Reassimilation of Leaf Internal CO2 Contributes to Isoprene Emission in the Neotropical Species Inga edulis Mart.
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
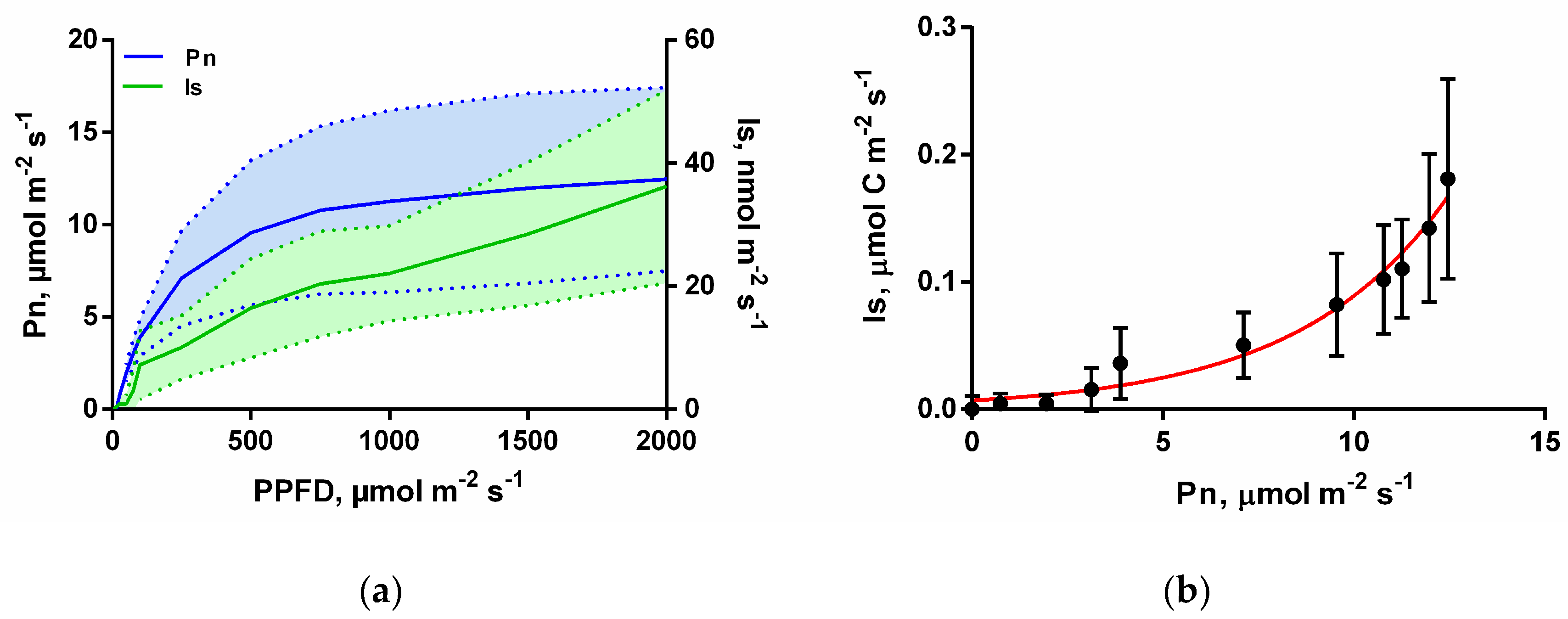
2.2. Isoprene Emissions and Net Photosynthesis
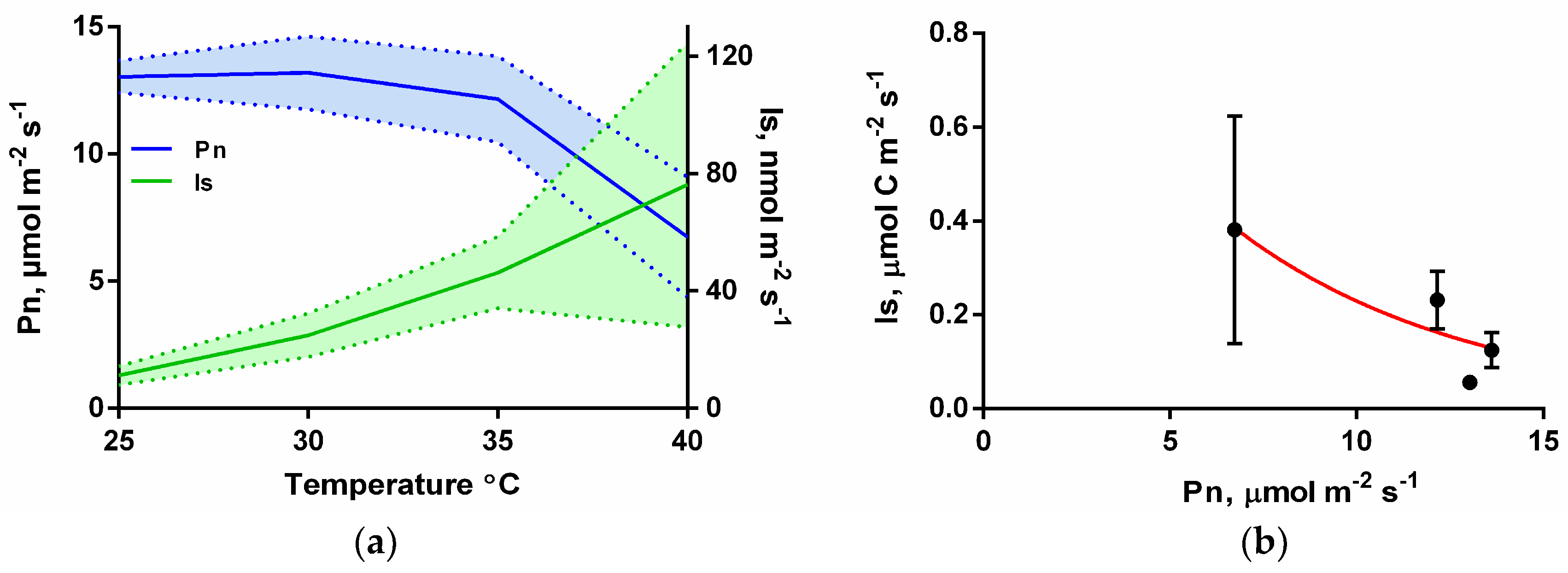
2.3. Light and Temperature Response of Isoprene
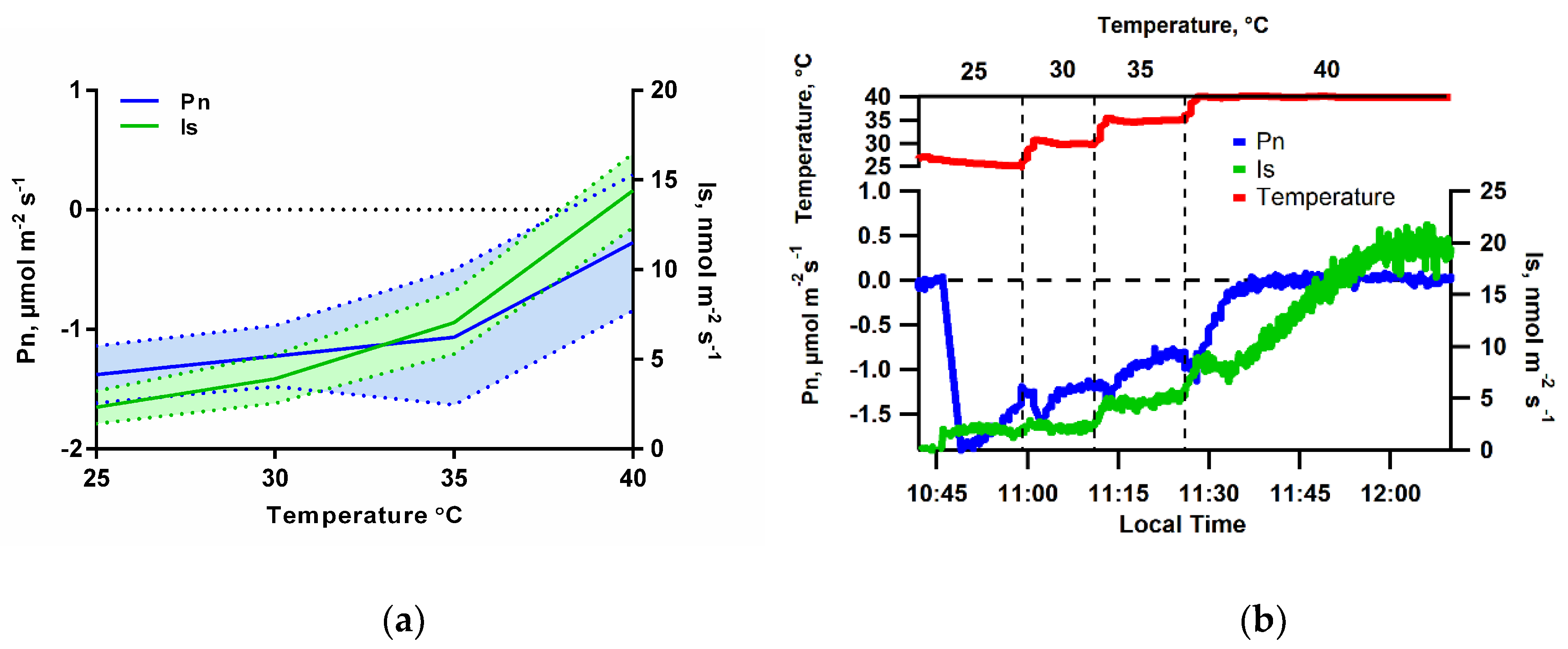
2.4. Isoprene Emission under Limiting Conditions for Net Photosynthesis
2.5. 13C-labeling of Leaf Isoprene Emissions Using Sodium Bicarbonate 13C Delivered through the Transpiration Stream
3. Results
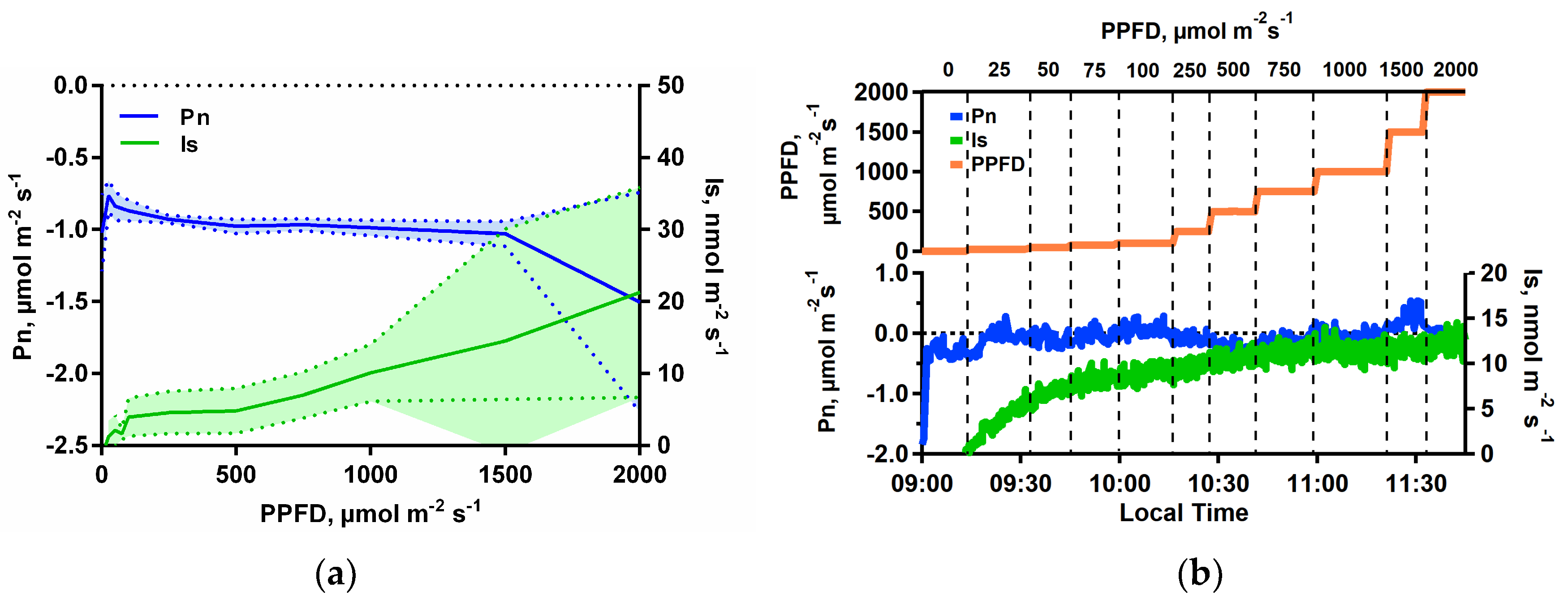
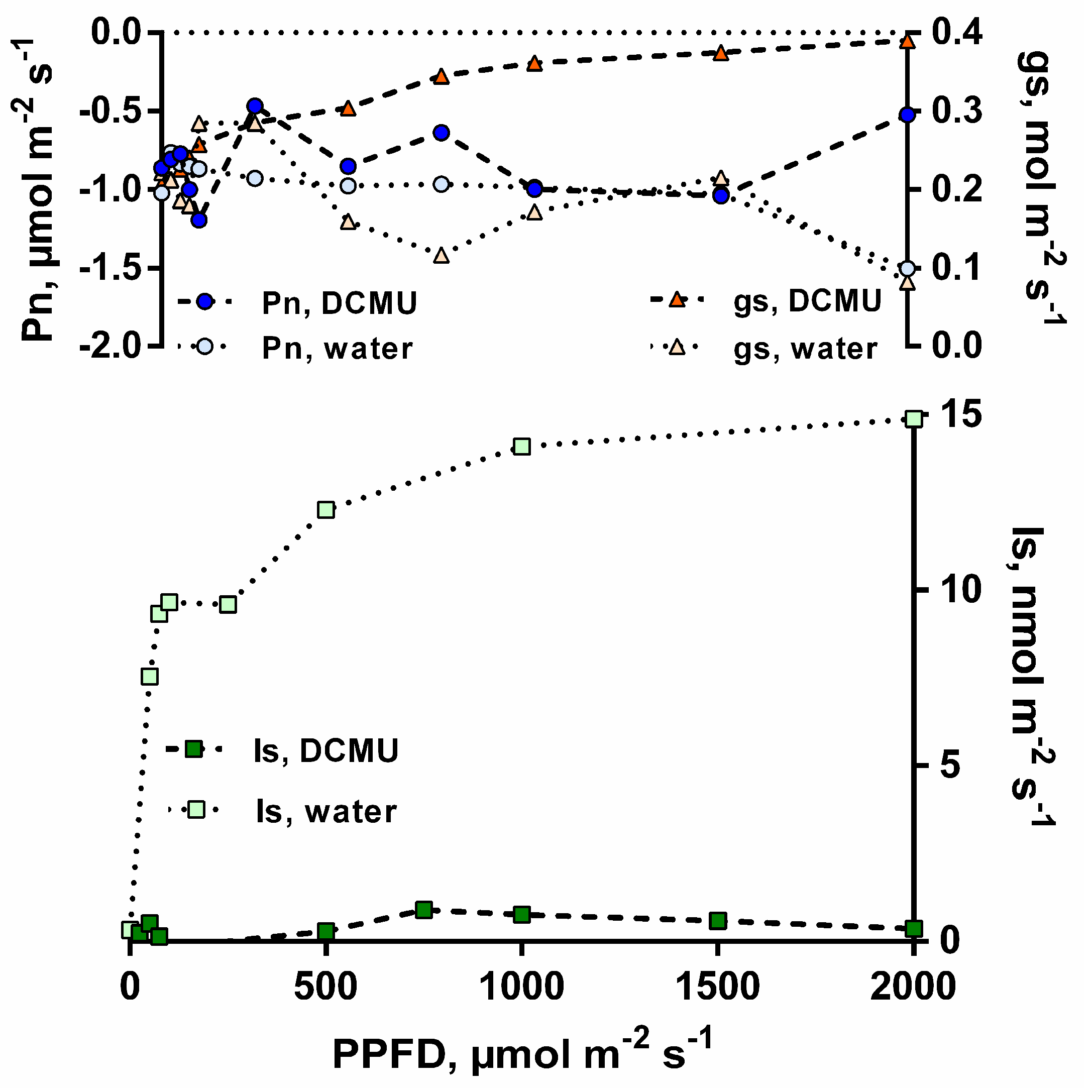
3.1. Isoprene Emissions under CO2-Free Atmosphere are Stimulated by Light but are Blocked When Photosynthesis is Inhibited
3.2. Isoprene Emissions under CO2-Free Atmosphere are Strongly Stimulate by Leaf Temperature Increase but are Eliminated in the Dark
3.3. Sodium Bicarbonate 13C Leaf Feeding
4. Discussion
4.1. Under CO2-Free Air, Isoprene Emissions Display a Similar Light and Temperature Response Pattern to That under Ambient Conditions, But Is Eliminated when Photosynthesis Is Inhibited
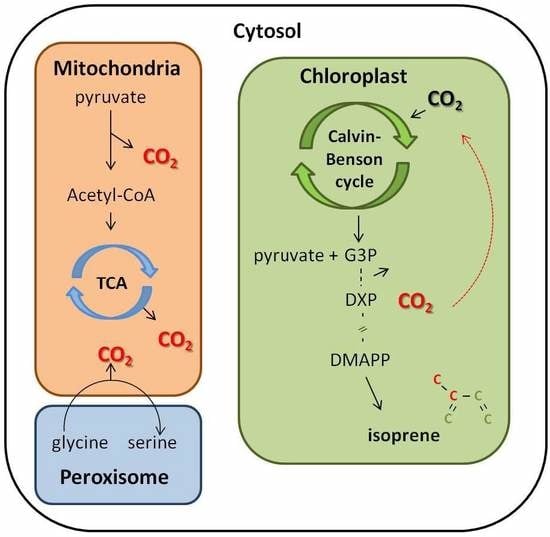
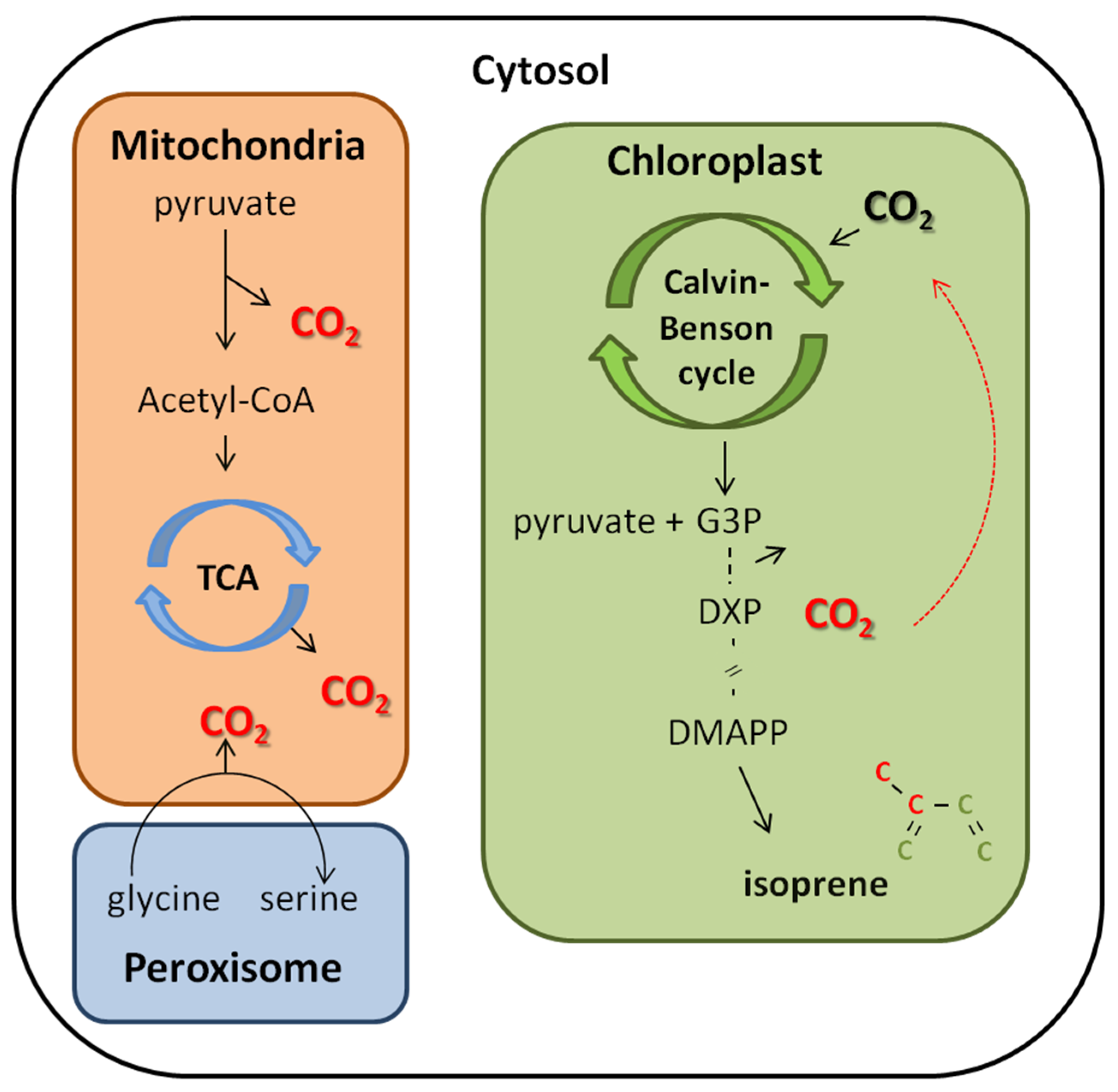
4.2. CO2 Reassimilation from Decarboxylation Process as an Alternative Carbon Source to Isoprene Synthesis
4.3. The Reassimilation of CO2 in the Cell Interior Linked to Isoprene Emission: Its Importance for Plant Physiological Functioning under Changing Environmental Factors
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rasmussem, R.A. Survey of plant species that release isoprene to atmosphere. In Abstracts of Papers of The American Chemical Society; American Chemical Society: Washington, DC, USA, 1972. [Google Scholar]
- Lerdau, M.T.; Harley, P.C.; Monson, R.K. Ecological and evolutionary aspects of isoprene emission from plants. Oecologia 1999, 118, 109–123. [Google Scholar]
