Chemical Composition and Thermal Behavior of Kraft Lignins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Black Liquor Characterization and Lignin Sample Denomination
2.2. Lignin Recovery Using a Precipitation with Sulfuric Acid
2.3. Lignin Recovery Using Ultrafiltration and Precipitation with Acetic Acid
2.4. Determination of Lignin Higher Heating Values and the Content of Methoxyl Groups
2.5. Thermogravimetric Analysis
2.6. Kinetic Analyses
2.7. Molecular Weight Distribution of Lignin
3. Results and Discussions
3.1. Elemental Composition of Lignins
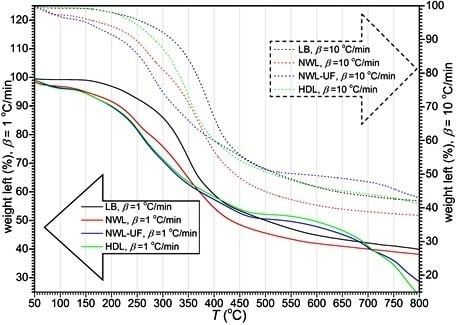
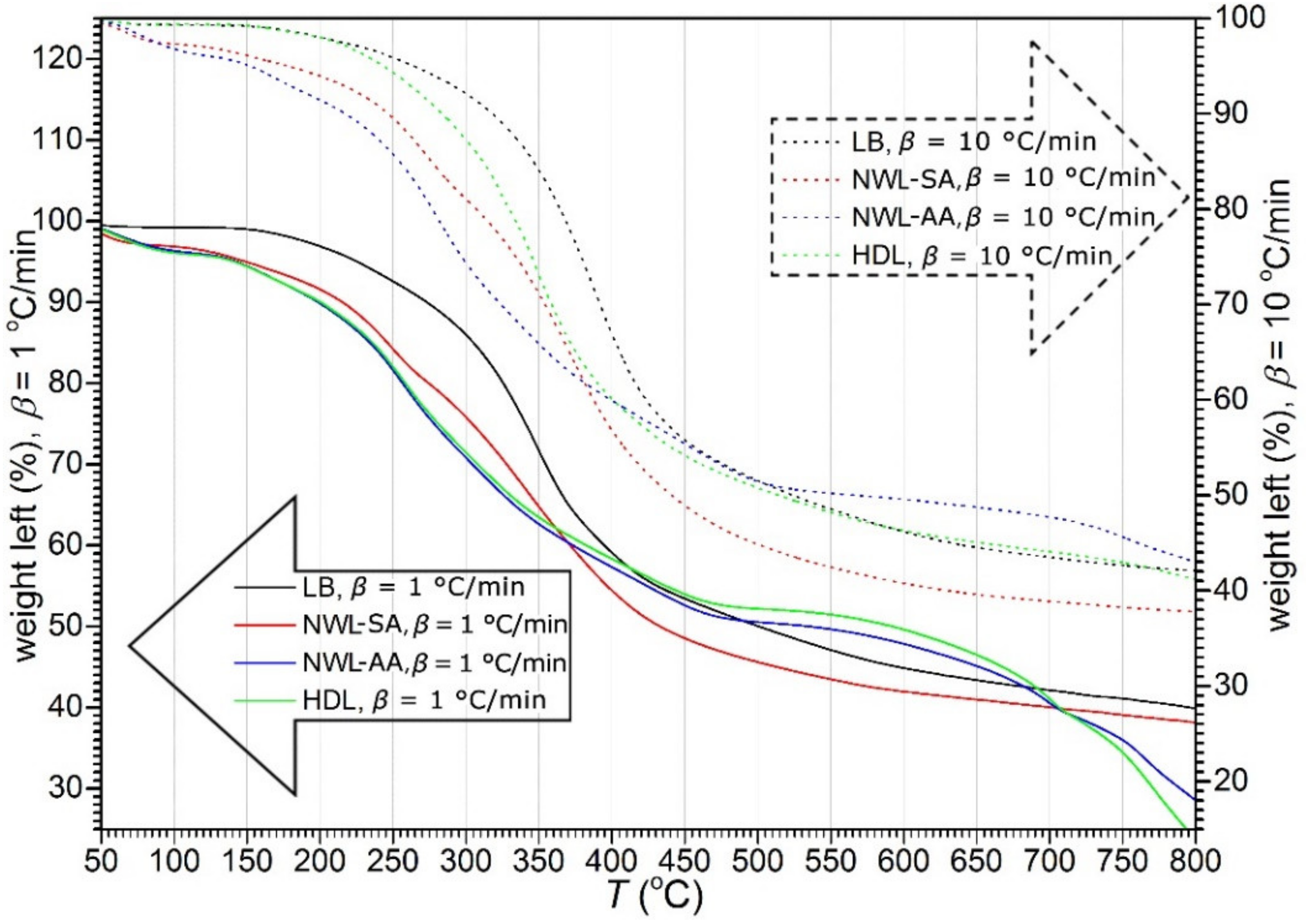
3.2. Thermal Decomposition of Lignins
3.3. Molecular Weight Distribution of Lignins
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Branco, R.H.R.; Serafim, L.S.; Xavier, A.M.R.B. Second Generation Bioethanol Production: On the Use of Pulp and Paper Industry Wastes as Feedstock. Fermentation 2019, 5, 4. [Google Scholar] [CrossRef]
- Mattinen, M.-L.; Riviere, G.; Henn, A.; Nugroho, R.W.N.; Leskinen, T.; Nivala, O.; Valle-Delgado, J.J.; Kostiainen, M.A.; Österberg, M. Colloidal Lignin Particles as Adhesives for Soft Materials. Nanomaterials 2018, 8, 1001. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.K.; Thakur, M.K. Recent advances in green hydrogels from lignin: A review. Int. J. Biol. Macromol. 2015, 72, 834–847. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, V.K.; Nguyen, D.D.; Dharmaraja, J.; Shobana, S.; Banu, J.R.; Saratale, R.G.; Chang, S.W.; Kumar, G. A review on lignin structure, pretreatments, fermentation reactions and biorefinery potential. Bioresour. Technol. 2019, 271, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Sathitsuksanoh, N.; Holtman, K.M.; Yelle, D.J.; Morgan, T.; Stavila, V.; Pelton, J.; Blanch, H.; Simmons, B.A.; George, A. Lignin fate and characterization during ionic liquid biomass pretreatment for renewable chemicals and fuels production. Green Chem. 2014, 16, 1236–1247. [Google Scholar] [CrossRef]
- Wang, H.; Pu, Y.; Ragauskas, A.; Yang, B. From lignin to valuable products—Strategies, challenges, and prospects. Bioresour. Technol. 2019, 271, 449–461. [Google Scholar] [CrossRef]
- Basso, M.C.; Pizzi, A.; Delmotte, L.; Abdalla, S. Analysis of the Cross-Linking Reaction of Lignin with Triethyl Phosphate by MALDI-TOF and 13C NMR. Polymers 2017, 9, 206. [Google Scholar] [CrossRef]
- Tribot, A.; Amer, G.; Alio, M.A.; de Baynast, H.; Delattre, C.; Pons, A.; Mathias, J.-D.; Callois, J.-M.; Vial, C.; Michaud, P.; et al. Wood-lignin: Supply, extraction processes and use as bio-based material. Eur. Polym. J. 2019, 112, 228–240. [Google Scholar] [CrossRef]
