Abstract
Isoprene (C5H8) is a hydrocarbon gas emitted by many tree species and has been shown to protect photosynthesis under abiotic stress. Under optimal conditions for photosynthesis, ~70%–90% of carbon used for isoprene biosynthesis is produced from recently assimilated atmospheric CO2. While the contribution of alternative carbon sources that increase with leaf temperature and other stresses have been demonstrated, uncertainties remain regarding the biochemical source(s) of isoprene carbon. In this study, we investigated leaf isoprene emissions (Is) from neotropical species Inga edulis Mart. as a function of light and temperature under ambient (450 µmol m−2 s−1) and CO2-free (0 µmol m−2 s−1) atmosphere. Is under CO2-free atmosphere showed light-dependent emission patterns similar to those observed under ambient CO2, but with lower light saturation point. Leaves treated with the photosynthesis inhibitor DCMU (3-(3,4-dichlorophenyl)-1,1-dimethylurea) failed to produce detectable Is in normal light under a CO2-free atmosphere. While strong temperature-dependent Is were observed under CO2-free atmosphere in the light, dark conditions failed to produce detectable Is even at the highest temperatures studied (40 °C). Treatment of leaves with 13C-labeled sodium bicarbonate under CO2-free atmosphere resulted in Is with over 50% containing at least one 13C atom. Is under CO2-free atmosphere and standard conditions of light and leaf temperature represented 19% ± 7% of emissions under ambient CO2. The results show that the reassimilation of leaf internal CO2 contributes to Is in the neotropical species I. edulis. Through the consumption of excess photosynthetic energy, our results support a role of isoprene biosynthesis, together with photorespiration, as a key tolerance mechanism against high temperature and high light in the tropics.
1. Introduction
Isoprene (2-methyl-1,3-butadiene, C5H8) is a reactive hydrocarbon gas emitted in large amounts to the atmosphere by many plants [1,2]. Approximately 500 Tg C year−1 is released as isoprene by vegetation [3]. Tropical rainforests are an important source of isoprene to the atmosphere and estimates suggest that they are responsible for 80% of global isoprene emissions (Is) [4]. Isoprene has an important role in atmospheric chemistry involving air quality and climate [5]. Due to its high chemical reactivity with respect to photooxidation, isoprene impacts the atmospheric concentrations of ozone, methane, and secondary organic aerosols, resulting in strong effects on the radiation balance of the Earth [6,7,8]. Despite great advances in our knowledge on the roles of plant Is on atmospheric chemistry, much less is known about the physiological roles that isoprene plays in plants [9,10,11]. The emerging view is that isoprene production can protect photosynthesis during abiotic stress through mechanisms including excess photosynthetic energy consumption, physical stability of biological membranes, and direct roles as an antioxidant through reactions with reactive oxygen species that accumulate under stress conditions [12,13]. However, recent work has noted that protection of photosynthesis through isoprene via the physical stabilization of membranes may not be possible and that the mechanisms of protection from oxidative stress are still unclear [14].
Isoprene is synthesized in the chloroplast by the 1-deoxy-D-xylulose 5-phosphate/2-C-methyl-D-erythritol 4-phosphate (DOXP/MEP) pathway [15,16,17,18]. The synthesis is initiated by the condensation of two primary precursors; pyruvate and glyceraldehyde 3-phosphate (G3P), a direct product of the Calvin–Benson cycle [15,19,20]. The fact that isoprene cannot be stored in the leaves results in Is from production, with the majority of carbon derived from recent photosynthesis [21]. Under steady-state conditions of light and temperature (1000 µmol m−2 s−1 and 30 °C) ~70%–90% of the carbon used for isoprene synthesis is produced from recently assimilated atmospheric CO2 [21,22,23]. It was observed that plants exposed to 13CO2 rapidly incorporate 13C into isoprene molecules [21,23,24,25]. Despite this rapid incorporation of 13C, ~10%–30% of the carbon atoms in the isoprene molecules emitted have been reported to remain unlabeled, even when the leaves are continuously exposed to 13CO2 [26]. This indicates a contribution of carbon sources other than the recently assimilated CO2. Those alternative carbon sources are important under stress conditions [27] and increase under high temperatures, while net photosynthesis (Pn) is reduced at the expense of photorespiration [25]. Therefore, under stressful situations that reduce the uptake of atmospheric CO2 due to partial stomatal closure while stimulating internal sources of CO2 (e.g., respiration and photorespiration), Is can be increased even if Pn is substantially reduced [28,29,30].
Several studies have identified that potential alternative carbon sources for Is can be greater when environmental conditions are limiting Pn, including xylem-transported carbohydrates [26,31], starch degradation [26,32], pyruvate [33], stored carbon pools [17], and extrachloroplastic intermediates [34]. In addition, the incorporation of CO2 released by intercellular decarboxylations, e.g., during mitochondrial respiration, photorespiration or decarboxylation of pyruvate during formation of MEP was suggested [25,26,27]. For example, photorespiratory CO2 release is strongly stimulated under high temperatures and stomatal closure due to the decrease of atmospheric CO2 uptake and the relative increase of O2 in relation to CO2. Therefore, under stressful conditions that promote stomatal closure, such as drought and high temperature, an increase in the release of photorespiratory CO2 inside the leaf could provide alternative carbon sources for isoprene production [35]. However, the identity and quantitative importance of alternative carbon sources for isoprene synthesis in tropical species under abiotic stress is still unclear.
In this study, we investigated the incorporation of CO2 from decarboxylation process into isoprene molecules by quantifying Is under CO2-free reference air in temperature- and light response curves from a neotropical specie Inga edulis Mart. Taking off the CO2 from the leaf chamber, we stimulated the photorespiration process and the release of internal CO2 in the leaf. We then compared leaf Is in CO2-free reference air with Is in ambient CO2 under standard conditions of light and temperature. We further investigated the potential for the reassimilation of CO2 release by internal decarboxylation processes using an inhibitor of photosynthesis under CO2-free reference air. Finally, we directly evaluated the potential of internal leaf CO2 to be incorporated into Is by providing 13C-sodium bicarbonate (NaH13CO3) solutions to detached leaves under CO2-free reference air. We suggest that CO2 released by internal decarboxylation processes is a quantitatively important source of alternative carbon for isoprene formation. Together with photorespiration, isoprene production may be a key tolerance mechanism under plant stress that leads to a decrease in stomatal conductance and CO2 uptake. Under these conditions, isoprene could still be synthesized using the CO2 released by photorespiration, offering a protective mechanism by consuming excess photosynthetic energy and reducing equivalents. These results deepen our understanding about Is by tropical species under different environmental conditions commonly experienced in the tropics, such as high irradiance and temperature, and contributes to the modeling of Is from terrestrial ecosystems in the tropics under climate change.