- Guenther, A. The contribution of reactive carbon emissions from vegetation to the carbon balance of terrestrial ecosystems. Chemosphere 2002, 49, 837–844. [Google Scholar] [CrossRef]
- Guenther, A.; Karl, T.; Harley, P.; Wiedinmyer, C.; Palmer, P.I.; Geron, C. Estimates of global terrestrial isoprene emissions using MEGAN (Model of Emissions of Gases and Aerosols from Nature). Atmos. Chem. Phys. Discuss. 2006, 6, 107–173. [Google Scholar] [CrossRef]
- Claeys, M.; Graham, B.; Vas, G.; Wang, W.; Vermeylen, R.; Pashynska, V.; Cafmeyer, J.; Guyon, P.; Andreae, M.O.; Artaxo, P.; et al. Formation of Secondary Organic Aerosols Through Photooxidation of Isoprene. Science 2004, 303, 1173–1176. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R.; Arey, J. Atmospheric Degradation of Volatile Organic Compounds. Chem. Rev. 2003, 103, 4605–4638. [Google Scholar] [CrossRef]
- Kroll, J.H.; Ng, N.L.; Murphy, S.M.; Flagan, R.C.; Seinfeld, J.H. Secondary Organic Aerosol Formation from Isoprene Photooxidation. Environ. Sci. Technol. 2006, 40, 1869–1877. [Google Scholar] [CrossRef]
- Unger, N. Global Climate Forcing by Criteria Air Pollutants. Annu. Resour. 2012, 37, 1–24. [Google Scholar] [CrossRef]
- Monson, R.K.; Jones, R.T.; Rosenstiel, T.N.; Schnitzler, J.P. Why only some plants emit isoprene. Plant. Cell Environ. 2013, 36, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, T.D. Is it useful to ask why plants emit isoprene? Plant Cell Environ. 2013, 36, 517–520. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Monson, R.K. Isoprene research—60 years later, the biology is still enigmatic. Plant Cell Environ. 2017, 40, 1671–1678. [Google Scholar] [CrossRef]
- Velikova, V.; Loreto, F. On the relationship between isoprene emission and thermotolerance in Phragmites australis leaves exposed to high temperatures and during the recovery from a heat stress. Plant Cell Environ. 2005, 28, 318–327. [Google Scholar] [CrossRef]
