Abstract
Research Highlights: Direct comparison of leaf litter decomposition rates between harsh soil conditions of degraded lands and adjacent “closer to natural” forest areas has not been done before. Background and Objectives: We aimed to fill this knowledge gap by determining the differences in amounts of carbon and nitrogen released by species-specific litter depending on decomposition rates in various stand and habitat conditions, which enables selection of the most ecologically and economically appropriate (for fast soil organic layer development) tree species for afforestation of reclaimed lands. Materials and Methods: The study was conducted on the external spoil heap of the “Bełchatów” lignite mine (Central Poland) and adjacent forests. In December 2013, we established a litterbag experiment beneath the canopies of birch and pine stands. We used litter of Alnus glutinosa (Gaertn.), Betula pendula (Roth), Pinus sylvestris (L.), and Quercus robur (L.) collected ex situ, which we installed (after oven-drying) beneath the canopies of eight stands. The experiment lasted for three years (with sampling of three-month intervals). Results: Harsh soil conditions of degraded lands are unfavorable for litter mineralization. It was found that 23%–74% of decomposed materials were mineralized in spoil heap stands, whereas in forest stands these amounts ranged from 35%–83%. Litter of Q. robur in birch stands on the spoil heap is predicted to take 12 years longer for total decomposition than in forest stands of the same species. This hinders organic carbon turnover and could result in elongation of the time for full biological and economic reclamation of degraded lands. On the other hand, decomposition of relatively fast decomposable litter (A. glutinosa and B. pendula) in pine stands on the spoil heap was faster than in pine stands in forest sites (17% and 13% faster, respectively). We did not observe this trend for decomposition of more recalcitrant litter types of P. sylvestris and Q. robur. Conclusions: The results show the value of selective choice of tree species for afforestation of post-mining areas to accelerate the development of technogenic soil substrates. We recommend introducing all tree species studied in the cluster form of admixtures as all of them could bring some profits in ecological and economical reclamation.
1. Introduction
Novel terrestrial ecosystems are often influenced by harsh initial soil conditions which may result in uncertainty about their sustainability or at least hamper their successional development [1,2]. Their appearance nowadays is very frequent due to high human impacts on the environment [3]. In many countries, degraded lands resulting from industrial activity in a broad sense are subject to re-naturalization after exploitation stops, which results in improvement of their visual aesthetics [4] as well as their biodiversity (e.g., [5]). However, restoration of biological function is not the only reason to restore degraded areas. Numerous reclamation actions are undertaken to shorten the time for crossing a threshold to self-sustenance of autogenic succession or/and habitat productivity for economic reasons [6]. Scientists and practitioners agree that soil substrate development plays a crucial role in attaining both aims [7,8,9,10]. Nevertheless, our recent knowledge allows well informed efforts to carry out reclamation projects, primarily with reference to the first assumption [11]. Artificial fertilization, spreading previously collected topsoil and/or introducing appropriate plant species may directly or indirectly influence soil development ([12,13,14,15], but see [16]). Moreover, even specified modes of admixtures can increase autogenic repair of the soil [17,18].
Techniques of reclamation and monitoring their effectiveness are highly important to verify and improve reclamation efforts [11,19,20]. A very common method of reclamation is afforestation [21,22]. However, relatively long time periods are needed to evaluate this method. Nevertheless, many indirect efforts for monitoring reclamation processes have been developed. For instance, Ludwig et al. [23] proposed a traditional measurement of vegetation community development and also two original landscape surface indicators as a post-mining habitat complexity index. Pietrzykowski et al. [24,25], in turn suggested assessment of particular parameters of upper mineral soil layers of reclaimed areas. The knowledge of this is relatively broad and comes from many post-mining projects (e.g., [20,26,27]). It gives us information about the current state of soil substrate. Assessing the rate of litter decomposition goes one step further—taken together with knowledge about biomass production (as an outcome the Ludwig’s et al. [23] proposal), annual litterfall (as an outcome of biomass production) and elemental contents of litter fall, can provide a tool to estimate the pattern of reconstruction of the organic soil layer in the near future, or at least the direction of its development or retrogression [17].
Despite the commonness of studies on decomposition rates in recent years [28,29], only rarely were these studies conducted on post-mining sites [17]. Nevertheless, some studies are available. For instance, Lawrey [30] used litterbags to determine leaf litter decay rate of three tree species on an abandoned surface coal mine in the USA. Dutta and Agrawal [7] examined five exotic tree species on mine spoils in India, in terms of their litterfall and nutrient input via decomposition. Horodecki and Jagodziński [17] and Horodecki et al. [31] conducted similar research with a set of temperate species litter on a spoil heap in Poland. There were also a few studies conducted on post-mining spoil heaps concerning decomposition processes on sites left for natural succession [8,32,33]. Comparison of all of these results to those from “closer to natural” sites, could indicate the development of ecosystems on degraded lands. These indirect comparisons show general differences in decomposition rates between different habitats. Nevertheless, they can lead to some inaccuracies, because general conditions prevailing during the studies compared can never be the same.
The aim of our study was to determine the rates of leaf litter decay of four tree species (Alnus glutinosa (Gaertn.), Betula pendula (Roth), Pinus sylvestris (L.), and Quercus robur (L.) and to compare them between two different stand and habitat conditions—forests of the spoil heap and nearby closer to natural forests area. Additionally, we have determined the differences in amounts of carbon and nitrogen release by species-specific litter depending on decomposition rates in various stand and habitat conditions. When planning the experimental design, we hypothesized that the decomposition rates will be higher in forest sites than on the spoil heap for litter of all tree species investigated (H1). Moreover, we assumed that differences found will be lower for A. glutinosa and B. pendula (these litters are relatively easily decomposed) than for P. sylvestris and Q. robur (these litters are relatively recalcitrant) (H2). We also assumed that the rate of humification (resulting from the dynamics of litter accumulation) will be higher in the stands growing on the spoil heap than on the forest site, and beneath pine stands compared to birch stands (H3). Additionally, we stated that organic carbon (C) turnover will be slower beneath pine than birch canopies due to both relatively slower litter decomposition rate and slower C concentration decrease with ongoing decomposition in pine stands (H4). Direct comparison of decomposition rates in two different but nearby habitats will advance knowledge of decomposition and provide a better understanding of patterns of initial soil development on degraded lands.
2. Materials and Methods
2.1. Study Site
The study was conducted on the external spoil heap of the “Bełchatów” lignite mine (Mount Kamieńsk; Central Poland; 51.1247° N, 19.2540° E) and adjacent forests (approximately 6.5 km north). The average annual air temperature for a 20-year period (1996–2015) was 8.65 °C, whereas the average annual precipitation was 665 mm (Institute of Meteorology and Water Management report, 2016) for the nearest meteorological station (51.4319° N, 19.2353° E, 179 m a.s.l.). The growing season lasts 210–220 days [34].
Mount Kamieńsk was created during the years 1977–1993 using material originating from nearby open pit overburden. The raw rock used was very diversified—mostly quaternary but, to a lesser extent, also tertiary strata [35,36]. Phytotoxic tertiary deposits were usually located inside the heap beyond the range of plant roots but those lying in the outer layers were neutralized with lake chalk or alkaline ash [35]. Before afforestation, the plateau of the heap was fertilized with N (60 kg ha−1), K (60 kg ha−1), and P (70 kg ha−1) and also sown with mixed grass species and legumes [37]. Target tree species with a significant share of admixtures were planted during afforestation [38]. B. pendula and P. sylvestris stands occur most often on the spoil heap (27% and 25% of all afforested area, respectively; [17]). A. glutinosa and Q. robur (together with Q. petraea) stands covered 11% and 5.6% of total forest area of the spoil heap, respectively. Comparable stands in forest habitats grow in the nearby vicinity of Mount Kamieńsk (approximately 6.5 km north). To enable comparison with stands on the spoil heap, they had to meet restricted criteria: same species, similar age, identical soil granulometry, and comparable stand structure (Table A1).
2.2. Experimental Details
Eight stands—four on the spoil heap and four in forest habitat—were selected for study. These included two B. pendula and two P. sylvestris stands on each of the two habitat types. Within these stands we measured all diameters at breast height (i.e., 1.3 m, henceforth DBH) and height (H) for at least 18% of trees. The soil surface layers were analyzed based on soil pit excavation (1.5 m deep) in the center of each study site. Soil characteristics are displayed in Table A2.
We also measured upper soil layer temperature (around 3 cm beneath the mineral soil surface) at one hour intervals using HOBO data-loggers (HOBO U22-001 Water Temperature Pro v2 and/or HOBO U23-001 Pro v2 Temperature/Relative Humidity) for each plot (Figure A1).
To characterize light availability on the forest floor, during 2015 (April–October) we measured canopy light absorption (characterized by diffuse non-interceptance—DIFN; Figure A2) using an LAI-2200 plant canopy analyzer (Li-Cor Inc., Lincoln, NE, USA).