2. Materials and Methods
2.1. Plant Material and Experimental Design
Six I. edulis trees with a height ranging from 5 to 10 m were used in this study. The experimental trees occur naturally near the Laboratory of Plant Physiology and Biochemistry at the National Institute for Amazonian Research (INPA) campus III in Manaus, Brazil. This tropical species was selected because of its high reported Is and for its ability to maintain high transpiration rates for many hours following branch detachment from the tree [25]. From October 2014 to February 2016 Pn and Is rates were quantified from healthy mature leaves, located in the upper third of the canopy. For each day of the study, one branch near the top of one of the plants was cut and placed in tap water before being recut and transported to the laboratory. The leaf gas exchange measurements occurred between 9:00 AM and 5:00 PM, with the branch exposed to ambient light and temperature conditions.
2.2. Isoprene Emissions and Net Photosynthesis
Pn and Is rates were quantified from I. edulis leaves using a portable open gas exchange system (IRGA) (LI-6400XT, LI-COR Inc., Lincoln, NE, USA) with an artificial light source 6400-02B Red Blue. The flow rate entering the LI-6400XT leaf chamber was set to 537 mL min−1. A fraction of air exiting the leaf chamber was used to determine Is using two methods: quantified in real-time using a high sensitivity quadrupole proton transfer reaction-mass spectrometry (PTR-MS, Ionicon Analytik, Innsbruck, Austria) or thermal desorption (TD) gas chromatography–mass spectrometry (GC-MS, 5975C series, Agilent Technologies, Santa Clara, CA, USA). Using four-way junction fitting, air exiting the leaf chamber was delivered or to the PTR-MS (40 mL min−1) or to the TD tube (100 mL min−1 when collecting), with the remainder of the flow diverted to the vent/match valve within the LI6400XT. The excess flow entering the vent/match valve was maintained to at least 200 mL min−1 [25]. Background measurements were performed with an empty leaf chamber, at the beginning and at the end of each experiment.
2.3. Light and Temperature Response of Isoprene
For each I. edulis leaf, either a light or temperature response curve was generated. Leaves were evaluated for their response of Pn and Is to changes in PPFD (Photosynthetic Photon Flux Density) at 0, 25, 50, 75, 100, 250, 500, 750, 1000, 1500, and 2000 µmol m−2 s−1 under constant leaf temperature (30 °C) in both a CO2-free atmosphere (0 µmol mol−1, 4 leaves) and ambient CO2 (450 µmol mol−1, 7 leaves). To achieve a CO2-free atmosphere inside the LI-6400XT leaf chamber, the CO2 mixer was turned off and the soda lime was put to fully scrub. Leaf temperature response curves (25→ 30→ 35→ 40 °C) were measured under constant PPFD (1000 µmol m−2 s−1), again in both CO2-free atmosphere (n = 3) and ambient CO2 (n = 4). Before each series of measurements, the leaves were acclimated for ~15 min in the chamber, until the stomatal conductance and photosynthesis were stable.
To quantify the ratio of Is between CO2-free atmosphere (0 µmol mol−1 of CO2, 8 leaves) and ambient CO2 concentrations (450 µmol mol−1 of CO2, 8 leaves), gas exchange was measured with leaves of I. edulis under standard conditions of light and leaf temperature (1000 µmol m−2 s−1 PPFD, 30 °C leaf temperature).
2.4. Isoprene Emission under Limiting Conditions for Net Photosynthesis
To evaluate Is under limiting conditions for Pn we measured gas exchange under two limiting situations: (a) using a specific photosynthesis inhibitor, DCMU (3-(3,4-dichlorophenyl)-1,1-dimethylurea) or (b) in the dark. DCMU blocks photosynthetic electron flow from photosystem II to the plastoquinone pool [36], inhibiting photosynthesis.
For the inhibitor experiment, a 180 µM DCMU solution, was prepared and adjusted to pH 7.5. A leaflet was cut from a leaf and placed in the DCMU solution for two hours, until the inhibitor took effect. This inhibition time was determined during previous experiments to be sufficient to decrease Pn to near 0 µmol m−2 s−1 in leaflets under standard conditions (i.e., with CO2, PPFD and leaf temperature at 450 µmol mol−1, 1000 µmol m−2 s−1, and 30 °C, respectively). Following uptake of the DMCU solution by the transpiration stream, a light response curve was measured under CO2-free atmosphere and constant leaf temperature (30 °C).
For the dark experiment, a temperature response curve was established in the absence of light. During the measurements the leaves were kept under CO2-free atmosphere and zero PPFD. The samples were collected in TD tubes, using the LI-6400XT/GC-MS methodology [25].
2.5. 13C-labeling of Leaf Isoprene Emissions Using Sodium Bicarbonate 13C Delivered through the Transpiration Stream
In order to observe possible reassimilation of internal CO2 by photosynthesis through isoprene labeling molecules, measurements of Is under CO2-free atmosphere were carried out on detached fully expanded I. edulis leaflets in a solution of 13C labeled sodium bicarbonate (NaH13CO3) (n = 5). Fresh solutions of sodium bicarbonate 13C (20, 60, 120 mM) were prepared with the pH adjusted to 7.0. For each concentration five leaflets were cut and recut in the sodium bicarbonate 13C solution, and leaf gas exchange measurements were initiated. In sequence, the measurements were made for two hours, under CO2-free atmosphere at constant PPFD (1000 µmol m−2 s−1) and temperature (30 °C) using the LI-6400XT/GC-MS methodology. Isoprene 13C-labeling is reported as the 13C/12C isotope ratio of [13C1]isoprene/[12C5]isoprene, [13C2]isoprene/[12C5]isoprene, [13C3]isoprene/[12C5]isoprene, [13C4]isoprene/[12C5]isoprene, and [13C5]isoprene/[12C5]isoprene by calculating the peak area ratios (7.1 min retention time) of m/z 69/68, 70/68, 71/68, 72/68, and 73/68, respectively.
3. Results
3.1. Isoprene Emissions under CO2-Free Atmosphere are Stimulated by Light but are Blocked When Photosynthesis is Inhibited
Under ambient CO2 and leaf temperature of 30 °C Is and Pn were stimulated together as PPFD increased (Figure 1a). However, while Pn saturated at roughly 1000 μmol m−2 s−1 PPFD, Is showed no sign of saturation up to the highest fluxes of PPFD applied (2000 μmol m−2 s−1) (Figure 1a). When Is was expressed in µmol C m−2 s−1 (IsC) and plotted against Pn (Figure 1b) the results show that during high PPFD intensities, Is keeps increasing, representing a higher fraction of Pn relative to lower PPFD intensities.
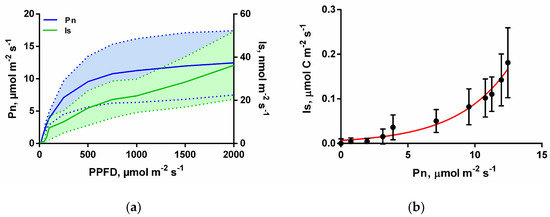
Figure 1.
