Ground-Dwelling Arthropod Community Responses to Recent and Repeated Wildfires in Conifer Forests of Northern New Mexico, USA
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design and Arthropod Sampling
2.3. Understory Environmental Assessment
2.4. Data Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bowman, D.M.J.S.; Balch, J.; Artaxo, P.; Bond, W.J.; Cochrane, M.A.; D’antonio, C.M.; DeFries, R.; Johnston, F.H.; Keeley, J.E.; Krawchuk, M.A.; et al. The human dimension of fire regimes on Earth. J. Biogeogr. 2011, 38, 2223–2236. [Google Scholar] [CrossRef] [PubMed]
- Baker, W.L. Spatially heterogeneous multi-scale response of landscapes to fire suppression. Oikos 1993, 66–71. [Google Scholar] [CrossRef]
- Abatzoglou, J.T.; Balch, J.K.; Bradley, B.A.; Kolden, C.A. Human-related ignitions concurrent with high winds promote large wildfires across the USA. Int. J. Wildland Fire 2018, 27, 377–386. [Google Scholar] [CrossRef]
- Allen, C.D.; Savage, M.; Falk, D.A.; Suckling, K.F.; Swetnam, T.W.; Schulke, T.; Stacey, P.B.; Morgan, P.; Hoffman, M.; Klingel, J.T. Ecological restoration of southwestern ponderosa pine ecosystems: A broad perspective. Ecol. Appl. 2002, 12, 1418–1433. [Google Scholar] [CrossRef]
- Westerling, A.L.; Hidalgo, H.G.; Cayan, D.R.; Swetnam, T.W. Warming and earlier spring increase western US forest wildfire activity. Science 2006, 313, 940–943. [Google Scholar] [CrossRef] [PubMed]
- Dennison, P.E.; Brewer, S.C.; Arnold, J.D.; Moritz, M.A. Large wildfire trends in the western United States, 1984–2011. Geophys. Res. Lett. 2014, 41, 2928–2933. [Google Scholar] [CrossRef]
- Abatzoglou, J.T.; Williams, A.P. Impact of anthropogenic climate change on wildfire across western US forests. Proc. Natl. Acad. Sci. USA 2016, 113, 11770–11775. [Google Scholar] [CrossRef] [PubMed]
- Stephens, S.L.; Collins, B.M.; Fettig, C.J.; Finney, M.A.; Hoffman, C.M.; Knapp, E.E.; North, M.P.; Safford, H.; Wayman, R.B. Drought, tree mortality, and wildfire in forests adapted to frequent fire. BioScience 2018. [Google Scholar] [CrossRef]
- Pastro, L.A.; Dickman, C.R.; Letnic, M. Burning for biodiversity or burning biodiversity? Prescribed burn vs. wildfire impacts on plants, lizards, and mammals. Ecol. Appl. 2011, 21, 3238–3253. [Google Scholar] [CrossRef]
- Tingley, M.W.; Ruiz-Gutiérrez, V.; Wilkerson, R.L.; Howell, C.A.; Siegel, R.B. Pyrodiversity promotes avian diversity over the decade following forest fire. Proc. R. Soc. B 2016, 283, 1703. [Google Scholar] [CrossRef]
- Buddle, C.M.; Langor, D.W.; Pohl, G.R.; Spence, J.R. Arthropod responses to harvesting and wildfire: Implications for emulation of natural disturbance in forest management. Biol. Conserv. 2006, 128, 346–357. [Google Scholar] [CrossRef]
- Ferrenberg, S.M.; Schwilk, D.W.; Knapp, E.E.; Groth, E.; Keeley, J.E. Fire decreases arthropod abundance but increases diversity: Early and late season prescribed fire effects in a Sierra Nevada mixed-conifer forest. Fire Ecol. 2006, 2, 79–101. [Google Scholar] [CrossRef]
- Ferrenberg, S.; Martinez, A.S.; Faist, A.M. Aboveground and belowground arthropods experience different relative influences of stochastic versus deterministic community assembly processes following disturbance. PeerJ 2016, 4, e2545. [Google Scholar] [CrossRef] [PubMed]
- Moretti, M.; Duelli, P.; Obrist, M.K. Biodiversity and resilience of arthropod communities after fire disturbance in temperate forests. Oecologia 2006, 149, 312–327. [Google Scholar] [CrossRef] [PubMed]
- Lessard, J.P.; Sackett, T.E.; Reynolds, W.N.; Fowler, D.A.; Sanders, N.J. Determinants of the detrital arthropod community structure: The effects of temperature and resources along an environmental gradient. Oikos 2011, 120, 333–343. [Google Scholar] [CrossRef]
