The Impacts of Vegetation Types and Soil Properties on Soil Microbial Activity and Metabolic Diversity in Subtropical Forests
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Sample Collection and Preparation
2.3. Chemical Analysis and Biolog® ECO-plate Technique
2.4. Biolog® ECO-plate Analysis
2.5. Statistical Analysis
3. Results
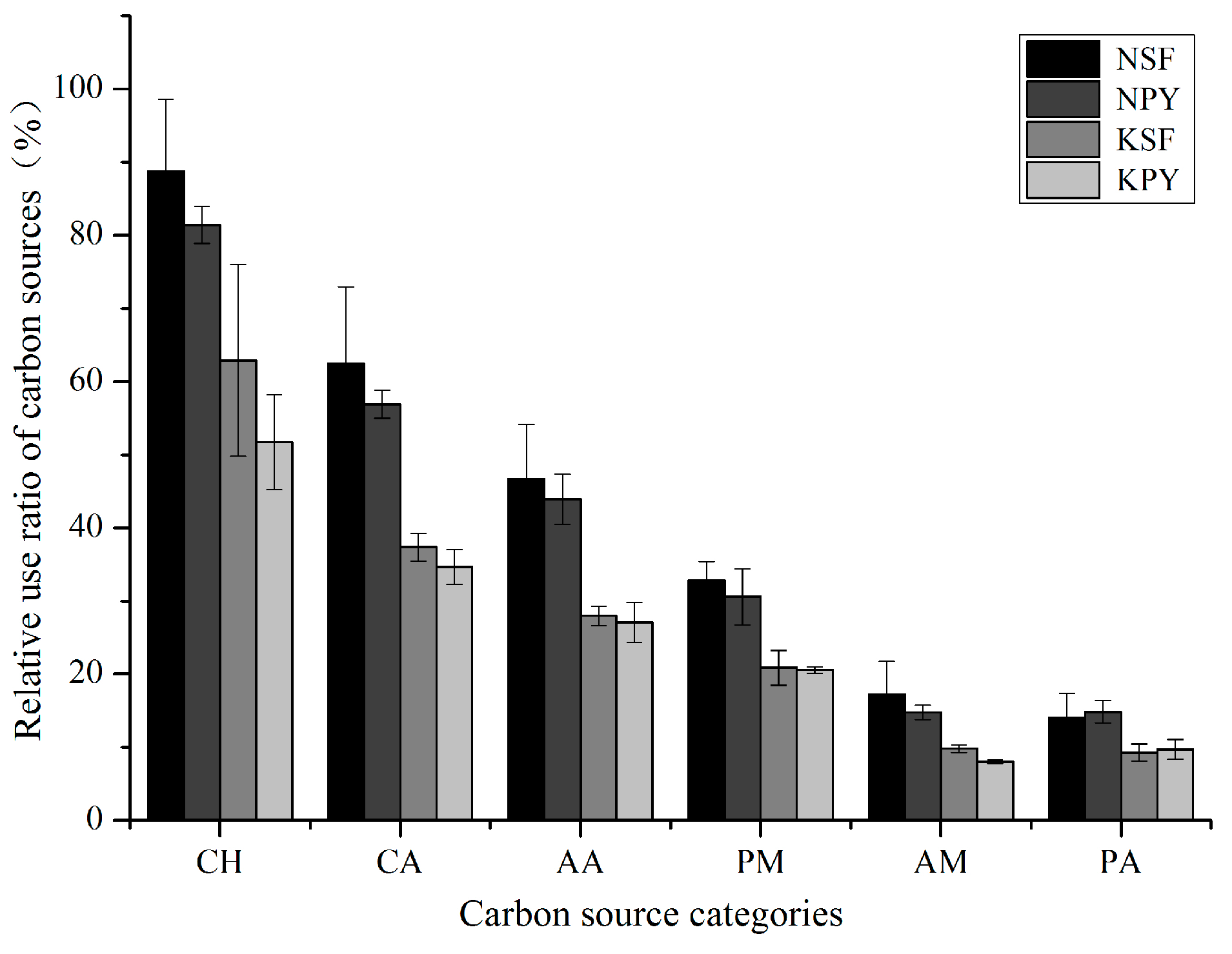
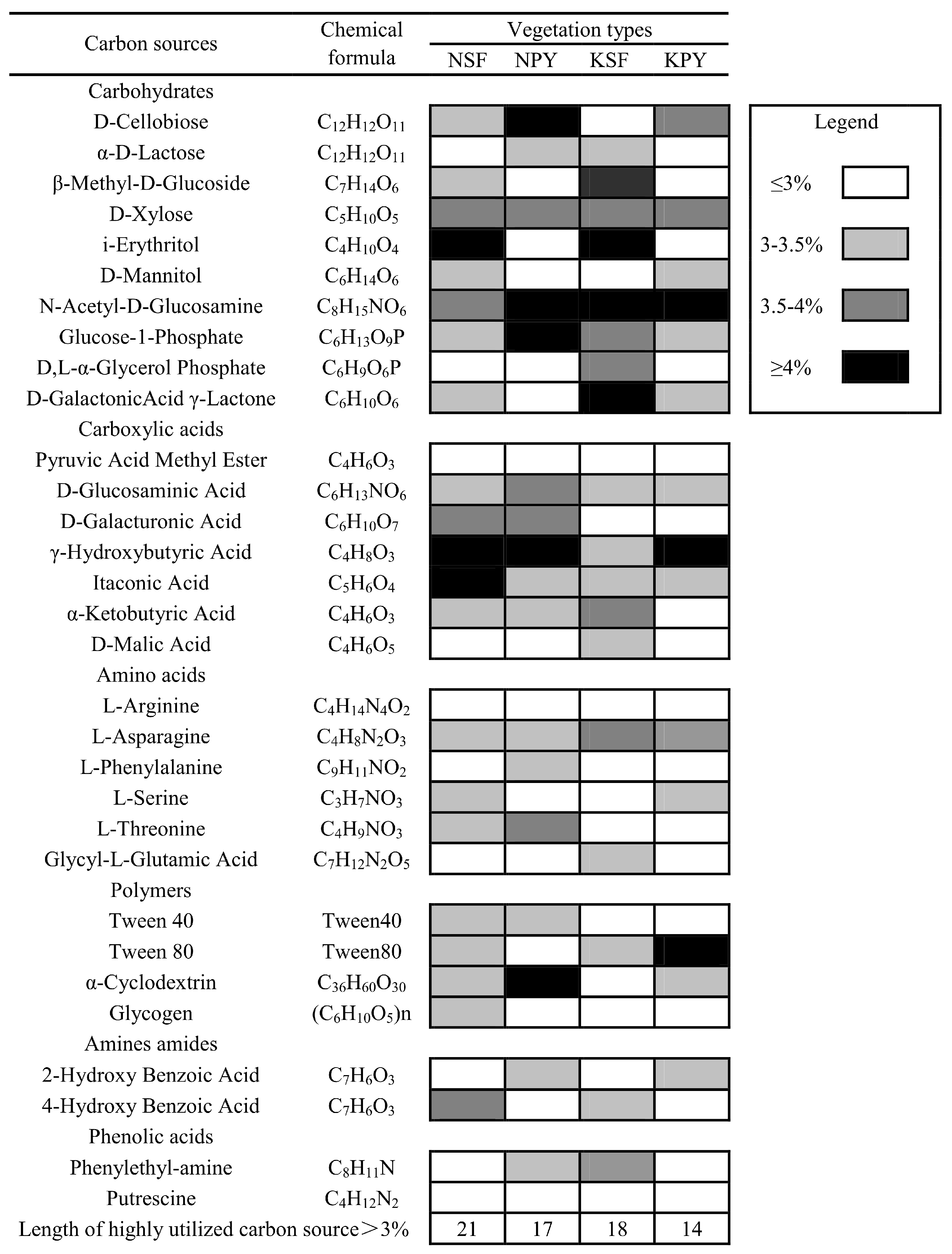
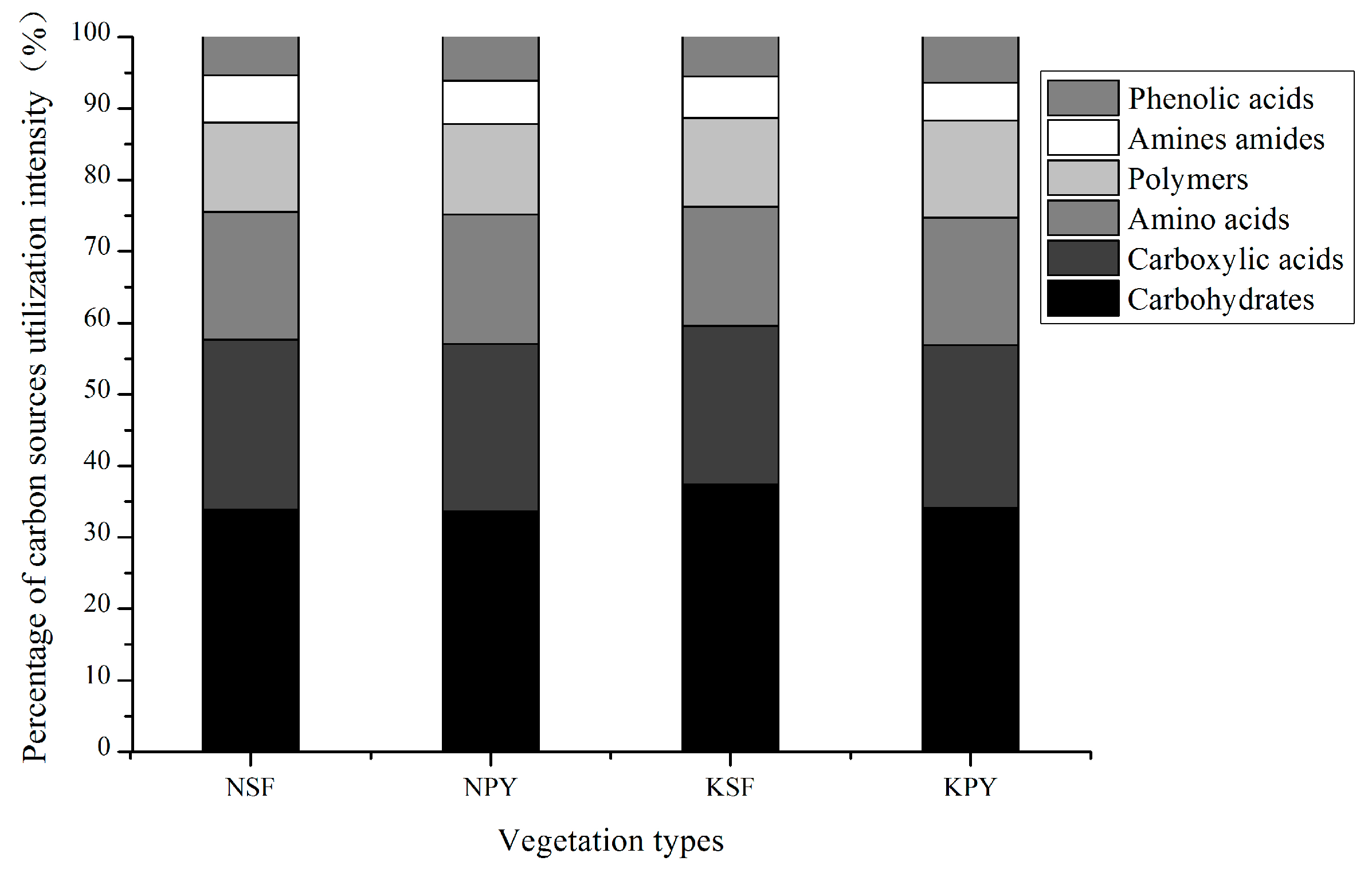
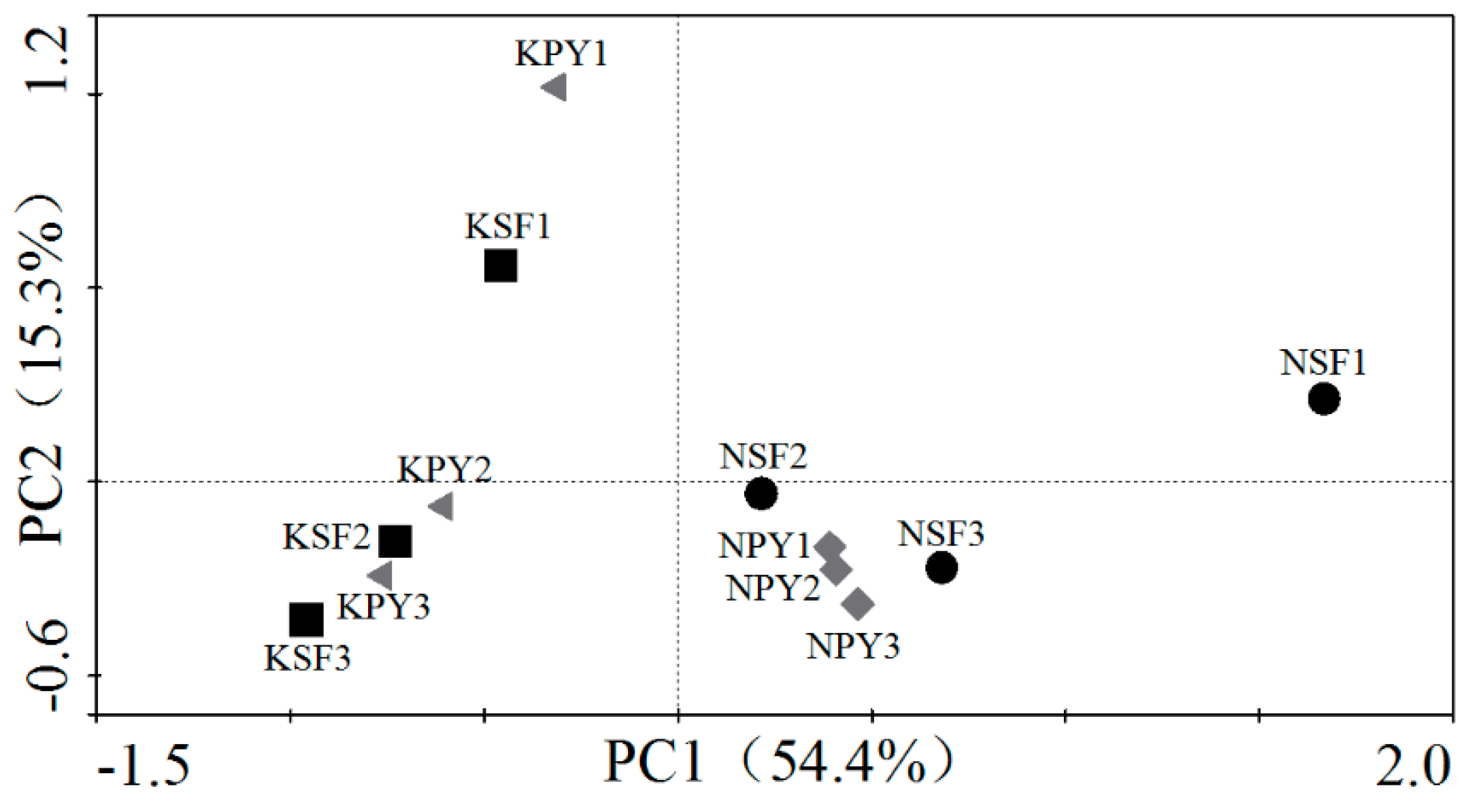
3.1. Soil Microbial Community Characteristics of the Two Different Vegetation Types
3.2. Soil Microbial Community Characteristics between Non-Karst and Karst Areas
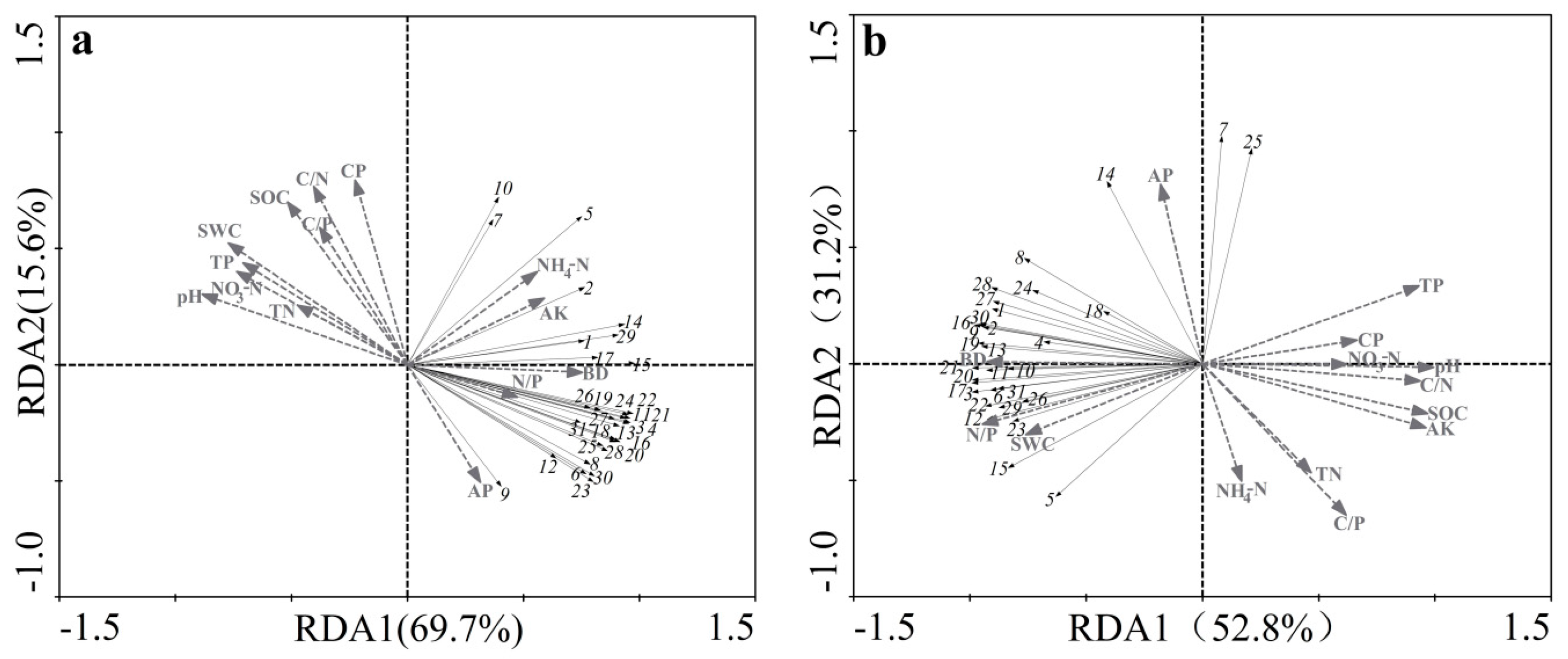
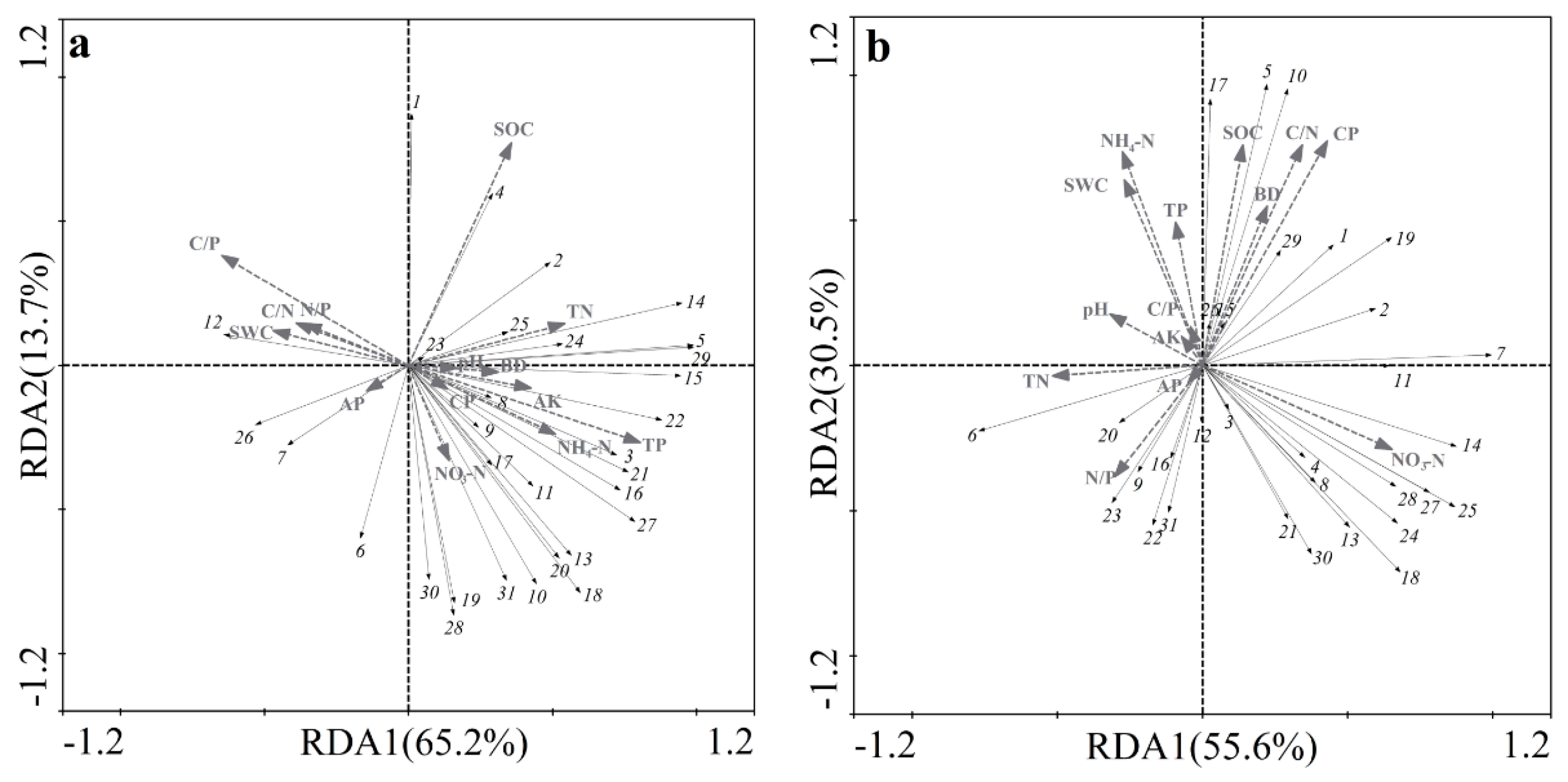
3.3. Relationship between Soil Microbial Community Characteristics and Soil Physicochemical Properties
4. Discussion
4.1. Differences in Soil Microbial Community Characteristics under Both Vegetation Types
4.2. Differences in Soil Microbial Characteristics between Non-Karst and Karst Areas
4.3. Impacts of Soil Physico-Chemical Properties on Microbial Communities
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CLPP | Community level physiological profile |
References
- Winding, A.; Hund-Rinke, K.; Rutgers, M. The use of microorganisms in ecological soil classification and assessment concepts. Ecotoxicol. Environ. Saf. 2005, 62, 230–248. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, B.J.; Srivastava, D.S.; Duffy, J.E.; Wright, J.P.; Downing, A.L.; Sankaran, M.; Jouseau, C. Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature 2006, 443, 989–992. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.L.; Paixao, S.M.; Cacador, I.; Carolino, M. CLPP and EEA profiles of microbial communities in salt marsh sediments. J. Soils Sediments 2007, 7, 418–425. [Google Scholar] [CrossRef]
- Strickland, M.S.; Rousk, J. Considering fungal: Bacterial dominance in soils emethods, controls, and ecosystem implications. Soil Biol. Biochem. 2010, 42, 1385–1395. [Google Scholar] [CrossRef]
- Amini, S.; Ghadiri, H.; Chen, C.; Marschner, P. Salt-affected soils, reclamation, carbon dynamics, and biochar: A review. J. Soils Sediments 2016, 16, 939–953. [Google Scholar] [CrossRef]
- Liang, J.; Lu, Z.; Yu, Z.D.; Wang, J.C.; Wang, X.A. Effects of leaf litter extraction fluid from dominant forest tree species on functional characteristics of soil microbial communities. J. For. Res. 2016, 27, 81–90. [Google Scholar] [CrossRef]
- Schimel, J. Soil carbon: Microbes and global carbon. Nat. Clim. Chang. 2013, 3, 867–868. [Google Scholar] [CrossRef]
- Chodak, M.; Klimek, B.; Niklinska, M. Composition and activity of soil microbial communities in different types of temperate forests. Biol. Fertil. Soils 2016, 52, 1093–1104. [Google Scholar] [CrossRef]
- Pierce, M.L.; Ward, J.E.; Dobbs, F.C. False positives in Biolog EcoPlates™ and MT2 MicroPlates™ caused by calcium. J. Microbiol. Methods 2014, 97, 20–24. [Google Scholar] [CrossRef]
- Klimek, B.; Chodak, M.; Jazwa, M.; Solak, A.; Tarasek, A.; Niklinska, M. The relationship between soil bacteria substrate utilisation patterns and the vegetation structure in temperate forests. Eur. J. For. Res. 2016, 135, 179–189. [Google Scholar] [CrossRef]