At the beginning of December 2013, we established the litterbag experiment beneath the canopies of selected stands. Freshly shed leaves of the four most common native tree species on the spoil heap (i.e., A. glutinosa, B. pendula, P. sylvestris, Q. robur) were collected from the ground in the Babki Forest District and dried in the laboratory. This made the plant material unified for both types of tree stands and both types of sites. Leaf samples with precisely determined weight (4.60–7.19 g) were installed in litterbags (15 × 15 cm) made from 1 mm wide mesh fiberglass netting. Every litterbag (1536 in total) was exactly labeled and installed in the field at the beginning of December 2013. Material collections (12) were done at three-month intervals, with a total research period of three years. After each interval, four litterbags (4) of each species (4) were harvested from each sample plot (8). Collected samples were dried for at least 72 h (at 65 °C), after which any intrusive material (vegetation, insects, sand, etc.) was removed manually using tweezers. Samples were then weighed with an accuracy of 0.001 g, to determine leaf mass loss for each litterbag.
Using an ECS CHNS−O 4010 Elemental Combustion System (Costech Analytical Technologies Inc., Valencia, CA, USA), we determined C and N contents in samples of initial material and after 3, 6, 12, 24, and 36 months of exposure in the field.
2.3. Statistical Analyses
The influences of leaf litter type, collection time, stand type, and site type on mass loss were tested via analysis of variance (ANOVA). To visualize real mass losses during all collection periods we used cubic splines with lambda levels of 0.1. To visualize changes in C and N contents during decomposition courses we used quadratic models with 95% confidence intervals for the predicted values.
We also calculated decomposition rates after Olson [39] as (Equation (1))
where m is remaining mass at interval t, im is initial mass at time t = 0, and ln is natural logarithm. The k value was estimated from values of remaining mass for each species × stand type × site type using non-linear regression. Non-linear regression models were further used for inversional calculation of half decay time (hd) (Equation (2))
and total decay time (assumed as 95% of initial mass loss; td) (Equation (3))
Additionally, we evaluated limit values (LV) after Berg and Ekbohm [40] (Equation (4))
where ml is the accumulated mass loss (%), t is time in days, m is maximum accumulated mass loss, and k is the initial decomposition rate. We also estimated litter-to-humus (LH) factors by modifying the simple relationships described by Berg et al. [41] to (Equation (5))
All analyses were performed in JMP Pro 13.0.0 (http://www.jmp.com; SAS Institute Inc. Cary, NC, USA).
3. Results
3.1. Decomposition
Testing statistical relationships, we found significant influences of leaf litter type, collection time, stand type, site type, and most of the interactions between these factors, on decomposition rates of the species studied in the experiment (Table 1).

Table 1.
Effect of leaf litter type (LITTER), collection time (TIME), stand type (STAND), site type (SITE), and their interactions on decomposition rates as a result of analysis of variance (ANOVA). DF—degrees of freedom.
For all litter types tested beneath the birch canopies, we recorded visibly faster decomposition on forest sites than on the spoil heap during all collection periods (Figure 1). For leaf litter of P. sylvestris and Q. robur, differences were statistically significant at every collection time, whereas for B. pendula and A. glutinosa only for nine and six of them, respectively (Table A3). Leaves of A. glutinosa decomposed the fastest among all investigated tree species. They lost 84.98% (±3.50), 91.21% (±1.63), and 91.86% (±2.46) of initial mass in forest sites after one, two, and three years of exposure, respectively. Analogous mass losses on the spoil heap site were 68.54% (±2.69), 87.97% (±1.91), and 85.64% (±1.75), respectively. Leaves of B. pendula lost 70.88% (±4.89), 78.04% (±2.63), and 83.64% (±2.20) of initial mass after one, two, and three years of the experiment under parent canopies of forest sites, whereas on the spoil heap site analogous values were 52.40% (±2.70), 74.06% (±2.56), and 77.74% (±1.34). Mass losses of P. sylvestris needles after analogous periods were 50.50% (±4.17), 66.21% (±3.78), and 78.47% (±3.42) on forest sites, and 24.67% (±0.59), 40.76% (±6.03), and 44.26% (±2.84) on the spoil heap site. Decomposition of Q. robur leaves were relatively the slowest. However, the differences in mass losses between site types were the highest: 51.74% (±6.02) on forest site vs. 13.61% (±0.63) on the spoil heap after one year, 66.03 (±3.39) vs. 36.79% (±5.55) after two years and 76.43% (±3.90) vs. 40.61% (±6.56) after three years.
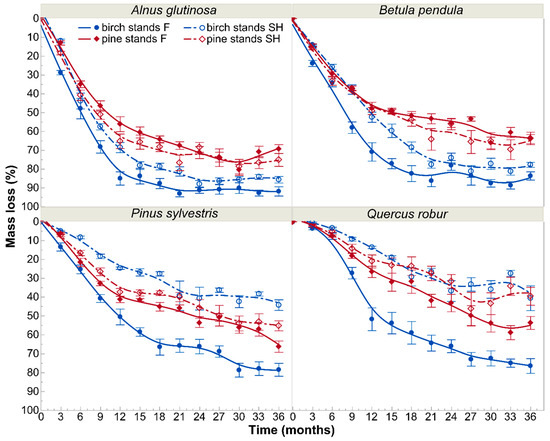
Figure 1.
Decomposition rates of particular tree species leaf litter in birch and pine stands growing on two different site types—post-mining external spoil heap (SH) or forest sites (F). Decay lines were smoothed by cubic splines with lambda of 0.1. The markers on the graph show mean values (±SE) of mass loss of particular tree species at particular collection times.
Differences in species-specific litter decomposition rates between site types were even more pronounced when presented as k constants (year−1) calculated based on the whole experimental period (Figure 2). K values for litter decay under birch canopies were 41% (for A. glutinosa), 47% (B. pendula), 176% (P. sylvestris), and 215% (Q. robur) higher on forest than on spoil heap sites. These differences, together with empirical decay rates (k year−1) reflect much different periods needed for specific leaf litter types in specific site conditions to reach the half and the total decay levels (Figure 3). Leaf litter of A. glutinosa will decompose to the half level of initial mass on the spoil heap 72 days later than on forest sites. Foliage of B. pendula, P. sylvestris and Q. robur will reach this level 117, 727, and 1012 days later on the spoil heap than on forest sites, respectively. The same litter types will be completely decomposed on the spoil heap 312, 505, 3141, and 4375 days later than on forest sites, respectively. Different k constants resulted also in different mineralization and humification levels on both site types. Under birch canopies, about 9% (A. glutinosa), 11% (B. pendula), 30% (P. sylvestris), and 32% (Q. robur) more of litterfall mass will be mineralized on forest sites than on the spoil heap (Figure 4).
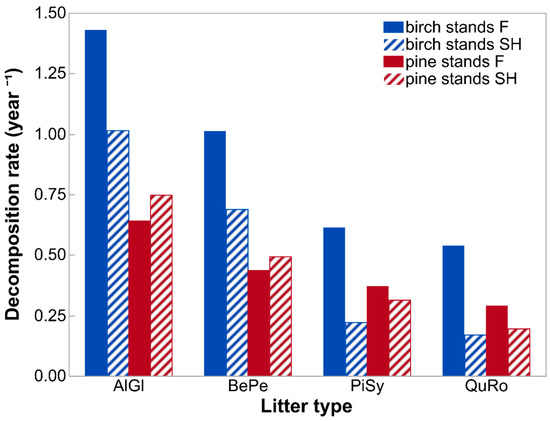
Figure 2.
Decomposition rates (k constants) of litter of four species decomposed in birch and pine stands growing on two different site types—post-mining external spoil heap (SH) or forest sites (F). Abbreviations: AlGl—Alnus glutinosa; BePe—Betula pendula; PiSy—Pinus sylvestris; QuRo—Quercus robur.
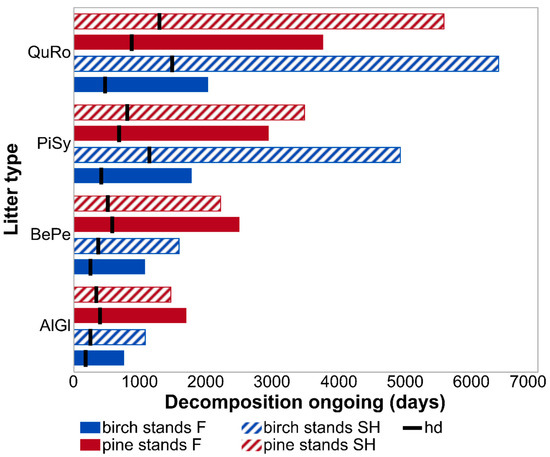
Figure 3.
Total (bars) and half (black line) decomposition levels reached by specific leaf litter in specific stand-site conditions. Abbreviations: AlGl—Alnus glutinosa; BePe—Betula pendula; PiSy—Pinus sylvestris; QuRo—Quercus robur; SH—post-mining external spoil heap site, F—forest site.
