Plant Pathogenic Fungi Associated with Coraebus florentinus (Coleoptera: Buprestidae) Attacks in Declining Oak Forests
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites, Field Surveys, and Sampling Procedure
2.2. Fungal Isolation and Identification
2.3. DNA Extraction, PCR Amplification, and Sequencing
2.4. Data Analysis
3. Results
3.1. Field Survey
3.2. Fungal Isolation
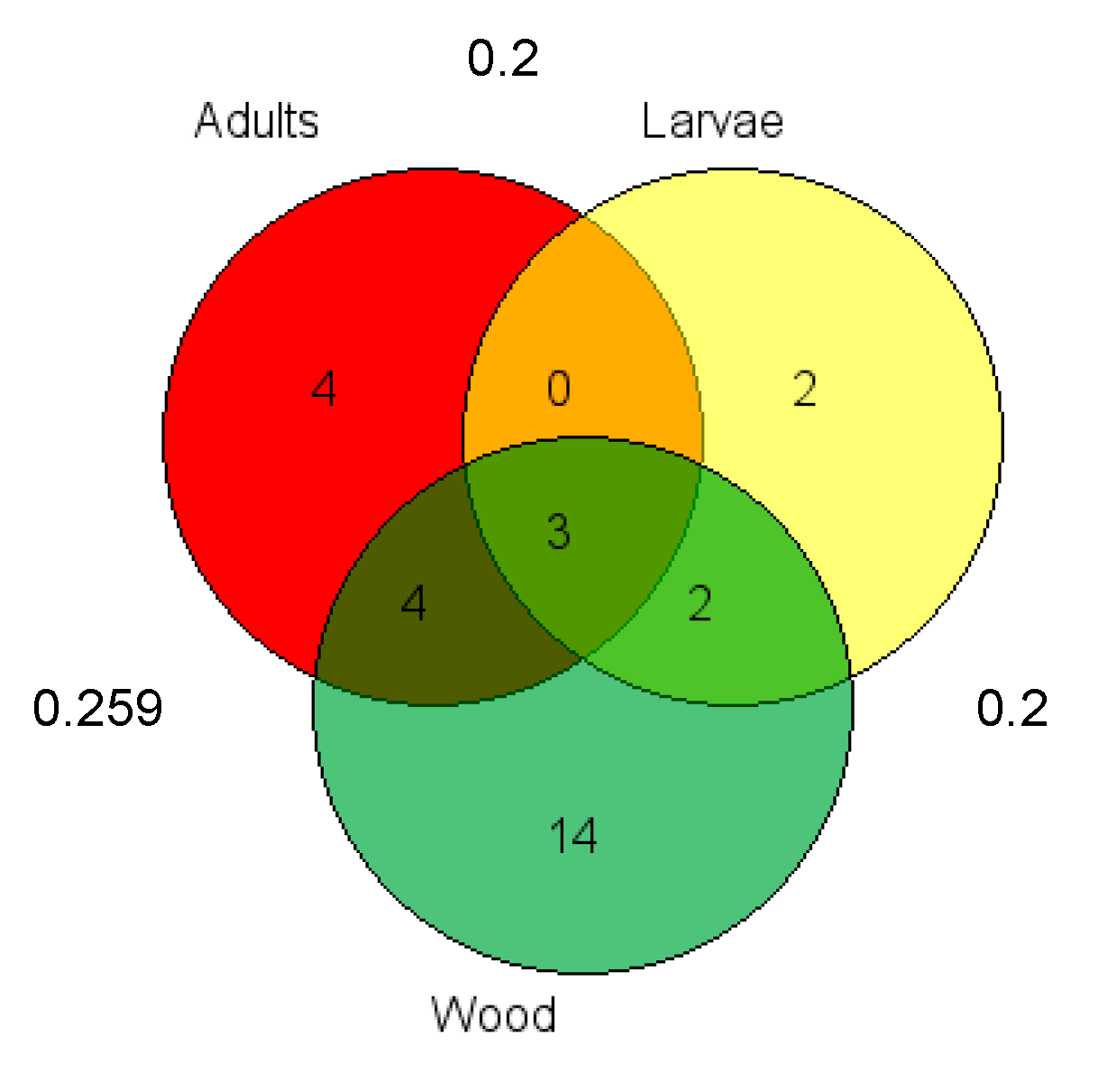
3.3. Fungal Community Diversity
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brasier, C.M. Oak tree mortality in Iberia. Nature 1992, 360, 539. [Google Scholar] [CrossRef]
- Sallé, A.; Nageleisen, L.M.; Lieutier, F. Bark and wood boring insects involved in oak declines in Europe: Current knowledge and future prospects in a context of climate change. For. Ecol. Manag. 2014, 328, 79–93. [Google Scholar] [CrossRef]
- Moricca, S.; Linaldeddu, B.T.; Ginetti, B.; Scanu, B.; Franceschini, A.; Ragazzi, A. Endemic and Emerging Pathogens Threatening Cork Oak Trees: Management Options for Conserving a Unique Forest Ecosystem. Plant Dis. 2016, 100, 2183–2193. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Aparicio, L.; Ibáñez, B.; Serrano, M.S.; De Vita, P.; Ávila, J.M.; Pérez-Ramos, I.M.; García, L.V.; Sánchez, M.E.; Marañón, T. Spatial patterns of soil pathogens in declining Mediterranean forests: Implications for tree species regeneration. New Phytol. 2012, 194, 1014–1024. [Google Scholar] [CrossRef] [PubMed]
- Haavik, L.J.; Billings, S.A.; Guldin, J.M.; Stephen, F.M. Emergent insects, pathogens and drought shape changing patterns in oak decline in North America and Europe. For. Ecol. Manag. 2015, 354, 190–205. [Google Scholar] [CrossRef]
- Domínguez-Begines, J.; De Deyn, G.B.; García, L.V.; Eisenhauer, N.; Gómez-Aparicio, L. Cascading spatial and trophic impacts of oak decline on the soil food web. J. Ecol. 2018, 107, 1199–1214. [Google Scholar] [CrossRef]
- Avila, J.M.; Gallardo, A.; Gómez-Aparicio, L. Pathogen-induced tree mortality interacts with predicted climate change to alter soil respiration and nutrient availability in Mediterranean systems. Biogeochemistry 2019, 142, 53–71. [Google Scholar] [CrossRef]
- Ghobad-Nejhad, M.; Meyn, R.; Langer, E. Endophytic fungi isolated from healthy and declining Persian oak (Quercus brantii) in western Iran. Nova Hedwig. 2018, 107, 273–290. [Google Scholar] [CrossRef]
- Moreira, A.C.; Martins, J.M.S. Influence of site factors on the impact of Phytophthora cinnamomi in cork oak stands in Portugal. For. Pathol. 2005, 35, 145–162. [Google Scholar] [CrossRef]
- Henriques, J.; Inácio, M.L.; Sousa, E. Ambrosia fungi in the insect-fungi symbiosis in relation to cork oak decline. Rev. Iberoam. Micol. 2006, 23, 185–188. [Google Scholar] [CrossRef]