- Ober, H.K.; DeGroote, L.W. Effects of litter removal on arthropod communities in pine plantations. Biodivers. Conserv. 2011, 20, 1273–1286. [Google Scholar] [CrossRef]
- Armitage, A.R.; Ho, C.K.; Quigg, A. The interactive effects of pulsed grazing disturbance and patch size vary among wetland arthropod guilds. PloS One 2013, 8, e76672. [Google Scholar] [CrossRef]
- Arnan, X.; Cerdá, X.; Retana, J. Partitioning the impact of environment and spatial structure on alpha and beta components of taxonomic, functional, and phylogenetic diversity in European ants. PeerJ 2015, 3, e1241. [Google Scholar] [CrossRef]
- Delph, R.J.; Clifford, M.J.; Cobb, N.S.; Ford, P.L.; Brantley, S.L. Pinyon pine mortality alters communities of ground-dwelling arthropods. West. North Am. Nat. 2014, 74, 162–185. [Google Scholar] [CrossRef]
- Williams, R.S.; Marbert, B.S.; Fisk, M.C.; Hanson, P.J. Ground-dwelling beetle responses to long-term precipitation alterations in a hardwood forest. Southeast. Nat. 2014, 13, 138–155. [Google Scholar] [CrossRef]
- Brunbjerg, A.K.; Jørgensen, G.P.; Nielsen, K.M.; Pedersen, M.L.; Svenning, J.C.; Ejrnæs, R. Disturbance in dry coastal dunes in Denmark promotes diversity of plants and arthropods. Biol. Conserv. 2015, 182, 243–253. [Google Scholar] [CrossRef]
- Moretti, M.; Obrist, M.K.; Duelli, P. Arthropod biodiversity after forest fires: Winners and losers in the winter fire regime of the southern Alps. Ecography 2004, 27, 173–186. [Google Scholar] [CrossRef]
- Radea, C.; Arianoutsou, M. Soil arthropod communities and population dynamics following wildfires in Pine forests of the Mediterranean basin: A review. Isr. J. Ecol. Evol. 2012, 58, 137–149. [Google Scholar]
- Vasconcelos, H.L.; Pacheco, R.; Silva, R.C.; Vasconcelos, P.B.; Lopes, C.T.; Costa, A.N.; Bruna, E.M. Dynamics of the leaf-litter arthropod fauna following fire in a neotropical woodland savanna. PloS ONE 2009, 4, e7762. [Google Scholar] [CrossRef] [PubMed]
- Wenninger, A.; Hollingsworth, T.; Wagner, D. Predatory hymenopteran assemblages in boreal Alaska: Associations with forest composition and post-fire succession. Écoscience 2019, 26, 205–220. [Google Scholar] [CrossRef]
- Parr, C.L.; Robertson, H.G.; Biggs, H.C.; Chown, S.L. Response of African savanna ants to long-term fire regimes. J. Appl. Ecol. 2004, 41, 630–642. [Google Scholar] [CrossRef]
- Ratchford, J.S.; Wittman, S.E.; Jules, E.S.; Ellison, A.M.; Gotelli, N.J.; Sanders, N.J. The effects of fire, local environment and time on ant assemblages in fens and forests. Divers. Distrib. 2005, 11, 487–497. [Google Scholar] [CrossRef]
- Sackmann, P.; Farji-Brener, A. Effect of fire on ground beetles and ant assemblages along an environmental gradient in NW Patagonia: Does habitat type matter? Ecoscience 2006, 13, 360–371. [Google Scholar] [CrossRef]
- Andersen, A.N.; Ribbons, R.R.; Pettit, M.; Parr, C.L. Burning for biodiversity: Highly resilient ant communities respond only to strongly contrasting fire regimes in Australia’s seasonal tropics. J. Appl. Ecol. 2014, 51, 1406–1413. [Google Scholar] [CrossRef]
- Vasconcelos, H.L.; Maravalhas, J.B.; Cornelissen, T. Effects of fire disturbance on ant abundance and diversity: A global meta-analysis. Biodivers. Conserv. 2017, 26, 177–188. [Google Scholar] [CrossRef]
- MacArthur, R.H. On the relative abundance of bird species. Proc. Natl. Acad. Sci. USA 1957, 43, 293. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D. Resource Competition and Community Structure (mpb-17); Princeton University Press: Princeton, NJ, USA, 1982. [Google Scholar]
- MacArthur, R.H.; Wilson, E.O. The Theory of Island Biogeography; Princeton University Press: Princeton, NJ, USA, 2001; Volume 1. [Google Scholar]