- Gartzia-Bengoetxea, N.; Kandeler, E.; de Arano, I.M.; Arias-Gonzalez, A. Soil microbial functional activity is governed by a combination of tree species composition and soil properties in temperate forests. Appl. Soil Ecol. 2016, 100, 57–64. [Google Scholar] [CrossRef]
- Wang, Q.K.; Wang, S.L.; Fan, B.; Yu, X.J. Litter production, leaf litter decomposition and nutrient return in Cunninghamia lanceolata plantations in south China: Effect of planting conifers with broadleaved species. Plant Soil 2007, 297, 201–211. [Google Scholar] [CrossRef]
- De, D.G.B.; Cornelissen, J.H.C.; Bardgett, R.D. Plant functional traits and soil carbon sequestration in contrasting biomes. Ecol. Lett. 2008, 11, 516–531. [Google Scholar]
- Wang, Y.; Ouyang, Z.Y.; Zheng, H.; Wang, X.K.; Chen, F.L.; Jing, Z. Carbon metabolism of soil microbial communities of restored forests in Southern China. J. Soils Sediments 2011, 11, 789–799. [Google Scholar] [CrossRef]
- Chodak, M.; Klimek, B.; Azarbad, H.; Jazwa, M. Functional diversity of soil microbial communities under Scots pine, Norway spruce, silver birch and mixed boreal forests. Pedobiologia 2015, 58, 81–88. [Google Scholar] [CrossRef]
- Adamczyk, B.; Kitunen, V.; Smolander, A. Protein precipitation by tannins in soil organic horizon and vegetation in relation to tree species. Biol. Fertil. Soils 2008, 45, 55–64. [Google Scholar] [CrossRef]
- Kanerva, S.; Kitunen, V.; Loponen, J.; Smolander, A. Phenolic compounds and terpenes in soil organic horizon layers under silver birch, Norway spruce and scots pine. Biol. Fertil. Soils 2008, 44, 547–556. [Google Scholar] [CrossRef]
- Ushio, M.; Balser, T.C.; Kitayama, K. Effects of condensed tannins in conifer leaves on the composition and activity of the soil microbial community in a tropical montane forest. Plant Soil 2013, 365, 157–170. [Google Scholar] [CrossRef]
- Tripathi, B.M.; Kim, M.; Singh, D.; Lee-Cruz, L.; Lai-Hoe, A.; Ainuddin, A.N.; Go, R.; Rahim, R.A.; Husni, M.H.; Chun, J.; et al. Tropical soil bacterial communities in Malaysia: pH dominates in the Equatorial Tropics too. Microb. Ecol. 2012, 64, 474–484. [Google Scholar] [CrossRef]
- Kuramae, E.E.; Yergeau, E.; Wong, L.C.; Pijl, A.S.; Van, V.J.A.; Kowalchuk, G.A. Soil characteristics more strongly influence soil bacterial communities than land-use type. FEMS Microbiol. Ecol. 2012, 79, 12–24. [Google Scholar] [CrossRef]
- Moore, J.; Marjorie, J.L.; Schulz, M.S.; White, A.F.; Brantley, S.L. Shifting microbial community structure across a marine terrace grassland chronosequence, Santa Cruz, California. Soil Biol. Biochem. 2010, 42, 21–31. [Google Scholar] [CrossRef]
- Wagai, R.; Kitayama, K.; Satomura, T.; Fujinuma, R.; Balser, T. Interactive influences of climate and parent material on soil microbial community structure in Bornean tropical forest ecosystems. Ecol. Res. 2011, 26, 627–636. [Google Scholar] [CrossRef]