- Aboyade, A.O.; Hugo, T.J.; Carrier, M.; Meyer, E.L.; Stahl, R.; Knoetze, J.H.; Gorgens, J.F. Non-isothermal kinetic analysis of the devolatilization of corn cobs and sugar cane bagasse in an inert atmosphere. Thermochim. Acta 2011, 517, 81–89. [Google Scholar] [CrossRef]
- Šimon, P. Isoconversional methods—Fundamentals, meaning and application. J. Therm. Anal. Calorim. 2004, 76, 123–132. [Google Scholar] [CrossRef]
- Gašparovič, L.; Labovský, J.; Markoš, J.; Jelemenský, L. Calculation of Kinetic Parameters of the Thermal Decomposition of Wood by Distributed Activation Energy Model (DAEM). Chem. Biochem. Eng. Q. 2012, 26, 45–53. [Google Scholar]
- Jiang, G.; Nowakowski, D.J.; Bridgwater, A.V. A systematic study of the kinetic of lignin pyrolysis. Thermochim. Acta 2010, 498, 61–66. [Google Scholar] [CrossRef]
- Ramiah, M.V. Thermogravimetric and differential thermal analysis of cellulose, hemicellulose and lignin. J. Appl. Polym. Sci. 1970, 14, 1323–1337. [Google Scholar] [CrossRef]
- Chan, R.W.; Krieger, B.B. Kinetics of dielectric-loss microwave degradation of polymers—lignin. J. Appl. Polym. Sci. 1981, 26, 1533–1553. [Google Scholar] [CrossRef]
- Nunn, T.R.; Howard, J.B.; Longwell, J.P.; Peters, W.A. Product compositions and kinetics in the rapid pyrolysis of milled wood lignin. Ind. Eng. Chem. Process Des. Dev. 1985, 24, 844–852. [Google Scholar] [CrossRef]
- Ferdous, D.; Dalai, A.K.; Bej, S.K.; Thring, R.W. Pyrolysis of lignins: Experimental and kinetics studies. Energy Fuels 2002, 16, 1405–1412. [Google Scholar] [CrossRef]
- Avni, E.; Coughlin, R.W. Kinetic analysis of lignin pyrolysis using non-isothermal TGA data. Thermochim. Acta 1985, 90, 157–167. [Google Scholar] [CrossRef]
- Pasquali, L.C.E.; Herrera, H. Pyrolysis of lignin and IR analysis of residues. Thermochim. Acta 1997, 293, 39–46. [Google Scholar] [CrossRef]
- Rao, T.R.; Sharma, A. Pyrolysis rates of biomass materials. Energy 1998, 23, 973–978. [Google Scholar] [CrossRef]
- Dominguez, J.C.; Oliet, M.; Alonso, M.V.; Gilarranz, M.A.; Rodriguez, F. Thermal stability and pyrolysis kinetics of organosolv lignins obtained from Eucalyptus globulus. Ind. Crops Prod. 2008, 27, 150–156. [Google Scholar] [CrossRef]
- Svenson, J.; Pettersson, J.B.C.; Davidsson, K.O. Fast pyrolysis of the main components of birch wood. Combust. Sci. Technol. 2004, 176, 977–990. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, S.; Wang, S.; Guo, X. Thermogravimetric analysis of pyrolysis characteristics of alkali lignin. Trans. China Pulp Paper 2007, 22, 31–34. [Google Scholar]