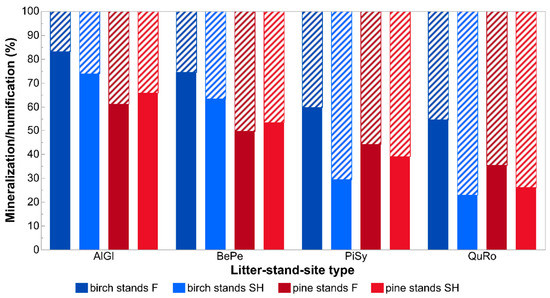
Figure 4.
Limit values (LV; full) and litter-to-humus factors (LH; striped) of particular tree species leaf litter in specific stand-site conditions. Abbreviations: AlGl—Alnus glutinosa, BePe—Betula pendula, PiSy—Pinus sylvestris, QuRo—Quercus robur, SH—post-mining external spoil heap site, F—forest site.
Beneath Scots pine canopies, decomposition courses of particular tree species litter proceeded similarly between the two site types (Figure 1). We did not find statistically significant differences in litter decomposition rates of B. pendula between forest and spoil heap sites at any of the collection periods. For A. glutinosa, P. sylvestris, and Q. robur we found such differences at one (at 1st collection period), three (3rd, 6th, 12th), or four (2nd, 7th, 11th and 12th) collection periods, respectively. Reversely, the losses of initial masses of A. glutinosa and B. pendula leaves in Scots pine stands were higher on the spoil heap than on forest sites (Figure 1). After one, two, and three years of exposure on the forest site, litter of A. glutinosa decomposed 56.11% (±3.96), 71.22% (±3.19), and 69.37% (±2.36) of initial mass, respectively. Its mass losses on the spoil heap reached 65.22% (±3.85), 68.46% (±2.64), and 75.09% (±3.47) after analogous periods, respectively. Litter of B. pendula lost 47.76% (±3.29), 55.60% (±2.27), and 64.03% (±2.39) of initial mass after one, two, and three years of exposure on the forest site, and 49.11% (±1.90), 56.06% (±3.07), and 63.70% (±3.31) on the spoil heap after analogous periods. Litter of P. sylvestris lost 41.33% (±1.63), 53.71% (±2.38), and 66.29% (±3.00) on the forest site and 37.44% (±2.05), 45.82% (±4.49), and 55.23% (±2.66) on the spoil heap after one, two, and three years, respectively. The slowest rates at analogous stages of the decomposition process were reflected by leaf litter of Q. robur—in forest stands it lost 26.59% (±2.98), 43.18% (±4.59), and 53.70% (±3.44) of initial mass, whereas in the spoil heap stands these values were 21.06% (±3.45), 31.66% (±3.63), and 39.58% (±4.72).
The differences in k constants showed increased similarity in decomposition courses between site types in Scots pine stands compared to birch stands. For A. glutinosa and B. pendula leaves, decomposition rates were 17% and 13% higher on the spoil heap, respectively, which resulted in attainment of halved initial mass in a period about two months shorter than on the forest site (Figure 2, Figure 3). Moreover, litters of the mentioned tree species will attain the total decay level 242 and 291 days earlier on spoil heap than on forest sites, respectively. For litter of P. sylvestris and Q. robur, k constants were higher on forest than on spoil heap sites—at levels of 18% and 48%, respectively (Figure 2). The half decay level of leaf litter of the mentioned tree species will be reached 123 and 420 days later on spoil heap than on forest sites, respectively. Their total mass loss will be reached 531 and 1814 days later on spoil heap than on forest sites (Figure 3). As a consequence of higher decomposition rates of A. glutinosa and B. pendula leaves on spoil heap than on forest sites, around 5% and 4% more of their litter, respectively, will be mineralized in spoil heap site conditions. For litter of P. sylvestris and Q. robur higher mineralization levels will be attained on the forest site (by 5% and 9%, respectively).
3.2. N and C Concentrations
Each of the litter types investigated initially had identical N and C concentrations in all stand and site types. For B. pendula, P. sylvestris, and Q. robur litters N concentration changes over time had similar trends (Figure 5). In all mentioned litter types N content increased faster on forest sites (here faster in birch than pine stands) than on spoil heap (here faster in pine than birch stands) during the decomposition process. For A. glutinosa, these relationships between N concentration courses were more intertwined.
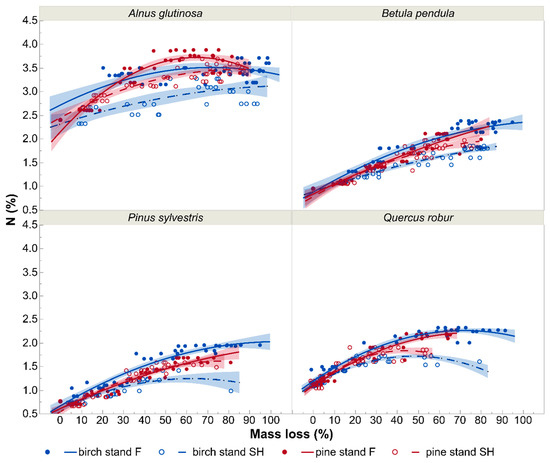
Figure 5.
Effects of decomposition level on nitrogen concentration (N; %) of particular tree species leaf litter in birch and pine stands growing in two different site types—post-mining external spoil heap (SH) or forest sites (F). Visualization is based on quadratic models with 95% confidence intervals for the predicted values. The markers on the graph show mean values of nitrogen concentrations of particular tree species at particular decomposition stages.
The C contents at particular decay stages in various stand and site types differed between litters of the tree species examined (Figure 6). However, they were generally lower on spoil heap (independently on stand type) than in forest stands.
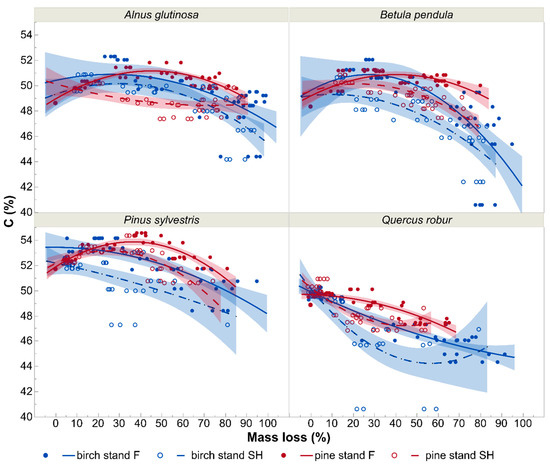
Figure 6.
Effects of decomposition level on carbon concentration (C; %) of particular tree species leaf litter in birch and pine stands growing in two different site types—post-mining external spoil heap (SH) or forest sites (F). Visualization is based on quadratic models with 95% confidence intervals for the predicted values. The markers on the graph show mean values of carbon concentrations of particular tree species at particular decomposition stages.
4. Discussion
Ecological restoration of degraded lands aims, first of all, towards habitat and ecosystem reconstruction [42]. However, recently, efforts have also been made to improve productivity functions of such ecosystems [6,43]. Soil improvement is a crucial element of mentioned efforts affecting both of the aims [44]. The differences in litter decomposition rates between two habitats and their consequences in improvement of upper soil layers were studied in our research. Soil properties are relatively easy to measure; however, this only gives us information about the present state of soil development. Our analyses of leaf litter decomposition rates, and empirical calculations based on those, contribute new information about patterns of development of upper soil layers. As far as we know, similar indirect comparisons of decomposition rates obtained on post-mining spoil heap and forest sites were not previously done.
Our previous papers (Horodecki and Jagodziński [17] and Horodecki et al. [31]) examined litter decomposition rates of 14 tree species on post-mining sites. Direct comparison of our results here with those obtained in previous papers should be done with special care because the decomposition processes have not been parallel in time. Nevertheless, contrasting our results from previous papers with results obtained by other authors from many disturbed and undisturbed habitats, in general, yielded similar conclusions to those of this paper, supporting the hypothesis that relatively harsh soil conditions on degraded lands play crucial roles in the course of litter decomposition (see the mentioned papers and literature cited therein). Moreover, according to the fact that, in the present experiment, we have used unified plant material, collected from the same stands, our direct comparisons give unbiased results. Due to the unification of study materials, we have inherently omitted the impacts of habitat conditions on structural form of leaves, and consequently differences in their decomposability [45,46], which could explain the differences if home material was used [47,48]. Environmental influence on decomposition rates could be visible in another way, i.e., by the state of development of soil faunal communities. Activities of microorganisms are (next to climate and structure and chemistry of litter) one of the main factors influencing decomposition [49,50]. They are dependent on soil organic layer development, its temperature and moisture. Based on previously conducted research we can state that after a short period of the reclamation (succession) process (in the case of “Bełchatów” lignite mine about 30 years), assemblages of microorganisms in such stands and soil conditions are inherently lower compared to more developed “older” soils [51,52,53,54].