- Imaya, A.; Ohta, S.; Tanaka, T.; Inagaki, Y. General chemical properties of brown forest soils developed from different parent materials in the submontane zone of the Kanto and Chubu districts, Japan. Soil Sci. Plant Nutr. 2005, 51, 873–884. [Google Scholar] [CrossRef]
- Appleton, J.D.; Adlam, K.A.M. Geogenic control on soil chemistry in urban areas: A novel method for urban geochemical mapping using parent material classified data. Appl. Geochem. 2012, 27, 161–170. [Google Scholar] [CrossRef]
- Gutknecht, J.L.M. Exploring Long-Term Microbial Responses to Simulated Global Change. Ph.D. Thesis, University of Wisconsin-Madison, Madison, WI, USA, 2007. [Google Scholar]
- Merila, P.; Malmivaara-Lamsa, M.; Spetz, P.; Vierikko, K. Soil organic matter quality as a link between microbial community structure and vegetation composition along a successional gradient in a boreal forest. Appl. Soil Ecol. 2010, 46, 259–267. [Google Scholar] [CrossRef]
- Faoro, H.; Alves, A.C.; Souza, E.M.; Rigo, L.U.; Cruz, L.M.; Al-Janabi, S.M.; Monteiro, R.A.; Baura, V.A.; Pedrosa, F.O. Influence of soil characteristics on the diversity of bacteria in the Southern Brazilian Atlantic forest. Appl. Environ. Microbiol. 2010, 76, 4744–4749. [Google Scholar] [CrossRef]
- Griffiths, R.I.; Thomson, B.C.; James, P.; Bell, T.; Bailey, M.; Whiteley, A.S. The bacterial biogeography of British soils. Environ. Microbiol. 2011, 13, 1642–1654. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Wei, Y.F.; Liu, Y.G.; Guo, K. Biomass of canopy and shrub layers of karst forests in Puding, Guizhou, China. J. Plant Ecol. 2009, 33, 698–705. [Google Scholar]
- Du, Y.X.; Pan, G.X.; Li, L.Q.; Hu, Z.L.; Wang, X.Z. Leaf N/P ratio and nutrient reuse between dominant species and stands: Predicting phosphorus deficiencies in Karst ecosystems, southwestern China. Environ. Earth Sci. 2011, 64, 299–309. [Google Scholar] [CrossRef]
- Efe, R. Ecological properties of vegetation formations on Karst terrains in the central Taurus Mountains (Southern Turkey). Proc. Soc. Behav. Sci. 2014, 120, 673–679. [Google Scholar] [CrossRef]
- Chen, F.; Wang, J.M.; Sun, B.G.; Chen, X.M.; Yang, Z.X.; Duan, Z.Y. Relationship between geographical distribution of Pinus yunnanensis and climate. J. For. Res. 2012, 25, 163–168. [Google Scholar]
- He, X.Y.; Wang, K.L.; Zhang, W.; Chen, Z.H.; Zhu, Y.G.; Chen, H.S. Positive correlation between soil bacterial metabolic and plant species diversity and bacterial and fungal diversity in a vegetation succession on Karst. Plant Soil. 2008, 307, 123–134. [Google Scholar] [CrossRef]
- Li, L.Q.; Wang, D.; Liu, X.Y.; Zhang, B.; Liu, Y.Z.; Xie, T.; Du, Y.X.; Pan, G.X. Soil organic carbon fractions and microbial community and functions under changes in vegetation: A case of vegetation succession in karst forest. Environ. Earth Sci. 2014, 71, 3727–3735. [Google Scholar] [CrossRef]
- Zhang, J.T.; Dong, Y.R. Factors affecting species diversity of plant communities and the restoration process in the loess area of China. Ecol. Eng. 2010, 36, 345–350. [Google Scholar] [CrossRef]
- Jiao, F.; Wen, Z.M.; An, S.S. Changes in soil properties across a chronosequence of vegetation restoration on the Loess Plateau of China. Catena 2011, 86, 110–116. [Google Scholar] [CrossRef]