- Linaldeddu, B.T.; Franceschini, A.; Alves, A.; Phillips, A.J. Diplodia quercivora sp. nov.: A new species of Diplodia on declining Quercus canariensis trees in Tunisia. Mycologia 2013, 105, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Camilo-Alves, C.S.P.; Clara, M.I.E.; Ribeiro, N.M.C.A. Decline of Mediterranean oak trees and its association with Phytophthora cinnamomi: A review. Eur. J. For. Res. 2013, 132, 411–432. [Google Scholar] [CrossRef]
- Tiberi, R.; Branco, M.; Bracalini, M.; Croci, F.; Panzavolta, T. Cork oak pests: A review of insect damage and management. Ann. For. Sci. 2016, 73, 219–232. [Google Scholar] [CrossRef]
- Cao, O.V.; Luciano, P. Severe infestations of Platypus cylindrus Fabricius (Coleoptera Platypodidae) in Sardinian cork oak forests. IOBC/WPRS Bull. 2005, 28, 145–146. [Google Scholar]
- Martín, J.; Cabezas, J.; Buyolo, T.; Paton, D. The relationship between Cerambyx spp. damage and subsequent Biscogniauxia mediterranum infection on Quercus suber forests. For. Ecol. Manag. 2005, 216, 166–174. [Google Scholar] [CrossRef]
- Gallardo, A.; Jiménez, A.; Antonietty, C.A.; Villagrán, M.; Ocete, M.E.; Soria, F.J. Forecasting infestation by Coraebus undatus (Coleoptera, Buprestidae) in cork oak forests. Int. J. Pest Manag. 2012, 58, 275–280. [Google Scholar] [CrossRef]
- Mirabolfathy, M.; Groenewald, J.Z.; Crous, P.W. The occurrence of charcoal disease caused by Biscogniauxia mediterranea on chestnut-leaved oak (Quercus castaneifolia) in the Golestan Forests of Iran. Plant Dis. 2011, 95, 876. [Google Scholar] [CrossRef] [PubMed]
- Linaldeddu, B.T.; Maddau, L.; Franceschini, A. First report of Diplodia corticola causing canker and dieback of Quercus ilex, Q. petraea and Q. suber in Corsica (France). Plant Dis. 2017, 101, 256. [Google Scholar] [CrossRef]
- Català, S.; Berbegal, M.; Pérez-Sierra, A.; Abad-Campos, P. Metabarcoding and development of new real-time specific assays reveal Phytophthora species diversity in holm oak forests in eastern Spain. Plant Pathol. 2016, 66, 115–123. [Google Scholar] [CrossRef]
- Frisullo, S.; Lima, G.; Magnano di San Lio, G.; Camele, I.; Melissano, L.; Puglisi, I.; Pane, A.; Agosteo, G.E.; Prudente, L.; Cacciola, S.O. Phytophthora cinnamomi Involved in the Decline of Holm Oak (Quercus ilex) Stands in Southern Italy. For. Sci. 2018, 64, 290–298. [Google Scholar] [CrossRef]
- Franceschini, A.; Corda, P.; Maddau, L.; Marras, F. Observations sur Diplodia mutila, pathogene du chêne-liege en Sardaigne. IOBC/WPRS Bull. 1999, 22, 5–12. [Google Scholar]
- Linaldeddu, B.T.; Scanu, B.; Maddau, L.; Franceschini, A. Diplodia corticola and Phytophthora cinnamomi: The main pathogens involved in holm oak decline on Caprera Island (Italy). For. Pathol. 2014, 44, 191–200. [Google Scholar] [CrossRef]
- Luciano, P.; Lentini, A.; Cao, O.V. Gravi danni da Coroebus florentinus in sugherete sarde. Notiziario sulla protezione delle piante 2007, 21, 215–217. [Google Scholar]