- Velikova, V.; Várkonyi, Z.; Szabo, M.; Maslenkova, L.; Nogues, I.; Kovács, L.; Peeva, V.; Busheva, M.; Garab, G.; Sharkey, T.D.; et al. Increased Thermostability of Thylakoid Membranes in Isoprene-Emitting Leaves Probed with Three Biophysical Techniques. Plant Physiol. 2011, 157, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Harvey, C.M.; Li, Z.; Tjellström, H.; Blanchard, G.J.; Sharkey, T.D. Concentration of isoprene in artificial and thylakoid membranes. J. Bioenerg. Biomembr. 2015, 47, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. The 1-Deoxy-D-Xylulose-5-Phosphate Pathway of Isoprenoid Biosynthesis in Plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 47–65. [Google Scholar] [CrossRef] [PubMed]
- Affek, H.P.; Yakir, D. Natural Abundance Carbon Isotope Composition of Isoprene Reflects Incomplete Coupling between Isoprene Synthesis and Photosynthetic Carbon Flow. Plant Physiol. 2003, 131, 1727–1736. [Google Scholar] [CrossRef] [PubMed]
- Funk, J.L.; Mak, J.E.; Lerdau, M. Stress-induced changes in carbon sources for isoprene production in Populus deltoides. Plant Cell Environ. 2004, 27, 747–755. [Google Scholar] [CrossRef]
- Brilli, F.; Barta, C.; Fortunati, A.; Lerdau, M.; Loreto, F.; Centritto, M. Response of isoprene emission and carbon metabolism to drought in white poplar (Populus alba) saplings. New Phytol. 2007, 175, 244–254. [Google Scholar] [CrossRef]
- Silver, G.M.; Fall, R. Characterization of Aspen Isoprene Synthase, an Enzyme Responsible for Leaf Isoprene Emission to the Atmosphere. J. Boil. Chem. 1995, 270, 13010–13016. [Google Scholar] [CrossRef]
- Schnitzler, J.-P.; Arenz, R.; Steinbrecher, R.; Lehning, A. Characterization of an Isoprene Synthase from Leaves of Quercus petraea (Mattuschka) Liebl. Bot. Acta 1996, 109, 216–221. [Google Scholar] [CrossRef]
- Delwiche, C.F.; Sharkey, T.D. Rapid appearance of 13C in biogenic isoprene when 13CO2 is fed to intact leaves. Plant Cell Environ. 1993, 16, 587–591. [Google Scholar] [CrossRef]