- Chen, F.L.; Zheng, H.; Zhang, K.; Ouyang, Z.Y.; Wu, Y.F.; Shi, Q.; Li, H.L. Non-linear impacts of Eucalyptus plantation stand age on soil microbial metabolic diversity. J. Soils Sediments 2013, 13, 887–894. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D. An extraction method for measuring microbial biomass carbon. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Wu, J.; Joergensen, R.G.; Pommerening, B.; Chaussod, R.; Brookes, P.C. Measurement of soil microbial biomass C by fumigation–extraction—An automated procedure. Soil Biol. Biochem. 1990, 22, 1167–1169. [Google Scholar] [CrossRef]
- Hu, S.; Bruggen, A.H.C.V. Microbial dynamics associated with multiphasic decomposition of 14c-labeled cellulose in soil. Microb. Ecol. 1997, 33, 134–143. [Google Scholar] [CrossRef]
- Anderson, T.H.; Domsch, K.H. The metabolic quotient for CO2 (qCO2) as a specific activity parameter to assess the effect of environmental conditions such as pH, on the microbial biomass of forest soils. Soil Biol. Biochem. 1993, 25, 393–395. [Google Scholar] [CrossRef]
- Zhang, C.; Xue, S.; Liu, G.B.; Song, Z.L. A comparison of soil qualities of different revegetation types in the Loess Plateau China. Plant Soil. 2011, 347, 163–178. [Google Scholar] [CrossRef]
- Garland, J.L.; Mills, A.L. Classification and characterization of heterotrophic microbial communities on the basis of patterns of community-level sole-carbon-source utilization. Appl. Environ. Microb. 1991, 57, 2351–2359. [Google Scholar]
- Chen, L.J.; Feng, Q.; Wei, Y.P.; Li, C.S.; Zhao, Y.; Li, H.Y.; Zhang, B.G. Effects of saline water irrigation and fertilization regimes on soil microbial metabolic activity. J. Soils Sediments 2017, 17, 376–383. [Google Scholar] [CrossRef]
- Classen, A.T.; Boyle, S.I.; Haskins, K.E.; Overby, S.T.; Hart, S.T. Community-level physiological profiles of bacteria and fungi: Plate type and incubation temperature influences on contrasting soils. FEMS Microbiol. Ecol. 2003, 44, 319–328. [Google Scholar] [CrossRef]
- Pang, D.B.; Cao, J.H.; Dan, X.Q.; Guan, Y.H.; Peng, X.W.; Cui, M.; Wu, X.Q.; Zhou, J.X. Recovery approach affects soil quality in fragile karst ecosystems of southwest China: Implications for vegetation restoration. Ecol. Eng. 2018, 123, 151–160. [Google Scholar] [CrossRef]
- Larkin, R.P. Characterization of soil microbial communities under different potato cropping systems by microbial population dynamics, substrate utilization, and fatty acid profiles. Soil Biol. Biochem. 2003, 35, 1451–1466. [Google Scholar] [CrossRef]
- Landesman, W.J.; Dighton, J. Response of soil microbial communities and the production of plant-available nitrogen to a two-year rainfall manipulation in the New Jersey Pinelands. Soil Biol. Biochem. 2010, 42, 1751–1758. [Google Scholar] [CrossRef]
- Ren, C.; Zhao, F.; Shi, Z.; Chen, J.; Han, X.; Yang, G.; Feng, Y.; Ren, G. Differential responses of soil microbial biomass and carbon-degrading enzyme activities to altered precipitation. Soil Biol. Biochem. 2017, 115, 1–10. [Google Scholar] [CrossRef]