- Murugan, P.; Mahinpey, N.; Johnson, K.E.; Wilson, M. Kinetics of the pyrolysis of lignin using thermogravimetric and differential scanning calorimetry methods. Energy Fuels 2008, 22, 2720–2724. [Google Scholar] [CrossRef]
- Wang, G.; Li, W.; Li, B.; Chen, H. TG study on pyrolysis of biomass and its three components under syngas. Fuel 2008, 87, 552–558. [Google Scholar] [CrossRef]
- Mani, T.; Murugan, P.; Mahinpey, N. Determination of distributed activation energy model kinetic parameters using simulated annealing optimization method for nonisothermal pyrolysis of lignin. Ind. Eng. Chem. Res. 2009, 48, 1464–1467. [Google Scholar] [CrossRef]
- Beis, S.H.; Mukkamala, S.; Hill, N.; Joseph, J.; Baker, C.; Jensen, B.; Stemmler, E.A.; Wheeler, M.C.; Frederick, B.G.; van Heiningen, A.; et al. Fast pyrolysis of lignins. BioResources 2010, 5, 1408–1428. [Google Scholar]
- Yang, Q.; Wu, S.B. Thermogravimetric characteristics of wheat straw lignin. Cellulose Chem. Technol. 2009, 43, 4–6. [Google Scholar]
- Poletto, M.; Zattera, A.J. Materials produced from plant biomass: Part III: Degradation kinetics and hydrogen bonding in lignin. Mat. Res. 2013, 16, 1065–1070. [Google Scholar] [CrossRef]
- Domburg, G.E.; Sergeeva, V.N. Thermal degradation of sulphuric acid lignins of hard wood. J. Therm. Anal. 1969, 1, 53–62. [Google Scholar] [CrossRef]
- Jablonský, M.; Ház, A.; Orságová, A.; Botková, M.; Šmatko, L.; Kočiš, J. Relationships between elemental carbon contents and heating values of lignins. In Proceedings of the 4th International Conference Renewable Energy Sources 2013, Tatranské Matliare, High Tatras, Slovakia, 21–23 May 2013; pp. 67–72. [Google Scholar]
- Jablonsky, M.; Botkova, M.; Adamovska, J. Prediction of methoxyl groups content in lignin based on ultimate analysis. Cellul. Chem. Technol. 2015, 49, 165–168. [Google Scholar]
- NREL. Determination of Ash in Biomass; Technical Report NREL/TP-510-42622; National Renewable Energy Laboratory: Golden, CO, USA, 2005. [Google Scholar]
- Kačík, F.; Ďurkovič, J.; Kačíková, D. Structural characterization of lignin by syringyl to guaiacyl ratio and molecular mass determination. In Lignin: Structural Analysis, Applications in Biomaterials and Ecological Significance; Nova Science Publishers: Hauppauge, NY, USA, 2014; pp. 67–89. ISBN 978-1-63117-452-0. [Google Scholar]
- Pereira, B.L.C.; de Carneiro, A.C.O.; Carvalho, A.M.M.L.; Colodette, J.L.; Oliveira, A.C.; Fontes, M.P.F. Influence of chemical composition of eucalyptus wood on gravimetric yield and charcoal properties. Bioresources 2013, 8, 4574–4592. [Google Scholar] [CrossRef]
- Haykiri-Acma, H.; Yaman, S.; Kucukbayrak, S. Effect of heating rate on the pyrolysis yields of rapeseed. Renew. Energy 2006, 31, 803–810. [Google Scholar] [CrossRef]