- Jurc, M.; Bojović, S.; Komjanc, B.; Krč, J. Xylophagous entomofauna in branches of oaks (Quercus spp.) and its significance for oak health in the Karst region of Slovenia. Biologia 2009, 64, 130–138. [Google Scholar] [CrossRef]
- Cárdenas, A.M.; Gallardo, P. The effect of temperature on the preimaginal development of the Jewel beetle, Coraebus florentinus (Coleoptera: Buprestidae). Eur. J. Entomol. 2012, 109, 21–28. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenies. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. ISBN 9780123721815. [Google Scholar]
- Linaldeddu, B.T.; Alves, A.; Phillips, A.J.L. Sardiniella urbana gen. et sp. nov., a new member of the Botryosphaeriaceae isolated from declining Celtis australis trees in Sardinian streetscapes. Mycosphere 2016, 7, 893–905. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 2010, 215, 403–410. [Google Scholar] [CrossRef]
- Morris, E.K.; Caruso, T.; Buscot, F.; Fischer, M.; Hancock, C.; Maier, T.S. Choosing and using diversity indices: Insights for ecological applications from the German biodiversity Exploratories. Ecol. Evol. 2014, 4, 3514–3524. [Google Scholar] [CrossRef]
- Jaccard, P. The distribution of the flora in the alpine zone. New Phytol. 1912, 11, 37–50. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Wanasinghe, D.N.; Phukhamsakda, C.; Hyde, K.D.; Jeewon, R.; Lee, H.B.; Jones, E.G.; Tibpromma, S.; Tennakoon, D.S.; Dissanayake, A.J.; Jayasiri, S.C.; et al. Fungal diversity notes 709–839: Taxonomic and phylogenetic contributions to fungal taxa with an emphasis on fungi on Rosaceae. Fungal Divers. 2018, 89, 1–236. [Google Scholar] [CrossRef]
- Kwaśna, H.; Ward, E.; Kosiak, B. Lewia hordeicola sp. nov. from barley grain. Mycologia 2006, 98, 662–668. [Google Scholar] [CrossRef]
- Crous, P.W.; Groenewald, J.Z. A phylogenetic re-evaluation of Arthrinium. IMA Fungus 2013, 4, 133–154. [Google Scholar] [CrossRef] [PubMed]
- Zalar, P.; Gostinčar, C.; De Hoog, G.S.; Uršič, V.; Sudhadham, M.; Gunde-Cimerman, N. Redefinition of Aureobasidium pullulans and its varieties. Stud. Mycol. 2008, 61, 21–38. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, M.L.; Lops, F.; Carlucci, A. Charcoal canker of pear, plum, and quince trees caused by Biscogniauxia rosacearum sp. nov. in Southern Italy. Plant Dis. 2016, 100, 1813–1822. [Google Scholar] [CrossRef] [PubMed]
- Slippers, B.; Crous, P.W.; Denman, S.; Coutinho, T.A.; Wingfield, B.D.; Wingfield, M.J. Combined multiple gene genealogies and phenotypic characters differentiate several species previously identified as Botryosphaeria dothidea. Mycologia 2004, 96, 83–101. [Google Scholar] [CrossRef]
- Vu, D.; Groenewald, M.; de Vries, M.; Gehrmann, T.; Stielow, B.; Eberhardt, U.; Al-Hatmi, A.; Groenewald, J.Z.; Cardinali, G.; Houbraken, J.; et al. Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Stud. Mycol. 2019, 92, 135–154. [Google Scholar] [CrossRef]