- Schimel, J.; Balser, T.C.; Wallenstein, M. Microbial stress-response physiology and its implications for ecosystem function. Ecology 2007, 88, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Cheng, S.; Wang, Y.; Yu, G.; Xu, M.; Dang, X.; Li, L.; Wang, L. Changes in soil heterotrophic respiration, carbon availability, and microbial function in seven forests along a climate gradient. Ecol. Res. 2014, 29, 1077–1086. [Google Scholar] [CrossRef]
- Prescott, C.E.; Grayston, S.J. Tree species influence on microbial communities in litter and soil: Current knowledge and research needs. For. Ecol. Manag. 2013, 309, 19–27. [Google Scholar] [CrossRef]
- Arshad, M.; Shaharoona, B.; Mahmood, T. Inoculation with Pseudomonas spp. Containing ACC-Deaminase Partially Eliminates the Effects of Drought Stress on Growth, Yield, and Ripening of Pea (Pisum sativum L.). Pedosphere 2008, 18, 611–620. [Google Scholar] [CrossRef]
- Rosenvald, K.; Ostonen, I.; Truu, M.; Truu, J.; Uri, V.; Vares, A.; Lohmus, K. Fine-root rhizosphere and morphological adaptations to site conditions in interaction with tree mineral nutrition in young silver birch (Betula pendula Roth.) stands. Eur. J. For. Res. 2011, 130, 1055–1066. [Google Scholar] [CrossRef]
- Li, Z.Q.; Zhao, B.Z.; Hao, X.Y.; Zhang, J.B. Effects of residue incorporation and plant growth on soil labile organic carbon and microbial function and community composition under two soil moisture levels. Environ. Sci. Pollut. Res. 2017, 24, 18849–18859. [Google Scholar] [CrossRef]
- Chen, H.; Li, D.J.; Xiao, K.C.; Wang, K.L. Soil microbial processes and resource limitation in karst and non-karst forests. Funct. Ecol. 2018, 32, 1400–1409. [Google Scholar] [CrossRef]
- Lamarche, J.; Bradley, R.L.; Hooper, E.; Shipley, B.; Simao, B.A.M.; Beaulieu, C. Forest Floor Bacterial Community Composition and Catabolic Profiles in Relation to Landscape Features in Quebec’s Southern Boreal Forest. Microb. Ecol. 2007, 54, 10–20. [Google Scholar] [CrossRef]
- Rumpel, C.; Kogel-Knabner, I. Deep soil organic matter—A key but poorly understood component of terrestrial C cycle. Plant Soil 2011, 338, 143–158. [Google Scholar] [CrossRef]
- Sheng, R.; Qin, H.L.; O’Donnell, A.G.; Huang, S.; Wu, J.S.; Wei, W.X. Bacterial succession in paddy soils derived from different parent materials. J. Soils Sediments 2015, 15, 982–992. [Google Scholar] [CrossRef]
- Shi, X.Z.; Wang, H.J.; Yu, D.S.; Weindorf, D.C.; Cheng, X.F.; Pan, X.Z.; Sun, W.X.; Chen, J.M. Potential for soil carbon sequestration of eroded areas in subtropical China. Soil Till. Res. 2009, 105, 322–327. [Google Scholar] [CrossRef]
- Ulrich, A.; Becker, R. Soil parent material is a key determinant of the bacterial community structure in arable soils. FEMS Microbiol. Ecol. 2006, 56, 430–443. [Google Scholar] [CrossRef]
- Hu, C.J.; Fu, B.J.; Liu, G.H.; Jin, T.T.; Guo, L. Vegetation patterns influence on soil microbial biomass and functional diversity in a hilly area of the Loess Plateau, China. J. Soils Sediments 2010, 10, 1082–1091. [Google Scholar] [CrossRef]
- Fu, P.L.; Liu, W.J.; Fan, Z.X.; Cao, K.F. Is fog an important water source for woody plants in an Asian tropical karst forest during the dry season? Ecohydrology 2015, 9, 964–972. [Google Scholar] [CrossRef]
