Abstract
The effect of wood modification on wood-water interactions in modified wood is poorly understood, even though water is a critical factor in fungal wood degradation. A previous review suggested that decay resistance in modified wood is caused by a reduced wood moisture content (MC) that inhibits the diffusion of oxidative fungal metabolites. It has been reported that a MC below 23%–25% will protect wood from decay, which correlates with the weight percent gain (WPG) level seen to inhibit decay in modified wood for several different kinds of wood modifications. In this review, the focus is on the role of water in brown rot decay of chemically and thermally modified wood. The study synthesizes recent advances in the inhibition of decay and the effects of wood modification on the MC and moisture relationships in modified wood. We discuss three potential mechanisms for diffusion inhibition in modified wood: (i) nanopore blocking; (ii) capillary condensation in nanopores; and (iii) plasticization of hemicelluloses. The nanopore blocking theory works well with cell wall bulking and crosslinking modifications, but it seems less applicable to thermal modification, which may increase nanoporosity. Preventing the formation of capillary water in nanopores also explains cell wall bulking modification well. However, the possibility of increased nanoporosity in thermally modified wood and increased wood-water surface tension for 1.3-dimethylol-4.5-dihydroxyethyleneurea (DMDHEU) modification complicate the interpretation of this theory for these modifications. Inhibition of hemicellulose plasticization fits well with diffusion prevention in acetylated, DMDHEU and thermally modified wood, but plasticity in furfurylated wood may be increased. We also point out that the different mechanisms are not mutually exclusive, and it may be the case that they all play some role to varying degrees for each modification. Furthermore, we highlight recent work which shows that brown rot fungi will eventually degrade modified wood materials, even at high treatment levels. The herein reviewed literature suggests that the modification itself may initially be degraded, followed by an increase in wood cell wall MC to a level where chemical transport is possible.
1. Introduction
When wood is exposed to humid conditions, it becomes highly vulnerable to fungal attack [1,2]. It is estimated that 10% of the lumber harvested each year is used to replace timber decayed by fungi [3]. Higher temperatures and humidity due to climate change will likely improve conditions for fungal decay and further shorten the service life of wood products in exterior applications [4]. Most wood used in constructions in the northern hemisphere is from coniferous wood species and brown rot fungi are the most common and most destructive organisms involved in the degradation of softwood products [1,5,6]. Brown rot fungi preferentially attack and rapidly depolymerize the structural carbohydrates in the cell wall, cellulose and hemicelluloses, leaving behind highly modified lignin residues [7,8,9]. The efficient degradation of polysaccharides causes brittleness in the wood and loss of mechanical strength [7] even before mass loss can be detected [10,11,12,13]. Thus, brown rot fungi are particularly relevant in the built environment where they can rapidly compromise the strength of structural wood products.
1.1. Brown Rot Wood Degradation
Brown rot fungi degrade wood through a very efficient process. Initially, the fungus produces hydrogen peroxide and reducing/chelating compounds within the wood cell lumen and the chemicals diffuse into the cell wall, sequestering ferric iron. Once in the cell wall, pH conditions favor the reduction of ferric iron and it reacts with the hydrogen peroxide to form hydroxyl (OH) radicals via the Fenton reaction [14,15]. The radicals depolymerize polysaccharides and simultaneously modify lignin [14,15,16]. This chelator-mediated Fenton (CMF) degradation is followed by enzymatic degradation, in which cellulases and hemicellulases hydrolyse the polysaccharides [14,15,16]. Water in the wood cell wall is crucial for the diffusion of the CMF metabolites [14,17] and fungi are sensitive to water stress. Low water content is often the limiting factor restricting the establishment of decay communities and lowering rates of decay [18].
Wood is hygroscopic, meaning it freely absorbs and exchanges water molecules with its surroundings. In untreated wood, at or below its fiber saturation point (FSP), wood moisture is primarily found as bound water inside the wood cell wall [19,20,21]. Bound water forms hydrogen bonds mainly with the OH groups of the wood constituents [19,22]. Most sorption sites are found in hemicelluloses followed by cellulose and lignin [19,23,24]. However, OH groups in cellulose inside microfibrils are already bound to one another in crystal formations and, thus, these groups remain inaccessible to water even in saturated samples [25,26]. Above the FSP, water starts to accumulate in macrovoids such as lumina and pits, through capillary condensation [19,20,21]. Under normal conditions, wood reaches its FSP at 97%–98% relative humidity (RH), which corresponds to the intersection between the hygroscopic RH region (0–97%–98% RH) and the over-hygroscopic RH region (above 97%–98% RH) [27]. It has been reported that moisture content (MC) below 23%–25% will protect wood from decay, which at 22 °C corresponds to an RH of ~95% [28,29,30].
Using X-ray fluorescent microscopy, Kirker et al. [31] visualized ion movements in the wood cell wall of untreated wood during degradation by the brown rot fungus Serpula lacrymans. They concluded that the fungus actively controlled the distribution of metal ions in the wood, in particular the transport of iron ions into the wood cell wall and calcium ions into the lumen. With the same technique, Zelinka et al. [32] showed that metal ions were able to diffuse through the wood cell wall at an MC above 14%–16%, depending on the ion. This is significantly lower than the MC at which fungi are able to degrade wood (23%–25%) [28,29,33], but in accordance with recent results by Meyer and Brischke [34] showing that Gloeophyllum trabeum was able to degrade Pinus sylvestris sapwood at 15% MC.
1.2. Wood Modification
The biodegradation of wooden structures is traditionally prevented with biocidal chemical preservatives that can be toxic to the environment and hazardous for human health [1]. In the EU, USA and Japan, chromated copper arsenate (CCA) has been phased out for residential use and for use in wood in contact with water [2,35,36,37]. Wood modification provides a non-biocidal, environmentally benign alternative. Modified wood is defined as chemically or physically altered wood materials which have increased decay resistance without being toxic under service conditions or at the end of service life [38]. Life cycle assessments have shown that acetylated wood has a considerably lower environmental impact than steel, concrete and tropical hardwoods [39]. Acetylation, furfurylation, cross-linking agents such as 1.3-dimethylol-4.5-dihydroxyethyleneurea (DMDHEU), and several variations of thermal modifications are common wood modification methods. Acetylation of wood is a modification where acetic anhydride reacts with the OH-groups on the wood polysaccharides causing them to be substituted with acetyl groups [40,41]. Furfurylation is an impregnation where furfuryl alcohol is polymerized inside the wood cell wall [42,43,44,45] and thermal modification is a heat treatment using mild pyrolysis [46,47,48,49].
1.3. Wood-Water Relationships in Modified Wood
The correlation between increased weight percent gain (WPG), reduced equilibrium moisture content (EMC), and decay resistance in modified wood materials has long been acknowledged [43,46,50,51,52,53]. A reduction of EMC by 40% was shown to correspond to the level of treatment that significantly improved decay resistance for all of the herein studied modified wood materials [30]. However, the mechanistic relationship between the reduced EMC and the increased decay resistance is less well understood. The importance of reduction in EMC for increased durability has been recognized e.g., through incorporation of moisture exclusion efficiency analysis in the durability standard for modified wood (No 2, part 4) issued by the Nordic Wood Preservation Council [54].
Moisture relationships in acetylated wood have been studied extensively. During acetylation, acetyl groups replace the OH-groups on the accessible polysaccharides, thereby reducing the number of accessible OH sorption sites [55,56,57,58]. The negative correlation between WPG and MC in acetylated wood was recently confirmed using differential scanning calorimetry (DSC) and low-field nuclear magnetic resonance (NMR) [59,60]. Specifically, water inside the cell wall of saturated samples was shown to decrease with increasing WPG. The reduction in water uptake of acetylated wood compared to untreated wood was also shown to be unaffected by RH [61].
Several studies have been conducted to investigate whether steric hindrance of water molecules by acetyl groups plays a role in MC reduction in acetylated wood [60,62,63,64,65]. In a recent study, Beck et al. [66] showed that acetylation reduced OH accessibility to a greater extent than would be predicted if OH substitution was solely responsible for accessibility reduction. This suggests that steric hindrance further reduces the accessibility of water molecules to unmodified OH groups in acetylated wood.
A reduction in EMC with increasing WPG has also been seen in furfurylated wood in the hygroscopic region [51]. However, Meyer et al. suggested that the furfurylation slows-down moisture diffusion rather than reducing the EMC [67]. Bulking has been proposed to be the main mechanism behind the reduced EMC in furfurylated wood, i.e., steric hindrance of water molecules to bind to the wood polymers [68]. In a low-field NMR study, furfurylation was shown to reduce the amount of water in the cell wall but did not change the interaction of water with the cell wall surface [69]. In the over-hygroscopic region, however, furfurylated wood took up more water than untreated wood [69]. The authors suggested that this may be due to voids and cracks in the cell wall that were created during the furfurylation process. Increased decay resistance in spite of the increased EMC under these conditions may be explained by the fact that the water is in an inadequate location or form; droplets of free water inside the wood cell wall may not allow for diffusion if the level of bound water is still too low.
Cross-linking agents, such as DMDHEU, reduce the EMC by constraining the swelling capacity of the wood. However, bulking and the subsequent exclusion of moisture from the smaller voids have been suggested to be the most important mechanisms behind decay resistance in DMDHEU-treated wood [70,71]. An interesting property of DMDHEU is the presence of OH-groups on this molecule, leading to an increase in sorption sites in the treated wood [71]. The increase in decay resistance conferred by DMDHEU illustrates that the number of sorption sites in itself is not a measure of decay resistance.
In thermally modified wood, OH-groups are reduced in number through the degradation of hemicelluloses in the modification process [72]. However, this characteristic does not correlate with decay resistance [47,73]. Instead, it has been suggested that the cross-links formed during the modification process lead to decay resistance [53,74]. In a study comparing thermal modification with pressurized hot water extraction, it was shown that even though cellulose aggregation and cell wall shrinkage took place in both cases, reduction in swelling only took place during thermal modification [74]. This was suggested to be due to the formation of cross-links in this process. Furthermore, thermal modification led to a reduction in EMC but pressurized hot water extraction did not. In another study, disruption of cross-links with KOH in thermally modified wood led to both a decrease in swelling and in fungal resistance [53]. Therefore, even though the reduction in sorption sites may contribute to a reduced EMC in thermally modified wood, the primary mechanism may be cross-linking.
Reduced EMC in thermally modified wood compared to untreated wood was seen throughout the hygroscopic RH range [75]. In the over-hygroscopic RH region, however, thermally modified wood took up more water than untreated wood in Hoffmeyer et al. [75]. The authors suggested that this may be due to the creation of voids during the thermal modification, just as for furfurylated wood. It should, however, be noted that in certain cases the EMC is not reduced during thermal modification. In a study by [76], wood treated at a temperature of 210 °C showed a decrease in the water absorption coefficient with 80% in the longitudinal direction while wood treated at 190 °C in fact showed an increase with 127%. The authors suggest that this is due to the differences in structural and chemical changes in the wood substance due to treatment temperature. At temperatures just below 200 °C, cracks appear in inside the wood cell wall, possibly leading to increased capillary water formation [76,77,78]. Above 200 °C, the thermoplastic flow of the torus may lead to filling of pores, thus reducing capillary water formation [76]. Furthermore, chemical alterations in the lignin at these temperatures may lead to a decrease in OH-groups.