- Braganca, H.; Rigling, D.; Diogo, E.; Capelo, J.; Phillips, A.; Tenreiro, R. Cryphonectria naterciae: A new species in the Cryphonectria-Endothia complex and diagnostic molecular markers based on microsatellite-primed PCR. Fungal Biol. 2011, 115, 852–861. [Google Scholar] [CrossRef]
- Luque, J.; Garcia-Figueres, F.; Legorburu, F.J.; Muruamendiaraz, A.; Armengol, J.; Trouillas, F.P. Species of Diatrypaceae associated with grapevine trunk diseases in Eastern Spain. Phytopathol. Mediterr. 2012, 51, 528–540. [Google Scholar] [CrossRef]
- Senanayake, I.C.; Crous, P.W.; Groenewald, J.Z.; Maharachchikumbura, S.S.N.; Jeewon, R.; Phillips, A.J.L.; Bhat, J.D.; Perera, R.H.; Li, Q.R.; Li, W.J.; et al. Families of Diaporthales based on morphological and phylogenetic evidence. Stud. Mycol. 2017, 86, 217–296. [Google Scholar] [CrossRef]
- Lawrence, D.P.; Travadon, R.; Nita, M.; Baumgartner, K. TrunkDiseaseID. org: A molecular database for fast and accurate identification of fungi commonly isolated from grapevine wood. Crop Prot. 2017, 102, 110–117. [Google Scholar] [CrossRef]
- Alves, A.; Correia, A.; Luque, J.; Phillips, A.J.L. Botryosphaeria corticola, sp. nov. on Quercus species, with notes and description of Botryosphaeria stevensii and its anamorph, Diplodia mutila. Mycologia 2004, 96, 598–613. [Google Scholar] [CrossRef]
- Phillips, A.J.L.; Alves, A.; Correia, A.; Luque, J. Two new species of Botryosphaeria with brown, 1-septate ascospores and Dothiorella anamorphs. Mycologia 2005, 97, 513–529. [Google Scholar] [CrossRef] [PubMed]
- Sogonov, M.V.; Castlebury, L.A.; Rossman, A.Y.; Mejía, L.C.; White, J.F. Leaf-inhabiting genera of the Gnomoniaceae, Diaporthales. Stud. Mycol. 2008, 62, 1–77. [Google Scholar] [CrossRef] [PubMed]
- Ariyawansa, H.A.; Tanaka, K.; Thambugala, K.M.; Phookamsak, R.; Tian, Q.; Camporesi, E.; Hongsanan, S.; Monkai, J.; Wanasinghe, D.N.; Chukeatirote, E.; et al. A molecular phylogenetic reappraisal of the Didymosphaeriaceae (=Montagnulaceae). Fungal Divers. 2014, 68, 69–104. [Google Scholar] [CrossRef]
- Valenzuela-Lopez, N.; Cano-Lira, J.F.; Guarro, J.; Sutton, D.A.; Wiederhold, N.; Crous, P.W.; Stchigel, A.M. Coelomycetous Dothideomycetes with emphasis on the families Cucurbitariaceae and Didymellaceae. Stud. Mycol. 2018, 90, 1–69. [Google Scholar] [CrossRef] [PubMed]
- Hirooka, Y.; Rossman, A.Y.; Zhuang, W.Y.; Salgado-Salazar, C.; Chaverri, P. Species delimitation for Neonectria coccinea group including the causal agents of beech bark disease in Asia, Europe, and North America. Mycosystema 2013, 32, 485–517. [Google Scholar]
- Wang, M.; Liu, F.; Crous, P.W.; Cai, L. Phylogenetic reassessment of Nigrospora: Ubiquitous endophytes, plant and human pathogens. Persoonia 2017, 39, 118–142. [Google Scholar] [CrossRef]
- Réblová, M.; Jaklitsch, W.M.; Réblová, K.; Štěpánek, V. Phylogenetic Reconstruction of the Calosphaeriales and Togniniales Using Five Genes and Predicted RNA Secondary Structures of ITS, and Flabellascus tenuirostris gen. et sp. nov. PLoS ONE 2015, 10, e0144616. [Google Scholar] [CrossRef]