- Affek, H.P.; Yakir, D. Protection by Isoprene against Singlet Oxygen in Leaves. Plant Physiol. 2002, 129, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Karl, T.; Rosenstiel, T.; Prazeller, P.; Seufert, G.; Lindinger, W.; Fall, R.; Larsen, B. On-line analysis of the 13CO2 labeling of leaf isoprene suggests multiple subcellular origins of isoprene precursors. Planta 2002, 215, 894–905. [Google Scholar]
- Loreto, F.; Pinelli, P.; Brancaleoni, E.; Ciccioli, P. 13C Labeling Reveals Chloroplastic and Extrachloroplastic Pools of Dimethylallyl Pyrophosphate and Their Contribution to Isoprene Formation1. Plant Physiol. 2004, 135, 1903–1907. [Google Scholar] [CrossRef] [PubMed]
- Jardine, K.; Chambers, J.; Alves, E.G.; Teixeira, A.; Garcia, S.; Holm, J.A.; Higuchi, N.; Manzi, A.; Abrell, L.; Fuentes, J.D.; et al. Dynamic Balancing of Isoprene Carbon Sources Reflects Photosynthetic and Photorespiratory Responses to Temperature Stress. Plant Physiol. 2014, 166, 2051–2064. [Google Scholar] [CrossRef]
- Schnitzler, J.-P.; Graus, M.; Kreuzwieser, J.; Heizmann, U.; Rennenberg, H.; Wisthaler, A.; Hansel, A.; Schnitzler, J.-P. Contribution of Different Carbon Sources to Isoprene Biosynthesis in Poplar Leaves. Plant Physiol. 2004, 135, 152–160. [Google Scholar] [CrossRef]
- Ghirardo, A.; Gutknecht, J.; Zimmer, I.; Brüggemann, N.; Schnitzler, J.-P.; Schnitzler, J.-P. Biogenic Volatile Organic Compound and Respiratory CO2 Emissions after 13C-Labeling: Online Tracing of C Translocation Dynamics in Poplar Plants. PLoS ONE 2011, 6, e17393. [Google Scholar] [CrossRef]
- E Vickers, C.; Gershenzon, J.; Lerdau, M.T.; Loreto, F.; Vickers, C. A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nat. Methods 2009, 5, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Loreto, F.; Schnitzler, J.P. Abiotic stresses and induced BVOCs. Trends Plant. Sci. 2010, 15, 154–166. [Google Scholar] [CrossRef]
- Niinemets, U.; Arneth, A.; Kühn, U.; Monson, R.K.; Penuelas, J.; Staudt, M. The emission factor of volatile isoprenoids: stress, acclimation, and developmental responses. Biogeosci. Discuss. 2010, 7, 1529–1574. [Google Scholar] [CrossRef]