(a) Leaf isoprene emissions (Is, nmol m−2 s−1) and net photosynthesis rates (Pn, µmol m−2 s−1) from I. edulis leaves as a function of photosynthetic photon flux density (PPFD) intensity under constant leaf temperature (30 °C) and ambient CO2 (450 µmol mol−1); the solid lines are the averages and the hatching area the standard deviation for n = 7. (b) Isoprene emissions expressed in µmol C m−2 s−1 (IsC) against Pn (µmol m−2 s−1) as PPFD intensity increases in the same conditions; means, and standard deviation for n = 7.
The light response curve in a CO2-free atmosphere shows that Pn remained negative, while light stimulated Is in a similar pattern to that observed under ambient conditions of CO2 (Figure 2a). However, under CO2-free atmosphere, average Is at maximum PPFD reached 59% of the observed under ambient CO2 conditions (21.3 nmol m−2s−1 at CO2-free atmosphere vs. 36 nmol m−2s−1 at ambient CO2, respectively). The fact that Pn remained negative while the leaf was in the chamber demonstrated that the enclosure CO2 concentrations were below that of the CO2 compensation point, and that the leaf was a net source of CO2 to the air, likely due in-part to the stimulation of photorespiration. When real-time Is data was collected, Is saturated at lower PPFD intensity (1500 µmol m−2 s−1 PPFD, using average values) under CO2-free atmosphere relative to ambient CO2 conditions (Figure 2a vs. Figure 1a), indicating a limitation of carbon for isoprene production.
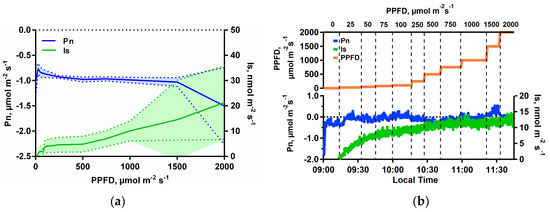
Figure 2.
(a) Leaf isoprene emissions (Is, nmol m−2 s−1, measured by gas chromatography-mass spectrometry (GC–MS)) and net photosynthesis rates (Pn, µmol m−2 s−1) from I. edulis leaves as a function of PPFD intensity under constant leaf temperature (30 °C) and CO2-free atmosphere (0 µmol mol−1). The solid lines are the averages and the hatching area the standard deviation (n = 4); (b) Example of leaf isoprene emissions (Is, nmol m−2 s−1) measured by high sensitivity quadrupole proton transfer reaction-mass spectrometry (PTR-MS) and net photosynthesis rates (Pn, µmol m−2 s−1) from an I. edulis leaf under constants leaf temperature (30 °C) and CO2-free atmosphere, as a function of time with PPFD increases at the marked intervals.
To extend the study of light responses of Is and Pn, a specific inhibitor of photosynthesis (DCMU—3-(3,4-dichlorophenyl)-1,1-dimethylurea) was provided to a I. edulis leaflet under CO2-free air. Although light-stimulated Is could be observed from the detached leaf placed in water as a control (up to 15 nmol m−2 s−1), Is were small to negligible and did not increase as a function of PPFD when DCMU was provided to the detached leaflet (Figure 3). As stomatal conductance (gs) in the DCMU fed leaflet was similar or higher than gs in the water control leaf, this effect could not be attributed to a potential stomatal closure induced by DCMU application.
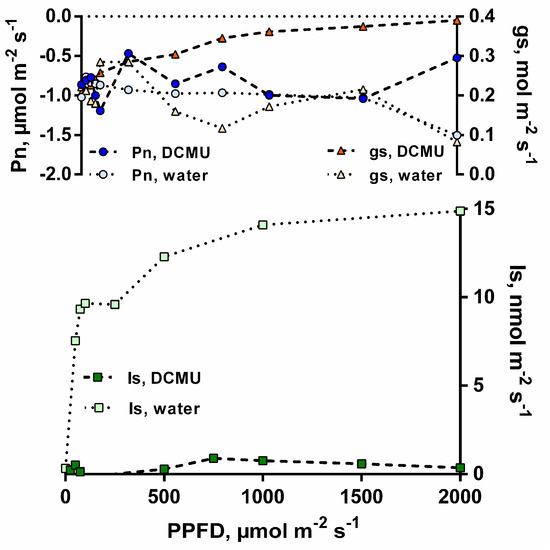
Figure 3.
Leaf isoprene emissions (Is, nmol m−2 s−1), net photosynthesis rates (Pn, µmol m−2 s−1), and stomatal conductance (gs, mol m−2 s−1) from an I. edulis leaflet under constant leaf temperature (30 °C) as a function of PPFD intensity under CO2-free atmosphere with and without the addition of the photosynthesis inhibitor DCMU in the transpiration stream (n = 1).
3.2. Isoprene Emissions under CO2-Free Atmosphere are Strongly Stimulate by Leaf Temperature Increase but are Eliminated in the Dark
Under temperature response curves with constant light and CO2 conditions (1000 μmol m−2 s−1 and 450 μmol mol−1) Is was strongly stimulated as leaf temperature increased and did not show a clear observable saturation point while reaching an average of 76 nmol m−2 s−1 at the highest leaf temperature (40 °C, Figure 4a). When IsC was plotted against Pn there was a negative relationship between these two parameters (Figure 4b), which represents the uncoupling between the Pn and Is as leaf temperature increases. This is driven by both the decrease in Pn and the increase in Is with increasing leaf temperature.
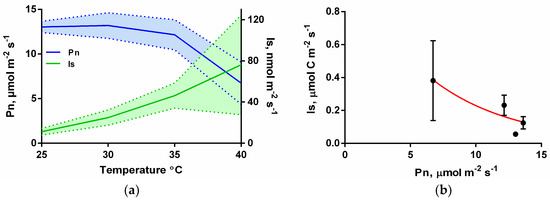
Figure 4.
(a) Leaf isoprene emissions (Is, nmol m−2 s−1) and net photosynthesis rates (Pn, µmol m−2 s−1) from I. edulis leaves as a function of temperature increases under constant PPFD intensity (1000 μmol m−2 s−1) and ambient CO2 (450 µmol mol−1); the solid lines are the averages and the hatching area the standard deviation (n = 4). (b) Isoprene emissions expressed in µmol C m−2 s−1 (IsC) against Pn (µmol m−2 s−1) as temperature intensity increases in the same conditions; means and standard deviation with n = 4.
When the same measurements were made under a CO2-free atmosphere, Is continued to increase with leaf temperature up to 14–20 nmol m−2 s−1 at 40 °C while Pn remained near-zero or slightly negative (e.g., −1.0 μmol m−2 s−1) (Figure 5).
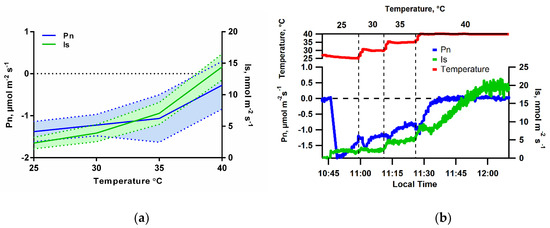
Figure 5.