- Wijayawardene, N.N.; Hyde, K.D.; Bhat, D.J.; Camporesi, E.; Schumacher, R.K.; Thilini Chethana, K.W.; Wikee, S.; Bahkali, A.H.; Wang, Y. Camarosporium-like species are polyphyletic in Pleosporales; introducing Paracamarosporium and Pseudocamarosporium gen. nov. in Montagnulaceae. Cryptogam. Mycol. 2014, 35, 177–198. [Google Scholar] [CrossRef]
- Crous, P.W.; Groenewald, J.Z. The Genera of Fungi–G 4: Camarosporium and Dothiora. IMA Fungus 2017, 8, 131–152. [Google Scholar] [CrossRef] [PubMed]
- Woudenberg, J.H.C.; Hanse, B.; van Leeuwen, G.C.M.; Groenewald, J.Z.; Crous, P.W. Stemphylium revisited. Stud. Mycol. 2017, 87, 77–103. [Google Scholar] [CrossRef] [PubMed]
- Nagy, L.G.; Petkovits, T.; Kovács, G.M.; Voigt, K.; Vágvölgyi, C.; Papp, T. Where is the unseen fungal diversity hidden? A study of Mortierella reveals a large contribution of reference collections to the identification of fungal environmental sequences. New Phytol. 2011, 191, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Torres-Vila, L.M.; Echave-Sanabria, A.C.; Mendiola-Díaz, F.J.; Moral-García, F.J. Mapping oak shoot browning in SW Spain using online imagery as virtual prospecting tool. Ann. For. Sci. 2019, 76, 32. [Google Scholar] [CrossRef]
- Aćimović, S.G.; Harmon, C.L.; Bec, S.; Wyka, S.; Broders, K.; Doccola, J.J. First report of Diplodia corticola causing decline of red oak (Quercus rubra) trees in Maine. Plant Dis. 2016, 100, 649. [Google Scholar] [CrossRef]
- Barradas, C.; Phillips, A.J.L.; Correia, A.; Diogo, E.; Bragança, H.; Alves, A. Diversity and potential impact of Botryosphaeriaceae species associated with Eucalyptus globulus plantations in Portugal. Eur. J. Plant Pathol. 2016, 146, 245–257. [Google Scholar] [CrossRef]
- Smahi, H.; Belhoucine-Guezouli, L.; Bouhraoua, R.T.; Franceschini, A.; Linaldeddu, B.T. First report of branch canker and dieback caused by Cryphonectria naterciae on Quercus suber in Algeria. Plant Dis. 2018, 102, 251. [Google Scholar] [CrossRef]
- Luque, J.; Pera, J.; Parladé, J. Evaluation of fungicides for the control of Botryosphaeria corticola on cork oak in Catalonia (NE Spain). For. Pathol. 2008, 38, 147–155. [Google Scholar] [CrossRef]
- Serrano, M.S.; Romero, M.A.; Jimenez, J.J.; De Vita, P.; Avila, A.; Trapero, A.; Sanchez, M.E. Preventive control of Botryosphaeria canker affecting Quercus suber in southern Spain. Forestry 2015, 88, 500–507. [Google Scholar] [CrossRef]
- Cimmino, A.; Maddau, L.; Masi, M.; Evidente, M.; Linaldeddu, B.T.; Evidente, A. Further secondary metabolites produced by Diplodia corticola, a fungal pathogen involved in cork oak decline. Tetrahedron 2016, 72, 6788–6793. [Google Scholar] [CrossRef]
- Inácio, M.L.; Henriques, J.; Sousa, E. Contribution of Symbiotic Fungi to Cork Oak Colonization by Platypus cylindrus (Coleoptera: Platypodidae). Silva Lusit. 2011, 19, 89–99. [Google Scholar]