- Kreuzwieser, J.; Graus, M.; Wisthaler, A.; Hansel, A.; Rennenberg, H.; Schnitzler, J.-P. Xylem-transported glucose as an additional carbon source for leaf isoprene formation in Quercus robur. New Phytol. 2002, 156, 171–178. [Google Scholar] [CrossRef]
- Monson, R.K.; Fall, R. Isoprene Emission from Aspen Leaves: Influence of Environment and Relation to Photosynthesis and Photorespiration. Plant Physiol. 1989, 90, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Jardine, K.J.; Sommer, E.D.; Saleska, S.R.; Huxman, T.E.; Harley, P.C.; Abrell, L. Gas Phase Measurements of Pyruvic Acid and Its Volatile Metabolites. Environ. Sci. Technol. 2010, 44, 2454–2460. [Google Scholar] [CrossRef]
- Trowbridge, A.M.; Asensio, D.; Eller, A.S.D.; Way, D.A.; Wilkinson, M.J.; Schnitzler, J.-P.; Jackson, R.B.; Monson, R.K.; Schnitzler, J.-P. Contribution of Various Carbon Sources Toward Isoprene Biosynthesis in Poplar Leaves Mediated by Altered Atmospheric CO2 Concentrations. PLoS ONE 2012, 7, e32387. [Google Scholar] [CrossRef] [PubMed]
- Gerbaud, A.; Andre, M. An Evaluation of the Recycling in Measurements of Photorespiration. Plant Physiol. 1987, 83, 933–937. [Google Scholar] [CrossRef] [PubMed]
- Salem, K.; Van Waasbergen, L.G. Photosynthetic Electron Transport Controls Expression of the High Light Inducible Gene in the Cyanobacterium Synechococcus elongatus Strain PCC 7942. Plant Cell Physiol. 2004, 45, 651–658. [Google Scholar] [CrossRef]
- Tingey, D.T.; Manning, M.; Grothaus, L.C.; Burns, W.F. The Influence of Light and Temperature on Isoprene Emission Rates from Live Oak. Physiol. Plant. 1979, 47, 112–118. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Loreto, F. Water stress, temperature, and light effects on the capacity for isoprene emission and photosynthesis of kudzu leaves. Oecologia 1993, 95, 328–333. [Google Scholar] [CrossRef]