- Connell, J.H. Diversity in tropical rain forests and coral reefs. Science 1978, 199, 1302–1310. [Google Scholar] [CrossRef] [PubMed]
- Hubbell, S.P. The Unified Neutral Theory of Biodiversity and Biogeography (MPB-32); Princeton University Press: Princeton, NJ, USA, 2001. [Google Scholar]
- Chave, J. Neutral theory and community ecology. Ecol. lett. 2004, 7, 241–253. [Google Scholar] [CrossRef]
- Adler, P.B.; HilleRisLambers, J.; Levine, J.M. A niche for neutrality. Ecol. let. 2007, 10, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Hart, D.D. Community organization in streams: The importance of species interactions, physical factors, and chance. Oecologia 1992, 91, 220–228. [Google Scholar] [CrossRef]
- Thompson, R.; Townsend, C. A truce with neutral theory: Local deterministic factors, species traits and dispersal limitation together determine patterns of diversity in stream invertebrates. J. Anim. Ecol. 2006, 75, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Chase, J.M. Drought mediates the importance of stochastic community assembly. Proc. Natl. Acad. Sci. USA 2007, 104, 17430–17434. [Google Scholar] [CrossRef]
- Farnon Ellwood, M.D.; Manica, A.; Foster, W.A. Stochastic and deterministic processes jointly structure tropical arthropod communities. Ecol. lett. 2009, 12, 277–284. [Google Scholar] [CrossRef]
- Rominger, A.J.; Miller, T.E.; Collins, S.L. Relative contributions of neutral and niche-based processes to the structure of a desert grassland grasshopper community. Oecologia 2009, 161, 791–800. [Google Scholar] [CrossRef]
- Lepori, F.; Malmqvist, B. Deterministic control on community assembly peaks at intermediate levels of disturbance. Oikos 2009, 118, 471–479. [Google Scholar] [CrossRef]
- Nemergut, D.R.; Schmidt, S.K.; Fukami, T.; O’Neill, S.P.; Bilinski, T.M.; Stanish, L.F.; Knelman, J.E.; Darcy, J.L.; Lynch, R.C.; Wickey, P.; et al. Patterns and processes of microbial community assembly. Microbiol. Mol. Biol. Rev. 2013, 77, 342–356. [Google Scholar] [CrossRef] [PubMed]
- Ferrenberg, S.; O’neill, S.P.; Knelman, J.E.; Todd, B.; Duggan, S.; Bradley, D.; Robinson, T.; Schmidt, S.K.; Townsend, A.R.; Williams, M.W.; et al. Changes in assembly processes in soil bacterial communities following a wildfire disturbance. ISME J. 2013, 7, 1102. [Google Scholar] [CrossRef] [PubMed]
- Barber, N.A.; Marquis, R.J. Leaf quality, predators, and stochastic processes in the assembly of a diverse herbivore community. Ecology 2011, 92, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Frizzo, T.L.; Campos, R.I.; Vasconcelos, H.L. Contrasting effects of fire on arboreal and ground-dwelling ant communities of a neotropical savanna. Biotropica 2012, 44, 254–261. [Google Scholar] [CrossRef]
- Ingwell, L.L.; Miller-Pierce, M.; Trotter, R.T.; Preisser, E.L. Vegetation and invertebrate community response to Eastern Hemlock decline in southern New England. Northeast. Nat. 2012, 19, 541–559. [Google Scholar] [CrossRef]
- Savage, M.; Mast, J.N.; Feddema, J.J. Double whammy: High-severity fire and drought in ponderosa pine forests of the Southwest. Can. J. For. Res. 2013, 43, 570–583. [Google Scholar] [CrossRef]
- Holden, Z.A.; Morgan, P.; Hudak, A.T. Burn severity of areas reburned by wildfires in the Gila National Forest, New Mexico, USA. Fire Ecol. 2010, 6, 77–85. [Google Scholar] [CrossRef]
- Coop, J.D.; Parks, S.A.; McClernan, S.R.; Holsinger, L.M. Influences of prior wildfires on vegetation response to subsequent fire in a reburned southwestern landscape. Ecol. Appl. 2016, 26, 346–354. [Google Scholar] [CrossRef]