Results of this experiment confirmed our first hypothesis only partially. Indeed, decomposition rates were generally higher on forest than on spoil heap sites, with the exception of the somewhat surprising case of A. glutinosa and B. pendula litters examined in pine stands, where the decomposition trends were reversed (Figure 1). Even though the differences (between forest and spoil heap sites) in decomposition rates of these two litter types in pine stands were statistically insignificant in 96% of cases (Table A3), these relationships were quite unexpected. Any efforts to explain it by the differences in stand structures failed because this unexpected trend concerned only half of the litter types investigated in the same stand conditions (litter of P. sylvestris and Q. robur decayed in predictable trends). The differences in light availability between site types are also useless in this case because in general, various light conditions did not affect upper soil layer temperatures in pine stands in the experiment (see Figure A1 and Figure A2). If the temperatures had been related to light (which did not occur in our experiment), then this could be interpreted as one of the factors affecting the differences in activity of soil organism communities in both site types [55,56]. The explanation of faster decomposition of A. glutinosa and B. pendula litters in pine stands on spoil heap than on forest sites could likely be connected with their susceptibility to decomposition (palatability for decomposers; [45,46]), which is higher than litters of P. sylvestris and Q. robur in homogeneous stand conditions [31]. Because of the relatively short period of soil development on the spoil heap investigated, soil organism communities are in very initial successional stages (see previous paragraph), which is even more visible in coniferous stands [57]. Decomposer specialists are likely not yet well developed and the web of soil organisms is dominated by bacteria. Therefore, in turn, decomposer generalists are primarily “focused” on the destruction of relatively easily decomposable organic material [51]. It is widely known that due to their chemical composition, first of all relatively high concentrations of hardly-decomposable cellulose and tannins or waxes (in our study litters of Q. robur and P. sylvestris), some litter types are more recalcitrant for decomposition than others [58].
Additionally, some authors concluded that birch litter displays some kind of resistance to environmental conditions, having similar decomposition rates in various habitats. For instance, Gao et al. [59] found that litter of B. platyphylla decomposed at the same rate in mixed and aspen stands (opposite to other litter types tested—oak and aspen). Conclusions based on our previous papers [17,31] contrast with Gao et al. [59]—the differences in decomposition rates (displayed as a k constants) between home and mixed stands were the highest for B. pendula litter (and relatively low for A. glutinosa, P. sylvestris, and Q. robur). However, we need to bear in mind (which we underlined in the cited papers [17,31]) that stand structures of home stands of B. pendula and the associated features of the upper soil layers were unfavorable for rapid decay of organic matter.
Due to the unexpected results for litter decomposition of A. glutinosa and B. pendula in pine stands, our second hypothesis was also only partially confirmed. Beneath the birch canopies, the differences in decomposition rates of P. sylvestris and Q. robur litters in two different site types were visibly higher than decomposition rates of A. glutinosa and B. pendula litters (Figure 1 and Figure 2, Table A3). Under pine stands, these differences were also higher for two recalcitrant litter types (in 10 and 8 collection periods for Q. robur and P. sylvestris, respectively). However, the reversed (compared to expected) trends in litter decomposition courses for the two remaining litter types in pine stands (see previous paragraph) deny our second hypothesis in the stand conditions mentioned.
Our third hypothesis was also only partially confirmed. Decomposition rates and essentially their courses in time enabled us to calculate the amount of litter that was mineralized and the remaining share accumulated on the forest floor as slowly degradable humus—humification rate [60,61]. Relatively higher shares of mineralization of most litters examined under birch than pine canopies is likely connected with the humus type created in the two stand conditions (see Table A2), which supports faster decomposition, especially in reference to relatively easy-decomposable material [62,63]. The soil environment created by pine litter is relatively more unfavorable for fast mineralization. Its acidic reaction hinders development of the soil fauna community [64], especially the abundance of earthworms which accelerate mineralization [56,65]. The relatively low share of mineralization of P. sylvestris and Q. robur litters in birch stands on the spoil heap was likely connected with low soil organic layer development in these stands (cf. [17]). Additionally, the lowest limit values obtained in our research on spoil heap were even smaller than the lowest given in other publications. For instance, Berg [60] stated that calculated limit values ranged so far between c. 42 and 100% decomposition.
High initial concentration of nitrogen in litter accelerates initial stages of the decomposition process [66,67]. However, later on, its influence is more complicated and should be examined together with concentrations of other, mainly insoluble substances [61,66]. As we used unified litters in all stand and site conditions, the nitrogen and carbon concentrations were initially identical. However, during ongoing decomposition their concentrations differentiated at various rates in disparate stand and site conditions. Nevertheless, it is likely not connected with initial N concentration in decomposed litter, but more likely with total N contents in surrounding soil environments, which are higher in birch than pine stands (Table A2). Decomposition rate increases with increasing nitrogen concentration, which is especially visible in disturbed forest [68]. Litters of three tree species (B. pendula, P. sylvestris, and Q. robur) released organic carbon faster in birch than pine stands in both investigated sites (see Figure 6). The only exception was linked with A. glutinosa litter, which released carbon during the decomposition processes faster in pine than birch stands on the forest site. All relationships between C concentration lines in various stands and site conditions (Figure 6) correspond (here positively) with analogous lines for N contents (Figure 5), which indicates that C release depended on N contents in soil rather than in decomposing litter.
5. Conclusions
Our results based on direct comparison of decomposition rates of four litter types in pine and birch stands growing on forest and spoil heap sites, generally showed that harsh soil conditions (of the latter) were unfavorable for fast organic matter mineralization. In some cases (birch stands on the spoil heap), organic soil layers were poorly developed, hindering organic carbon turnover and elongating the time for full biological and economic reclamation of degraded lands. On the other hand, pine stands on the spoil heap supported fast litter decomposition more than pine stands on the forest site, in reference to relatively fast decomposable litter (A. glutinosa and B. pendula). This, in turn, showed the value of selective choice of tree species for afforestation of post-mining areas to accelerate the development of technogenic soil substrates. We are aware of the various habitat requirements of the species studied. However, due to the specificity of post-industrial areas related to the high variation of soil substrates, we recommend introducing all tree species studied in the cluster form of admixtures as all of them could bring some benefits in ecological and economical reclamation. Due to fast litter decomposition and atmospheric N fixing, A. glutinosa enriches upper soil layers. B. pendula due to transpicuous tree crown could increase soil temperatures affecting activity of soil microorganisms. Q. robur increases upper soil layer thickness. P. sylvestris, in turn, as a beneficiary of other tree species presence and due to its fast growing rate plays a crucial role in the economic aim of reclamation.
Author Contributions
Conceptualization, A.M.J. and P.H.; Methodology, A.M.J. and P.H.; Validation, A.M.J. and P.H.; Formal analysis, P.H.; Investigation, P.H.; Resources, A.M.J. and P.H.; Data curation, A.M.J. and P.H.; Writing—original draft preparation, P.H.; Writing—review and editing, A.M.J. and P.H.; Visualization, P.H.; Supervision, A.M.J.; Project administration, A.M.J.; Funding acquisition, A.M.J.
Funding
This study was financially supported by the General Directorate of State Forests, Warsaw, Poland (research project: “Environmental and genetic factors affecting productivity of forest ecosystems on forest and post-industrial habitats”) and the Institute of Dendrology, Polish Academy of Sciences, Kórnik, Poland.
Acknowledgments
We thank Mirosław Nowiński (Faculty of Forestry, Poznań University of Life Sciences, Poland) for valuable help with field work and Lee E. Frelich (University of Minnesota, USA) for linguistic support and valuable comments to the early draft of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Appendix A
Microhabitat Conditions
The average upper soil layer temperatures under the birch canopy were higher on the forest site during all autumns and winters as well as during spring 2015 (Figure A1). Almost all recorded differences between site types (excluding spring and autumn 2015) were statistically significant. During summers and the two remaining springs (2014 and 2016), forest floors were warmer in birch stands growing on the spoil heap. In most seasons examined (excluding spring 2016) these differences were statistically significant. Under the pine stands, the differences in average soil temperatures were not as noticeable as in birch stands—they were statistically significant in only four of 12 cases. Forest floor in autumn 2014 and winters of 2015 and 2016 were warmer in forest stands, but warmer in autumn 2015 in spoil heap stands (Figure A1).
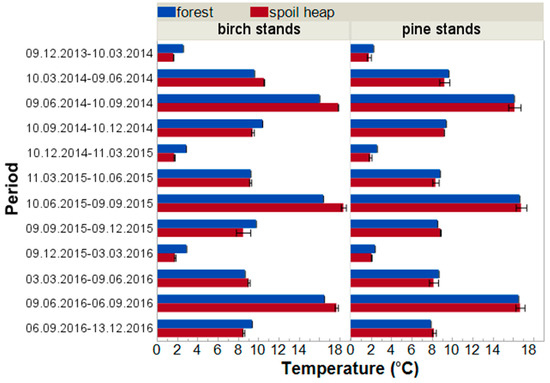
Figure A1.
The average temperatures (°C, ±SE) of upper soil layers in birch and pine stands growing on two different site types—post-mining external spoil heap (red) or forest sites (blue). Measurement periods were appropriate to decomposition time intervals.