When comparing the above findings on moisture interactions in modified wood, it is evident that the mechanisms behind the reduced EMC in modified wood materials differ fundamentally. Sorption site reduction, cell wall bulking and crosslinking all affect water uptake, and each has a varying degree of importance for the different modifications. It is important to keep this in mind when discussing the mechanism behind decay protection in modified wood generally.
1.4. Aim of the Review
Ringman et al. [79] evaluated the main established theories on the mode of action of modified wood based on the brown rot degradation process. It was concluded that the decay resistance must target the CMF degradation, since inhibition of the enzymatic degradation alone would lead to substantial strength loss. Inhibition of diffusion through the wood cell wall due to reduction of cell wall moisture was suggested to be the most essential parameter that delays the onset of wood decay [64,80,81]. Other mechanisms, such as enzyme non-recognition, micropore blocking, unavailability of easily accessible nutrients, and a reduced number of free OH-groups, were proposed to affect the rate of degradation after water uptake is initiated [57,62,70,81,82].
This review focuses on the role of water in brown rot decay of chemically and thermally modified wood. The study synthesizes recent advances in the inhibition of decay, the effects of wood modification on wood-water interactions and how the moisture relationship in modified wood is altered during decay. Potential mechanisms of chemical transport through the wood cell wall are critically discussed in relation to different wood modifications. Crucial open questions that need to be addressed in order to understand the wood water relationship in modified wood are highlighted.
2. Fungal Response to Wood Modification and Inhibition of Decay
Multiple studies over the years have shown that brown rot fungi are not killed by wood modification and are capable of expressing the genes needed for both the oxidative and enzymatic wood degradation processes that also take place in modified wood [83,84,85,86,87,88]. It should be noted that gene expression studies on modified wood have mainly been performed in Rhodonia placenta and may therefore not be representative for all brown rot fungi. Even though the fungi express the genes needed for degradation, the wood remains intact for a prolonged period of time, compared to untreated wood materials [83,84,85,86,87,88]. Since the fungi express genes involved in wood degradation in modified wood before mass loss occurs at similar levels as in untreated wood where degradation has begun, it is evident that the fungi are attempting to degrade the wood but fail [83,84,85,86,87,88]. The question is: why?
In vitro studies mimicking both the oxidative and the enzymatic brown rot degradation processes have shown that even though the wood modification provides some protection against degradation by Fenton derived OH radicals, the radicals were able to depolymerise wood polysaccharides in acetylated, DMDHEU-treated and thermally modified wood [89,90,91,92]. Furthermore, fungal cellulases were able to degrade the wood polysaccharides in acetylated and DMDHEU-treated materials but at a slower rate than in untreated wood [85,89]. Therefore, it can be concluded that the fungal metabolites and enzymes involved in wood degradation are still functional in at least some modified wood materials. However, in the in vitro experiments, Fenton’s reagent was added in the supernatant which means that OH radicals mainly formed outside of the wood cell wall. It is therefore still possible that e.g., altered pH inside the wood cell wall may inhibit the Fenton reaction in modified wood. Furthermore, recent results indicate that mimicking the Fenton reaction in vitro may be difficult, at least to achieve the same efficiency as in brown rot degradation [93]. Hence, it is possible that there are components in the brown rot fungi oxidative degradation process that are yet to be discovered.
OH radicals depolymerise wood polysaccharides in untreated wood, leading to the characteristic strength loss seen in brown rot [94]. In Ringman et al. [95], wood specimens treated to high levels of acetylation and furfurylation did not lose strength over more than 200 days of the decay test. Together with the in vitro tests above, these results indicate that the fungus was not able to induce OH radical formation in these materials. Whether this is due to the fact that the CMF metabolites cannot be transported through the modified wood cell wall or that the Fenton reaction somehow is inhibited cannot be concluded from the current literature. Thybring et al. [96] suggest that reductions in cell wall MC prevent fungal decay by hindering the transport of fungal agents into the wood cell walls, presumably from a disruption of the continuous water network within cell walls. Zelinka et al. [97] also discussed the possible mechanisms behind the decay resistance seen in modified wood: (i) inhibition of diffusion through an increase in the glass transition temperature of hemicelluloses (assumed to be the medium of transport); (ii) inhibition of diffusion through nanopore blocking; (iii) no inhibition of diffusion but instead a lower rate of diffusion and/or inhibition of chemical reactions leading up to the Fenton reaction through e.g., alteration of the pH level. The first two are discussed in detail in the following section.
In future research it would be of value to investigate whether CMF metabolites diffuse into the wood cell wall in modified wood during the time frame where degradation is inhibited. This could be done with a number of methods, that need to be selected for each metabolite. Fungal iron reductants may be tagged with immunogold antibodies and detected with electron microscopy [98]. Hydrogen peroxide can be precipitated with CeCl3 and also detected with electron microscopy [99]. None of these methods have so far been conducted on modified wood. If CMF metabolites are able to diffuse through the wood cell wall but fail to react and form OH radicals, alterations in pH due to wood modification is the most likely mechanism. The effect of pH alterations on the CMF process should therefore also be investigated.
3. Diffusion in Modified Wood
Inhibition of diffusion of CMF metabolites was identified as the most likely mechanism for brown rot decay protection in modified wood [79]. Rowell et al. [100] showed that fungal decay in both untreated and acetylated wood with different WPGs was always preceded by swelling, i.e., the uptake of water to such an extent that the wood cell wall is enlarged; however, the time taken to reach swelling was increased by an increasing WPG. In a recent study, the RH threshold for the diffusion of potassium ions through the wood cell wall of acetylated wood was shown to increase with an increasing WPG [101]. The threshold in acetylated pine with WPG = 20% was >90% RH, compared to 60%–65% RH in untreated pine under the same conditions. Even though the RH thresholds may be different for CMF metabolites, this study clearly shows that acetylation of wood has a negative effect on diffusion through the wood cell wall. This is in accordance with Hosseinpourpia and Mai, who showed that diffusion of iron was inhibited in acetylated wood with a high WPG [102]. A similar experiment was performed on thermally modified wood, which showed a significant reduction in iron diffusion in this material but no threshold [91].
The exact mechanism of chemical transport through the wood cell wall below the FSP is not known; however, several theories have been proposed [103,104,105]. In the following sections we discuss possible mechanisms for inhibition of diffusion through the wood cell wall in modified wood. It is important to keep in mind that different wood modifications may affect MC and diffusion in different ways, and some may have modes of action that are not at all related to moisture. On the other hand, comparing the mechanisms of different wood modifications may also lead to an improved understanding of the moisture relationships in untreated wood.
3.1. Nanopore Blocking
One proposed means of diffusion inhibition in modified wood is nanopore blocking [62]. In this review, we use the term nanopore to refer to nanoscale voids within the wood cell wall, since this terminology is better suited to wood anatomy than the IUPAC definition for pore size classes [61]. The nanopore blocking theory postulates that ingress of fungal degradation agents into the modified wood cell wall is prevented by the physical bulking the modifications provide. By filling space within the cell wall nanopore network, chemical modification may block the penetration of the degradation chemicals.
Chemical wood modifications, such as acetylation, furfurylation and DMDHEU, increase the dry volume of wood [30,106,107,108], suggesting the modification chemicals are present within the wood cell wall. An increase in cell wall volume as a result of acetylation has also been demonstrated using helium pycnometry [29,109]. The change in cell wall volume indicates that these modifications fill nanopores within the cell wall matrix and may close some nanopores completely. Thus, the reduced nanoporosity may block the degradation chemicals from entering the cell wall. However, nanopore blocking seems an unlikely explanation for the decay resistance provided by thermally modified wood where mild pyrolysis removes material from the wood cell wall, rather than bulking it.
To test the nanopore blocking theory, cell wall accessibility can be determined by performing solute exclusion experiments. This method assesses porosity of water-swollen samples by measuring the penetration of solute probe molecules of various sizes into the substrate. The concentration of a probe solution is determined before and after exposure to a water-saturated wood sample and the accessible volume is determined from the measured reduction in probe solution concentration. To the authors’ knowledge, only two solute exclusion studies have been conducted on chemically modified wood, both of which focus on anhydride modification [110,111]. Higher WPGs reduced accessibility of the wood to probe molecules of 1.2 nm diameter and smaller. This indicates the filling of nanopores in anhydride modified wood. However, this method does not provide an absolute determinant of nanopore size distribution in the sample because it relies on the assumption that the concentration of solute in the nanopores is the same as the concentration in the bulk solution. This is not the case as the size of the pores approaches the size of the probe molecules [112,113]. Also, the degree of polarity and hydrogen bonding capability of the probe molecule will affect the result [114]. Furthermore, solute exclusion studies of wood vary widely in the amount of time allotted for the samples to come to equilibrium with the probe solution [115]. Only “several minutes” were used for pulp samples [20] while solid wood samples were equilibrated for 1–14 days [110,111,116].
The solute exclusion technique is a suitable method to investigate this theory, yet more research is needed to determine the appropriate experimental procedure. Specifically, the necessary time for samples to come to equilibrium must be established. Once a satisfactory method has been determined, solute exclusion should be used in future studies to investigate cell wall nanopore accessibility for other chemical modifications. Additionally, the experiment could be performed with probe solutions of chemicals known to be involved in CMF degradation, similar to the work of Hosseinpourpia and Mai [91,102,117] involving iron ion uptake in modified wood.
3.2. Media of Transport and Percolating Network
Diffusion inhibition may also be achieved by disruption of the continuous percolating network in the wood cell wall. Percolation theory is used to represent the connectivity of randomly distributed elements containing a percolation threshold; that is, a fraction of sites that need to be occupied before a continuous network exists [103]. The proposed percolation model for conductivity in wood was shown to fit experimental data and indicates that there is a continuous path of conducting water at 16% MC [103]. The medium of transport for the percolation network was originally proposed to be freezing bound water, however, with the discovery that freezing bound water appears to be an artefact of sample preparation [118], other possible transport media have been explored.
Two different transport media are discussed below: capillary water in nanopores and water associated with plasticized hemicelluloses. Although they are presented here in separate sections, both theories affect one another, and they are not mutually exclusive. Plasticization of hemicelluloses will change nanoporosity as the hemicellulose sheath surrounding cellulose microfibrils is loosened and water adsorption to hemicellulose will be affected by capillary condensation within nanopores.
3.2.1. Capillary Water Formation in Nanopores
The wood cell wall has a complex structure in which highly ordered, semi-crystalline cellulose microfibrils are embedded in a porous matrix of amorphous cellulose, hemicellulose and lignin. The nanopores in the cell wall structure provide enclosures in which capillary water formation can take place. The Kelvin equation relates vapor pressure to the curvature of a liquid-vapor interface and predicts that undersaturated vapors will condense in capillaries of sufficiently small dimensions [119]. This means that at a given RH, capillary condensation will form liquid water in pores of a particular size range. As described in Thybring et al. [96], the minimum RH required for fungal growth is in the range of 92%–97% [120,121,122,123]. Using the Kelvin equation and assuming the surface tension between water and wood is 0.1 J/m2 [124], capillary water should only form in nanopores of 34–96 nm diameter or smaller at the brown rot growth RH threshold. Thus, the presence of nanopores in this size range may be the critical factor for brown rot decay if capillary water is indeed the transport medium for degradation agents.