- Ridout, A.J.; Carrier, M.; Görgens, J. Fast pyrolysis of low and high ash paper waste sludge: Influence of reactor temperature and pellet size. J. Anal. Appl. Pyrolysis 2015, 111, 64–75. [Google Scholar] [CrossRef]
- Carrier, M.; Auret, L.; Bridgwater, A.; Knoetze, J.H. Using apparent activation energy as a reactivity criterion for biomass pyrolysis. Energy Fuels 2016, 30, 7834–7841. [Google Scholar] [CrossRef]
- Wen, J.L.; Sun, S.L.; Xue, B.L.; Sun, R.C. Quantitative structures and thermal properties of birch lignins after ionic liquid pretreatment. J. Agric. Food Chem. 2013, 61, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.L.; Sheng, Y.; Sun, S.L.; Yuan, T.Q.; Sun, R.C. Structural characterization and thermal properties of enzymatic hydrolysis lignins. In Lignin: Structural Analysis, Applications in Biomaterials & Ecological Significance; Nova Science Publisher: New York, NY, USA, 2014; p. 109. ISBN 978-1-63117-452-0. [Google Scholar]
- Serrano, L.; Toledano, A.; García, A.; Labidi, J. Obtain lignins for specific applications. In Lignin: Properties and Applications in Biotechnology and Bioenergy; Nova Science Publishers: Hauppauge, NY, USA, 2012; pp. 139–182. ISBN 978-1-61122-907-3. [Google Scholar]
- El Mansouri, N.-E.; Salvadó, J. Structural characterization of technical lignins for the production of adhesives: Application to lignosulfonate, kraft, soda-anthraquinone, organosolv and ethanol process lignins. Ind. Crops Prod. 2006, 24, 8–16. [Google Scholar] [CrossRef]
- Paterson, J.R. Lignin: Properties and Applications in Biotechnology and Bioenergy; Nova Science Publisher: New York, NY, USA, 2012; p. 558. ISBN 978-1-61122-907-3. [Google Scholar]

| Ref. | Ea (kJ/mol) | n | ln(A) (1/min) | |
|---|---|---|---|---|
| Alcell lignin (hardwood) | [12] | 159.5 | 1.07 ± 0.11 | 28.50 ± 0.13 |
| Asian lignin (straw and grass) | [12] | 133.9 | 1.06 ± 0.11 | 23.92 ± 0.24 |
| Organosolv lignin (hardwood) | [12] | 144.2 | 1.01 ± 0.16 | 25.68 ± 0.29 |
| Etek lignin (softwood) | [12] | 151.9 | 1.04 ± 0.17 | 27.37 ± 0.37 |
| Klason lignin (beech, hardwood) | [12] | 151.1 | 1.43 ± 0.18 | 26.80 ± 0.20 |
| Klason lignin (cassava rhizine) | [12] | 172.2 | 1.58 ± 0.12 | 29.87 ± 0.24 |
| Klason lignin (cassava stalk) | [12] | 171.7 | 1.53 ± 0.16 | 29.81 ± 0.26 |
| Klason lignin (mixed softwood) | [12] | 145.2 | 1.44 ± 0.08 | 24.78 ± 0.10 |
| Klason lignin (willow) | [12] | 156.5 | 1.53 ± 0.08 | 27.82 ± 0.17 |
| Klason lignin (Douglas fir) | [13] | 79.8 ± 4.2 | 1 | |
| Periodate lignin (spruce) | [13] | 54.6 ± 4.2 | 1 | |