(a) Leaf isoprene emissions (Is, nmol m−2 s−1) and net photosynthesis rates (Pn, µmol m−2 s−1) from I. edulis leaves as a function of temperature under constant PPFD (1000 μmol m−2 s−1) and CO2-free atmosphere (0 µmol mol−1); the solid lines are the averages and the hatching area the standard deviation (n = 3). (b) Example of leaf isoprene emissions (Is, nmol m−2 s−1) measured by PTR-MS and net photosynthesis rates (Pn, µmol m−2 s−1) from an I. edulis leaf under constants PPFD intensity (1000 μmol m−2 s−1) and CO2-free atmosphere, as a function of time with temperature increases at the marked intervals.
In order to further evaluate the dependence on Is and Pn, a measurement of these variables was made as a function of leaf temperature, but in the dark to eliminate photosynthesis. When a temperature response curve was performed in the dark under a CO2-free atmosphere, Is was not detectable, even at the highest temperatures studied (40 °C) (Figure 6). In contrast, in the light, increases in leaf temperature stimulated Is, which continued to increase up to the highest leaf temperature studied (40 °C).
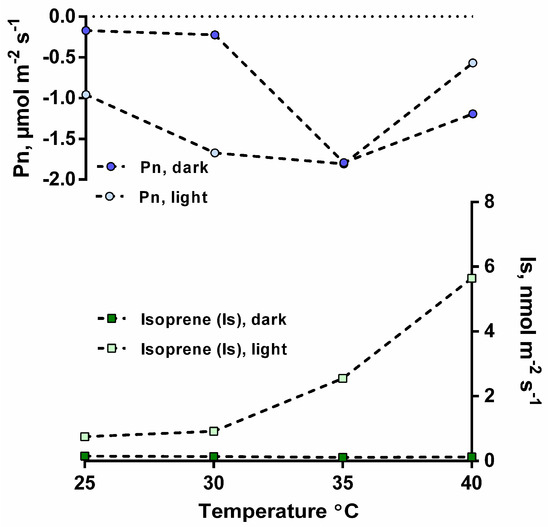
Figure 6.
Leaf isoprene emissions (Is, nmol m−2 s−1) and net photosynthesis rates (Pn, µmol m−2 s−1) from an I. edulis leaf as a function of temperature under CO2-free atmosphere, in the presence and absence of light (PPFD intensity 1000 μmol m−2 s−1) (n = 1).
As it was determined that Is are stimulated by increases in light and leaf temperature under CO2-free air, we calculated Is under a CO2-free atmosphere relative to ambient CO2 under standard conditions of light and temperature (1000 μmol m−2 s−1 PPFD, 30 °C, n = 8). When Is emissions under a CO2-free atmosphere were quantified from eight I. edulis leaves, it was determined that those emissions amounted to 19% ± 7% of emissions under ambient CO2.
3.3. Sodium Bicarbonate 13C Leaf Feeding
In order to verify the contribution of CO2 reassimilation from the decarboxylation process in isoprene molecules, sodium 13C-bicarbonate solutions were provided to I. edulis leaflets under CO2-free atmosphere with PPFD and temperature constant (1000 μmol m−2 s−1, 30 °C). Within minutes of placing the leaflet in the solution, emissions of 13C-labeled isoprene could be observed (Figure 7).
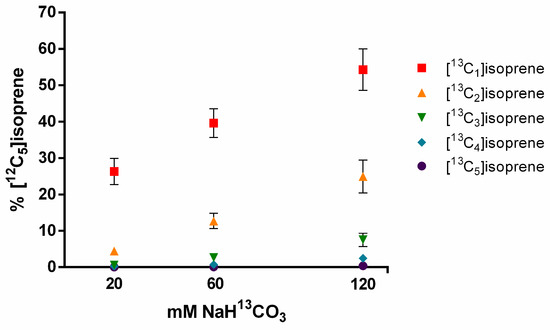
Figure 7.
Percentage of 13C-labeled isoprene emissions (13C1-5) relative to unlabeled isoprene emissions (12C5) from detached I. edulis leaves fed with three concentrations of NaH13CO3 solutions (20, 60, and 120 mM) through the transpiration stream; means and standard deviation for n = 5.
When leaflets were placed in the 20 mM solution of sodium bicarbonate 13C they emitted Is with one labeled carbon, representing 26% of the emissions of unlabeled isoprene molecules. Emissions of completely labeled molecules [13C5]isoprene were nearly undetectable. With the increase of the molarity (20–120 mM), the percentage of Is with 13C-labeling also increased relative to the unlabeled Is, as did the number of carbon atoms 13C-labeled per molecule. In the solution with 120 mM of sodium bicarbonate 13C, the percentage of molecules with one 13C atom reached 54%, with two labeled carbons 25%, with three labeled carbons 8%, with four labeled carbons 2%, and with all labeled carbons reaching up 0.35% (relative to unlabeled Is).
4. Discussion
4.1. Under CO2-Free Air, Isoprene Emissions Display a Similar Light and Temperature Response Pattern to That under Ambient Conditions, But Is Eliminated when Photosynthesis Is Inhibited
The high correlation between Is and Pn as a function of light (Figure 1) is widely discussed in literature, since isoprene production via the MEP pathway utilizes, as the first precursor, a product of the Calvin–Benson cycle, glyceraldehydes-3-phosphate (G3P), which is responsible for three of the five carbons in the isoprene skeleton and requires 14 mol of NADPH and 20 mol of ATP for each mole of isoprene synthesized [37,38,39,40,41,42] (Figure 8). Thus, it is expected that the relationship between Pn and Is be close under ambient CO2 conditions (Figure 1b).
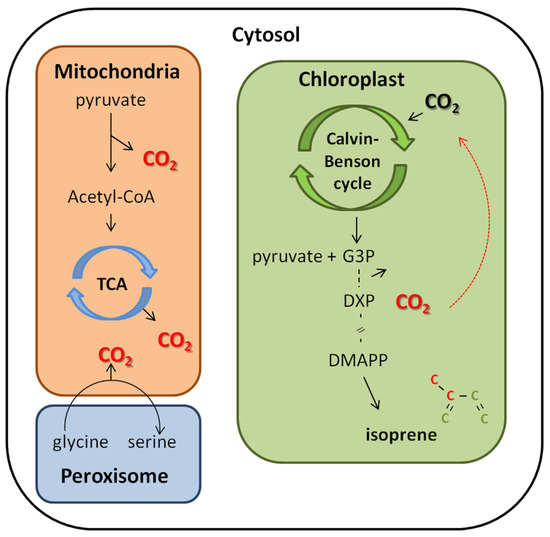
Figure 8.
Simplified scheme of the main decarboxylation processes that may contribute as alternative sources for isoprene synthesis: respiration, photorespiration, and the first reaction of the DOXP/MEP pathway.