- Chen, Y.J.; Cao, K.F.; Schnitzer, S.A.; Fan, Z.X.; Zhang, J.L.; Bongers, F. Water-use advantage for lianas over trees in tropical seasonal forests. New Phytol. 2015, 205, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Bongers, F.; Tomlinson, K.; Fan, Z.X.; Lin, H.; Zhang, S.B.; Zheng, Y.L.; Li, Y.P.; Cao, K.F.; Zhang, J.L. Time lags between crown and basal sap flows in tropical lianas and co-occurring trees. Tree Physiol. 2016, 36, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Hamer, U.; Marschner, B. Priming effects in soils after combined and repeated substrate additions. Geoderma 2005, 128, 38–51. [Google Scholar] [CrossRef]
- Hu, J.L.; Lin, X.G.; Wang, J.H.; Dai, J.; Chen, R.R.; Zhang, J.B.; Wong, M.H. Microbial functional diversity, metabolic quotient, and invertase activity of a sandy loam soil as affected by long-term application of organic amendment and mineral fertilizer. J. Soils Sediments 2011, 11, 271–280. [Google Scholar] [CrossRef]
- Zheng, S.; Hu, J.; Chen, K.; Yao, J.; Yu, Z.; Lin, X. Soil microbial activity measured by microcalorimetry in response to long-term fertilization regimes and available phosphorous on heat evolution. Soil Biol. Biochem. 2009, 41, 2094–2099. [Google Scholar] [CrossRef]
- Han, W.X.; Fang, J.Y.; Guo, D.L.; Zhang, Y. Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New Phytol. 2005, 168, 377–385. [Google Scholar] [CrossRef]
- Pang, D.B.; Wang, G.Z.; Li, G.J.; Sun, Y.L.; Liu, Y.G.; Zhou, J.X. Ecological Stoichiometric Characteristics of Two Typical Plantations in the Karst Ecosystem of Southwestern China. Forests 2018, 9, 56. [Google Scholar] [CrossRef]
- Griffiths, B.S.; Spilles, A.; Bonkowski, M. C:N:P stoichiometry and nutrient limitation of the soil microbial biomass in a grazed grassland site under experimental P limitation or excess. Ecol. Process. 2012, 1, 6. [Google Scholar] [CrossRef]
- Liu, M.; Liu, J.; Jiang, C.Y.; Wu, M.; Song, R.S.; Gui, R.Y.; Jia, J.X.; Li, Z.P. Improved nutrient status affects soil microbial biomass, respiration, and functional diversity in a Lei bamboo plantation under intensive management. J. Soils Sediments 2016, 17, 917–926. [Google Scholar] [CrossRef]
- Zhou, X.Q.; Wu, H.W.; Koetz, E.; Xu, Z.H.; Chen, C.R. Soil labile carbon and nitrogen pools and microbial metabolic diversity under winter crops in an arid environment. Appl. Soil Ecol. 2012, 53, 49–55. [Google Scholar] [CrossRef]
- Yan, F.; McBratney, A.B.; Copeland, L. Functional substrate biodiversity of cultivated and uncultivated A horizons of vertisols in NW New South Wales. Geoderma 2000, 96, 321–343. [Google Scholar] [CrossRef]
- Wang, M.; Qu, L.Y.; Ma, K.M.; Yuan, X. Soil microbial properties under different vegetation types on Mountain Han. Life Sci. 2013, 56, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Chodak, M.; Gołebiewski, M.; Morawska-Ploskonka, J.; Kuduk, K.; Niklinska, M. Diversity of microorganisms from forest soils differently polluted with heavy metals. Appl. Soil Ecol. 2013, 64, 7–14. [Google Scholar] [CrossRef]