As mentioned in Section 3.1, cell wall bulking modification chemicals penetrate the wood cell wall and reduce nanoporosity. Thus, if the modification chemicals fill nanopores in the size range relevant for capillary condensation at the decay threshold RH, they may prevent a continuous network of capillary water from forming and thereby hinder diffusion of fungal degradation agents into the wood cell wall. However, this theory assumes that the chemical modification does not create new nanopores in the relevant size range. Mercury intrusion porosimetry has been used to determine pore size distribution in thermally modified wood [125] and wood modified with phenol-formaldehyde [126]. Both studies found that the modifications tended to increase the number of pores of <100 nm. For thermally modified wood, the increase in nanopores was attributed to the formation of cell wall cracks and the opening of intercellular spaces, while, for phenol-formaldehyde modification, the increase in the small pore fraction may have occurred due to partial filling of larger pores. However, Zauer et al. [127] found contradictory results for thermally modified wood when they observed an increase in nanopores from 4–400 nm due to thermal modification using thermoporosimetry with DSC. They suggested that the mechanism for the reduction in nanoporosity could be shrinkage of the wood cell wall or flowing of lignin into the pores. More studies on pore size distribution in modified wood are needed to clarify the effect of the modifications, but the first two studies suggest that, based on nanopore size distribution alone, modification may increase capillary water formation.
However, nanopore size distribution is not the only factor determining capillary water condensation in wood—the surface tension between water and the wood surface is another important variable. As mentioned previously, acetylation, furfurylation and thermal modification decrease the interaction of water with the wood surface. Bastani et al. [128] measured the contact angle of water on furfurylated and thermally modified Scots pine and used the Owens–Wendt approach to calculate surface tension from contact angle. They found the modifications reduced surface tension by circa 20%. Thus, the less hydrophilic nature of these modified wood surfaces may lead to reduced capillary condensation in the modified material. However, the additional sorption sites contributed by DMDHEU modification [129] suggest that the affinity of water for DMDHEU treated wood may not be reduced. The effect of crosslinking on nanoporosity may be the more dominant factor contributing to capillary condensation and decay resistance in DMDHEU modified wood.
Further investigations are needed to better understand the effect of wood modification on both cell wall nanoporosity and surface tension between water and the wood surface, since these are the critical parameters for capillary condensation. The previously mentioned disagreement between mercury intrusion porosimetry and thermoporosimetry determined values for pore size distribution of thermally modified wood should be further investigated [125,127]. The discrepancy highlights the need for porosimetry methods to be verified against one another, as each method has its own drawbacks. Mercury intrusion porosimetry is limited by the accessibility and connectivity of the pores. The high pressures required to force mercury into the smallest pores will compress the wood causing nanopores to collapse [130]. Thus, mercury intrusion can only reliably determine nanopores greater than 10 nm in size [125,131]. Thermoporosimetry techniques utilize the fact that water in nanopores will exhibit a depressed melting point compared with bulk water. The melting temperature of water in saturated wood samples can be determined with either DSC [125,132] or low-field NMR [133]. However, both DSC and low-field NMR thermoporosimetry require water saturation of the sample which may increase nanoporosity [19]. A third porosimetry technique which has not yet been used on modified wood is nitrogen adsorption with super critical drying. Nitrogen adsorption utilizes the Brunauer–Emmet–Teller theory for multilayer gas absorption to derive values for the surface area and pore size distribution of solids. Using this technique on wood can be problematic because drying processes create surface tension forces at the liquid-vapor interface that can collapse the nanopores of the wood [132]. However, this problem can be circumvented by using carbon dioxide critical point drying. Nitrogen adsorption with critical point drying has been used to measure surface area and pore size distribution of wood pulp [134,135] and pretreated eucalyptus wood [136] but has yet to be implemented on modified wood materials. Unfortunately, the equations used to determine porosity for all of these techniques (the Washburn equation for mercury intrusion, the Gibbs–Thompson equation for thermoporosimetry, and the Kelvin equation for nitrogen adsorption) assume the material has cylindrical pores and this is not likely to be the case for the tortuous nanopore network present in wood. There are more powerful techniques to characterize nanoporous materials that do not rely on a cylindrical pore assumption, such as 2D T1T2 low-field NMR [137], x-ray and neutron small angle scattering [138], electron tomography [139], positron annihilation lifetime spectroscopy [140], and molecular dynamic simulations [141], however, analysis and interpretation of results using these methods is complicated.
The previously mentioned techniques indirectly determine capillary condensation from the Kelvin equation by measuring nanoporosity and surface tension. However, gravimetric techniques can directly measure capillary condensation if the RH of interest can be stably provided. Unfortunately, this is difficult at the high RH levels relevant for the brown rot decay threshold. As discussed in Thybring et al. [96], conditioning wood samples to near saturation is problematic using traditional methods for controlling RH, like sorption balances, saturated salt solutions and climate chambers. It is difficult to obtain stable saturated water vapor because of temperature differences between the vapor and the surrounding surfaces. Cooler surfaces will cause the vapor to condense while warmer surfaces will lead to non-saturated vapor. Temperature fluctuations as small as 0.5 °C are an issue. However, the pressure plate technique can be used to achieve stable equilibrium at near saturation vapor pressure [20]. Water-saturated samples are placed on a porous supporting substrate and pressure is applied using dry gas to press water out of pores in which capillary forces are lower than the applied pressure. The technique has been used to measure desorption from the water-saturated state in acetylated and furfurylated wood [61] as well as thermally modified wood [142]. The studies indicate that during desorption, capillary condensed water does not contribute to sorption below 99.9% RH. Yet, the adsorption process is more relevant for the decay threshold. Fredriksson and Johansson [143] have developed a pressure plate method which can measure adsorption, but the technique has yet to be used on modified wood.
It should be noted that this discussion of capillary condensation presumes that the nanopores are preexistent at the instant that moisture is being taken up by the wood matrix. The physical adsorption principles which cause capillary condensation assume the vapor is contained within a rigid, porous network. However, the nanopores in wood are transient and can collapse as the wood dries [132]. It may be more accurate to think of water vapor sorption in wood as a situation where water molecules are engulfed by the wood matrix, pushing the wood polymers away to the extent needed to accommodate the new water molecules and forming the nanopores as they do so [144].
3.2.2. Plasticised Hemicelluloses
Jakes et al. [104] recently proposed that diffusion through the wood cell wall at an MC below the FSP occurs in plasticized hemicelluloses. This model stipulates that above 15% MC, the glass transition temperature of hemicelluloses is reduced to below room temperature, leading to the formation of an interconnecting network of plasticized hemicelluloses. On a molecular scale, as the MC increases, intra-/intermolecular bonds between hemicelluloses are disrupted by newly formed hydrogen bonds with water molecules, thereby loosening up the structure. It is theorized that the water adsorbed to the softened polymers can solvate ions and allow diffusion. The model is based on the observation that room temperature glass transition of hemicelluloses occurs at 60%–80% RH [145,146,147], corresponding to approximately 11%–15% MC in wood, which is slightly lower than the measured MC threshold for ionic movement in wood [32].
Zelinka et al. [97] suggested that wood modification may alter the glass transition temperature for the hemicelluloses in such a way that they will not plasticize at room temperature. If softened hemicelluloses are needed for a percolating network to form, this alteration may inhibit chemical transport through the wood cell wall.
The different wood modifications affect hemicelluloses in different ways. In acetylated wood, OH-groups on the wood polymers are substituted with an acetyl group, resulting in fewer sorption sites on the acetylated hemicelluloses compared to the untreated hemicelluloses [82]. With fewer sorption sites, water may not affect the plasticity of acetylated hemicelluloses as strongly as it does in untreated wood, resulting in an increased glass transition temperature [97]. Furthermore, Beck et al. [66] showed that the acetyl groups block more OH-groups than they substitute due to steric hindrance, which further reduces the number of accessible sorption sites. A dynamic vapor sorption (DVS) based study showed that the slow sorption process is more heavily affected by acetylation than the fast sorption process as described by the parallel exponential kinetics (PEK) model [56]. These results suggest that the effect of moisture on plasticization of hemicelluloses will be reduced in acetylated wood. In thermally modified wood, most of the hemicelluloses are degraded during the modification process [73,148].
The glass transition temperature of wood polymers can be measured with DSC, dynamic mechanical analysis (DMA) and dynamic Fourier-transform infrared spectroscopy (FTIR) [146,149]. Gröndahl et al. [150] used DSC to determine the glass transition of acetylated glucuronoxylan ex situ. The samples were dry prior to analysis and the DSC scan ranged from 100–220 °C. No glass transition was observed for untreated samples, likely because the transition occurred below 100 °C. However, acetylated samples showed a clear glass transition between 160–200 °C. Thus, it seems acetylation increases the glass transition temperature of this hemicellulose ex situ. Willems [151] argued that fewer hemicelluloses in thermally modified wood are plasticized than in untreated wood, due to increased crosslinking between the thermally modified wood polymers. The slow sorption process is also highly affected in thermally modified wood compared to untreated wood [152], which may suggest a change in the glass transition temperature of the wood polymers. Salmén et al. [153] used dynamic FTIR to investigate the effect of thermal modification on wood polymer plasticity. They found an increasing degree of thermal modification led to greater load bearing contributions from xylan and lignin. They attributed this finding to increased crosslinking between the wood polymers resulting from hemiacetal forming reactions catalyzed by thermal degradation products. Thus, thermal modification also appears to increase the glass transition temperature of hemicellulose.
In DMDHEU-treated wood, cross-links are formed between DMDHEU and the wood polymers thereby making the wood matrix stiffer [108,154]. The change in glass transition temperature of the hemicelluloses might therefore be similar to that in thermally modified wood. The simultaneous introduction of OH-groups may not be sufficient for keeping the glass transition temperature below room temperature. Furfurylation has been shown to be associated with lignin rather than hemicelluloses. Furfuryl alcohol binds a lignin model molecule and has been shown to be highest in concentration in the lignin rich parts of the wood cell wall [155,156]. Whether furfurylation also affects hemicelluloses through modification or cross-linking is not known. However, poly(furfuryl alcohol) may sterically hinder the water molecules from binding to the hemicelluloses, which might reduce the number of accessible sorption sites in the same manner as described above for acetylated wood. Herold et al. [157] measured the plasticity of furfurylated maple veneers at 65% RH using DMA and found that furfurylation led to increased plasticity of the veneer as a whole. This suggests that, unlike acetylation and thermal modification, furfurylation may in fact increase hemicellulose plasticity. However, it is questionable whether the softening interpretation of the macroscopic DMA result also applies at the nanoscale where moisture sorption takes place.
The previously mentioned studies assessing hemicellulose plasticity in modified wood have only investigated the glass transition of the material at a single MC. However, to test the plastic hemicellulose theory for diffusion, the effect of humidity on glass transition must be explored. Both DMA and dynamic FTIR have been used to determine the effect of moisture on wood polymer plasticity in untreated wood [146,149]. For these analyses, the temperature is held constant while a mechanical property is assessed and the RH is gradually increased. Alternatively, DSC could be performed with many samples climatized to different MC’s to determine the effect of moisture on glass transition. Another potential method is nanoindentation with RH control. This technique involves the indentation of a sample by a sharp tip with well-defined geometry. Nanoindentation in combination with atomic force microscopy has been used to determine the effect of moisture on the plasticity of pulp fibers and thin cellulose films [158]. All these analyses have the potential to reveal if wood modification does increase the RH at which hemicellulose becomes plastic, yet, to the authors’ knowledge, no such studies have been performed on modified wood.