- Barton, A.M.; Poulos, H.M. Pine vs. oaks revisited: Conversion of Madrean pine-oak forest to oak shrubland after high-severity wildfire in the Sky Islands of Arizona. For. Ecol. Manag. 2018, 414, 28–40. [Google Scholar] [CrossRef]
- Swetnam, T.W.; Baisan, C.H. Historical fire regime patterns in the southwestern United States since AD 1700. In Fire Effects in Southwestern Forests, Proceedings of the Second La Mesa Fire Symposium, Los Alamos, New Mexico, 29–31 March 1994; Allen, C.D., Ed.; Rocky Mountain Research Station. USDA Forest Service: Fort Collins, CO, USA, 1996; pp. 11–32. [Google Scholar]
- Brown, P.M.; Kaye, M.W.; Huckaby, L.S.; Baisan, C.H. Fire history along environmental gradients in the Sacramento Mountains, New Mexico: Influences of local patterns and regional processes. Ecoscience 2001, 8, 115–126. [Google Scholar] [CrossRef]
- Odion, D.C.; Hanson, C.T.; Arsenault, A.; Baker, W.L.; DellaSala, D.A.; Hutto, R.L.; Klenner, W.; Moritz, M.A.; Sherriff, R.L.; Veblen, T.T.; et al. Examining historical and current mixed-severity fire regimes in ponderosa pine and mixed-conifer forests of western North America. PloS ONE 2014, 9, e87852. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.W.; Cobb, N.S.; Sommer, S.; Delph, R.J.; Brantley, S.L. Ground-dwelling arthropod responses to succession in a pinyon-juniper woodland. Ecosphere 2014, 5, 1–29. [Google Scholar] [CrossRef]
- Touchan, R.; Allen, C.D.; Swetnam, T.W. Fire history and climatic patterns in ponderosa pine and mixed-conifer forests of the Jemez Mountains, northern New Mexico. Fire Eff. Southwest. 1994, 33–46. [Google Scholar]
- Querner, P.; Bruckner, A. Combining pitfall traps and soil samples to collect Collembola for site scale biodiversity assessments. Appl. Soil Ecol. 2010, 45, 293–297. [Google Scholar] [CrossRef]
- Woodcock, B.A. Pitfall trapping in ecological studies. Insect Sampl. For. Ecosyst. 2005, 37–57. [Google Scholar]
- Brown, J.K. Handbook for Inventorying Downed Woody Material; Gen. Tech. Rep. INT-16; US Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station: Ogden, UT, USA, 1974; p. 24.
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’hara, R.B.; Simpson, G.L.; Stevens, M.H.H.; Wagner, H.; Oksanen, M.J. Package ‘Vegan’. Community Ecology Package, Version 2.5–5, 2013, 2. Available online: https://cran.r-project.org/web/packages/vegan/vegan.pdf (accessed on 7 August 2019).
- Roberts, D.W. Ordination on the basis of fuzzy set theory. Vegetatio 1986, 66, 123–131. [Google Scholar] [CrossRef]
- Roberts, D.W. fso: Fuzzy Set Ordination. R Package Version 2.1-1. 2018. Available online: https://cran.r-project.org/web/packages/fso/fso.pdf (accessed on 7 August 2019).
- Dallas, T. metacom: An R package for the analysis of metacommunity structure. Ecography 2014, 37, 402–405. [Google Scholar] [CrossRef]
- Cáceres, M.D.; Legendre, P. Associations between species and groups of sites: Indices and statistical inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. Available online: https://arxiv.org/abs/1406.5823 (accessed on 8 August 2019).
- Liaw, A.; Wiener, M. Classification and regression by randomForest. R News 2002, 2, 18–22. [Google Scholar]
- Kuhn, M. Building Predictive Models in R Using the caret Package. J. Stat. Softw. 2008, 28, 1–26. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest package: Tests in linear mixed effects models. J. Stat. Softw. 2017, 82. [Google Scholar] [CrossRef]
- Barton, B.K. MuMIn: Multi-Model Inference. R Package Version 1.15. 6. 2016. Available online: https://cran.r-project.org/web/packages/MuMIn/MuMIn.pdf (accessed on 7 August 2019).
- Wheeler, R.E. Permutation Tests for Linear Models in R. Available online: https://cran.r-project.org/web/packages/lmPerm/vignettes/lmPerm.pdf. (accessed on 7 August 2019).