Canopy light absorption did not differ statistically significantly between pine stands on both site types at any of the recording months; however, the size effect is visible and shows higher DIFN (diffuse non-interceptance) values in spoil heap than in forest stands during whole growing season (Figure A2). Similar trends were noted beneath birch stands; however, in this case, the differences in DIFN values were statistically significant in almost all recording months (except April). In general, light availability on forest floors of birch stands were much higher than in pine stands (except June at forest sites). Absolute differences in that parameter were much higher on spoil heap than on forest sites (on average 73% vs. 186%).
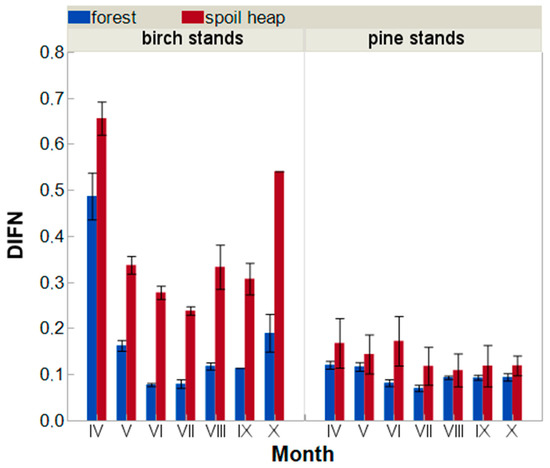
Figure A2.
The average canopy openness index (diffuse non-interceptance; DIFN) measured during the 2015 growing season (±SE) in birch and pine stands growing in two different site types—post-mining external spoil heap (red) or forest sites (blue).

Table A1.
Basic characteristics of sample plots. Abbreviations: BePe—Betula pendula; PiSy—Pinus sylvestris; SH—spoil heap; F—forest sites; CL—clay loam; S—sand; SL—sandy loam; BA—basal area at breast height; DBH—diameter at breast height; H—tree height; SE—standard error.
Table A1.
Basic characteristics of sample plots. Abbreviations: BePe—Betula pendula; PiSy—Pinus sylvestris; SH—spoil heap; F—forest sites; CL—clay loam; S—sand; SL—sandy loam; BA—basal area at breast height; DBH—diameter at breast height; H—tree height; SE—standard error.
| Plot Number | Coordinates | Plot Area (m2) | Stand Type | Site Type | Year of Measurements | Soil Texture | Stand Age | Average DBH (cm) * ±SE | Average H (m) * ±SE | BA (m2 ha−1) *, ** | Stocking Density (individuals ha−1) *, ** |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 | 51.2207, 19.4339 | 604.5 | BePe | SH | 2011 | clay loam | 22 | 8.87 (0.30) | 9.21 (0.21) | 8.54 (98.31) | 1274 (74.76) |
| 2015 | 26 | 10.14 (0.36) | 10.61 (0.26) | 11.45 (98.22) | 1290 (62.40) | ||||||
| 7 | 51.2208, 19.4339 | 604.5 | 2011 | clay loam | 22 | 8.96 (0.34) | 9.09 (0.22) | 7.4 (95.55) | 1075 (69.15) | ||
| 2015 | 26 | 10.73 (0.39) | 11.26 (0.25) | 10.35 (95.24) | 1059 (57.66) | ||||||
| 15 | 51.2105, 19.4384 | 900 | PiSy | 2011 | sand | 18 | 5.49 (0.07) | 4.86 (0.04) | 12.76 (99.83) | 5022 (99.12) | |
| 2015 | 22 | 6.52 (0.09) | 6.58 (0.05) | 18.18 (99.80) | 5022 (98.69) | ||||||
| 16 | 51.2117, 19.4265 | 810 | 2011 | sand | 17 | 6.81 (0.11) | 6.05 (0.05) | 20.61 (99.96) | 5123 (99.28) | ||
| 2015 | 21 | 7.92 (0.13) | 8.05 (0.06) | 27.50 (99.94) | 5049 (99.27) | ||||||
| 105 | 51.2744, 19.4324 | 646.52 | BePe | F | 2012 | sandy loam | 20 | 10.40 (0.21) | 13.66 (0.11) | 15.79 (99.95) | 1779 (92.00) |
| 2015 | 23 | 10.76 (0.28) | 14.83 (0.23) | 17.57 (98.95) | 1794 (73.42) | ||||||
| 106 | 51.2747, 19.4326 | 450 | 2012 | clay loam | 20 | 10.10 (0.20) | 13.44 (0.12) | 18.32 (99.99) | 2200 (95.19) | ||
| 2015 | 23 | 10.45 (0.29) | 14.93 (0.25) | 20.05 (99.46) | 2178 (72.06) | ||||||
| 107 | 51.1738, 19.4323 | 750 | PiSy | 2012 | sand | 21 | 8.02 (0.15) | 8.90 (0.08) | 23.41 (100.00) | 4200 (99.68) | |
| 2015 | 24 | 8.99 (0.17) | 10.21 (0.08) | 28.76 (99.64) | 4093 (81.65) | ||||||
| 108 | 51.1746, 19.4321 | 750 | 2012 | sand | 21 | 8.46 (0.16) | 9.16 (0.08) | 21.22 (99.81) | 3467 (99.24) | ||
| 2015 | 24 | 9.30 (0.19) | 10.09 (0.11) | 26.12 (99.22) | 3467 (54.74) |
* including only the main tree species in the stand. ** in the brackets, the share of BA/stocking density of the main tree species in the stand in total BA/stocking density is given.

Table A2.
Basic characteristics of organic soil layers of sample plots. Abbreviations: BePe—Betula pendula; PiSy—Pinus sylvestris; SH—spoil heap; F—forest sites; V—thickness; Corg—organic carbon; Ntot—nitrogen; Hum—humus content, Ca, Mg, K, Na contents (cmol(+)/kg); FPMD—fresh protomoder; FPM—fresh protomor; FMD—fresh moder; FMM—fresh modermor; FM—fresh mor.
Table A2.
Basic characteristics of organic soil layers of sample plots. Abbreviations: BePe—Betula pendula; PiSy—Pinus sylvestris; SH—spoil heap; F—forest sites; V—thickness; Corg—organic carbon; Ntot—nitrogen; Hum—humus content, Ca, Mg, K, Na contents (cmol(+)/kg); FPMD—fresh protomoder; FPM—fresh protomor; FMD—fresh moder; FMM—fresh modermor; FM—fresh mor.
| Plot Number | Stand Type | Site Type | Humus Type | V (cm) | Corg. (%) | Ntot (%) | C/N | Hum (%) | pH H2O | Ca | Mg | K | Na |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 | BePe | SH | FPMD | 1 | 28.20 | 1.07 | 30.65 | 48.62 | 6.10 | 14.7300 | 1.5600 | 0.6600 | 0.0300 |
| 7 | FPMD | 1 | 32.83 | 1.36 | 28.09 | 56.59 | 6.12 | 14.0100 | 2.5100 | 0.9800 | 0.0200 | ||
| 15 | PiSy | FPM | 1 | 38.43 | 0.88 | 52.05 | 66.26 | 5.01 | 11.8420 | 1.0250 | 0.4390 | 0.0200 | |
| 16 | FPM | 2 | 46.20 | 0.96 | 56.21 | 79.65 | 4.93 | 9.2460 | 1.4910 | 1.1225 | 0.0300 | ||
| 105 | BePe | F | FMD | 3 | 34.17 | 1.33 | 36.20 | 58.91 | 5.55 | 13.6667 | 3.4367 | 2.5633 | 0.0300 |
| 106 | FMD | 2 | 37.23 | 1.29 | 37.98 | 64.19 | 5.57 | 13.8100 | 3.3850 | 2.2650 | 0.0300 | ||
| 107 | PiSy | FMM | 4 | 45.09 | 1.30 | 41.94 | 77.74 | 4.68 | 10.1025 | 1.2125 | 1.7075 | 0.0375 | |
| 108 | FM | 6 | 32.64 | 1.07 | 37.03 | 56.27 | 4.37 | 6.7410 | 0.8805 | 0.7256 | 0.0420 |

Table A3.
Average mass loss (%, ±SE) of particular tree species leaf litter at every collection time in two different site and stand types. ANOVA was performed to examine the influence of site type on leaf litter decomposition rates for every litter type at every collection time. Abbreviations: SH—spoil heap; F—forest sites.
Table A3.