4. Moisture Relationships in Modified Wood during Decay
The literature on the development of decay in modified wood is scarce. Wood modification is intended to increase decay resistance and, thus, even at low modification levels, long time frames are often required to obtain substantial decay. However, a few recent studies shed light on decay induced changes in modified wood materials and, together with older literature, they give a rough picture of the mechanisms involved.
Hill et al. [62] suggested that wood modification does not inhibit decay but rather delays it. In traditional durability tests that are run over a specific timeframe, wood materials modified to high levels often perform showing full resistance to fungal decay. However, rather than fully inhibiting decay, the modifications may only delay degradation past the duration of the standard tests. Several recent studies have shown that modified wood materials with high treatment levels may eventually degrade given enough time [85,87]. These studies also indicate that once degradation has started in the modified wood, it seems to proceed in a similar way as in untreated wood, only slower [85,87,159]. Beck et al. [159] showed that the order in which the wood polymers are degraded is similar in acetylated and untreated wood, where hemicelluloses are degraded at a faster rate than cellulose and holocellulose accumulates compared to lignin. In both acetylated and thermally modified wood exposed to brown rot fungi and undergoing mass loss, it has been seen that the onset of decay was delayed in the modified wood materials and the rate of degradation once started was lower [87]. The intensity of these phenomena was shown to be dependent on WPG [87]. Furthermore, R. placenta gene expression patterns in thermally modified wood undergoing mass loss were similar to those in untreated wood [85].
These results suggest that alterations of moisture relationships in modified wood during decay may be similar to that seen in untreated wood. However, since wood modification affects wood-water relationships, whether or not the wood modification in itself is affected by brown rot decay will have an effect on how the moisture relationships change in the modified wood during decay. Beck et al. [159] showed that acetylated pine earlywood (WPG = 21.4%) had lost 30% of total bound acetyl at 10% mass loss. At higher levels of mass loss, the acetyl content flattened out. Whether the deacetylation was active or a result of that the polysaccharides most accessible for acetylation are also the most accessible for degradation and hence are degraded first could not be concluded from this study. The reason that the acetyl levels flattened out after 10% mass loss was suggested to be due to acetyl groups on the lignin which is not degraded by brown rot fungi. These results contradict those of Ringman et al. [95] in which it was concluded that total bound acetyl in acetylated pine (WPG = 23%) was not degraded during exposure to R. placenta. However, the results of that study may have been misinterpreted since acetyl content was presented as a function of exposure time and not mass loss. The same acetyl content values from Ringman et al. [95] are shown in Figure 1, this time displayed as a function of mass loss. When presented this way, the data suggest that acetyl content in these samples may have also been degraded once mass loss occurred. The variation of the results of the non-exposed controls (incubated on soil for 8 weeks but without fungi), however, make the results difficult to interpret.
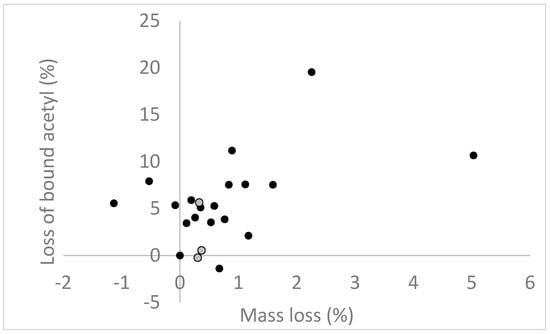
Figure 1.
Loss of bound acetyl in acetylated pine samples exposed to R. placenta for up to 200 days, in percent of acetyl content after acetylation and related to mass loss due to degradation. Black circles: samples exposed to R. placenta; grey circles: non-exposed controls incubated on sterile soil for 8 weeks.
Very little research regarding whether other wood modifications are degraded during brown rot decay has been published. In Ringman et al. [89], the massive increase in aldehydes after in vitro treatment with Fenton’s reagent of furfurylated wood was suggested to be due to degradation of the poly(furfuryl alcohol). For DMDHEU, the results are inconsistent. In an in vitro experiment where DMDHEU treated veneers were subjected to Fenton’s reagent, very little loss of nitrogen, as a measure of the DMDHEU content, was recorded [92]. DMDHEU treated pine mini-blocks exposed to C. puteana for 12 weeks, on the other hand, lost 50% of their initial N content [160]. This was estimated to account for almost all of the 18% mass lost during fungal exposure; i.e., very little wood mass was lost. It is possible that loss of nitrogen in the in vitro test would have been larger with a longer and/or more powerful Fenton treatment. Nonetheless, loss of DMDHEU in the fungal test indicates that brown rot fungi can degrade the modification polymer in DMDHEU treated wood, provided that the polymer was not leached out during the decay test.
Comparisons of literature data by Thybring [161] also indicated that once degradation starts in modified wood the moisture relationships change in a similar manner as in untreated wood. The literature data used were mainly conventional decay tests, i.e., all samples run over a specified number of weeks. In Beck et al. [159] moisture interactions throughout R. placenta decay were characterized for untreated and acetylated samples with OH group accessibility and low-field NMR. In this study, accessible OH groups in acetylated samples were shown to initially increase during early decay and subsequently decrease back to the initial level at later stages. In a similar manner, low-field NMR T2 relaxations times for both cell wall water and void water initially decreased to a similar level as in untreated wood, after which a slight increase could be seen. The initial increase in accessible OH groups and decrease in T2 relaxation time, could both be explained by the recorded de-acetylation. De-acetylation of polysaccharides would theoretically increase the number of sorption sites in the wood material. An increase of the number of sorption sites would increase the affinity of water to the wood cell wall and thereby decrease relaxation times. Low-field NMR was also used to determine the proportion of water in the wood cell wall in water saturated samples. This value provides information on the rate of cell wall nanoporosity development versus erosion of the lumen-cell wall interface. Whereas there was no clear trend for untreated samples, strongly increasing values in acetylated samples during early decay indicate substantial nanoporosity development while decreasing values in heavily degraded, acetylated samples suggest lumen erosion at this later stage. The authors propose that more thorough oxidative degradation occurs in acetylated wood once degradation begins and causes increased nanoporosity development.
In Ringman et al. [81], it was suggested that decay resistance in modified wood is achieved by reduction of the MC and consequently a reduction or inhibition of diffusion of CMF metabolites through the wood cell wall. The experimental data reviewed here indicate that the wood modification in itself may initially be degraded to such an extent that the MC in the wood cell wall may increase to levels at which diffusion can occur. However, further research is needed to confirm this. It is also of great importance to find out where and how the initial degradation of the wood modification and/or the wood constituents takes place. Theoretically, a locally low modification level (due to uneven treatment) may lead to onset of decay in this area and a subsequent spreading of decay from this point of entry.
Study of the decay induced changes in the wood structure in modified wood may lead to increased understanding of the decay resistance in these materials. The structure and chemical composition of the wood cell wall, including the wood modification itself, during decay should be investigated. Ideally, these measurements should be done on the microscale, using methods like FT-IR microscopy and X-ray fluorescence microscopy. However, bulk measurements of chemical composition and cell wall moisture in decayed, modified material is still useful and such data is scarce in the literature. Furthermore, although solute exclusion has been used to determine porosity development during wood decay in unmodified material [116], no studies have been performed on modified wood during degradation. Future experiments should strive to fill this knowledge gap.
Finally, even though repeating key experiments, such as analyses of diffusion and moisture relationships, with other modified wood materials is important, including other fungi may be even more critical. Brown rot fungi diverged from their white rot ancestors at least at four different timepoints in evolution [162]. Therefore, all brown rot fungi are not closely related and may use somewhat different mechanisms for degradation. To give a full picture of the decay resistance of modified wood against brown rot fungi, representative fungi from each sub-group should be investigated. The occurrence of fungal species in rot damaged constructions should also influence the choice of fungal species to examine (for Europe, see e.g., Gabriel and Svejk [6]).
In addition to increasing the understanding of the decay resistance in modified wood materials, investigations of moisture conditions in modified wood during decay may lead to increased knowledge on water requirements for fungal decay also in untreated wood. In untreated wood at a RH where degradation is possible, water is mainly present as bound cell wall water, but some capillary water appears too; it is therefore hard to determine whether capillary water is needed for decay or not. The differences in wood moisture location in modified wood materials compared to untreated wood may be used to investigate the importance of the location of wood moisture in fungal decay.
5. Controlling the Moisture Conditions during Decay Tests: A Challenge
In the study of moisture relationships in modified wood and its effect on fungal decay, controlling the moisture conditions in the samples is of utmost importance. Performing decay tests under controlled moisture conditions is a challenge, as pointed out by Thybring et al. [96]. As mentioned above, the threshold RH for brown rot decay is >95% and maintaining such a high RH is hard to achieve with conventional methods. Therefore, a combination of approaches have been used in single strain decay tests such as EN 113, ASTM E10 and the miniblock test, to ensure appropriate moisture conditions for decay throughout the test: (i) conditioning of the samples prior to the decay test at 20 °C and 65% RH to create an initial MC of approximately 12%; (ii) incubating the samples at 22 °C and 75% RH to further increase the MC during the test; and (iii) addition of moisture in the soil or agar to both increase air humidity and wood MC [163,164]. However, for the study of moisture relationships during decay, this approach is not optimal. Since the RH in the climate chamber is higher than that at which the samples are conditioned, a moisture gradient will be established in the samples. Furthermore, moisture in the agar or soil will evaporate to the surrounding air and gradually dry out, which means that less water evaporates at the end of the test than at the beginning and, hence, RH will decrease. For the long-term decay tests needed for modified wood, drying of the growth medium may also affect fungal growth, rate of degradation etc. Agar plates have visual signs of drying-out after eight weeks and soil plates need to be watered every 2–4 weeks to prevent drying [84,85,95].
Incubating the samples at the same RH as that of the decay test will be run at will decrease the effect of the moisture gradient in the samples. However, conditioning at such high RH may lead to mold contamination issues. Furthermore, subsequent sterilization must be carried out in a way that leads to minimal change in sample MC.
Even if the samples are pre-conditioned at 75% RH instead of 65%, a moisture gradient will still be created in the samples during the decay test because the agar/soil moisture evaporates during the decay test and thereby increases the RH of the surrounding air above. This problem may be overcome by incubating the samples at the RH needed for decay, i.e., >95%. One way to do this is to use saturated salt solutions or pressure plates to create a steady, high RH. However, as mentioned previously, temperature fluctuations as small as 0.5 °C will affect the RH [96]. Additionally, the sealed vessels used in such analyses create another issue. As the fungi metabolize the wood carbohydrates, they consume oxygen and produce water and carbon dioxide. Therefore, in order to maintain stable conditions throughout decay, oxygen must be added to the vessel and water and carbon dioxide removed. This must happen at a rate which matches the rate of decay, thus, sensors within the vessel would be required to constantly monitor and adjust the levels of these vapors.
At the microscale, moisture gradients seem unavoidable. Since fungal metabolism produces water, the moisture conditions may differ in different parts of the sample depending on the local degree of degradation. In the case of modified wood materials, uneven treatment and/or local degradation of the wood modification may also contribute to wood moisture heterogeneity. Thus, methods measuring wood-water relationships at the macroscale may give a false picture of the situation. One way to overcome this problem is to use a decay set-up that in itself separates the different stages of decay from each other, such as the wafer test [165]. In this set-up, a thin rectangular wood wafer, with the cross-section on the biggest surface, is placed with the small edge on a feeder strip and the top side leaning on the inside of the jar. In this way, the fungi will grow up the sample and mycelium of different ages, and in different stages of decay, may be separated through horizontal sectioning of the wafer. It may still not be possible to achieve segments on the microscale, but this method is still an improvement compared to using whole mini-block samples.
