260 * |
| Herbaceous cover (%) | −0.0368 | 0.0155 | −2.380 | 0.0269 * |
| Basal area (m2) | −10.6060 | 6.4488 | −1.645 | 0.1149 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Lim, J.-H.; Yun, C. Dynamics of Abies nephrolepis Seedlings in Relation to Environmental Factors in Seorak Mountain, South Korea. Forests 2019, 10, 702. https://doi.org/10.3390/f10080702
Kim J, Lim J-H, Yun C. Dynamics of Abies nephrolepis Seedlings in Relation to Environmental Factors in Seorak Mountain, South Korea. Forests. 2019; 10(8):702. https://doi.org/10.3390/f10080702
Chicago/Turabian StyleKim, JiDong, Jong-Hwan Lim, and ChungWeon Yun. 2019. "Dynamics of Abies nephrolepis Seedlings in Relation to Environmental Factors in Seorak Mountain, South Korea" Forests 10, no. 8: 702. https://doi.org/10.3390/f10080702
APA StyleKim, J., Lim, J.-H., & Yun, C. (2019). Dynamics of Abies nephrolepis Seedlings in Relation to Environmental Factors in Seorak Mountain, South Korea. Forests, 10(8), 702. https://doi.org/10.3390/f10080702