- Maindonald, J.H.; Braun, W.J. Package: DAAG. Version 1.22.1. Available online: https://cran.r-project.org/web/packages/DAAG/DAAG.pdf (accessed on 7 August 2019).
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S. Fourth Edition; Springer: New York, NY, USA, 2002; ISBN 0-387-95457-0. [Google Scholar]
- Niemelä, J.; Haila, Y.; Punttila, P. The importance of small-scale heterogeneity in boreal forests: Variation in diversity in forest-floor invertebrates across the succession gradient. Ecography 1996, 19, 352–368. [Google Scholar] [CrossRef]
- Kaila, L.; Martikainen, P.; Punttila, P. Dead trees left in clear-cuts benefit saproxylic Coleoptera adapted to natural disturbances in boreal forest. Biodivers. Conserv. 1997, 6, 1–18. [Google Scholar] [CrossRef]
- Schiegg, K. Effects of dead wood volume and connectivity on saproxylic insect species diversity. Ecoscience 2000, 7, 290–298. [Google Scholar] [CrossRef]
- Kehler, D.; Bondrup-Nielsen, S.; Corkum, C. Beetle diversity associated with forest structure including deadwood in softwood and hardwood stands in Nova Scotia. Proc. N. S. Inst. Sci. 2004, 42, 227–239. [Google Scholar] [CrossRef][Green Version]
- Silveira, J.M.; Barlow, J.; Louzada, J.; Moutinho, P. Factors affecting the abundance of leaf-litter arthropods in unburned and thrice-burned seasonally-dry Amazonian forests. PloS ONE 2010, 5, e12877. [Google Scholar] [CrossRef]
- Apigian, K.O.; Dahlsten, D.L.; Stephens, S.L. Fire and fire surrogate treatment effects on leaf litter arthropods in a western Sierra Nevada mixed-conifer forest. For. Ecol. Manag. 2006, 221, 110–122. [Google Scholar] [CrossRef]
- Apigian, K.O.; Dahlsten, D.L.; Stephens, S.L. Biodiversity of Coleoptera and the importance of habitat structural features in a Sierra Nevada mixed-conifer forest. Environ. Entomol. 2006, 35, 964–975. [Google Scholar] [CrossRef]