| Kraft lignin (pine) | [14] | 25.2 | 1 | 6.15 |
| Milled wood lignin (sweetgum hardwood) | [15] | 82.0 | 1 | 16.81 |
| Kraft lignin (unknown species) | [16] | 129–361 | 1 | 17.31–21.31 |
| Alcell lignin (unknown species) | [16] | 80–158 | 1 | 27.15–52.89 |
| Steam-exploded lignin (aspen) | [17] | 58.6–291.6 | 1.09 | 19.41 |
| Klason lignin (three types of hardwood) | [18] | 12.5; 39.4; 42.6 | 0.5 | |
| Lignin (unknown type and species) | [19] | 70.7 | 1 | 16.34 |
| Organosolv lignin (Eucalyptus) | [20] | 19.1–42.5 | 0.30–0.74 | |
| Lignin (unknown type, birch) | [21] | 75 ± 11 | 1 | 13.99 |
| Alkali lignin (bamboo + hardwood) | [22] | 47.9–54.5 | 6.52–11.10 | |
| Alcell lignin (hardwood) | [23] | 8.5–67.9 | 1 | |
| Lignin (unknown type and species) | [24] | 120.7–197.3 | 1 | 18.42–29.34 |
| Alcell lignin (hardwood) | [25] | 83–195 | 1 | |
| LignoBoost lignin | [26] | 193 | ||
| Acetocell | [26] | 193 | ||
| Indulin AT | [26] | 192 | ||
| Enzymatic acidolysis lignin | [27] | 103.92–107.69 | 19.21–20.60 | |
| Klason lignins from flooded gum | [28] | 158.43 | 1.48 ± 0.07 | 26.38 ± 0.43 |
| Klason lignins from loblolly pine | [28] | 165.69 | 1.42 ± 0.02 | 27.44 ± 0.13 |
| Birch acid-insoluble lignin | [29] | 82–107.95 | ||
| Birch acid-soluble lignin | [29] | 100–160.25 | ||
| Aspen acid-insoluble lignin | [29] | 79.1–102.51 | ||
| Aspen acid-soluble lignin | [29] | 71.5–74.55 | ||
| Oak acid-insoluble lignin | [29] | 109.2 | ||
| Oak acid-soluble lignin | [29] | 77.8 |
| Sample | Liquor #1 | Liquor #2 |
|---|---|---|
| Dry matter (wt %) | 36.80 ± 0.62 | 54.48 ± 0.06 |
| C (wt %) | 36.24 ± 0.09 | 31.45 ± 0.09 |
| H (wt %) | 4.93 ± 0.05 | 4.42 ± 0.05 |
| N (wt %) | 1.13 ± 0.01 | 0.11 ± 0.01 |
| S (wt %) | 0.24 ± 0.04 | 3.68 ± 0.04 |
| Ash (wt %) | 45.75 ± 0.32 | 51.69 ± 0.32 |
| pH | 12.90 ± 0.30 | 12.80 ± 0.40 |
| Density (g/mL) | 1.24 | 1.36 |
| Sample | Elemental Analysis (wt %) | Ratio O/C | Ratio H/C | Ash (%) | OCH3 | HHV | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | C | H | S | O | (wt %) | (MJ/kg) | ||||
| LB | 0.22 | 65.23 | 5.83 | 1.37 | 27.13 | 0.42 | 0.09 | 0.22 ± 0.03 | 14.5 | 26.5 |
| NWL-SA | 1.12 | 63.64 | 6.54 | 0.07 | 27.97 | 0.44 | 0.10 | 0.66 ± 0.07 | 17.7 | 25.9 |
| NWL-AA | 1.05 | 51.13 | 5.58 | <0.05 | 40.94 | 0.80 | 0.11 | 1.30 ± 0.05 | 18.3 | 20.8 |
| HDL | 0.28 | 55.68 | 4.62 | 3.91 | 31.66 | 0.57 | 0.08 | 3.85 ± 0.11 | 11.1 | 22.6 |
| Sample | Heating Rate (°C/min) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 5 | 7 | 9 | 10 | 12 | 15 | Average | ||
| LB | Tm (°C) | 349.5 | 373.3 | 381.1 | 387.7 | – | 393.6 | – | 401.5 | |
| Tdehyd (°C) | 76.7 | 97.5 | 94.9 | 73.6 | – | 74.7 | – | 78.6 | 82.7 ± 9.7 | |