- Sun, Z.; Niinemets, Ü.; Hüve, K.; Noe, S.; Rasulov, B.; Copolovici, L.; Vislap, V. Enhanced isoprene emission capacity and altered light responsiveness in aspen grown under elevated atmospheric CO2 concentration. Chang. Boil. 2012, 18, 3423–3440. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Sun, Z. How light, temperature, and measurement and growth [CO2] interactively control isoprene emission in hybrid aspen. J. Exp. Bot. 2014, 66, 841–851. [Google Scholar]
- Sharkey, T.; Chen, X.; Yeh, S. Isoprene Increases Thermotolerance of Fosmidomycin-Fed Leaves. Plant Physiol. 2001, 125, 2001–2006. [Google Scholar] [CrossRef]
- De Souza, V.F.; Niinemets, Ü.; Rasulov, B.; Vickers, C.E.; Júnior, S.D.; Araújo, W.L.; Gonçalves, J.F.D.C. Alternative Carbon Sources for Isoprene Emission. Trends Plant Sci. 2018, 23, 1081–1101. [Google Scholar] [CrossRef]
- Loreto, F. Emission of Isoprene from Salt-Stressed Eucalyptus globulus Leaves. Plant Physiol. 2000, 123, 1605–1610. [Google Scholar] [CrossRef] [PubMed]
- Voss, I.; Sunil, B.; Scheibe, R.; Raghavendra, A.S. Emerging concept for the role of photorespiration as an important part of abiotic stress response. Plant Boil. 2013, 15, 713–722. [Google Scholar] [CrossRef]
- Monson, R.K.; Jaeger, C.H.; Adams, W.W.; Driggers, E.M.; Silver, G.M.; Fall, R. Relationships among Isoprene Emission Rate, Photosynthesis, and Isoprene Synthase Activity as Influenced by Temperature. Plant Physiol. 1992, 98, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Rasulov, B.; Hüve, K.; Bichele, I.; Laisk, A.; Niinemets, Ü. Temperature Response of Isoprene Emission in Vivo Reflects a Combined Effect of Substrate Limitations and Isoprene Synthase Activity: A Kinetic Analysis. Plant Physiol. 2010, 154, 1558–1570. [Google Scholar] [CrossRef] [PubMed]
- Jardine, K.J.; Jardine, A.B.; Souza, V.F.; Carneiro, V.; Ceron, J.V.; Gimenez, B.O.; Soares, C.P.; Durgante, F.M.; Higuchi, N.; Manzi, A.O.; et al. Methanol and isoprene emissions from the fast growing tropical pioneer species Vismia guianensis (Aubl.) Pers. (Hypericaceae) in the central Amazon forest. Atmos. Chem. Phys. Discuss. 2016, 16, 6441–6452. [Google Scholar] [CrossRef]