- Kemmitt, S.J.; Wright, D.; Goulding, K.W.T.; Jones, D.L. pH regulation of carbon and nitrogen dynamics in two agricultural soils. Soil Biol. Biochem. 2006, 38, 898–911. [Google Scholar] [CrossRef]
- Taylor, D.L.; Herriott, I.C.; Stone, K.E.; McFarland, J.W.; Booth, W.G.; Leigh, M.B. Structure and resilience of fungal communities in Alaskan boreal forest soils. Can. J. For. Res. 2010, 40, 1288–1301. [Google Scholar] [CrossRef]
- Hogberg, M.N.; Baath, E.; Nordgren, A.; Arnebrant, K.; Hogberg, P. Contrasting effects of nitrogen availability on plant carbon supply to mycorrhizal fungi and saprotrophs—A hypothesis based on field observations in boreal forest. New Phytol. 2003, 160, 225–238. [Google Scholar] [CrossRef]
- Ingwersen, J.; Poll, C.; Streck, T.; Kandeler, E. Micro-scale modelling of carbon turnover driven by microbial succession at a biogeochemical interface. Soil Biol. Biochem. 2008, 40, 864–878. [Google Scholar] [CrossRef]
- Fanin, N.; Fromin, N.; Buatois, B.; Hattenschwiler, S. An experimental test of the hypothesis of non-homeostatic consumer stoichiometry in a plant litter microbe system. Ecol. Lett. 2013, 16, 764–772. [Google Scholar] [CrossRef]
- Ma, X.; Liu, M.; Li, Z. Shifts in microbial biomass and community composition in subtropical paddy soils under a gradient of manure amendment. Biol. Fertil. Soils 2016, 52, 775–787. [Google Scholar] [CrossRef]
- Kaiser, C.; Franklin, O.; Dieckmann, U.; Richter, A. Microbial community dynamics alleviate stoichiometric constraints during litter decay. Ecol. Lett. 2014, 17, 680–690. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Non-Karst Area | Karst Area | |||
|---|---|---|---|---|---|
| Pinus yunnanensis | Secondary Forest | Pinus yunnanensis | Secondary Forest | ||
| Shrub | Species number (S) | 3.333 ± 0.577 | 6.667 ± 0.577 | 3.667 ± 0.182 | 8.667 ± 0.577 |
| Shannon–Wiener index (H) | 0.977 ± 0.260 | 0.713 ± 0.132 | 0.875 ± 0.376 | 1.541 ± 0.094 | |
| Evenness index (E) | 0.409 ± 0.054 | 0.379 ± 0.090 | 0.457 ± 0.351 | 0.714 ± 0.022 | |
| Herb | Species number (S) | 15.000 ± 1.000 | 11.000 ± 3.606 | 12.667 ± 2.082 | 12.333 ± 1.528 |
| Shannon–Wiener index (H) | 2.074 ± 0.153 | 2.079 ± 0.341 | 2.091 ± 0.181 | 1.828 ± 0.267 | |
| Evenness index (E) | 0.767 ± 0.066 | 0.881 ± 0.010 | 0.828 ± 0.081 | 0.733 ± 0.138 | |
| Parameters | Non-Karst Area | Karst Area | ||
|---|---|---|---|---|
| Pinus yunnanensis | Secondary Forest | Pinus yunnanensis | Secondary Forest | |
| pH | 4.633 ± 0.091 | 4.850 ± 0.052 | 5.983 ± 0.099 | 5.917 ± 0.133 |
| SWC (%) | 25.456 ± 0.000 | 24.835 ± 0.000 | 23.481 ± 2.179 | 34.933 ± 3.043 |
| BD (g cm−3) | 1.269 ± 0.049 | 1.413 ± 0.059 | 1.073 ± 0.046 | 1.126 ± 0.088 |
| CP (%) | 47.9 ± 0.012 | 46.7 ± 0.020 | 45.540 ± 3.260 | 50.202 ± 2.563 |
| SOC (g kg−1) | 6.709 ± 0.753 | 7.569 ± 1.195 | 28.271 ± 4.020 | 42.348 ± 5.808 |
| TN (g kg−1) | 4.225 ± 1.058 | 3.233 ± 1.727 | 4.975 ± 0.135 | 5.275 ± 1.267 |
| TP (g kg−1) | 0.182 ± 0.025 | 0.528 ± 0.209 | 0.598 ± 0.070 | 1.341 ± 0.143 |
| AP (mg kg−1) | 1.160 ± 0.418 | 8.107 ± 4.565 | 1.267 ± 0.303 | 3.247 ± 0.660 |