The production of isoprene from the tropical species I. edulis under a CO2-free atmosphere in this study is consistent with previous research on several temperate plant species including myrtle (Myrtus communis), buckthorn (Rhamnus alaternus), velvet bean (Mucuna pruriens), gray poplar (Populus x canescens), aspen (Populus tremuloides), and tasmanian bluegum (Eucalyptus globulus) [16,27,32,43]. We demonstrate for the first time that Is from I. edulis under CO2-free air are stimulated by light and temperature in a similar fashion to emissions under ambient CO2, albeit with important differences (Figure 2b and Figure 5b). Relative to emissions under ambient CO2, Is under a CO2-free atmosphere were reduced and saturated at low light levels (e.g., 100 μmol m−2 s−1 PPFD) versus no clear light saturation under ambient CO2 up to 2000 μmol m−2 s−1 PPFD. Other studies that observed Is under limited or zero CO2 have suggested that alternative carbon sources, that do not come directly from photosynthesis, such as stored starch and carbohydrates transported in the xylem stream help to maintain the carbon supply for isoprene when a reduced atmospheric CO2 source is limiting G3P formation, while NADPH and ATP are still producing by the photochemical phase of photosynthesis [26]. In this study, we provide quantitative evidence that most carbon alternatives for isoprene production are derived from the reassimilation of internal CO2 sources.
Previous studies have sought to understand how these alternative carbon sources can maintain high rates of Is when Pn is reduced under stress [25,34]. However, the decrease in Pn does not necessarily mean that gross photosynthesis rates are decreasing, but rather that decarboxylation processes are being stimulated (especially photorespiration), increasing the CO2 released directly into the cell. At increasing temperatures the oxygenating reaction of RuBisCO has a relative advantage over the carboxylation reactions because (1) stomatal closure reduces the uptake of atmospheric CO2, (2) the solubility of CO2 declines stronger than the solubility of O2, and (3) the kinetic properties of RuBisCO decrease the affinity for CO2 more strongly than that for O2, causing a proportional increase in photorespiration [44].
Indeed, the Is is stimulated by elevated temperatures (Figure 4), and this dependence is a consequence of high temperature optimum for isoprene synthase and the use of excess energy not used in the Calvin–Benson cycle by the enzymatic reactions of the MEP pathway [45,46,47].
However, when leaves were fed with the photosynthesis inhibitor DCMU under a light response curve it was not possible to observe Is anymore (Figure 3). The same result occurred when the light was turned off during a temperature response curve (Figure 6). This confirms that Is is dependent on the photochemical products of photosynthesis (NADPH and ATP). Once these energetic cofactors are no longer produced, it is not possible to maintain the isoprene synthesis by the MEP pathway demonstrating that the Is is dependent on these energetic cofactors products but can be maintain without the external carbon supply during several hours.
4.2. CO2 Reassimilation from Decarboxylation Process as an Alternative Carbon Source to Isoprene Synthesis
With respect to the molecular composition, 60% of carbon that comprises the isoprene molecule is assigned to G3P, as one of initial precursors of the MEP pathway [15,48]. When the external CO2 source was removed from the reference air stream, 19% ± 7% of Is continued relative to Is observed under ambient CO2. This percentage is in the range observed by studies that analyzed the contribution of alternative carbon sources to isoprene synthesis by providing atmospheres of 13CO2 to leaves. In these studies, it was observed that ~10%–30% of the carbon atoms were not from atmospheric CO2, but instead originated from other sources [23,26,27]. Although the possibility of reassimilated CO2 as a source of carbon for isoprene has been discussed in the literature, our results support a possibility that it represents the main alternative carbon source for isoprene and is largely responsible for maintaining high Is rates under abiotic stress conditions like high temperatures, which decrease Pn.
A study carried out in leaves of Populus sp. under 0 μmol mol−1 CO2 observed that Is rates were little affected relative to the measures carried out under ambient CO2 [32]. However, the authors observed that, when photorespiration was inhibited by the absence of O2, it was no longer possible to observe Is. In our study, we observed that the Is rates are high under photorespiratory conditions by stimulating the photorespiratory process by removing the CO2 from the reference air.
In agreement with these results, a previous study provided a [2−13C]glycine (a photorespiratory intermediate) solution to I. edulis leaves under high temperature and observed Is with labeled carbon atoms [25]. The authors suggested that, in situations where photorespiration is stimulated, reassimilation of photorespiratory CO2 by photosynthesis acts as a protective mechanism to avoid oxidative stress by consuming excess ATP and NADHP produced by the light reactions. Likewise, our results suggest that increasing the carbon flux through the MEP pathway, which also consumes excess photosynthetic ATP and NADPH, further increases plant tolerance to abiotic stress including high temperatures and drought. Thus, the coupled activity of enhanced photorespiration and the MEP pathway together with reassimilation of internal CO2 sources may offer plants protection against abiotic stress by helping balance the sources and sinks of photosynthetic ATP and NADPH [44] (Figure 8).
When sodium bicarbonate (NaHCO3) was provided to I. edulis leaves under CO2-free air, the bicarbonate could be decarboxylated by carbonic anhydrase enzyme. The CO2 released by the decarboxylation could be fixed by the Calvin–Benson cycle, constituting a G3P molecule, being able to enter into the DOXP/MEP pathway and compose isoprene molecules as observed (Figure 7).
Under conditions of limiting CO2, a potential alternative source to isoprene synthesis is the CO2 released into the cell by the decarboxylation process. A previous study estimated that the refixation rate of mitochondrial respiration was related to the incomplete carbon labeling of Is, suggesting that respiratory CO2 can contribute to isoprene formation [24]. Another study in Populus deltoides Barr. leaves measured Is under N2 and 13CO2 atmosphere and observed that the inhibition of photorespiration did not remove the 12C in isoprene released from leaves under a 13CO2 atmosphere and suggested that the unlabeled carbon in isoprene molecules is not derived from photorespiratory carbon [23]. On the other hand, in I. edulis under [2−13C]glycine, feeding a strong labeling of isoprene molecules was observed, suggesting that the CO2 released from photorespiration can be incorporated into isoprene molecules [25]. In addition to (photo)respiration, other decarboxylation processes could be acting as alternative carbon sources to isoprene synthesis, including biosynthetic pathways such as the DOXP/MEP, fatty acid, mevalonate, fermentation, and the oxidative pentose phosphate pathway [48,49,50].
The incorporation of labeled CO2 released by bicarbonate decarboxylation into isoprene molecules was observed within minutes after placing the leaf in a 13C sodium bicarbonate solution (Figure 7). This confirms the contribution of CO2 reassimilation to isoprene synthesis and suggests that other decarboxylation processes can contribute to isoprene synthesis as a primary carbon source under reduced atmospheric CO2 availability. These results have relevance for the next decades of climate change predictions [51], since higher temperatures increase respiration and photorespiration and consequently the amount of CO2 released in to the leaf. This CO2 can contribute to Is in stress situations that could decrease the stomatal conductance and the carbon uptake.