- Vranová, E.; Coman, D.; Gruissem, W. Network Analysis of the MVA and MEP Pathways for Isoprenoid Synthesis. Annu. Rev. Plant Biol. 2013, 64, 665–700. [Google Scholar] [CrossRef]
- Kruger, N.J.; Von Schaewen, A. The oxidative pentose phosphate pathway: structure and organisation. Plant Boil. 2003, 6, 236–246. [Google Scholar] [CrossRef]
- Sweetlove, L.J.; Williams, T.C.R.; Cheung, C.Y.M.; Ratcliffe, R.G. Modelling metabolic CO2 evolution—A fresh perspective on respiration. Plant Cell Environ. 2013, 36, 1631–1640. [Google Scholar] [CrossRef]
- Lean, J.L.; Rind, D.H. How will Earth’s surface temperature change in future decades? Geophys. Res. Lett. 2009, 36, 15. [Google Scholar] [CrossRef]
- Busch, F.A.; Sage, T.L.; Cousins, A.B.; Sage, R.F. C3 plants enhance rates of photosynthesis by reassimilating photorespired and respired CO2. Plant Cell Environ. 2013, 36, 200–212. [Google Scholar] [CrossRef]
- Delfine, S.; Di Marco, G.; Loreto, F. Estimation of photorespiratory carbon dioxide recycling during photosynthesis. Aust. J. Plant Physiol. 1999, 26, 733. [Google Scholar] [CrossRef]
- Sage, T.L.; Sage, R.F. The Functional Anatomy of Rice Leaves: Implications for Refixation of Photorespiratory CO2 and Efforts to Engineer C4 Photosynthesis into Rice. Plant Cell Physiol. 2009, 50, 756–772. [Google Scholar] [CrossRef] [PubMed]
- Bloemen, J.; McGuire, M.A.; Aubrey, D.P.; O Teskey, R.; Steppe, K. Internal recycling of respired CO2 may be important for plant functioning under changing climate regimes. Plant Signal. Behav. 2013, 8, 197–555. [Google Scholar] [CrossRef] [PubMed]
- Haupt-herting, S.; Klug, K.; Fock, H.P. A New Approach to Measure Gross CO2 Fluxes in Leaves. Gross CO2 Assimilation, Photorespiration, and Mitochondrial Respiration in the Light in Tomato under Drought Stress. Plant. Physiol. 2001, 126, 388–396. [Google Scholar] [CrossRef]