6. Conclusions
Current literature indicates that Fenton derived OH radicals can degrade polysaccharides in modified wood [89,90]. In addition, the brown rot fungus R. placenta has been shown to express genes needed for the oxidative degradation in modified wood [84,85,86,87,88,166]. Still the modified wood is protected from decay for a prolonged period compared to untreated wood [41,85,87,95,107,160,167]. One explanation is that brown rot fungi fail to produce OH radicals in modified wood materials. This could be due to inhibition of diffusion of CMF metabolites or inhibition of the Fenton reaction itself. The reduction in EMC seen in modified wood materials and its correlation to decay resistance indicates that inhibition of diffusion is of importance.
The exact mechanism for chemical transport through the wood cell wall is not known. A model based on percolation theory fit well with experimental results [103]. Different types of medium of transport for the percolating network have been proposed [103,104,105]. Inhibition of percolation in modified wood may occur through the filling of nanopores and physical blocking of the solute molecules [62,63,64,65,66,67,68,69,70]. Solute exclusion was identified as an appropriate technique to test this theory. Alternatively, modification may affect the medium of transport itself. We discussed capillary water condensation in nanopores and absorbed water in plasticised hemicelluloses as possible diffusion media as described by Zelinka et al. [103] and Jakes et al. [104]. The capillary condensation theory works well with acetylated and furfurylated wood where both nanoporosity and wood-water surface tension are reduced [29,56,107,128]. Increased OH content in DMDHEU modified wood and potentially increased nanoporosity in thermally modified wood obscure whether the capillary water theory fits with these modifications [71,125]. The plasticized hemicellulose theory also works well with acetylated wood since acetylation has been shown to increase hemicellulose glass transition temperature [150]. Increased crosslinking in thermally and DMDHEU modified wood is also likely to increase hemicellulose stiffness, however, furfurylation may have the opposite effect and increase hemicellulose plasticity [151,157]. Currently, it remains unclear which of these mechanisms is the dominant factor for inhibiting diffusion in modified wood. However, the different theories are not mutually exclusive, and each may play more or less of a role for each of the different wood modifications. Various methods were pointed out that could assess the effect of wood modifications on these means of transport. We also discussed the importance of controlling moisture conditions during decay experiments and highlighted the challenges associated with maintaining stable high RH.
Recent studies have shown that modified wood is not permanently protected from brown rot decay even at high modification levels [84,86,87]. Current literature indicates that the wood modification itself may initially be degraded, at least locally, generating areas of sufficiently low modification levels to allow for diffusion/degradation [87,89,90]. More long-term degradation experiments with modified wood are needed to confirm this theory, but the potential implications are large. A better understanding of how degradation eventually occurs in modified wood will help improve existing modification procedures and guide development of future, environmentally friendly wood protection systems.
Author Contributions
All authors contributed to the conceptualization, original draft preparation, review and editing of this manuscript. Data curation for Figure 1 was handled by R.R.
Acknowledgments
R.R. and A.P. gratefully acknowledge financial support from The Swedish Research Council Formas 942-2015-530.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Eaton, R.A.; Hale, M.D.C. Wood: Decay, Pests and Protection; Chapman & Hall: Cambridge, UK, 1993. [Google Scholar]
- Ibach, R. Biological properties. In Wood Chemistry and Wood Composites; Rowell, R.M., Ed.; Taylor & Francis: Boca Raton, FL, USA, 2005. [Google Scholar]
- Morrell, J.J. Protection of wood based materials. In Handbook of Environmental Degradation of Materials; Kutz, M., Ed.; William Andrew: Norwich, NY, USA, 2005; pp. 299–317. [Google Scholar]
- IPCC. Climate Change 2013; IPCC: Budapest, Hungary, 2013. [Google Scholar]
- Alfredsen, G.; Solheim, H.; Jenssen, K. Råtesopp i norske bygninger. Agarica 2006, 26, 78–86. [Google Scholar]
- Gabriel, J.; Švec, K. Occurrence of indoor wood decay basidiomycetes in Europe. Fungal Biol. Rev. 2017, 31, 212–217. [Google Scholar] [CrossRef]
- Schwarze, F.W.M.R. Wood decay under the microscope. Fungal Biol. Rev. 2007, 21, 133–170. [Google Scholar] [CrossRef]
- Yelle, D.J.; Wei, D.; Ralph, J.; Hammel, K.E. Multidimensional NMR analysis reveals truncated lignin structures in wood decayed by the brown rot basidiomycete Postia Placenta. Environ. Microbiol. 2011, 13, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Arantes, V.; Milagres, A.M.F.; Filley, T.R.; Goodell, B. Lignocellulosic polysaccharides and lignin degradation by wood decay fungi: The relevance of nonenzymatic Fenton-based reactions. J. Ind. Microbiol. Biotechnol. 2011, 38, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Winandy, J.E.; Morrell, J.J. Relationship between incipient decay, strength, and chemical composition of wood during incipient brown rot decay. Wood Fiber Sci. 1992, 25, 278–288. [Google Scholar]
- Schultze-Dewitz, G. Relations between elasticity and static and impact bending strength of pinewood after exposure to basidiomycetes. Holz Als Roh-Und Werkst. 1966, 24, 506–512. [Google Scholar] [CrossRef]
- Bariska, M.; Osuk, A.; Bosshard, H.H. Änderung der mechanischen Eigenschaften von Holz nach Abbau durch Basidiomyceten. Holz Als Roh-Und Werkst. 1983, 41, 241–245. [Google Scholar] [CrossRef]
- Venäläinen, M.; Partanen, H.; Harju, A. The strength loss of Scots pine timber in an accelerated soil contact test. Int. Biodeterior. Biodegrad. 2013, 86, 150–152. [Google Scholar] [CrossRef]
- Goodell, B.; Jellison, J.; Liu, G.; Daniel, A.; Paszcynski, F.; Fekete, S.; Krishnamurthy, L.; Jun, L.; Xu, G. Low molecular weight chelators and phenolic compounds isolated from wood decay fungi and their role in the fungal biodegradation of wood. J. Biotechnol. 1997, 53, 133–162. [Google Scholar] [CrossRef]
- Arantes, V.; Jellison, J.; Goodell, B. Peculiarities of brown-rot fungi and biochemical Fenton reaction with regard to their potential as a model for bioprocessing biomass. Appl. Microbiol. Biotechnol. 2012, 94, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Baldrian, P.; Valaskova, V. Degradation of cellulose by basidiomycetous fungi. Fems Microbiol. Rev. 2008, 32, 501–521. [Google Scholar] [CrossRef] [PubMed]
- Goodell, B.; Zhu, Y.; Kim, S.; Kafle, K.; Eastwood, D.; Daniel, G.; Jellison, J.; Yoshida, M.; Groom, L.; Pingali, S.; et al. Biotechnology for Biofuels Modification of the nanostructure of lignocellulose cell walls via a non-enzymatic lignocellulose deconstruction system in brown rot wood—Decay fungi. Biotechnol. Biofuels 2017, 10, 179. [Google Scholar] [CrossRef] [PubMed]
- Dix, N.J. Fungal Ecology; Chapman & Hall: London, UK, 1995. [Google Scholar]
- Engelund, E.T.; Thygesen, L.G.; Svensson, S.; Hill, C.A.S. A critical discussion of the physics of wood–water interactions. Wood Sci. Technol. 2012, 47, 141–161. [Google Scholar] [CrossRef]
- Stone, J.E.; Scallan, A.M. A structural model for the cell wall of swollen wood pulp fibres based on accessibility to macromolecules. Cellul. Chem. Technol. 1968, 2, 343–358. [Google Scholar]
- Hoffmeyer, P.; Engelund, E.T.; Thygesen, L.G. Equilibrium moisture content (EMC) in Norway spruce during the first and second desorptions. Holzforschung 2011, 65, 875–882. [Google Scholar] [CrossRef]
- Simpson, W. Sorption theories applied to wood. Wood Fiber 1980, 12, 183–195. [Google Scholar]
- Christensen, G.N.; Kelsey, K.E. The rate of sorption of water vapor by wood. Holz Roh Werkst 1959, 17, 178–188. [Google Scholar] [CrossRef]
- Thybring, E.E.; Thygesen, L.G.; Burgert, I. Hydroxyl accessibility in wood cell walls as affected by drying and re-wetting procedures. Cellulose 2017, 24, 2375–2384. [Google Scholar] [CrossRef]
- Maréchal, Y.; Chanzy, H. The hydrogen bond network in Iβ cellulose as observed by infrared spectrometry. J. Mol. Struct. 2000, 523, 183–196. [Google Scholar] [CrossRef]
- Hofstetter, K.; Hinterstoisser, B.; Salmén, L. Moisture uptake in native cellulose—The roles of different hydrogen bonds: A dynamic FT-IR study using Deuterium exchange. Cellulose 2006, 13, 131–145. [Google Scholar] [CrossRef]
- Fredriksson, M.; Thybring, E.E. Scanning or desorption isotherms? Characterising sorption hysteresis of wood. Cellulose 2018, 25, 4477–4485. [Google Scholar] [CrossRef]
- Cardias Williams, F.; Hale, M.D. The resistance of wood chemically modified with isocyanates: The role of moisture content in decay suppression. Int. Biodeterior. Biodegrad. 2003, 52, 215–221. [Google Scholar] [CrossRef]
- Hill, C.A.S.; Ormondroyd, G.A. Dimensional changes in Corsican pine (Pinus nigra Arnold) modified with acetic anhydride measured using a helium pycnometer. Holzforschung 2004, 58, 544–547. [Google Scholar] [CrossRef]
- Thybring, E.E. The decay resistance of modified wood influenced by moisture exclusion and swelling reduction. Int. Biodeterior. Biodegrad. 2013, 82, 87–95. [Google Scholar] [CrossRef]