- Sasal, Y.; Raffaele, E.; Farji-Brener, A.G. Succession of ground-dwelling beetle assemblages after fire in three habitat types in the Andean forest of NW Patagonia, Argentina. J. Insect Sci. 2010, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Arnan, X.; Cerdá, X.; Rodrigo, A.; Retana, J. Response of ant functional composition to fire. Ecography 2013, 36, 1182–1192. [Google Scholar] [CrossRef]
- Stinner, R.E.; Barfield, C.S.; Stimac, J.L.; Dohse, L. Dispersal and movement of insect pests. Annu. Rev. Entomol. 1983, 28, 319–335. [Google Scholar] [CrossRef]
- Ranius, T. Measuring the dispersal of saproxylic insects: A key characteristic for their conservation. Popul. Ecol. 2006, 48, 177–188. [Google Scholar] [CrossRef]
- Leibold, M.A.; Holyoak, M.; Mouquet, N.; Amarasekare, P.; Chase, J.M.; Hoopes, M.F.; Holt, R.D.; Shurin, J.B.; Law, R.; Tilman, D.; et al. The metacommunity concept: A framework for multi-scale community ecology. Ecol. Lett. 2004, 7, 601–613. [Google Scholar] [CrossRef]
- Mouquet, N.; Loreau, M. Community patterns in source-sink metacommunities. Am. Nat. 2003, 162, 544–557. [Google Scholar] [CrossRef] [PubMed]
- Farji-Brener, A.G.; Corley, J.C.; Bettinelli, J. The effects of fire on ant communities in north-western Patagonia: The importance of habitat structure and regional context. Divers. Distrib. 2002, 8, 235–243. [Google Scholar] [CrossRef]
- Košulič, O.; Michalko, R.; Hula, V. Impact of canopy openness on spider communities: Implications for conservation management of formerly coppiced oak forests. PLoS ONE 2016, 11, e0148585. [Google Scholar] [CrossRef]
- Gonçalves-Souza, T.; Romero, G.Q.; Cottenie, K. Metacommunity versus biogeography: A case study of two groups of neotropical vegetation-dwelling arthropods. PloS ONE 2014, 9, e115137. [Google Scholar] [CrossRef]