| AK (mg kg−1) | 20.000 ± 0.000 | 103.333 ± 5.774 | 78.667 ± 6.855 | 83.333 ± 16.116 |
| NO3−-N (mg kg−1) | 10.880 ± 1.924 | 10.433 ± 0.424 | 11.907 ± 0.551 | 12.527 ± 0.243 |
| NH4+-N (mg kg−1) | 4.987 ± 2.039 | 28.813 ± 6.128 | 4.755 ± 2.072 | 20.350 ± 5.324 |
| Parameter | Non-Karst Area | Karst Area | ||
|---|---|---|---|---|
| Pinus yunnanensis | Secondary Forest | Pinus yunnanensis | Secondary Forest | |
| Shannon H′ | 2.214 ± 0.076 | 2.110 ± 0.578 | 2.468 ± 0.700 | 2.735 ± 0.380 |
| Pielou E | 0.648 ± 0.018 | 0.751 ± 0.222 | 0.925 ± 0.324 | 1.179 ± 0.385 |
| Simpson D | 0.856 ± 0.040 | 0.806 ± 0.054 | 0.869 ± 0.076 | 0.890 ± 0.051 |
| McIntosh U | 0.210 ± 0.120 | 0.509 ± 0.329 | 0.369 ± 0.272 | 0.478 ± 0.181 |
| Parameters | Non-Karst Area | Karst Area | ||||||
|---|---|---|---|---|---|---|---|---|
| Axes | I | II | III | IV | I | II | III | IV |
| Eigenvalues | 0.652 | 0.137 | 0.090 | 0.070 | 0.556 | 0.305 | 0.057 | 0.030 |
| Soil microbial-physicochemical properties | 1.000 | 0.987 | 0.999 | 0.965 | 0.983 | 1.000 | 1.000 | 1.000 |
| Cumulative percentage variance of species data (%) | 65.2 | 79.0 | 88.0 | 95.0 | 55.6 | 86.1 | 91.8 | 94.9 |
| Cumulative percentage variance of soil Microbial-physicochemical properties (%) | 68.7 | 83.1 | 92.6 | 100 | 58.6 | 90.8 | 96.8 | 100 |
| Sum of all canonical eigenvalues | 0.950 | 0.949 | ||||||
| Sum of all eigenvalues | 1.000 | 1.000 | ||||||
| Parameter | Non-Karst Area | Karst Area | ||
|---|---|---|---|---|
| Importance Ranking | Physicochemical Properties | Explained Variation (%) | Physicochemical Properties | Explained Variation (%) |
| 1 | TP | 45.2 | CP | 30.2 |
| 2 | C/P | 31.8 | NO3−-N | 29.4 |
| 3 | TN | 25.4 | C/N | 26.2 |
| 4 | NH4+-N | 22.9 | NH4+-N | 22.8 |
| 5 | SWC | 20.9 | SOC | 20.5 |
| 6 | SOC | 19.3 | SWC | 19.4 |
| 7 | AK | 18.7 | TN | 17.7 |
| 8 | N/P | 17.2 | BD | 14.9 |
| 9 | C/N | 16.3 | N/P | 13.1 |
| 10 | BD | 14.1 | pH | 11.5 |
| 11 | pH | 10.1 | TP | 11.4 |
| 12 | CP | 9.9 | C/P | 5.8 |
| 13 | AP | 9.7 | AP | 5.5 |
| 14 | NO3−-N | 8.8 | AK | 5.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, D.; Wang, G.; Liu, Y.; Cao, J.; Wan, L.; Wu, X.; Zhou, J. The Impacts of Vegetation Types and Soil Properties on Soil Microbial Activity and Metabolic Diversity in Subtropical Forests. Forests 2019, 10, 497. https://doi.org/10.3390/f10060497
Pang D, Wang G, Liu Y, Cao J, Wan L, Wu X, Zhou J. The Impacts of Vegetation Types and Soil Properties on Soil Microbial Activity and Metabolic Diversity in Subtropical Forests. Forests. 2019; 10(6):497. https://doi.org/10.3390/f10060497
Chicago/Turabian StylePang, Danbo, Genzhu Wang, Yuguo Liu, Jianhua Cao, Long Wan, Xiuqin Wu, and Jinxing Zhou. 2019. "The Impacts of Vegetation Types and Soil Properties on Soil Microbial Activity and Metabolic Diversity in Subtropical Forests" Forests 10, no. 6: 497. https://doi.org/10.3390/f10060497
APA StylePang, D., Wang, G., Liu, Y., Cao, J., Wan, L., Wu, X., & Zhou, J. (2019). The Impacts of Vegetation Types and Soil Properties on Soil Microbial Activity and Metabolic Diversity in Subtropical Forests. Forests, 10(6), 497. https://doi.org/10.3390/f10060497