- Kostovcik, M.; Bateman, C.C.; Kolařík, M.; Stelinski, L.L.; Jordal, B.H.; Hulcr, J. The ambrosia symbiosis is specific in some species and promiscuous in others: Evidence from community pyrosequencing. ISME J. 2015, 9, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, A.J.; Camporesi, E.; Hyde, K.D.; Phillips, A.J.L.; Fu, C.Y.; Yan, J.Y.; Li, X.H. Dothiorella species associated with woody hosts in Italy. Mycosphere 2016, 7, 51–63. [Google Scholar] [CrossRef]
- Lynch, S.C.; Eskalen, A.; Zambino, P.J.; Mayorquin, J.S.; Wang, D.H. Identification and pathogenicity of Botryosphaeriaceae species associated with coast live oak (Quercus agrifolia) decline in southern California. Mycologia 2013, 105, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Smahi, H.; Belhoucine-Guezouli, L.; Berraf-Tebbal, A.; Chouih, S.; Arkam, M.; Franceschini, A.; Linaldeddu, B.T.; Phillips, A.J.L. Molecular characterization and pathogenicity of Diplodia corticola and other Botryosphaeriaceae species associated with canker and dieback of Quercus suber in Algeria. Mycosphere 2017, 8, 1261–1272, doi 105943/mycosphere/8/2/10. [Google Scholar] [CrossRef]
- Linaldeddu, B.T.; Sirca, C.; Spano, D.; Franceschini, A. Variation of endophytic cork oak-associated fungal communities in relation to plant health and water stress. For. Pathol. 2011, 41, 193–201. [Google Scholar] [CrossRef][Green Version]
- Lynch, S.C.; Zambino, P.J.; Scott, T.A.; Eskalen, A. Occurrence, incidence and associations among fungal pathogens and Agrilus auroguttatus, and their roles in Quercus agrifolia decline in California. For. Pathol. 2014, 44, 62–74. [Google Scholar] [CrossRef]
- Campos, P.; Daly-Hassen, H.; Ovando-Pol, P. Cork oak forest management in Spain and Tunisia: Two case studies of conflicts between sustainability and private income. Int. For. Rev. 2007, 9, 610–626. [Google Scholar] [CrossRef]
- Costa, A.; Pereira, H.; Madeira, M. Analysis of spatial patterns of oak decline in cork oak woodlands in Mediterranean conditions. Ann. For. Sci. 2010, 67, 204. [Google Scholar] [CrossRef]

| Study Sites | Locality | Elevation (m a.s.l.) | Coordinates (°N, °E) | Number of Branches Sampled | |
|---|---|---|---|---|---|
| 1 | Gavoi | 710 | 40° 07′ 43.6″ | 9° 11′ 14.1″ | 0 (c), 13 (h), 2 (p) |
| 2 | Buddusò | 780 | 40° 34′ 01.4″ | 9° 19′ 06.9″ | 19 (c), 11 (h), 0 (p) |
| 3 | Pattada | 630 | 40° 33′ 48.6″ | 9° 08′ 58.2″ | 6 (c), 0 (h), 0 (p) |
| 4 | Monte Lerno | 600 | 40° 35′ 17.2″ | 9° 10′ 10.4″ | 12 (c), 8 (h), 0 (p) |
| 5 | Bottidda | 310 | 40° 20′ 56.8″ | 9° 04′ 11.6″ | 2 (c), 0 (h), 0 (p) |
| 6 | Abbasanta | 370 | 40° 08′ 28.9″ | 8° 45′ 40.1″ | 60 (c), 0 (h), 0 (p) |
| Study Sites | Locality | N° of oak Tree Monitored | N° of Tree Damaged | Infestation Level (%) | Bored Branches per Tree (Mean ± SD) |
|---|---|---|---|---|---|