4.3. The Reassimilation of CO2 in the Cell Interior Linked to Isoprene Emission: Its Importance for Plant Physiological Functioning under Changing Environmental Factors
In our experiment, high temperatures and light intensities under a CO2-free atmosphere caused a remarkable depression of Pn and substantial increase in Is, suggesting that other sources than recently assimilated carbon are used for isoprene formation. Subsequently, when labeled sodium bicarbonate was provided, the emissions of labeled isoprene indicate that the reassimilation of CO2 released in the chloroplast can contribute as a carbon source to isoprene synthesis. This is in line with evidence from Loreto et al. [24] who showed refixation of unlabeled CO2 derived from respiration is an alternative source of carbon for isoprene in nonstressed leaves.
Under normal conditions, the reassimilation of CO2 in the chloroplast can reach up to 40% [52] and increase to more than 80% under low CO2 conditions and high temperature [53]. The amount of photorespiratory or respiratory CO2 emitted or recycled by leaves have been associated with an improvement of the photosynthetic carbon gain [52,53]. Therefore, the reassimilation of CO2 in the chloroplast can contribute significantly for photosynthetic carboxylation efficiency by minimizing the loss of photosynthetic carbon caused by stimulus of photorespiration and respiration.
Previous report have pointed out that the stimulus of internal recycling of photorespired and respired CO2 in plants may become more important at conditions with high temperatures, low CO2, water stress, or high irradiance [54,55,56,57]. However, in some cases, the respiratory rate is dramatically inhibited by water stress or extreme temperature conditions, while the photorespiration rate increases significantly [58]. Efficient internal recycling of photorespired CO2 linked to isoprene synthesis might improve tolerance against high irradiance and temperature, commonly experienced in the tropics, besides increasing the overall carbon budget of plants. Nevertheless, the contribution of each of these decarboxylation processes to the CO2 balance is highly variable, depending on the species, stage of growth, biotic and abiotic factors [48,50,51,52]. In addition, there is little evidence for widespread uses of an effective mechanism to trap and reassimilate photorespired CO2 in tropical species, and particularly with relation with Is. Thus, subsequent research is needed to clarify the overall significance of trapping of CO2 in the cell interior and its flux to isoprene production, particularly in tropical forests, where the bulk of the world’s Is occur, and both temperature and drought risk are expected to increase according to most climate scenarios [59,60,61].
5. Conclusions
Previous studies have shown that plants can use nonatmospheric carbon sources to produce isoprene. Most studies have focused on stored carbon sources including carbohydrates (starch and glucose) and their metabolites (pyruvate and phosphoenolpyruvate) as potentially important alternative carbon sources and few studies are made with tropical species. In this study, we show that the CO2 released by decarboxylation processes is an important carbon source for I. edulis—a widespread tropical specie in Amazon forest. Once decarboxylation processes release CO2 within the leaf mesophyll, our observations suggest that this process acts as a quantitatively important carbon source for isoprene syntheses mainly during stress situations when the stomata are closed and the amount of internal CO2 decreases. This study enhances our knowledge of isoprene emission by tropical species under conditions that plants often experience in the tropics, where the amount of irradiance and temperature, the two main environmental drivers of isoprene fluxes, are high throughout the year. The reassimilation of released CO2 within the leaf and its use for isoprene synthesis may be a key tolerance mechanism against a specific type of stress, for example high irradiance and high temperature in tropical environments.
Author Contributions
Conceptualization: S.G., K.J. and V.F.d.S.; Methodology: K.J., R.A.F.d.S., S.D.J., and J.F.d.C.G. Writing and Editing: S.G., K.J. and V.F.d.S., and J.F.d.C.G.
Funding
This research was funded by the Office of Biological and Environmental Research of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231 as part of their Terrestrial Ecosystem Science Program, Coordination for the Improvement of Higher Level Personnel (CAPES, Brazil), Amazonas State Research Support Foundation (FAPEAM, Brazil, 024/2014), and by the National Council for Scientific and Technological Development (CNPq, Brazil) for their financial support of this study.
Acknowledgments
The authors are grateful to the National Institute for Amazonian Research (MCTIC-INPA)/ Laboratory of Plant Physiology and Biochemistry. Many thanks to the Large-Scale Biosphere-Atmosphere Experiment in Amazonia (LBA) and Green Ocean Amazon 2014/2015 project for logistical support. J.F.C. Gonçalves is a CNPq researcher.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rasmussem, R.A. Survey of plant species that release isoprene to atmosphere. In Abstracts of Papers of The American Chemical Society; American Chemical Society: Washington, DC, USA, 1972. [Google Scholar]
- Lerdau, M.T.; Harley, P.C.; Monson, R.K. Ecological and evolutionary aspects of isoprene emission from plants. Oecologia 1999, 118, 109–123. [Google Scholar]
- Guenther, A. The contribution of reactive carbon emissions from vegetation to the carbon balance of terrestrial ecosystems. Chemosphere 2002, 49, 837–844. [Google Scholar] [CrossRef]
- Guenther, A.; Karl, T.; Harley, P.; Wiedinmyer, C.; Palmer, P.I.; Geron, C. Estimates of global terrestrial isoprene emissions using MEGAN (Model of Emissions of Gases and Aerosols from Nature). Atmos. Chem. Phys. Discuss. 2006, 6, 107–173. [Google Scholar] [CrossRef]