- Pärnik, T.; Ivanova, H.; Keerberg, O. Photorespiratory and respiratory decarboxylations in leaves of C3plants under different CO2concentrations and irradiances. Plant Cell Environ. 2007, 30, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Wingler, A.; Lea, P.J.; Quick, W.P.; Leegood, R.C. Photorespiration: metabolic pathways and their role in stress protection. Philos. Trans. Soc. B Boil. Sci. 2000, 355, 1517–1529. [Google Scholar] [CrossRef]
- Zhou, X.; Fu, Y.; Li, B.; Luo, Y. An imperative need for global change research in tropical forests. Tree Physiol. 2013, 33, 903–912. [Google Scholar] [CrossRef]
- Cavaleri, M.A.; Reed, S.C.; Smith, W.K.; Wood, T.E. Urgent need for warming experiments in tropical forests. Chang. Boil. 2015, 21, 2111–2121. [Google Scholar] [CrossRef]
- Leitold, V.; Morton, D.C.; Longo, M.; Dos-Santos, M.N.; Keller, M.; Scaranello, M. El Niño drought increased canopy turnover in Amazon forests. New Phytol. 2018, 219, 959–971. [Google Scholar] [CrossRef]

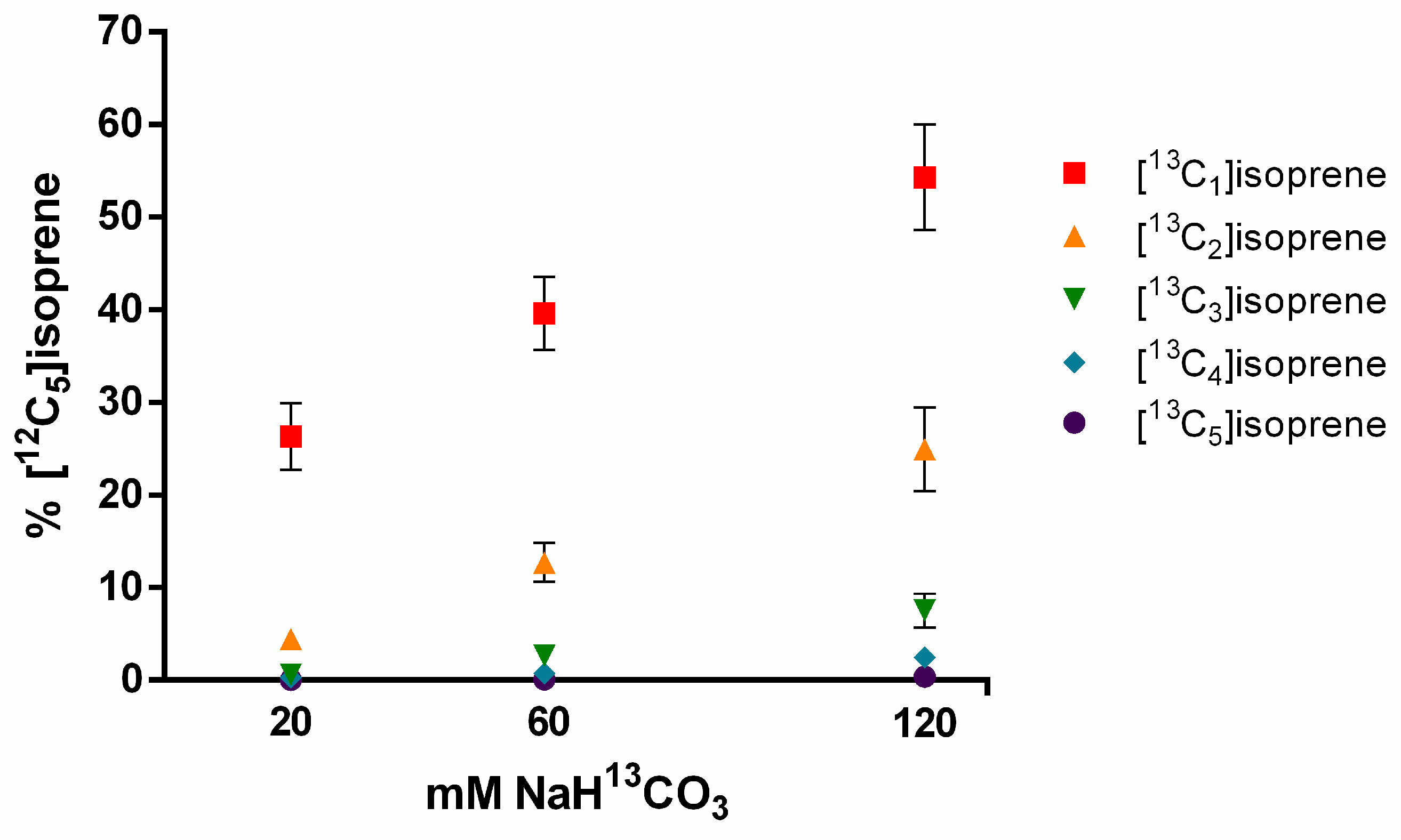
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia, S.; Jardine, K.; Souza, V.F.d.; Souza, R.A.F.d.; Duvoisin Junior, S.; Gonçalves, J.F.d.C. Reassimilation of Leaf Internal CO2 Contributes to Isoprene Emission in the Neotropical Species Inga edulis Mart. Forests 2019, 10, 472. https://doi.org/10.3390/f10060472
Garcia S, Jardine K, Souza VFd, Souza RAFd, Duvoisin Junior S, Gonçalves JFdC. Reassimilation of Leaf Internal CO2 Contributes to Isoprene Emission in the Neotropical Species Inga edulis Mart. Forests. 2019; 10(6):472. https://doi.org/10.3390/f10060472
Chicago/Turabian StyleGarcia, Sabrina, Kolby Jardine, Vinicius F. de Souza, Rodrigo A. F. de Souza, Sergio Duvoisin Junior, and José Francisco de C. Gonçalves. 2019. "Reassimilation of Leaf Internal CO2 Contributes to Isoprene Emission in the Neotropical Species Inga edulis Mart." Forests 10, no. 6: 472. https://doi.org/10.3390/f10060472
APA StyleGarcia, S., Jardine, K., Souza, V. F. d., Souza, R. A. F. d., Duvoisin Junior, S., & Gonçalves, J. F. d. C. (2019). Reassimilation of Leaf Internal CO2 Contributes to Isoprene Emission in the Neotropical Species Inga edulis Mart. Forests, 10(6), 472. https://doi.org/10.3390/f10060472