Average mass loss (%, ±SE) of particular tree species leaf litter at every collection time in two different site and stand types. ANOVA was performed to examine the influence of site type on leaf litter decomposition rates for every litter type at every collection time. Abbreviations: SH—spoil heap; F—forest sites.
| Alnus glutinosa | Betula pendula | Pinus sylvestris | Quercus robur | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| birch stands | pine stands | birch stands | pine stands | birch stands | pine stands | birch stands | pine stands | |||
| Collection time (months) | 3 | F | 28.77 (1.18) | 12.97 (1.12) | 23.84 (1.61) | 14.33 (0.90) | 13.4 (2.17) | 6.23 (0.68) | 3.66 (0.59) | 1.55 (0.46) |
| SH | 11.89 (0.99) | 18.38 (0.93) | 14.07 (0.38) | 15.71 (0.82) | 4.98 (0.30) | 7.17 (0.77) | 0.44 (0.30) | 2.03 (0.63) | ||
| ANOVA | <0.0001 | 0.0023 | <0.0001 | 0.2790 | 0.0010 | 0.3591 | 0.0071 | 0.4176 | ||
| 6 | F | 47.84 (5.43) | 34.93 (1.79) | 34.16 (2.13) | 29.42 (1.19) | 25.33 (2.3) | 21.58 (2.21) | 7.63 (1.66) | 7.55 (0.67) | |
| SH | 43.82 (4.19) | 40.78 (2.73) | 25.77 (1.47) | 33.23 (2.19) | 8.29 (0.73) | 16.61 (1.40) | 3.36 (0.46) | 5.15 (0.49) | ||
| ANOVA | 0.5959 | 0.0943 | 0.0051 | 0.1441 | <0.0001 | 0.0874 | 0.0007 | 0.0118 | ||
| 9 | F | 68.18 (3.28) | 46.40 (2.70) | 57.96 (3.06) | 36.97 (1.12) | 40.73 (2.08) | 32.77 (1.15) | 27.3 (3.04) | 18.15 (1.27) | |
| SH | 57.51 (1.67) | 51.06 (2.49) | 37.72 (2.48) | 37.19 (1.26) | 18.38 (1.20) | 26.58 (2.07) | 9.24 (1.01) | 14.25 (2.34) | ||
| ANOVA | 0.0089 | 0.2246 | 0.0002 | 0.9004 | <0.0001 | 0.0206 | <0.0001 | 0.1238 | ||
| 12 | F | 84.98 (3.50) | 56.11 (3.96) | 70.88 (4.89) | 47.76 (3.29) | 50.50 (4.17) | 41.33 (1.63) | 51.74 (6.02) | 26.59 (2.98) | |
| SH | 68.54 (2.69) | 65.22 (3.85) | 52.40 (2.70) | 49.11 (1.90) | 24.67 (0.59) | 37.44 (2.05) | 13.61 (0.63) | 21.06 (3.45) | ||
| ANOVA | 0.0021 | 0.1293 | 0.0049 | 0.7447 | <0.0001 | 0.1627 | <0.0001 | 0.2096 | ||
| 15 | F | 83.71 (4.16) | 60.53 (2.95) | 77.11 (2.71) | 49.35 (1.05) | 58.53 (2.27) | 41.56 (0.77) | 53.81 (5.67) | 32.09 (4.82) | |
| SH | 78.14 (2.02) | 65.66 (3.77) | 59.65 (3.58) | 49.19 (1.57) | 26.88 (1.93) | 37.79 (1.65) | 19.01 (0.92) | 23.22 (4.05) | ||
| ANOVA | 0.1881 | 0.2991 | 0.0015 | 0.9320 | <0.0001 | 0.0558 | <0.0001 | 0.1631 | ||
| 18 | F | 87.66 (2.68) | 64.23 (1.84) | 82.38 (4.2) | 51.64 (2.25) | 66.46 (4.21) | 45.11 (2.29) | 58.95 (5.98) | 31.80 (6.19) | |
| SH | 78.63 (1.76) | 68.26 (2.43) | 68.49 (2.91) | 53.84 (3.38) | 27.69 (1.15) | 37.85 (1.27) | 29.28 (4.82) | 23.57 (4.94) | ||
| ANOVA | 0.0108 | 0.2014 | 0.0167 | 0.6046 | <0.0001 | 0.0145 | 0.0024 | 0.2807 | ||
| 21 | F | 93.08 (1.63) | 67.48 (2.02) | 86.25 (3.03) | 53.25 (2.65) | 65.76 (3.54) | 45.90 (1.71) | 64.40 (4.22) | 41.96 (3.42) | |
| SH | 81.24 (2.46) | 76.70 (4.14) | 77.60 (1.88) | 64.19 (5.76) | 39.29 (7.82) | 39.99 (2.41) | 26.60 (1.88) | 27.42 (3.82) | ||
| ANOVA | 0.001 | 0.0584 | 0.0278 | 0.1020 | 0.0179 | 0.0651 | <0.0001 | 0.0117 | ||
| 24 | F | 91.21 (1.63) | 71.22 (3.19) | 78.04 (2.63) | 55.60 (2.27) | 66.21 (3.78) | 53.71 (2.38) | 66.03 (3.39) | 43.18 (4.59) | |
| SH | 87.97 (1.91) | 68.46 (2.64) | 74.06 (2.56) | 56.06 (3.07) | 40.76 (6.03) | 45.82 (4.49) | 36.79 (5.55) | 31.66 (3.63) | ||
| ANOVA | 0.2167 | 0.4931 | 0.2826 | 0.8945 | 0.0040 | 0.1549 | 0.0005 | 0.0666 | ||
| 27 | F | 90.86 (2.49) | 73.61 (2.60) | 83.54 (4.99) | 53.38 (1.14) | 68.81 (2.98) | 50.79 (2.12) | 72.95 (3.60) | 49.89 (5.39) | |
| SH | 86.37 (1.74) | 73.96 (4.69) | 81.21 (3.18) | 65.56 (6.12) | 36.35 (1.15) | 49.88 (5.46) | 33.29 (3.58) | 46.27 (8.75) | ||
| ANOVA | 0.1158 | 0.7914 | 0.4891 | 0.0828 | <0.0001 | 0.9572 | <0.0001 | 0.7405 | ||
| 30 | F | 90.10 (2.85) | 80.43 (2.88) | 87.54 (2.02) | 66.05 (3.08) | 78.67 (3.65) | 56.19 (2.98) | 72.44 (3.95) | 53.88 (4.65) | |
| SH | 85.59 (1.58) | 78.70 (3.88) | 77.18 (2.95) | 65.11 (4.48) | 42.42 (3.19) | 53.98 (3.94) | 35.94 (4.68) | 43.49 (5.77) | ||
| ANOVA | 0.1296 | 0.7918 | 0.0120 | 0.9147 | <0.0001 | 0.6730 | <0.0001 | 0.1784 | ||
| 33 | F | 92.71 (1.54) | 70.89 (3.05) | 88.66 (1.95) | 60.57 (2.73) | 77.87 (3.61) | 57.03 (3.65) | 74.92 (2.16) | 58.84 (3.71) | |
| SH | 84.09 (1.28) | 76.57 (3.16) | 81.22 (2.00) | 69.66 (5.27) | 38.36 (1.13) | 53.19 (4.36) | 27.48 (1.67) | 34.25 (4.85) | ||
| ANOVA | 0.0013 | 0.2216 | 0.0180 | 0.1363 | <0.0001 | 0.5305 | <0.0001 | 0.0013 | ||
| 36 | F | 91.86 (2.46) | 69.37 (2.36) | 83.64 (2.20) | 64.03 (2.39) | 78.47 (3.42) | 66.29 (3.00) | 76.43 (3.90) | 53.70 (3.44) | |
| SH | 85.64 (1.75) | 75.09 (3.47) | 77.74 (1.34) | 63.70 (3.31) | 44.26 (2.84) | 55.23 (2.66) | 40.61 (6.56) | 39.58 (4.72) | ||
| ANOVA | 0.4901 | 0.1763 | 0.7832 | 0.9527 | <0.0001 | 0.0162 | 0.0004 | 0.0300 | ||
References
- Jagodziński, A.M.; Kałucka, I.; Horodecki, P.; Oleksyn, J. Aboveground biomass allocation and accumulation in a chronosequence of young Pinus sylvestris stands growing on a lignite mine spoil heap. Dendrobiology 2014, 72, 139–150. [Google Scholar] [CrossRef]
- Jimenez, M.D.; Ruiz-Capillas, P.; Mola, I.; Pérez-Corona, E.; Casado, M.A.; Balaguer, L. Soil Development at the Roadside: A Case Study of a Novel Ecosystem. Land Degrad. Dev. 2013, 24, 564–574. [Google Scholar] [CrossRef]
- Hobbs, R.J.; Higgs, E.; Harris, J.A. Novel ecosystems: Implications for conservation and restoration. Trends Ecol. Evol. 2009, 24, 599–605. [Google Scholar] [CrossRef]
- Maiti, S.K. Bioreclamation of coalmine overburden dumps—With special empasis on micronutrients and heavy metals accumulation in tree species. Environ. Monit. Assess. 2007, 125, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Jagodziński, A.M.; Wierzcholska, S.; Dyderski, M.K.; Horodecki, P.; Rusińska, A.; Gdula, A.K.; Kasprowicz, M. Tree species effects on bryophyte guilds on a reclaimed post-mining site. Ecol. Eng. 2018, 110, 117–127. [Google Scholar] [CrossRef]
- Prach, K.; Hobbs, R.J. Spontaneous Succession versus Technical Reclamation in the Restoration of Disturbed Sites. Restor. Ecol. 2008, 16, 363–366. [Google Scholar] [CrossRef]
- Dutta, R.K.; Agrawal, M. Litterfall, litter decomposition and nutrient release in five exotic plant species planted on coal mine spoils. Pedobiologia 2001, 45, 298–312. [Google Scholar] [CrossRef]