| 1 | Gavoi | 30 | 20 | 66.7 | 4.8 ± 3.9 |
| 2 | Buddusò | 30 | 13 | 43.3 | 1.7 ± 1.1 |
| 3 | Pattada | 30 | 4 | 13.3 | 1.5 ± 0.6 |
| 4 | Monte Lerno | 30 | 23 | 76.7 | 2.6 ± 2.2 |
| 5 | Bottidda | 30 | 1 | 3.3 | 2 |
| 6 | Abbasanta | 30 | 26 | 86.7 | 5.5 ± 3.9 |
| The Closest Matching NCBI GenBank Entry | |||||
|---|---|---|---|---|---|
| Fungal taxa (Strain Number) | Accession Number | Taxon | Accession Number | (%) Identity | References |
| Alternaria doliconidium (cp54) | MK796128 | Alternaria doliconidium* | MG828864 | 100% | [32] |
| Alternaria hordeicola (cp72.1) | MK796129 | Alternaria hordeicola* | NR_136019 | 100% | [33] |
| Arthrinium sp. (cp62.2) | MK796130 | Arthrinium kogelbergense* | KF144892 | 96.1% | [34] |
| Aureobasidium pullulans (cp73.2) | MK796131 | Aureobasidium pullulans* | FJ150906 | 100% | [35] |
| Biscogniauxia rosacearum (cp73.1) | MK796132 | Biscogniauxia rosacearum* | KT253493 | 100% | [36] |
| Botryosphaeria dothidea (cp2) | MK796133 | Botryosphaeria dothidea* | AY236949 | 100% | [37] |
| Botrytis cinerea (cp67) | MK796134 | Botrytis cinerea | MH860108 | 100% | [38] |
| Cladosporium sp.§ | |||||
| Cryphonectria naterciae (cp71.1) | MK796135 | Cryphonectria naterciae* | NR_159875 | 100% | [39] |
| Cryptovalsa ampelina (cp12) | MK796136 | Cryptovalsa ampelina | JN975335 | 100% | [40] |
| Cytospora sp. (cp68.2) | MK796137 | Cytospora fraxinigena* | MF190134 | 99.04% | [41] |
| Diatrype sp. (cp11) | MK796138 | Diatrype stigma | KU721866 | 99.42% | [42] |
| Diplodia corticola (cp60) | MK796139 | Diplodia corticola* | AY259100 | 100% | [43] |
| Dothiorella iberica (cp89.1) | MK796140 | Dothiorella iberica* | AY573202 | 100% | [44] |
| Fusarium sp. (cp79.6) | MK796141 | Fusarium solani | MH864425 | 99.58% | [38] |
| Gnomoniopsis paraclavulata (cp71.5) | MK796142 | Gnomoniopsis paraclavulata* | EU254839 | 100% | [45] |
| Kalmusia sp. (cp46) | MK796143 | Kalmusia variispora* | NR_145165 | 99.6% | [46] |
| Neocucurbitaria cava (cp75.3) | MK796144 | Neocucurbitaria cava | AY853248 | 100% | [47] |
| Neonectria coccinea (cp70) | MK796145 | Neonectria coccinea* | KC660521 | 99.81% | [48] |
| Nigrospora osmanthi (cp22) | MK796146 | Nigrospora osmanthi* | KX986010 | 99.79% | [49] |
| Penicillium sp.§ | |||||
| Phaeoacremonium angustius (cp77.3) | MK796147 | Phaeoacremonium angustius* | KU060813 | 100% | [50] |
| Pseudocamarosporium piceae (cp48) | MK796148 | Pseudocamarosporium piceae* | KJ747046 | 100% | [51] |
| Querciphoma carteri (cp53) | MK796149 | Querciphoma carteri | KF251209 | 100% | [52] |
| Stemphylium amaranthi (cp73.3) | MK796150 | Stemphylium amaranthi* | KU850505 | 100% | [53] |
| Stemphylium vesicarium (cp78.1) | MK796151 | Stemphylium vesicarium | KU850565 | 100% | [53] |
| Stereum armeniacum (cp35) | MK796152 | Stereum armeniacum* | MH862626 | 100% | [38] |
| Trichoderma harzianum (cp25) | MK796153 | Trichoderma harzianum | MH865862 | 100% | [38] |