| αdehyd (%) | 0.7 | 0.9 | 1.2 | 1.0 | – | 0.7 | – | 1.0 | 0.9 ± 0.2 | |
| NWL-SA | Tm1 (°C) | 250.8 | 261.9 | 265.7 | 268.6 | – | 273.2 | – | 274.3 | |
| Tm2 (°C) | 336.8 | 350.7 | 360.3 | 366.0 | – | 374.2 | – | 378.9 | ||
| Tdehyd (°C) | 62.7 | 71.5 | 75.1 | 77.7 | – | 82.6 | – | 80.5 | 75.0 ± 6.6 | |
| αdehyd (%) | 2.7 | 3.3 | 3.0 | 3.1 | – | 2.5 | – | 2.7 | 2.9 ± 0.3 | |
| NWL-AA | Tm1 (°C) | 256.5 | 267.9 | 273.5 | 276.8 | – | 280.4 | – | 283.5 | |
| Tm2 (°C) | 323.0 | 335.1 | 343.4 | 349.3 | – | 352.8 | – | 353.6 | ||
| Tdehyd (°C) | 84.4 | 96.9 | 98.1 | 98.6 | – | 98.7 | – | 100.8 | 96.3 ± 5.4 | |
| αdehyd (%) | 3.3 | 3.5 | 3.5 | 3.1 | – | 3.4 | – | 5.3 | 3.7 ± 0.7 | |
| HDL | Tm1 (°C) | 255.8 | – | – | 306.3 | 312.9 | 316.4 | 319.9 | 325.0 | |
| Tm2 (°C) | 320.6 | 335.7 | 339.8 | – | 345.1 | 344.9 | 348.0 | 351.1 | ||
| Tdehyd (°C) | 86.7 | 95.4 | 100.0 | 69.8 | 70.3 | 75.7 | 72.5 | 72.8 | 80 ± 11 | |
| αdehyd (%) | 3.8 | 4.0 | 4.0 | 0.7 | 0.7 | 1.0 | 0.8 | 0.7 | 1.9 ± 1.5 | |
| Sample | ln(A) (1/min) | Ea (kJ/mol) | R2 | Average Mass Loss (%) | |
|---|---|---|---|---|---|
| LB | – | 26.7 ± 1.4 | 174.9 ± 7.3 | 0.993 | 31.1 ± 1.6 |
| NWL-SA | 1st stage | 53.3 ± 3.6 | 260 ± 16 | 0.984 | 15.48 ± 0.83 |
| 2nd stage | 31.7 ± 1.8 | 194.8 ± 9.0 | 0.991 | 28.2 ± 9.7 | |
| NWL-AA | 1st stage | 46.8 ± 1.6 | 234.2 ± 7.2 | 0.996 | 22.0 ± 4.0 |
| 2nd stage | 43.1 ± 4.3 | 246 ± 21 | 0.967 | 36.4 ± 6.2 | |
| HDL | 1st stage | 13.8 ± 9.3 | 93 ± 15 | 0.997 | 17.6 ± 1.4 |
| 2nd stage | 50 ± 13 | 281 ± 25 | 0.980 | 29.1 ± 3.4 |
| Sample | Mn (g/mol) | Mw (g/mol) | PD |
|---|---|---|---|
| LB | 403 | 623 | 1.55 |
| NWL-SA | 1253 | 1933 | 1.54 |
| NWL-AA | 404 | 621 | 1.54 |
| HDL | 1044 | 1711 | 1.64 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ház, A.; Jablonský, M.; Šurina, I.; Kačík, F.; Bubeníková, T.; Ďurkovič, J. Chemical Composition and Thermal Behavior of Kraft Lignins. Forests 2019, 10, 483. https://doi.org/10.3390/f10060483
Ház A, Jablonský M, Šurina I, Kačík F, Bubeníková T, Ďurkovič J. Chemical Composition and Thermal Behavior of Kraft Lignins. Forests. 2019; 10(6):483. https://doi.org/10.3390/f10060483
Chicago/Turabian StyleHáz, Aleš, Michal Jablonský, Igor Šurina, František Kačík, Tatiana Bubeníková, and Jaroslav Ďurkovič. 2019. "Chemical Composition and Thermal Behavior of Kraft Lignins" Forests 10, no. 6: 483. https://doi.org/10.3390/f10060483
APA StyleHáz, A., Jablonský, M., Šurina, I., Kačík, F., Bubeníková, T., & Ďurkovič, J. (2019). Chemical Composition and Thermal Behavior of Kraft Lignins. Forests, 10(6), 483. https://doi.org/10.3390/f10060483