- Esperschütz, J.; Zimmermann, C.; Dümig, A.; Welzl, G.; Buegger, F.; Elmer, M.; Munch, J.C.; Schloter, M. Dynamics of microbial communities during decomposition of litter from pioneering plants in initial soil ecosystems. Biogeosciences 2013, 10, 5115–5124. [Google Scholar] [CrossRef]
- Jagodziński, A.M.; Kałucka, I. Fine roots biomass and morphology in a chronosequence of young Pinus sylvestris stands growing on a reclaimed lignite mine spoil heap. Dendrobiology 2010, 64, 19–30. [Google Scholar]
- Kałucka, I.L.; Jagodziński, A.M. Successional traits of ectomycorrhizal fungi in forest reclamation after surface mining and agricultural disturbances: A review. Dendrobiology 2016, 76, 91–104. [Google Scholar] [CrossRef]
- Pietrzykowski, M. Reclamation and Reconstruction of Terrestrial Ecosystems on Mine Sites-Ecological Effectiveness Assessment. In Energy Science and Technology; Sivakumar, S., Sharma, U.C., Prasad, R., Eds.; Coal Energy; Studium Press LLC: New Delhi, India, 2015; pp. 121–151. ISBN 1-62699-063-8. [Google Scholar]
- Frouz, J.; Livečková, M.; Albrechtová, J.; Chroňáková, A.; Cajthaml, T.; Pižl, V.; Háněl, L.; Starý, J.; Baldrian, P.; Lhotáková, Z.; et al. Is the effect of trees on soil properties mediated by soil fauna? A case study from post-mining sites. For. Ecol. Manag. 2013, 309, 87–95. [Google Scholar] [CrossRef]
- Helingerová, M.; Frouz, J.; Šantrůčková, H. Microbial activity in reclaimed and unreclaimed post-mining sites near Sokolov (Czech Republic). Ecol. Eng. 2010, 36, 768–776. [Google Scholar] [CrossRef]
- Laganière, J.; Paré, D.; Bradley, R.L. How does a tree species influence litter decomposition? Separating the relative contribution of litter quality, litter mixing, and forest floor conditions. Can. J. For. Res. 2010, 40, 465–475. [Google Scholar] [CrossRef]
- Pietrzykowski, M. Soil quality index as a tool for Scots pine (Pinus sylvestris) monoculture conversion planning on afforested, reclaimed mine land. J. For. Res. 2014, 25, 63–74. [Google Scholar] [CrossRef]
- Józefowska, A.; Pietrzykowski, M.; Woś, B.; Cajthaml, T.; Frouz, J. The effects of tree species and substrate on carbon sequestration and chemical and biological properties in reforested post-mining soils. Geoderma 2017, 292, 9–16. [Google Scholar] [CrossRef]
- Horodecki, P.; Jagodziński, A.M. Tree species effects on litter decomposition in pure stands on afforested post-mining sites. For. Ecol. Manag. 2017, 406, 1–11. [Google Scholar] [CrossRef]
- Sroka, K.; Chodak, M.; Klimek, B.; Pietrzykowski, M. Effect of black alder (Alnus glutinosa) admixture to Scots pine (Pinus sylvestris) plantations on chemical and microbial properties of sandy mine soils. Appl. Soil Ecol. 2018, 124, 62–68. [Google Scholar] [CrossRef]
- Doley, D.; Audet, P. Adopting novel ecosystems as suitable rehabilitation alternatives for former mine sites. Ecol. Process. 2013, 2, 1–11. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Maiti, S.K.; Masto, R.E. Development of mine soil quality index (MSQI) for evaluation of reclamation success: A chronosequence study. Ecol. Eng. 2014, 71, 10–20. [Google Scholar] [CrossRef]
- Macdonald, S.E.; Landhäusser, S.M.; Skousen, J.; Franklin, J.; Frouz, J.; Hall, S.; Jacobs, D.F.; Quideau, S. Forest restoration following surface mining disturbance: Challenges and solutions. New For. 2015, 46, 703–732. [Google Scholar] [CrossRef]
- Pietrzykowski, M.; Woś, B.; Haus, N. Scots pine needles macronutrient (N, P, K, Ca, Mg, and S) supply at different reclaimed mine soil substrates—As an indicator of the stability of developed forest ecosystems. Environ. Monit. Assess. 2013, 185, 7445–7457. [Google Scholar] [CrossRef]
- Ludwig, J.A.; Hindley, N.; Barnett, G. Indicators for monitoring minesite rehabilitation: Trends on waste-rock dumps, northern Australia. Ecol. Indic. 2003, 3, 143–153. [Google Scholar] [CrossRef]
- Pietrzykowski, M.; Pająk, M.; Krzaklewski, W. Assessment of soil-site conditions on the spoil heaps of the lignite mining plant (KWB) “Bełchatów” reclaimed to forest with the use of the site soil index (SIG). Pol. J. Soil Sci. 2011, 44, 81–88. [Google Scholar]
- Pietrzykowski, M.; Pająk, M.; Krzaklewski, W. Próba zastosowania metod liczbowej wyceny gleb na podstawie Indeksu Trofizmu Gleb Leśnych (ITGL) oraz Siedliskowego Indeksu Glebowego (SIG) do opisu zmienności warunków siedliskowych na zrekultywowanych dla leśnictwa zwałowiskach KWB “Bełchatów”. Gospod. Surowcami Miner. 2010, 26, 155–165. [Google Scholar]
- Asensio, V.; Guala, S.D.; Vega, F.A.; Covelo, E.F. A soil quality index for reclaimed mine soils. Environ. Toxicol. Chem. 2013, 32, 2240–2248. [Google Scholar] [CrossRef]
- Monokrousos, N.; Boutsis, G.; Diamantopoulos, J.D. Development of soil chemical and biological properties in the initial stages of post-mining deposition sites. Environ. Monit. Assess. 2014, 186, 9065–9074. [Google Scholar] [CrossRef]
- Berg, B. Decomposition patterns for foliar litter—A theory for influencing factors. Soil Biol. Biochem. 2014, 78, 222–232. [Google Scholar] [CrossRef]
- Frouz, J.; Roubíčková, A.; Heděnec, P.; Tajovský, K. Do soil fauna really hasten litter decomposition? A meta-analysis of enclosure studies. Eur. J. Soil Biol. 2015, 68, 18–24. [Google Scholar] [CrossRef]
- Lawrey, J.D. The relative decomposition potential of habitats variously affected by surface coal mining. Can. J. Bot. 1977, 55, 1544–1552. [Google Scholar] [CrossRef]
- Horodecki, P.; Nowiński, M.; Jagodziński, A.M. Advantages of mixed tree stands in restoration of upper soil layers on postmining sites: A five-year leaf litter decomposition experiment. Land Degrad. Dev. 2019, 30, 3–13. [Google Scholar] [CrossRef]
- Frouz, J. The effect of litter type and macrofauna community on litter decomposition and organic matter accumulation in post-mining sites. Biologia 2008, 63, 249–253. [Google Scholar] [CrossRef]
- Urbanová, M.; Šnajdr, J.; Brabcová, V.; Merhautová, V.; Dobiášová, P.; Cajthaml, T.; Vaněk, D.; Frouz, J.; Šantrůčková, H.; Baldrian, P. Litter decomposition along a primary post-mining chronosequence. Biol. Fertil. Soils 2014, 50, 827–837. [Google Scholar] [CrossRef]
- Zielony, R.; Kliczkowska, A. Regionalizacja Przyrodniczo-Leśna Polski 2010; Centrum Informacyjne Lasów Państwowych: Na zlec; Dyrekcji Generalnej Lasów Państwowych: Warszawa, Poland, 2012; ISBN 978-83-61633-62-4.