- Claeys, M.; Graham, B.; Vas, G.; Wang, W.; Vermeylen, R.; Pashynska, V.; Cafmeyer, J.; Guyon, P.; Andreae, M.O.; Artaxo, P.; et al. Formation of Secondary Organic Aerosols Through Photooxidation of Isoprene. Science 2004, 303, 1173–1176. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R.; Arey, J. Atmospheric Degradation of Volatile Organic Compounds. Chem. Rev. 2003, 103, 4605–4638. [Google Scholar] [CrossRef]
- Kroll, J.H.; Ng, N.L.; Murphy, S.M.; Flagan, R.C.; Seinfeld, J.H. Secondary Organic Aerosol Formation from Isoprene Photooxidation. Environ. Sci. Technol. 2006, 40, 1869–1877. [Google Scholar] [CrossRef]
- Unger, N. Global Climate Forcing by Criteria Air Pollutants. Annu. Resour. 2012, 37, 1–24. [Google Scholar] [CrossRef]
- Monson, R.K.; Jones, R.T.; Rosenstiel, T.N.; Schnitzler, J.P. Why only some plants emit isoprene. Plant. Cell Environ. 2013, 36, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, T.D. Is it useful to ask why plants emit isoprene? Plant Cell Environ. 2013, 36, 517–520. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Monson, R.K. Isoprene research—60 years later, the biology is still enigmatic. Plant Cell Environ. 2017, 40, 1671–1678. [Google Scholar] [CrossRef]
- Velikova, V.; Loreto, F. On the relationship between isoprene emission and thermotolerance in Phragmites australis leaves exposed to high temperatures and during the recovery from a heat stress. Plant Cell Environ. 2005, 28, 318–327. [Google Scholar] [CrossRef]
- Velikova, V.; Várkonyi, Z.; Szabo, M.; Maslenkova, L.; Nogues, I.; Kovács, L.; Peeva, V.; Busheva, M.; Garab, G.; Sharkey, T.D.; et al. Increased Thermostability of Thylakoid Membranes in Isoprene-Emitting Leaves Probed with Three Biophysical Techniques. Plant Physiol. 2011, 157, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Harvey, C.M.; Li, Z.; Tjellström, H.; Blanchard, G.J.; Sharkey, T.D. Concentration of isoprene in artificial and thylakoid membranes. J. Bioenerg. Biomembr. 2015, 47, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. The 1-Deoxy-D-Xylulose-5-Phosphate Pathway of Isoprenoid Biosynthesis in Plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 47–65. [Google Scholar] [CrossRef] [PubMed]
- Affek, H.P.; Yakir, D. Natural Abundance Carbon Isotope Composition of Isoprene Reflects Incomplete Coupling between Isoprene Synthesis and Photosynthetic Carbon Flow. Plant Physiol. 2003, 131, 1727–1736. [Google Scholar] [CrossRef] [PubMed]
- Funk, J.L.; Mak, J.E.; Lerdau, M. Stress-induced changes in carbon sources for isoprene production in Populus deltoides. Plant Cell Environ. 2004, 27, 747–755. [Google Scholar] [CrossRef]
- Brilli, F.; Barta, C.; Fortunati, A.; Lerdau, M.; Loreto, F.; Centritto, M. Response of isoprene emission and carbon metabolism to drought in white poplar (Populus alba) saplings. New Phytol. 2007, 175, 244–254. [Google Scholar] [CrossRef]
- Silver, G.M.; Fall, R. Characterization of Aspen Isoprene Synthase, an Enzyme Responsible for Leaf Isoprene Emission to the Atmosphere. J. Boil. Chem. 1995, 270, 13010–13016. [Google Scholar] [CrossRef]
- Schnitzler, J.-P.; Arenz, R.; Steinbrecher, R.; Lehning, A. Characterization of an Isoprene Synthase from Leaves of Quercus petraea (Mattuschka) Liebl. Bot. Acta 1996, 109, 216–221. [Google Scholar] [CrossRef]
- Delwiche, C.F.; Sharkey, T.D. Rapid appearance of 13C in biogenic isoprene when 13CO2 is fed to intact leaves. Plant Cell Environ. 1993, 16, 587–591. [Google Scholar] [CrossRef]
- Affek, H.P.; Yakir, D. Protection by Isoprene against Singlet Oxygen in Leaves. Plant Physiol. 2002, 129, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Karl, T.; Rosenstiel, T.; Prazeller, P.; Seufert, G.; Lindinger, W.; Fall, R.; Larsen, B. On-line analysis of the 13CO2 labeling of leaf isoprene suggests multiple subcellular origins of isoprene precursors. Planta 2002, 215, 894–905. [Google Scholar]
- Loreto, F.; Pinelli, P.; Brancaleoni, E.; Ciccioli, P. 13C Labeling Reveals Chloroplastic and Extrachloroplastic Pools of Dimethylallyl Pyrophosphate and Their Contribution to Isoprene Formation1. Plant Physiol. 2004, 135, 1903–1907. [Google Scholar] [CrossRef] [PubMed]
- Jardine, K.; Chambers, J.; Alves, E.G.; Teixeira, A.; Garcia, S.; Holm, J.A.; Higuchi, N.; Manzi, A.; Abrell, L.; Fuentes, J.D.; et al. Dynamic Balancing of Isoprene Carbon Sources Reflects Photosynthetic and Photorespiratory Responses to Temperature Stress. Plant Physiol. 2014, 166, 2051–2064. [Google Scholar] [CrossRef]
- Schnitzler, J.-P.; Graus, M.; Kreuzwieser, J.; Heizmann, U.; Rennenberg, H.; Wisthaler, A.; Hansel, A.; Schnitzler, J.-P. Contribution of Different Carbon Sources to Isoprene Biosynthesis in Poplar Leaves. Plant Physiol. 2004, 135, 152–160. [Google Scholar] [CrossRef]
- Ghirardo, A.; Gutknecht, J.; Zimmer, I.; Brüggemann, N.; Schnitzler, J.-P.; Schnitzler, J.-P. Biogenic Volatile Organic Compound and Respiratory CO2 Emissions after 13C-Labeling: Online Tracing of C Translocation Dynamics in Poplar Plants. PLoS ONE 2011, 6, e17393. [Google Scholar] [CrossRef]
- E Vickers, C.; Gershenzon, J.; Lerdau, M.T.; Loreto, F.; Vickers, C. A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nat. Methods 2009, 5, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Loreto, F.; Schnitzler, J.P. Abiotic stresses and induced BVOCs. Trends Plant. Sci. 2010, 15, 154–166. [Google Scholar] [CrossRef]
- Niinemets, U.; Arneth, A.; Kühn, U.; Monson, R.K.; Penuelas, J.; Staudt, M. The emission factor of volatile isoprenoids: stress, acclimation, and developmental responses. Biogeosci. Discuss. 2010, 7, 1529–1574. [Google Scholar] [CrossRef]
- Kreuzwieser, J.; Graus, M.; Wisthaler, A.; Hansel, A.; Rennenberg, H.; Schnitzler, J.-P. Xylem-transported glucose as an additional carbon source for leaf isoprene formation in Quercus robur. New Phytol. 2002, 156, 171–178. [Google Scholar] [CrossRef]
- Monson, R.K.; Fall, R. Isoprene Emission from Aspen Leaves: Influence of Environment and Relation to Photosynthesis and Photorespiration. Plant Physiol. 1989, 90, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Jardine, K.J.; Sommer, E.D.; Saleska, S.R.; Huxman, T.E.; Harley, P.C.; Abrell, L. Gas Phase Measurements of Pyruvic Acid and Its Volatile Metabolites. Environ. Sci. Technol. 2010, 44, 2454–2460. [Google Scholar] [CrossRef]