| Unidentified (Mortierellales) (cp91.1) | MK796154 | Mortierella hyalina | HQ630355 | 82% | [54] |
| Fungal taxa | Ecology * | Adults | Larvae | Wood Tissues | Number of Sites | |
|---|---|---|---|---|---|---|
| Male | Female | |||||
| Alternaria doliconidium | unk | 1 | 2 | 3 | 1 | |
| Alternaria hordeicola | unk | 8 | 3 | 2 | 1 | |
| Arthrinium sp. | unk | 1 | 1 | |||
| Aureobasidium pullulans | ocr | 8 | 1 | |||
| Biscogniauxia rosacearum | pp | 3 | 1 | |||
| Botryosphaeria dothidea | pp | 5 | 2 | |||
| Botrytis cinerea | pp | 1 | 2 | 2 | ||
| Cladosporium sp. | unk | 1 | 1 | |||
| Cryphonectria naterciae | pp | 5 | 19 | 4 | ||
| Cryptovalsa ampelina | pp | 1 | 1 | |||
| Cytospora sp. | unk | 1 | 1 | |||
| Diatrype sp. | unk | 5 | 2 | |||
| Diplodia corticola | pp | 5 | 10 | 1 | ||
| Dothiorella iberica | pp | 1 | 16 | 4 | ||
| Fusarium sp. | unk | 6 | 1 | |||
| Gnomoniopsis paraclavulata | unk | 1 | 6 | 2 | ||
| Kalmusia sp. | unk | 2 | 1 | |||
| Neocucurbitaria cava | pp | 7 | 4 | 1 | ||
| Neonectria coccinea | pp | 5 | 2 | |||
| Nigrospora osmanthi | unk | 2 | 1 | |||
| Penicillium sp. | unk | 1 | 1 | 1 | 1 | |
| Phaeoacremonium angustius | pp | 2 | 1 | |||
| Pseudocamarosporium piceae | ocr | 1 | 1 | |||
| Querciphoma carteri | unk | 1 | 1 | |||
| Stemphylium amaranthi | pp | 1 | 1 | |||
| Stemphylium vesicarium | pp | 1 | 1 | |||
| Stereum armeniacum | ocr | 1 | 1 | |||
| Trichoderma harzianum | ocr | 1 | 3 | 3 | ||
| Unidentified Mortierellales | unk | 1 | 1 | |||
| Index | Insect | Wood Tissues |
|---|---|---|
| Taxonomic richness | 15 | 23 |
| Shannon diversity index | 2.352 | 2.636 |
| Simpson dominance index | 0.119 | 0.101 |
| Jaccard similarity coefficient * | 0.31 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinna, C.; Linaldeddu, B.T.; Deiana, V.; Maddau, L.; Montecchio, L.; Lentini, A. Plant Pathogenic Fungi Associated with Coraebus florentinus (Coleoptera: Buprestidae) Attacks in Declining Oak Forests. Forests 2019, 10, 488. https://doi.org/10.3390/f10060488
Pinna C, Linaldeddu BT, Deiana V, Maddau L, Montecchio L, Lentini A. Plant Pathogenic Fungi Associated with Coraebus florentinus (Coleoptera: Buprestidae) Attacks in Declining Oak Forests. Forests. 2019; 10(6):488. https://doi.org/10.3390/f10060488
Chicago/Turabian StylePinna, Claudia, Benedetto T. Linaldeddu, Vitale Deiana, Lucia Maddau, Lucio Montecchio, and Andrea Lentini. 2019. "Plant Pathogenic Fungi Associated with Coraebus florentinus (Coleoptera: Buprestidae) Attacks in Declining Oak Forests" Forests 10, no. 6: 488. https://doi.org/10.3390/f10060488
APA StylePinna, C., Linaldeddu, B. T., Deiana, V., Maddau, L., Montecchio, L., & Lentini, A. (2019). Plant Pathogenic Fungi Associated with Coraebus florentinus (Coleoptera: Buprestidae) Attacks in Declining Oak Forests. Forests, 10(6), 488. https://doi.org/10.3390/f10060488