- Kirker, G.; Zelink, S.; Gleber, S.-G.; Vine, D.; Finney, L.; Chen, S.; Hong, Y.P.; Uyarte, O.; Vogt, S.; Jellison, J.; et al. Synchrotron-based X-ray fluorescence microscopy enables multiscale spatial visualization of ions involved in fungal lignocellulose deconstruction. Sci. Rep. 2017, 7, 41798. [Google Scholar] [CrossRef] [PubMed]
- Zelinka, S.L.; Gleber, S.-C.; Vogt, S.; Rodriguez Lopez, G.M.; Jakes, J. Threshold for ion movements in wood cell walls below fiber saturation observed by X-ray fluorescence microscopy (XFM). Holzforschung 2015, 69, 441–448. [Google Scholar] [CrossRef]
- Schmidt, O. Wood and Tree Fungi: Biology, Damage, Protection and Use; Springer: Berlin, Germany, 2006. [Google Scholar]
- Meyer, L.; Brischke, C. Fungal decay at different moisture levels of selected European-grown wood species. Int. Biodeterior. Biodegrad. 2015, 103, 23–29. [Google Scholar] [CrossRef]
- KIFS. Kemikalieinspektionens föreskrifter (KIFS 1998:8) om kemiska produkter och biotekniska organismer; KIFS: Sundbyberg, Sweden, 1998. [Google Scholar]
- Hingston, J.A.; Collins, C.D.; Murphy, R.J.; Lester, J.N. Leaching of chromated copper arsenate wood preservatives: A review. Environ. Pollut. 2000, 111, 53–66. [Google Scholar] [CrossRef]
- Townsend, T.; Dubey, B.; Tolaymat, T.; Solo-Gabriele, H. Preservative leaching from weathered CCA-treated wood. J. Environ. Manag. 2005, 75, 105–113. [Google Scholar] [CrossRef]
- Hill, C. Wood Modifications: Chemical, Thermal, and Other Processes; John Wiley and Sons, Ltd.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Van der Lugt, P.; Vogtländer, J.G. Wood acetylation: A potential route towards climate change mitigation. Wit Trans. Built Environ. 2014, 142, 241–252. [Google Scholar]
- Militz, H. Die Verbesserung das Schwind-und Quellverhaltens und der Daurhaftigkeit von Holz mittels Behnadlung mit unkatalysiertem Essigsäureanhydrid. Holz Als Roh-Und Werkst. 1991, 49, 147–152. [Google Scholar] [CrossRef]
- Larsson Brelid, P.; Simonson, R.; Bergman, Ö.; Nilsson, T. Resistance of acetylated wood to biological degradation. Holz Als Roh-Und Werkst. 2000, 58, 331–337. [Google Scholar] [CrossRef]
- Schneider, M.H. New cell wall and cell lumen wood polymer composites. J. Wood Sci. Technol. 1995, 36, 429–433. [Google Scholar] [CrossRef]
- Lande, S.; Eikenes, M.; Westin, M.; Schneider, M. Furfurylation of wood: Chemistry; properties and commerzialisation. Dev. Commer. Wood Preserv. 2008, 982, 337–355. [Google Scholar]
- Bryne, L.E.; Wålinder, M.E.P. Ageing of modified wood. Part 1: Wetting properties of acetylated, furfurylated, and thermally modified wood. Holzforschung 2010, 64, 295–304. [Google Scholar] [CrossRef]
- Thygesen, L.G.; Barsberg, S.; Venås, T.M. The fluorescence characteristics of furfurylated wood studied by fluorescence spectroscopy and confocal laser scanning microscopy. Wood Sci. Technol. 2010, 44, 51–65. [Google Scholar] [CrossRef]
- Tjeerdsma, B.F.; Boonstra, M.; Pizzi, A.; Militz, H. Characterisation of thermally modified wood: Molecular reasons for wood performance improvement. Holz Als Roh-Und Werkst. 1998, 56, 149–153. [Google Scholar] [CrossRef]
- Welzbacher, C.R. Verhalten Von Nach Neuen Thermischen Modifikationsverfahren Behandelter Fichte und Kiefer Unter Besonderer Berücksichtigung der Dauerhaftigkeit Gegenüber Holzzerstörenden Mikroorganismen—Performance of Spruce and Pine Timber, Treated by Novel Thermal Modification. Ph.D. Thesis, University of Hamburg, Hamburg, Germany, 2007. [Google Scholar]
- Windeisen, E.; Bächle, H.; Zimmer, B.; Wegener, G. Relations between chemical changes and mechanical properties of thermally treated wood. Holzforschung 2009, 63, 773–778. [Google Scholar]
- Pfriem, A.; Zauer, M.; Wagenführ, A. Alteration of the unsteady sorption behaviour of maple (Acer pseudoplatanus L.) and spruce (Picea abies (L.) Karst.) due to thermal modification. Holzforschung 2010, 64, 235–241. [Google Scholar] [CrossRef]
- Ibach, R.; Rowell, R.M. Improvements in Decay Resistance Based on Moisture Exclusion. Mol. Cryst. Liq. Cryst. 2000, 353, 22–33. [Google Scholar] [CrossRef]
- Epmeier, H.; Westin, M.; Rapp, A. Differently modified wood: Comparison of some selected properties. Scand. J. For. Res. 2004, 19, 31–37. [Google Scholar] [CrossRef]
- Rowell, R.M.; Ibach, R.; Nilsson, T. Influence of moisture on brown-rot fungal attack on wood. In Proceedings of the 3rd meeting of the Nordic-Baltic Network in Wood Material Science and Engineering (WSE), Helsinki, Finland, 29–30 October 2007; pp. 65–69. [Google Scholar]
- Rapp, A.O.; Brischke, C.; Welzbacher, C.R.; Jazayeri, L. Increased resistance of thermally modiied norway spruce timber (TMT) against brown rot decay by Oligoporus placenta—A study on the mode of protective action. Wood Res. 2008, 53, 13–26. [Google Scholar]
- Nordiska träskyddsföreningen. Conditions for Approval of Industrially Protected Wood in the Nordic Countries; No 2; Nordiska Träskyddsföreningen: Stockholm, Sweden, 2017. [Google Scholar]
- Hill, C.; Popescu, C.; Rautkari, L.; Ormondroyd, G.; Xie, Y.; Jalaludin, Z. The role of hydroxyl groups in determining the sorption properties of modified wood. In Proceedings of the Seventh European Conference on Wood Modification, Lisbon, Portugal, 10–12 March 2014. [Google Scholar]
- Popescu, C.-M.; Hill, C.A.S.; Curling, S.; Ormondroyd, G.; Xie, Y. The water vapour sorption behaviour of acetylated birch wood: How acetylation affects the sorption isotherm and accessible hydroxyl content. J. Mater. Sci. 2013, 49, 2362–2371. [Google Scholar] [CrossRef]
- Rowell, R.M.; Ibach, R.E.; McSweeny, J.; Nilsson, T. Understanding decay resistance, dimensional stability and strength changes in heat-treated and acetylated wood. Wood Mater. Sci. Eng. 2009, 4, 14–22. [Google Scholar] [CrossRef]
- Popescu, C.-M.; Hill, C.A.S.; Popescu, M.-C. Water adsorption in acetylated birch wood evaluated through near infrared spectroscopy. Int. Wood Prod. J. 2016, 7, 61–65. [Google Scholar] [CrossRef]
- Passarini, L.; Zelinka, S.L.; Glass, S.V.; Hunt, C.G. Effect of weight percent gain and experimental method on fiber saturation point of acetylated wood determined by differential scanning calorimetry. Wood Sci. Technol. 2017, 51, 1291–1305. [Google Scholar] [CrossRef]
- Beck, G.; Thybring, E.E.; Thygesen, L.G.; Hill, C. Characterization of moisture in acetylated and propionylated radiata pine using low-field nuclear magnetic resonance (LFNMR) relaxometry. Holzforschung 2017, 72, 225–233. [Google Scholar] [CrossRef]
- Thygesen, L.G.; Tang Engelund, E.; Hoffmeyer, P. Water sorption in wood and modified wood at high values of relative humidity. Part I: Results for untreated, acetylated, and furfurylated Norway spruce. Holzforschung 2010, 64, 315–323. [Google Scholar] [CrossRef]
- Hill, C.A.S.; Forster, S.C.; Farahani, M.R.M.; Hale, M.D.C.; Ormondroyd, G.A.; Williams, G.R. An investigation of cell wall micropore blocking as a possible mechanism for the decay resistance of anhydride modified wood. Int. Biodeterior. Biodegrad. 2005, 55, 69–76. [Google Scholar] [CrossRef]
- Hill, C.A.S.; Curling, S.F.; Kwon, J.H.; Marty, V. Decay resistance of acetylated and hexanoylated hardwood and softwood species exposed to Coniophora puteana. Holzforschung 2009, 63, 619–625. [Google Scholar] [CrossRef]
- Papadopoulos, A.N.; Hill, C.A.S. The biological effectiveness of wood modified with linear chain carboxylic acid anhydrides against Coniophora Puteana. Holz Als Roh-Und Werkst. 2002, 60, 329–332. [Google Scholar] [CrossRef]
- Papadopoulos, A.N.; Hill, C.A.S. The sorption of water vapour by anhydride modified softwood. Wood Sci. Tech. 2003, 37, 221–231. [Google Scholar] [CrossRef]
- Beck, G.; Strohbusch, S.; Larnøy, E.; Militz, H.; Hill, C. Accessibility of hydroxyl groups in anhydride modified wood as measured by deuterium exchange and saponification. Holzforschung 2017, 72, 17–23. [Google Scholar] [CrossRef]
- Meyer, L.; Brischke, C.; Pilgård, A. Moisture performance based wood durability testing. In Proceedings of the IRG Annual meeting (ISSN 2000-8953), Kuala Lumpur, Malaysia, 6–10 May 2012. IRG/WP 12-20495. [Google Scholar]
- Venås, T.M. A Study of Mechanisms Related to the Fungal Decay Protection Rendered by Wood Furfurylation. Ph.D. Thesis, University of Copenhagen, Copenhagen, Denmark, 2008. [Google Scholar]
- Thygesen, L.G.; Elder, T. Moisture in Untreated, Acetylated, and Furfurylated Norway Spruce Monitored During Drying Below Fiber Saturation Using Time Domain NMR. Wood Fiber Sci. 2009, 41, 194–200. [Google Scholar]
- Dieste, A.; Krause, A.; Mai, C.; Sèbe, G.; Grelier, S.; Militz, H. Modification of Fagus sylvatica L. with 1,3-dimethylol-4,5-dihydroxy ethylene urea (DMDHEU). Part 2: Pore size distribution determined by differential scanning calorimetry. Holzforschung 2009, 63, 89–93. [Google Scholar] [CrossRef]
- Dieste, A.; Krause, A.; Mai, C.; Militz, H. The calculation of EMC for the analysis of wood/water relations in Fagus sylvatica L. modified with 1,3-dimethylol-4,5-dihydroxyethyleneurea. Wood Sci. Technol. 2009, 44, 597–606. [Google Scholar] [CrossRef][Green Version]
- Phuong, L.X.; Takayama, M.; Shida, S.; Matsumoto, Y.; Aoyagi, T. Determination of the accessible hydroxyl groups in heat-treated Styrax tonkinensis (Pierre) Craib ex Hartwich wood by hydrogen-deuterium exchange and 2H NMR spectroscopy. Holzforschung 2007, 61, 488–491. [Google Scholar] [CrossRef]
- Hakkou, M.; Pétrissans, M.; Gérardin, P.; Zoulalian, A. Investigations of the reasons for fungal durability of heat-treated beech wood. Polym. Degrad. Stab. 2006, 91, 393–397. [Google Scholar] [CrossRef]
- Altgen, M.; Willems, W.; Hosseinpourpia, R.; Rautkari, L. Hydroxyl accessibility and dimensional changes of Scots pine sapwood affected by alterations in the cell wall ultrastructure during heat-treatment. Polym. Degrad. Stab. 2018, 52, 244–252. [Google Scholar] [CrossRef]