- Trowbridge, A.M.; Asensio, D.; Eller, A.S.D.; Way, D.A.; Wilkinson, M.J.; Schnitzler, J.-P.; Jackson, R.B.; Monson, R.K.; Schnitzler, J.-P. Contribution of Various Carbon Sources Toward Isoprene Biosynthesis in Poplar Leaves Mediated by Altered Atmospheric CO2 Concentrations. PLoS ONE 2012, 7, e32387. [Google Scholar] [CrossRef] [PubMed]
- Gerbaud, A.; Andre, M. An Evaluation of the Recycling in Measurements of Photorespiration. Plant Physiol. 1987, 83, 933–937. [Google Scholar] [CrossRef] [PubMed]
- Salem, K.; Van Waasbergen, L.G. Photosynthetic Electron Transport Controls Expression of the High Light Inducible Gene in the Cyanobacterium Synechococcus elongatus Strain PCC 7942. Plant Cell Physiol. 2004, 45, 651–658. [Google Scholar] [CrossRef]
- Tingey, D.T.; Manning, M.; Grothaus, L.C.; Burns, W.F. The Influence of Light and Temperature on Isoprene Emission Rates from Live Oak. Physiol. Plant. 1979, 47, 112–118. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Loreto, F. Water stress, temperature, and light effects on the capacity for isoprene emission and photosynthesis of kudzu leaves. Oecologia 1993, 95, 328–333. [Google Scholar] [CrossRef]
- Sun, Z.; Niinemets, Ü.; Hüve, K.; Noe, S.; Rasulov, B.; Copolovici, L.; Vislap, V. Enhanced isoprene emission capacity and altered light responsiveness in aspen grown under elevated atmospheric CO2 concentration. Chang. Boil. 2012, 18, 3423–3440. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Sun, Z. How light, temperature, and measurement and growth [CO2] interactively control isoprene emission in hybrid aspen. J. Exp. Bot. 2014, 66, 841–851. [Google Scholar]
- Sharkey, T.; Chen, X.; Yeh, S. Isoprene Increases Thermotolerance of Fosmidomycin-Fed Leaves. Plant Physiol. 2001, 125, 2001–2006. [Google Scholar] [CrossRef]
- De Souza, V.F.; Niinemets, Ü.; Rasulov, B.; Vickers, C.E.; Júnior, S.D.; Araújo, W.L.; Gonçalves, J.F.D.C. Alternative Carbon Sources for Isoprene Emission. Trends Plant Sci. 2018, 23, 1081–1101. [Google Scholar] [CrossRef]
- Loreto, F. Emission of Isoprene from Salt-Stressed Eucalyptus globulus Leaves. Plant Physiol. 2000, 123, 1605–1610. [Google Scholar] [CrossRef] [PubMed]
- Voss, I.; Sunil, B.; Scheibe, R.; Raghavendra, A.S. Emerging concept for the role of photorespiration as an important part of abiotic stress response. Plant Boil. 2013, 15, 713–722. [Google Scholar] [CrossRef]
- Monson, R.K.; Jaeger, C.H.; Adams, W.W.; Driggers, E.M.; Silver, G.M.; Fall, R. Relationships among Isoprene Emission Rate, Photosynthesis, and Isoprene Synthase Activity as Influenced by Temperature. Plant Physiol. 1992, 98, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Rasulov, B.; Hüve, K.; Bichele, I.; Laisk, A.; Niinemets, Ü. Temperature Response of Isoprene Emission in Vivo Reflects a Combined Effect of Substrate Limitations and Isoprene Synthase Activity: A Kinetic Analysis. Plant Physiol. 2010, 154, 1558–1570. [Google Scholar] [CrossRef] [PubMed]
- Jardine, K.J.; Jardine, A.B.; Souza, V.F.; Carneiro, V.; Ceron, J.V.; Gimenez, B.O.; Soares, C.P.; Durgante, F.M.; Higuchi, N.; Manzi, A.O.; et al. Methanol and isoprene emissions from the fast growing tropical pioneer species Vismia guianensis (Aubl.) Pers. (Hypericaceae) in the central Amazon forest. Atmos. Chem. Phys. Discuss. 2016, 16, 6441–6452. [Google Scholar] [CrossRef]
- Vranová, E.; Coman, D.; Gruissem, W. Network Analysis of the MVA and MEP Pathways for Isoprenoid Synthesis. Annu. Rev. Plant Biol. 2013, 64, 665–700. [Google Scholar] [CrossRef]
- Kruger, N.J.; Von Schaewen, A. The oxidative pentose phosphate pathway: structure and organisation. Plant Boil. 2003, 6, 236–246. [Google Scholar] [CrossRef]
- Sweetlove, L.J.; Williams, T.C.R.; Cheung, C.Y.M.; Ratcliffe, R.G. Modelling metabolic CO2 evolution—A fresh perspective on respiration. Plant Cell Environ. 2013, 36, 1631–1640. [Google Scholar] [CrossRef]
- Lean, J.L.; Rind, D.H. How will Earth’s surface temperature change in future decades? Geophys. Res. Lett. 2009, 36, 15. [Google Scholar] [CrossRef]
- Busch, F.A.; Sage, T.L.; Cousins, A.B.; Sage, R.F. C3 plants enhance rates of photosynthesis by reassimilating photorespired and respired CO2. Plant Cell Environ. 2013, 36, 200–212. [Google Scholar] [CrossRef]
- Delfine, S.; Di Marco, G.; Loreto, F. Estimation of photorespiratory carbon dioxide recycling during photosynthesis. Aust. J. Plant Physiol. 1999, 26, 733. [Google Scholar] [CrossRef]
- Sage, T.L.; Sage, R.F. The Functional Anatomy of Rice Leaves: Implications for Refixation of Photorespiratory CO2 and Efforts to Engineer C4 Photosynthesis into Rice. Plant Cell Physiol. 2009, 50, 756–772. [Google Scholar] [CrossRef] [PubMed]
- Bloemen, J.; McGuire, M.A.; Aubrey, D.P.; O Teskey, R.; Steppe, K. Internal recycling of respired CO2 may be important for plant functioning under changing climate regimes. Plant Signal. Behav. 2013, 8, 197–555. [Google Scholar] [CrossRef] [PubMed]
- Haupt-herting, S.; Klug, K.; Fock, H.P. A New Approach to Measure Gross CO2 Fluxes in Leaves. Gross CO2 Assimilation, Photorespiration, and Mitochondrial Respiration in the Light in Tomato under Drought Stress. Plant. Physiol. 2001, 126, 388–396. [Google Scholar] [CrossRef]
- Pärnik, T.; Ivanova, H.; Keerberg, O. Photorespiratory and respiratory decarboxylations in leaves of C3plants under different CO2concentrations and irradiances. Plant Cell Environ. 2007, 30, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Wingler, A.; Lea, P.J.; Quick, W.P.; Leegood, R.C. Photorespiration: metabolic pathways and their role in stress protection. Philos. Trans. Soc. B Boil. Sci. 2000, 355, 1517–1529. [Google Scholar] [CrossRef]
- Zhou, X.; Fu, Y.; Li, B.; Luo, Y. An imperative need for global change research in tropical forests. Tree Physiol. 2013, 33, 903–912. [Google Scholar] [CrossRef]
- Cavaleri, M.A.; Reed, S.C.; Smith, W.K.; Wood, T.E. Urgent need for warming experiments in tropical forests. Chang. Boil. 2015, 21, 2111–2121. [Google Scholar] [CrossRef]
- Leitold, V.; Morton, D.C.; Longo, M.; Dos-Santos, M.N.; Keller, M.; Scaranello, M. El Niño drought increased canopy turnover in Amazon forests. New Phytol. 2018, 219, 959–971. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).