- Haire, S.L.; Coop, J.D.; Miller, C. Characterizing Spatial Neighborhoods of Refugia Following Large Fires in Northern New Mexico USA. Land 2017, 6, 19. [Google Scholar] [CrossRef]
- Meddens, A.J.H.; Kolden, C.A.; Lutz, J.A.; Smith, A.M.S.; Cansler, C.A.; Abatzoglou, J.T.; Meigs, G.W.; Downing, W.M.; Krawchuk, M.A. Fire refugia: What are they, and why do they matter for global change? BioScience 2018, 68, 944–954. [Google Scholar] [CrossRef]
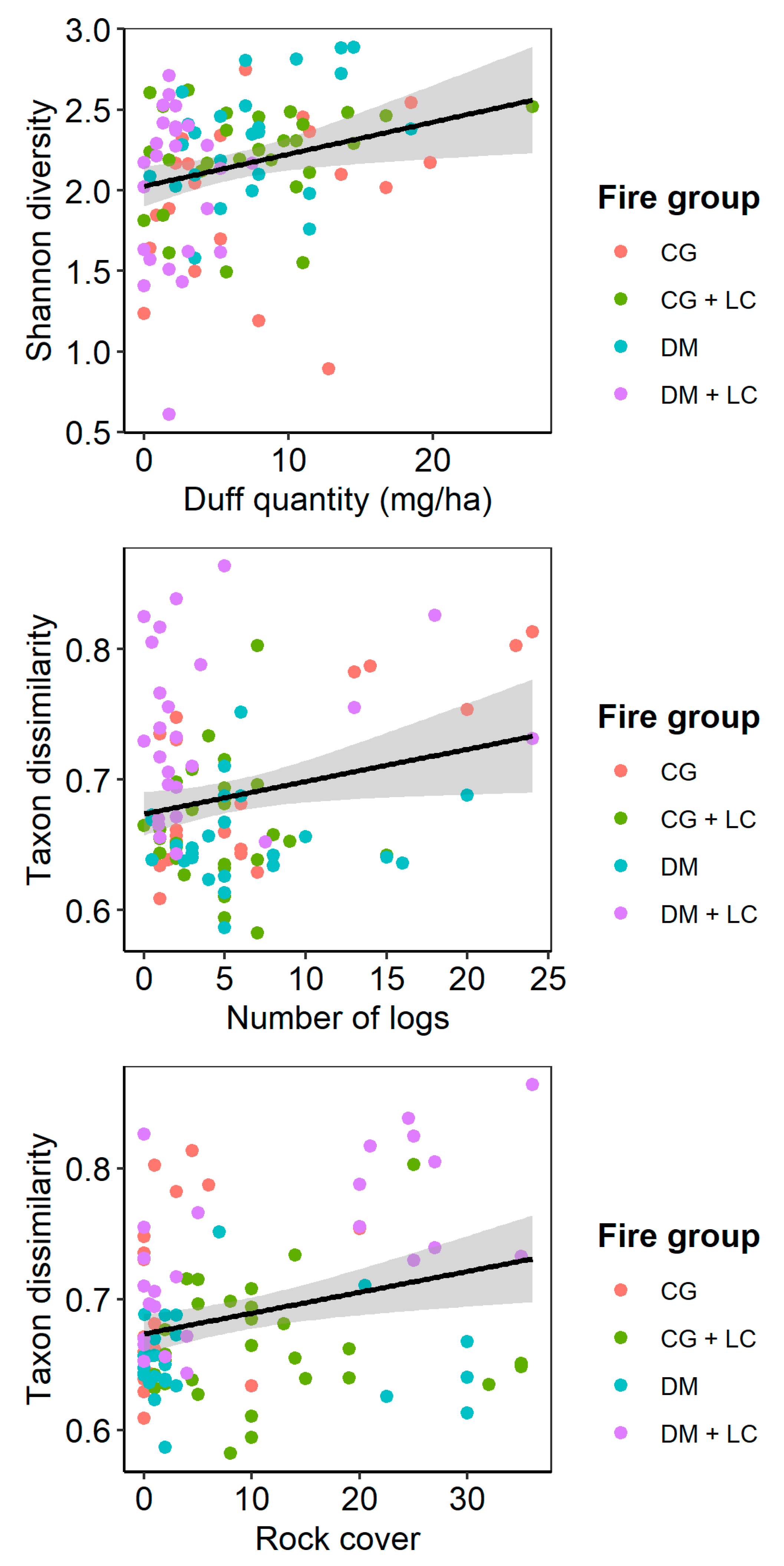
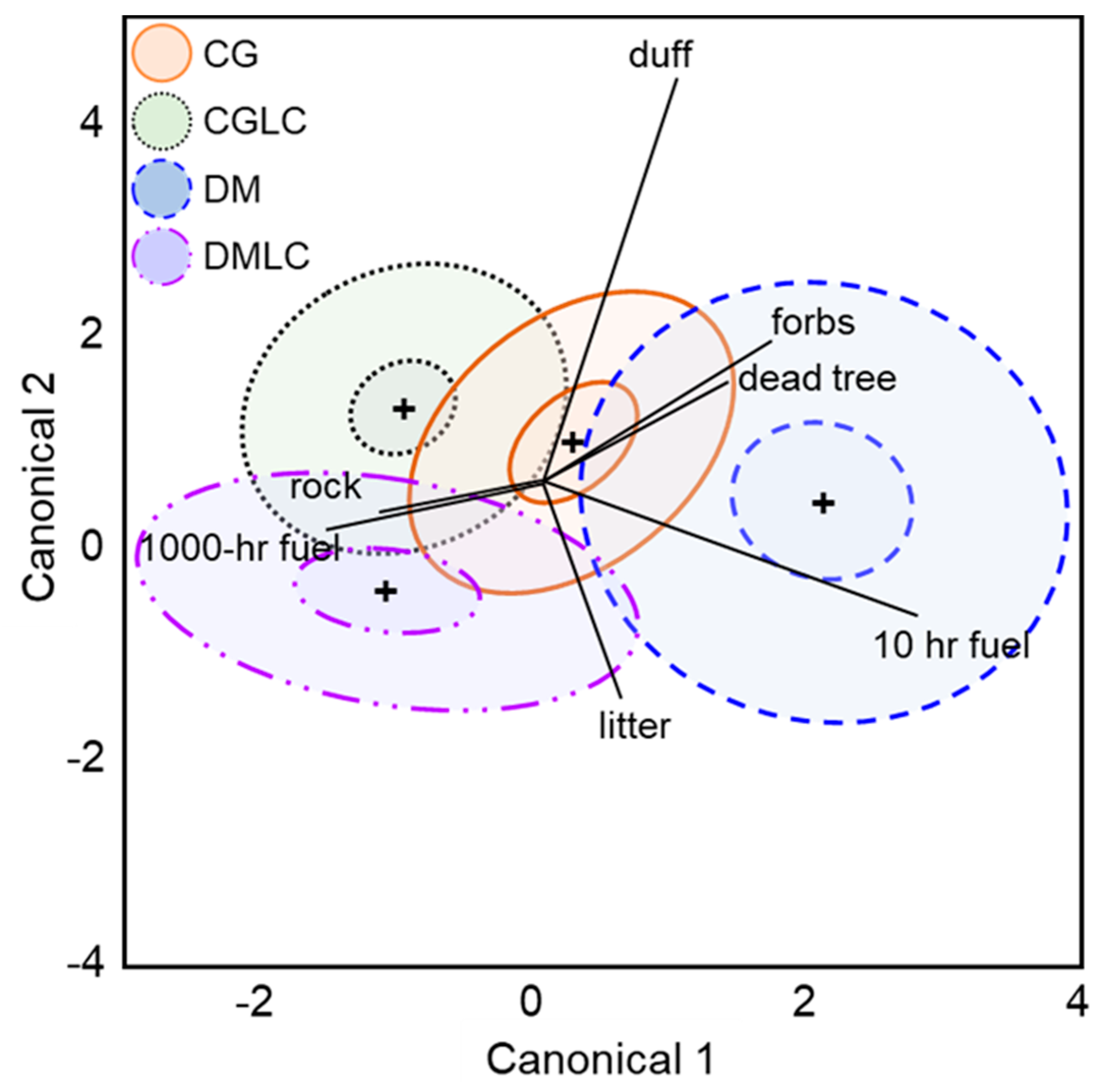

| Factor | DF | SS | Iterations | p-Value |
|---|---|---|---|---|
| Fire group | 3 | 0.113 | 5000 | <0.0001 |
| Logs (count) | 1 | 0.036 | 5000 | <0.0001 |
| Rock cover | 1 | 0.034 | 5000 | <0.0001 |
| Residuals | 94 | 0.204 |
| Fire group | Order | Suborder/Family | Spec. | Prob. | Stat | p |
|---|---|---|---|---|---|---|
| Cerro Grande (CG) | Coleoptera | Carabidae | 0.66 | 0.90 | 0.77 | 0.005 |
| Coleoptera | Unknown larvae | 0.55 | 0.65 | 0.60 | 0.035 | |
| Hemiptera | Nabidae | 0.65 | 0.40 | 0.51 | 0.005 | |
| Hemiptera | Miridae | 1.00 | 0.15 | 0.39 | 0.010 | |
| Cerro Grande + Las Conchas (CG + LC) | Hymenoptera | Tapinoma | 0.53 | 0.87 | 0.68 | 0.005 |
| Orthoptera | Ensifera | 0.51 | 0.83 | 0.65 | 0.005 | |
| Orthoptera | Rhaphidophoridae | 0.44 | 0.97 | 0.65 | 0.010 | |
| Araneae | Gnaphosidae | 0.39 | 0.93 | 0.60 | 0.025 | |
| Scolopendromorpha | 0.40 | 0.67 | 0.52 | 0.045 | ||
| Coleoptera | Scarabidae | 0.60 | 0.37 | 0.47 | 0.015 | |
| Coleoptera | Pselaphinae | 1.00 | 0.13 | 0.37 | 0.025 | |
| Dome (DM) | Opiliones | 0.55 | 0.88 | 0.69 | 0.005 | |
| Hemiptera | Cicadellidae | 0.50 | 0.76 | 0.61 | 0.015 | |
| Hemiptera | Aphidae | 0.54 | 0.60 | 0.57 | 0.005 | |
| Coleoptera | Byrrhidae | 0.74 | 0.40 | 0.55 | 0.005 | |
| Coleoptera | Curculionidae | 0.44 | 0.64 | 0.53 | 0.040 | |
| Hymenoptera | Myrmicinae | 0.96 | 0.24 | 0.48 | 0.010 | |