- Hoffmeyer, P.; Jensen, S.K.; Jones, D.; Klinke, H.B.; Felby, C. Sorption properties of steam treated wood and plant fibres. In Proceedings of the First European Conference on Wood Modification, Ghent, Belgium, 3–4 April 2003; pp. 177–189. [Google Scholar]
- Scheiding, W.; Direske, M.; Zauer, M. Water absorption of untreated and thermally modified sapwood and heartwood of Pinus sylvestris L. Eur. J. Wood Wood Prod. 2016, 74, 585–589. [Google Scholar] [CrossRef]
- Zauer, M.; Pfriem, A.; Wagenführ, A. Toward improved understanding of the cell-wall density and porosity of wood determined by gas pycnometry. Wood Sci. Technol. 2013, 6, 1197–1211. [Google Scholar] [CrossRef]
- Biziks, V.; Andersons, B.; Sansonetti, E.; Andersone, I.; Militz, H.; Grinins, J. One-stage thermo-hydro treatment (THT) of hardwoods: An analysis of form stability after five soaking-drying cycles. Holzforschung 2014, 69, 563–571. [Google Scholar] [CrossRef]
- Ringman, R.; Pilgard, A.; Brischke, C.; Richter, K. Mode of action of brown rot decay resistance in modified wood: A review. Holzforschung 2014, 68, 239–246. [Google Scholar] [CrossRef]
- Boonstra, M.J.; Tjeerdsma, B. Chemical analysis of heat treated softwoods. Holz Als Roh-Und Werkst. 2006, 64, 204–211. [Google Scholar] [CrossRef]
- Boonstra, M.J.; Van Acker, J.; Kegel, E.; Stevens, M. Optimisation of a two-stage heat treatment process: durabilityaspects. Wood Sci. Technol. 2007, 41, 31–57. [Google Scholar] [CrossRef]
- Rowell, R.M. Chemical modification of wood. In Wood Chemistry and Wood Composites; Taylor & Francis: Boca Raton, FL, USA, 2005. [Google Scholar]
- Schmöllerl, B.; Alfredsen, G.; Fossdal, C.G.; Westin, M.; Steitz, A. Molecular investigation of Postia placenta growing in modified wood. In Proceedings of the International Research Group on Wood Protection, Queenstown, New Zealand, 8–12 May 2011. Document No. IRG/WP 11-10756. [Google Scholar]
- Alfredsen, G.; Pilgård, A.; Fossdal, C.G. Characterisation of Postia placenta colonisation during 36 weeks in acetylated southern yellow pine sapwood at three acetylation levels including genomic DNA and gene expression quantification of the fungus. Holzforschung 2016, 70, 1055–1065. [Google Scholar] [CrossRef]
- Ringman, R.; Pilgård, A.; Kölle, M.; Brischke, C.; Richter, K. Effects of thermal modification on Postia placenta wood degradation dynamics: Measurements of mass loss, structural integrity and gene expression. Wood Sci. Technol. 2016, 50, 385–397. [Google Scholar] [CrossRef]
- Ringman, R.; Pilgård, A.; Richter, K. Effect of wood modification on gene expression during incipient Postia placenta decay. Int. Biodeterior. Biodegrad. 2014, 86, 86–91. [Google Scholar] [CrossRef]
- Beck, G.; Hegnar, O.A.; Fossdal, C.G.; Alfredsen, G. Acetylation of Pinus radiata delays hydrolytic depolymerisation by the brown-rot fungus Rhondonia placenta. Int. Biodeterior. Biodegrad. 2018, 135, 39–52. [Google Scholar] [CrossRef]
- Alfredsen, G.; Fossdal, C.G.; Nagy, N.E.; Jellison, J.; Goodell, B. Furfurylated wood: Impact on Postia placenta gene expression and oxalate crystal formation. Holzforschung 2016, 70, 947–962. [Google Scholar] [CrossRef]
- Ringman, R.; Pilgård, A.; Richter, K. In vitro oxidative and enzymatic degradation of modified wood. Int. Wood Prod. J. 2015, 6, 36–39. [Google Scholar] [CrossRef]
- Verma, P.; Mai, C. Hydrolysis of cellulose and wood powder treated with DMDHEU by a hydrolase enzyme complex, Fenton’s reagent, and in a liquid culture of Trametes Versicolor. Holzforschung 2010, 64, 69–75. [Google Scholar] [CrossRef]
- Hosseinpourpia, R.; Mai, C. Mode of action of brown rot decay resistance of thermally modified wood: Resistance to Fenton’s reagent. Holzforschung 2016, 70, 691–697. [Google Scholar] [CrossRef]
- Xie, Y.; Xiao, Z.; Mai, C. Degradation of chemically modified Scots pine (Pinus sylvestris L.) with Fenton reagent. Holzforschung 2014, 69, 153–161. [Google Scholar] [CrossRef]
- Hegnar, O.A.; Goodell, B.; Felby, C.; Johansson, L.; Labbé, N.; Kim, K.; Eijsink, V.G.H.; Alfredsen, G.; Várnai, A. Challenges and opportunities in mimicking non-enzymatic brown-rot decay mechanisms for pretreatment of Norway spruce. Wood Sci. Technol. 2019, 53, 291–311. [Google Scholar] [CrossRef]
- Curling, S.F.; Clausen, C.A.; Winandy, J.E. Relationships between mechanical properties, weight loss and chemical composition of wood during incipient brown-rot decay. For. Prod. J. 2002, 52, 34–39. [Google Scholar]
- Ringman, R.; Pilgård, A.; Brischke, C.; Windeisen, E.; Richter, K. Incipient brown rot decay in modified wood: Patterns of mass loss, structural integrity, moisture and acetyl content in high resolution. Int. Wood Prod. J. 2017, 8, 172–182. [Google Scholar] [CrossRef]
- Thybring, E.E.; Kymäläinen, M.; Rautkari, L. Moisture in modified wood and its relevance for fungal decay. IForest 2018, 11, 418–422. [Google Scholar] [CrossRef]
- Zelinka, S.L.; Ringman, R.; Pilgård, A.; Thybring, E.E.; Jakes, J.E.; Richter, K. The role of chemical transport in the brown-rot decay resistance of modified wood. Int. Wood Prod. J. 2016, 7, 35–43. [Google Scholar] [CrossRef]
- Jellison, J.; Chandhoke, V.; Goodell, B.; Fekete, F. A The isolation and immunolocalization of iron-binding compounds produced by Gloeophyllum trabeum. Appl. Microbiol. Biotechnol. 1991, 35, 805–809. [Google Scholar] [CrossRef]
- Kim, Y.S.; Wi, A.G.; Lee, K.H.; Singh, S.P. Cytochemical Localization of Hydrogen Peroxide Production during Wood Decay by Brown-Rot Fungi Tyromyces palustris and Coniophora puteana. Holzforschung 2002, 56, 7–12. [Google Scholar] [CrossRef]
- Rowell, R.M. Chemical modification of wood: A short review. Wood Mater. Sci. Eng. 2006, 1, 29–33. [Google Scholar] [CrossRef]
- Hunt, C.G.; Zelinka, S.L.; Frihart, C.R.; Lorenz, L.; Yelle, D.; Gleber, S.-C.; Vogt, S.; Jakes, J.E. Acetylation increases relative humidity threshold for ion transport in wood cell walls—A means to understanding decay resistance. Int. Biodeterior. Biodegrad. 2018, 133, 230–237. [Google Scholar] [CrossRef]
- Hosseinpourpia, R.; Mai, C. Mode of action of brown rot decay resistance of acetylated wood: Resistance to Fenton’s reagent. Wood Sci. Technol. 2016, 50, 413–426. [Google Scholar] [CrossRef]
- Zelinka, S.L.; Glass, S.V.; Stone, D.S. A percolation model for electrical conduction in wood with implications for wood-water relations. Wood Fiber Sci. 2008, 40, 544–552. [Google Scholar]
- Jakes, J.E.; Plaza, N.; Stone, D.S.; Hunt, C.G.; Glass, S.V.; Zelinka, S.L. Mechanism of Transport Through Wood Cell Wall Polymers. J. For. Prod. Ind. 2013, 2, 10–13. [Google Scholar]
- Hearle, J.W.S. The electrical resistance of textile materials: IV. Theory. J. Text. Inst. Trans. 1953, 44, T177–T198. [Google Scholar] [CrossRef]
- Obataya, E.; Minato, K. Potassium acetate-catalyzed acetylation of wood: Extraordinarily rapid acetylation at 120 degrees C. Wood Sci. Technol. 2008, 42, 567–677. [Google Scholar] [CrossRef]
- Lande, S.; Westin, M.; Schneider, M. Properties of furfurylated wood. Scand. J. For. Res. 2004, 19, 22–30. [Google Scholar] [CrossRef]
- Yasuda, R.; Minato, K.; Norimoto, M. Chemical modification of wood by non-formaldehyde cross-linking reagents. Wood Sci. Technol. 1994, 28, 209–218. [Google Scholar] [CrossRef]
- Kwon, J.H.; Hill, C.A.S.; Ormondroyd, G.A.; Karim, S. Changes in the cell wall volume of a number of wood species due to reaction with acetic anhydride. Holzforschung 2007, 61, 138–142. [Google Scholar] [CrossRef]
- Farahani, M.R.M. Decay Resistance of Modified Wood. Ph.D. Thesis, University of Wales, Bangor, UK, 2003. [Google Scholar]
- Forster, S.C. The Decay Resistance of Chemically Modified Softwood. Ph.D. Thesis, University of Wales, Bangor, UK, 1999. [Google Scholar]
- Allan, G.G.; Ritzenthaler, P. The microporosity of pulp: The nature of the pore size distribution. Tappi J. 1991, 74, 205–212. [Google Scholar]
- Alince, B. Comments on porosity or swollen pulp fibers analyzed by solute-exclusion. Tappi J. 1991, 74, 200–202. [Google Scholar]
- Rowland, S.P.; Bertoniere, N.R. Some interactions of water-soluble solutes with cellulose and Sephadex. Text. Res. J. 1976, 46, 770–775. [Google Scholar] [CrossRef]
- Thybring, E.E.; Kymäläinen, M.; Rautkari, L. Experimental techniques for characterising water in wood covering the range from dry to fully water-saturated. Wood Sci. Technol. 2018, 52, 297–329. [Google Scholar] [CrossRef]
- Flournoy, D.S.; Kirk, K.; Highley, T.L. Wood Decay by Brown-Rot Fungi: Changes in Pore Structure and Cell Wall Volume. Holzforschung 1991, 45, 383–388. [Google Scholar] [CrossRef]
- Hosseinpourpia, R.; Mai, C. Mode of action of brown rot decay resistance in phenol-formaldehyde-modified wood: Resistance to Fenton’s reagent. Holzforschung 2015, 70, 253–259. [Google Scholar] [CrossRef]
- Zelinka, S.L.; Lambrecht, M.J.; Glass, S.V.; Wiedenhoeft, A.C.; Yelle, D.J. Examination of water phase transitions in Loblolly pine and cell wall components by differential scanning calorimetry. Thermochim. Acta 2012, 533, 39–45. [Google Scholar] [CrossRef]
- Thomson, W. On the Equilibrium of Vapour at a Curved Surface of Liquid. Proc. R. Soc. Edinb. 1872, 7, 63–68. [Google Scholar] [CrossRef]
- Griffin, D.M. Water Potential and Wood-Decay Fungi. Annu. Rev. Phytopathol. 1977, 15, 319–329. [Google Scholar] [CrossRef]
- Clarke, R.W.; Jennings, D.H.; Coggings, C.R. Growth of Serpula lacrimans in relation to water potential of substrate. Trans. Br. Mycol. Soc. 1980, 75, 271–280. [Google Scholar] [CrossRef]