- Goździk, J.; Jończyk, W.; Niżnik, A.M. Kopalnia Węgla Brunatnego Bełchatów i Elektrownia Bełchatów—Zmiany środowiska geograficznego spowodowane ich działalnością. In Proceedings of the Obszary Metropolitalne We Współczesnym Środowisku Geograficznym, Łódź, Poland, 8–12 September 2010; Barwiński, M., Ed.; Wydawnictwo Triada: Łódź, Poland, 2010; Volume 2, pp. 397–422. [Google Scholar]
- Pietrzykowski, M. Scots pine (Pinus sylvestris L.) ecosystem macronutrients budget on reclaimed mine sites—Stand trees supply and stability. Nat. Sci. 2010, 2, 590–599. [Google Scholar] [CrossRef]
- Rawlik, M.; Kasprowicz, M.; Jagodziński, A.M. Differentiation of herb layer vascular flora in reclaimed areas depends on the species composition of forest stands. For. Ecol. Manag. 2018, 409, 541–551. [Google Scholar] [CrossRef]
- Wójcik, J.; Krzaklewski, W. Metody rekultywacji leśnej terenów bezglebowych w górnictwie odkrywkowym. Prz. Górniczy 2010, 10, 115–119. [Google Scholar]
- Olson, J.S. Energy Storage and the Balance of Producers and Decomposers in Ecological Systems. Ecology 1963, 44, 322–331. [Google Scholar] [CrossRef]
- Berg, B.; Ekbohm, G. Litter mass-loss rates and decomposition patterns in some needle and leaf litter types. Long-term decomposition in a Scots pine forest. VII. Can. J. Bot. 1991, 69, 1449–1456. [Google Scholar] [CrossRef]
- Berg, B.; McClaugherty, C.; Santo, A.V.D.; Johnson, D. Humus buildup in boreal forests: Effects of litter fall and its N concentration. Can. J. For. Res. 2001, 31, 988–998. [Google Scholar] [CrossRef]
- Benayas, J.M.R.; Newton, A.C.; Diaz, A.; Bullock, J.M. Enhancement of Biodiversity and Ecosystem Services by Ecological Restoration: A Meta-Analysis. Science 2009, 325, 1121–1124. [Google Scholar] [CrossRef]
- Audet, P.; Pinno, B.D.; Thiffault, E. Reclamation of boreal forest after oil sands mining: Anticipating novel challenges in novel environments. Can. J. For. Res. 2015, 45, 364–371. [Google Scholar] [CrossRef]
- Frouz, J. Effects of Soil Development Time and Litter Quality on Soil Carbon Sequestration: Assessing Soil Carbon Saturation with a Field Transplant Experiment along a Post-mining Chronosequence: Carbon Sequestration and Saturation in Post-mining Sites. Land Degrad. Dev. 2017, 28, 664–672. [Google Scholar] [CrossRef]
- Cornelissen, J.H.; Pérez-Harguindeguy, N.; Díaz, S.; Grime, J.P.; Marzano, B.; Cabido, M.; Vendramini, F.; Cerabolini, B. Leaf structure and defence control litter decomposition rate across species and life forms in regional floras on two continents. New Phytol. 1999, 143, 191–200. [Google Scholar] [CrossRef]
- Cornelissen, J.H.C.; Thompson, K. Functional leaf attributes predict litter decomposition rate in herbaceous plants. New Phytol. 1997, 135, 109–114. [Google Scholar] [CrossRef]
- Austin, A.T.; Vivanco, L.; González-Arzac, A.; Pérez, L.I. There’s no place like home? An exploration of the mechanisms behind plant litter-decomposer affinity in terrestrial ecosystems. New Phytol. 2014, 204, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Ayres, E.; Steltzer, H.; Simmons, B.L.; Simpson, R.T.; Steinweg, J.M.; Wallenstein, M.D.; Mellor, N.; Parton, W.J.; Moore, J.C.; Wall, D.H. Home-field advantage accelerates leaf litter decomposition in forests. Soil Biol. Biochem. 2009, 41, 606–610. [Google Scholar] [CrossRef]
- Aerts, R. Climate, Leaf Litter Chemistry and Leaf Litter Decomposition in Terrestrial Ecosystems: A Triangular Relationship. Oikos 1997, 79, 439–449. [Google Scholar] [CrossRef]
- Berg, B.; Staaf, H. Decomposition rate and chemical changes of Scots pine needle litter. II. Influence of chemical composition. Ecol. Bull. 1980, 32, 373–390. [Google Scholar]
- Frouz, J.; Thébault, E.; Pižl, V.; Adl, S.; Cajthaml, T.; Baldrián, P.; Háněl, L.; Starý, J.; Tajovský, K.; Materna, J.; et al. Soil Food Web Changes during Spontaneous Succession at Post Mining Sites: A Possible Ecosystem Engineering Effect on Food Web Organization? PLoS ONE 2013, 8, e79694. [Google Scholar] [CrossRef]
- Frouz, J.; Prach, K.; Pižl, V.; Háněl, L.; Starý, J.; Tajovský, K.; Materna, J.; Balík, V.; Kalčík, J.; Řehounková, K. Interactions between soil development, vegetation and soil fauna during spontaneous succession in post mining sites. Eur. J. Soil Biol. 2008, 44, 109–121. [Google Scholar] [CrossRef]
- Hopkins, D.W.; Badalucco, L.; English, L.C.; Meli, S.M.; Chudek, J.A.; Ioppolo, A. Plant litter decomposition and microbial characteristics in volcanic soils (Mt Etna, Sicily) at different stages of development. Biol. Fertil. Soils 2007, 43, 461–469. [Google Scholar] [CrossRef]
- Jangid, K.; Williams, M.A.; Franzluebbers, A.J.; Schmidt, T.M.; Coleman, D.C.; Whitman, W.B. Land-use history has a stronger impact on soil microbial community composition than aboveground vegetation and soil properties. Soil Biol. Biochem. 2011, 43, 2184–2193. [Google Scholar] [CrossRef]
- Jagodziński, A.M.; Dyderski, M.K.; Horodecki, P.; Rawlik, K. Limited dispersal prevents Quercus rubra invasion in a 14-species common garden experiment. Divers. Distrib. 2018, 24, 403–414. [Google Scholar] [CrossRef]
- Mueller, K.E.; Eisenhauer, N.; Reich, P.B.; Hobbie, S.E.; Chadwick, O.A.; Chorover, J.; Dobies, T.; Hale, C.M.; Jagodziński, A.M.; Kałucka, I.; et al. Light, earthworms, and soil resources as predictors of diversity of 10 soil invertebrate groups across monocultures of 14 tree species. Soil Biol. Biochem. 2016, 92, 184–198. [Google Scholar] [CrossRef]
- Frouz, J.; Keplin, B.; Pižl, V.; Tajovský, K.; Starý, J.; Lukešová, A.; Nováková, A.; Balík, V.; Háněl, L.; Materna, J.; et al. Soil biota and upper soil layer development in two contrasting post-mining chronosequences. Ecol. Eng. 2001, 17, 275–284. [Google Scholar] [CrossRef]
- Kraus, T.E.C.; Dahlgren, R.A.; Zasoski, R.J. Tannins in nutrient dynamics of forest ecosystems—A review. Plant Soil 2003, 256, 41–66. [Google Scholar] [CrossRef]
- Gao, J.; Kang, F.; Han, H. Effect of Litter Quality on Leaf-Litter Decomposition in the Context of Home-Field Advantage and Non-Additive Effects in Temperate Forests in China. Pol. J. Environ. Stud. 2016, 25, 1911–1920. [Google Scholar] [CrossRef]
- Berg, B. Decomposing litter; limit values; humus accumulation, locally and regionally. Appl. Soil Ecol. 2018, 123, 494–508. [Google Scholar] [CrossRef]
- Berg, B.; McClaugherty, C. Plant Litter. Decomposition, Humus Formation, Carbon Sequestration, 2nd ed.; Springer: Berlin, Germany, 2008; ISBN 978-3-540-74923-3. [Google Scholar]
- Bocock, K.L.; Gilbert, O.; Capstick, C.K.; Twinn, D.C.; Waid, J.S.; Woodman, M.J. Changes in Leaf Litter When Placed on the Surface of Soils with Contrasting Humus Types. J. Soil Sci. 1960, 11, 1–9. [Google Scholar] [CrossRef]
- Howard, D.M.; Howard, P.J.A. Effect of Species, Source of Litter, Type of Soil, and Climate on Litter Decomposition: Microbial Decomposition of Tree and Shrub Leaf Litter 3. Oikos 1980, 34, 115–124. [Google Scholar] [CrossRef]
- Smolander, A.; Kitunen, V. Soil microbial activities and characteristics of dissolved organic C and N in relation to tree species. Soil Biol. Biochem. 2002, 34, 651–660. [Google Scholar] [CrossRef]
- Meentemeyer, V.; Berg, B. Regional variation in rate of mass loss of Pinus sylvestris needle litter in Swedish pine forests as influenced by climate and litter quality. Scand. J. For. Res. 1986, 1, 167–180. [Google Scholar] [CrossRef]
- Berg, B.; Matzner, E. Effect of N deposition on decomposition of plant litter and soil organic matter in forest systems. Environ. Rev. 1997, 5, 1–25. [Google Scholar] [CrossRef]
- Zhang, W.; Gao, D.; Chen, Z.; Li, H.; Deng, J.; Qiao, W.; Han, X.; Yang, G.; Feng, Y.; Huang, J. Substrate quality and soil environmental conditions predict litter decomposition and drive soil nutrient dynamics following afforestation on the Loess Plateau of China. Geoderma 2018, 325, 152–161. [Google Scholar] [CrossRef]
- Mo, J.; Brown, S.; Xue, J.; Fang, Y.; Li, Z. Response of litter decomposition to simulated N deposition in disturbed, rehabilitated and mature forests in subtropical China. Plant Soil 2006, 282, 135–151. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).