| Coleoptera | Cryptophagidae | 0.61 | 0.36 | 0.47 | 0.005 | |
| Coleoptera | Ptilidae | 0.75 | 0.28 | 0.46 | 0.030 | |
| Dome + Las Conchas (DM + LC) | Hymenoptera | Pheidole | 0.75 | 0.68 | 0.72 | 0.005 |
| Coleoptera | Anthicidae | 0.73 | 0.52 | 0.62 | 0.005 | |
| Archaeognatha | 0.70 | 0.40 | 0.53 | 0.005 | ||
| Hymenoptera | Solenopsis | 0.42 | 0.64 | 0.52 | 0.040 |
| Coherence | Turnover | Bound. Clumping | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fire | Abs. | Z | p | SD | Rep. | Z | p | SD | Mor. Ind. | p | Pattern | ||
| CG | 365 | 4.0 | <0.01 | 484.5 | 5.5 | 11,935 | −4.6 | <0.01 | 6558.0 | 34.0 | 1.77 | <0.01 | Nested |
| CG + LC | 806 | 0.1 | 0.96 | 808.4 | 6.8 | Random | |||||||
| DM | 521 | 1.7 | 0.08 | 580.0 | 5.8 | Random | |||||||
| DM + LC | 532 | 3.5 | <0.01 | 660.7 | 6.1 | 17,285 | −4.0 | <0.01 | 10312.7 | 41.8 | 1.69 | <0.01 | Nested |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrenberg, S.; Wickey, P.; Coop, J.D. Ground-Dwelling Arthropod Community Responses to Recent and Repeated Wildfires in Conifer Forests of Northern New Mexico, USA. Forests 2019, 10, 667. https://doi.org/10.3390/f10080667
Ferrenberg S, Wickey P, Coop JD. Ground-Dwelling Arthropod Community Responses to Recent and Repeated Wildfires in Conifer Forests of Northern New Mexico, USA. Forests. 2019; 10(8):667. https://doi.org/10.3390/f10080667
Chicago/Turabian StyleFerrenberg, Scott, Philipp Wickey, and Jonathan D. Coop. 2019. "Ground-Dwelling Arthropod Community Responses to Recent and Repeated Wildfires in Conifer Forests of Northern New Mexico, USA" Forests 10, no. 8: 667. https://doi.org/10.3390/f10080667
APA StyleFerrenberg, S., Wickey, P., & Coop, J. D. (2019). Ground-Dwelling Arthropod Community Responses to Recent and Repeated Wildfires in Conifer Forests of Northern New Mexico, USA. Forests, 10(8), 667. https://doi.org/10.3390/f10080667