- Viitanen, H. Modelling the Time Factor in the Development of Mould Fungi-the Effect of Critical Humidity and Temperature Conditions on Pine and Spruce Sapwood. Holzforschung 1997, 51, 6–14. [Google Scholar] [CrossRef]
- Schmidt, O. Indoor wood-decay basidiomycetes: Damage, causal fungi, physiology, identification and characterization, prevention and control. Mycol. Prog. 2007, 6, 261–279. [Google Scholar] [CrossRef]
- Mantanis, G.I.; Young, R.A. Wetting of wood. Wood Sci. Technol. 1997, 31, 339. [Google Scholar] [CrossRef]
- Zauer, M.; Hempel, S.; Pfriem, A.; Mechtcherine, V.; Wagenführ, A. Investigations of the pore-size distribution of wood in the dry and wet state by means of mercury intrusion porosimetry. Wood Sci. Technol. 2014, 48, 1229–1240. [Google Scholar] [CrossRef]
- Wang, W.; Yan, N. Characterizing Liquid Resin Penetration In Wood Using A Mercury Intrusion Porosimeter. Wood Fiber Sci. 2005, 37, 505–513. [Google Scholar]
- Zauer, M.; Kretzschmar, J.; Großmann, L.; Pfriem, A.; Wagenführ, A. Analysis of the pore-size distribution and fiber saturation point of native and thermally modified wood using differential scanning calorimetry. Wood Sci. Technol. 2013, 48, 177–193. [Google Scholar] [CrossRef]
- Bastani, A.; Adamopoulos, S.; Militz, H. Water uptake and wetting behaviour of furfurylated, N-methylol melamine modified and heat-treated wood. Eur. J. Wood Wood Prod. 2015, 73, 627–634. [Google Scholar] [CrossRef]
- Dieste, A.; Krause, A.; Militz, H. Modification of Fagus sylvatica (L.) with 1,3-dimethylol-4,5-dihydroxyethylene urea (DMDHEU): Part 1. Estimation of heat adsorption by the isosteric method (Hailwood-Horrobin model) and by solution calorimetry. Holzforschung 2008, 62, 577–583. [Google Scholar] [CrossRef]
- Hill, C.A.S.; Papadopoulos, A.N. A Review of Methods Used to Determine the Size of the Cell Wall Microvoids of Wood. J. Inst. Wood Sci. 2001, 15, 337–345. [Google Scholar]
- Plötze, M.; Niemz, P. Porosity and pore size distribution of different wood types as determined by mercury intrusion porosimetry. Eur. J. Wood Wood Prod. 2011, 69, 649–657. [Google Scholar] [CrossRef]
- Park, S.; Venditti, R.A.; Jameel, H.; Pawlak, J.J. Changes in pore size distribution during the drying of cellulose fibers as measured by differential scanning calorimetry. Carbohydr. Polym. 2006, 66, 97–103. [Google Scholar] [CrossRef]
- Gao, X.; Zhuang, S.; Jin, J.; Cao, P. Bound Water Content and Pore Size Distribution in Swollen Cell Walls Determined by NMR Technology. BioResources 2015, 10, 8208–8224. [Google Scholar] [CrossRef]
- Weatherwax, R.C. Collapse of cell-wall pores during drying of cellulose. J. Colloid Interface Sci. 1977, 62, 432–446. [Google Scholar] [CrossRef]
- Weatherwax, R.C.; Caulfield, D.F. Cellulose aerogels: An improved method for preparing a highly expanded form of dry cellulose. Tappi J. 1971, 54, 985–986. [Google Scholar]
- Kang, K.-Y.; Hwang, K.-R.; Park, J.-Y.; Lee, J.-P.; Kim, J.-S.; Lee, J.-S. Critical Point Drying: An Effective Drying Method for Direct Measurement of the Surface Area of a Pretreated Cellulosic Biomass. Polymers 2018, 10, 676. [Google Scholar] [CrossRef]
- Cox, J.; McDonald, P.J.; Gardiner, B.A. A study of water exchange in wood by means of 2D NMR relaxation correlation and exchange. Holzforschung 2010, 64, 259–266. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Gidley, M.J.; Gilbert, E.P. Application of X-ray and neutron small angle scattering techniques to study the hierarchical structure of plant cell walls: A review. Carbohydr. Polym. 2015, 125, 120–134. [Google Scholar]
- Xu, P.; Donaldson, L.A.; Gergely, Z.R.; Staehelin, L.A. Dual-axis electron tomography: A new approach for investigating the spatial organization of wood cellulose microfibrils. Wood Sci. Technol. 2006, 41, 101–116. [Google Scholar] [CrossRef]
- Torstensen, J.Ø.; Liu, M.; Jin, S.-A.; Deng, L.; Hawaru, A.I.; Syverud, K.; Spontak, R.J.; Gregersen, Ø.W. Swelling and free-volume characteristics of TEMPO-oxidized cellulose nanofibril films. Biomacromolecules 2018, 19, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Kulasinski, K.; Guyer, R.A. Quantification of nanopore networks: Application to amorphous polymers. J. Phys. Chem. C 2016, 120, 28144–28151. [Google Scholar] [CrossRef]
- Zauer, M.; Meissner, F.; Plagge, R.; Wagenführ, A. Capillary pore-size distribution and equilibrium moisture content of wood determined by means of pressure plate technique. Holzforschung 2015, 70, 137–143. [Google Scholar] [CrossRef]
- Fredriksson, M.; Johansson, S.A. Method for Determination of Absorption Isotherms at High Relative Humidity Levels: Measurements on Lime-Silica Brick and Norway Spruce. Dry. Technol. 2016, 34, 132–141. [Google Scholar] [CrossRef]
- Katz, J.R. Die Quellung. In Ergebnisse der Exakten Naturwissenschaften; Springer: Berlin, Germany, 1924; Volume 3, p. 316. [Google Scholar]
- Cousins, W.J. Young’s modulus of hemicellulose as related to moisture content. Wood Sci. Technol. 1978, 12, 161–167. [Google Scholar] [CrossRef]
- Kelley, S.S.; Rials, T.G.; Glasser, W.G.; Al, E. Relaxation behaviour of the amorphous components of wood. J. Mater. Sci. 1987, 22, 617–624. [Google Scholar] [CrossRef]
- Olsson, A.M.; Salmén, L. The softening behavior of hemicelluloses related to moisture. ACS Symp. Ser. 2004, 864, 184–197. [Google Scholar]
- Paul, W.; Ohlmeyer, M.; Leithoff, H.; Boonstra, M.J.; Pizzi, A. Optimising the properties of OSB by a one-step heat pre-treatment process. Holz Als Roh-Und Werkst. 2006, 64, 227–234. [Google Scholar] [CrossRef]
- Åkerholm, M.; Salmen, L. Softening of wood polymers induced by moisture studied by dynamic FTIR spectroscopy. J. Appl. Polym. Sci. 2004, 94, 2032–2040. [Google Scholar] [CrossRef]
- Gröndahl, M.; Teleman, A.; Gatenholm, P. Effect of acetylation on the material properties of glucuronoxylan from aspen wood. Carbohydr. Polym. 2003, 52, 359–366. [Google Scholar] [CrossRef]
- Willems, W. Glassy state of wood polymers in native and thermally modified wood: Effects on long-term material performance in service. Int. Wood Prod. J. 2016, 7, 71–75. [Google Scholar] [CrossRef]
- Hill, C.A.S.; Ramsay, J.; Keating, B.; Laine, K.; RAutkari, L.; Hughes, M.; Constant, B. The water vapour sorption properties of thermally modified and densified wood. J. Mater. Sci. 2012, 47, 3191–3197. [Google Scholar] [CrossRef]
- Salmén, L.; Possler, H.; Stevanic, J.S.; Stanzl-Tschegg, S.E. Analysis of thermally treated wood samples using dynamic FT-IR-spectroscopy. Holzforschung 2008, 62, 676–678. [Google Scholar] [CrossRef]
- Krause, A.; Jones, D.; van der Zee, M.; Militz, H. Interlacement treatment—wood modification with n-methylol compounds. In Proceedings of the First European Conference on Wood Modification, Ghent, Belgium, 3–4 April 2003; pp. 317–327. [Google Scholar]
- Nordstierna, L.; Lande, S.; Westin, M.; Karlsson, O.; Furó, I. Towards novel wood-based materials: Chemical bonds between lignin-like model molecules and poly(furfuryl alcohol) studied by NMR. Holzforschung 2008, 62, 709–713. [Google Scholar] [CrossRef]
- Ehmcke, G.; Pilgård, A.; Koch, G.; Richter, K. Topochemical analyses of furfuryl alcohol-modified radiata pine (Pinus radiata) by UMSP, light microscopy and SEM. Holzforschung 2017, 71, 821–831. [Google Scholar] [CrossRef]
- Herold, N.; Grigsby, W.J.; Franich, R.A.; Pfriem, A. Investigations of wood veneer during furfuryl alcohol modification using DMTA. Eur. J. Wood Wood Prod. 2015, 73, 693–695. [Google Scholar] [CrossRef]
- Ganser, C.; Hirn, U.; Rohm, S.; Schennach, R. AFM nanoindentation of pulp fibers and thin cellulose films at varying relative humidity. Holzforschung 2013, 68, 53–60. [Google Scholar] [CrossRef]
- Beck, G.; Thybring, E.E.; Thygesen, L.G. Brown-rot fungal degradation and de-acetylation of acetylated wood. Int. Biodeterior. Biodegrad. 2018, 135, 62–70. [Google Scholar] [CrossRef]
- Verma, P.; Junga, U.; Militz, H.; Mai, C. Protection mechanisms of DMDHEU treated wood against white and brown rot fungi. Holzforschung 2009, 63, 371–378. [Google Scholar] [CrossRef]
- Thybring, E.E. Water relations in untreated and modified wood under brown-rot and white-rot decay. Int. Biodeterior. Biodegrad. 2017, 118, 134–142. [Google Scholar] [CrossRef]
- Eastwood, D.C.; Floudas, D.; Binder, M.; Majcherczyk, A.; Schneider, P.; Aerts, A.; Asiegbu, F.O.; Baker, S.E.; Barry, K.; Bendiksby, M.; et al. The plant cell wall-decomposing machinery underlies the functional diversity of forest fungi. Science 2011, 333, 762–765. [Google Scholar] [CrossRef] [PubMed]
- EN 113. Wood Preservatives—Test Method for Determining the Protective Effectiveness Against Wood Destroying Basidiomycetes: Determination of Toxic Values; European Committee for Standardization (CEN): Brussels, Belgium, 1996; p. 31. [Google Scholar]
- AWPA E10. American Wood-Preservers’ Association Standard E10-91. Standard Method for Testing Wood Preservatives by Laboratory Soil-Block Cultures; AWPA: Birmingham, AL, USA, 1991. [Google Scholar]
- Zhang, J.; Presley, G.N.; Hammel, K.E.; Ryu, J.-S.; Menke, J.R.; Figueroa, M.; Hu, D.; Orr, G.; Schilling, J.S. Localizing gene regulation reveals a staggered wood decay mechanism for the brown rot fungus Postia placenta. Proc. Natl. Acad. Sci. USA 2016, 113, 10968–10973. [Google Scholar] [CrossRef] [PubMed]
- Alfredsen, G.; Ringman, R.; Pilgård, A.; Fossdal, C.G. New insight regarding mode of action of brown rot decay of modified wood based on DNA and gene expression studies: A review. Int. Wood Prod. J. 2015, 6, 2008–2013. [Google Scholar] [CrossRef]
- Esteves, B.M.; Pereira, H.M. Wood modification by heat treatment: A review. BioResources 2009, 4, 370–404. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).